Abstract
We previously characterized multiple interactions between chemokine and opioid G-protein coupled receptors (GPCR), and we found both µ and δ-opioid receptors cross-desensitize CCR1, CCR2, CCR5, but not CXCR4. Here we report that the κ-opioid receptor (KOR) is able to cross-desensitize CXCR4, and this phenomenon is bi-directional. Chemotactic responses by KOR activation are diminished with prior activation of CXCR4. Additionally, calcium mobilization assays show these cross-desensitization processes occur within seconds of receptor activation, and target receptor internalization is not responsible for desensitization between these receptors. These results have implications for several essential processes including neuronal and lymphocyte development, inflammatory responses, and pain/sensitivity.
Keywords: Chemokines, Inflammation, Neuroimmunology
1. INTRODUCTION
Opioids have roles in the central and peripheral nervous systems for the control of pain sensations. They also function as regulators of cellular and humoral immune responses (Carr, Rogers, & Weber 1996;McCarthy et al. 2001) and have been shown to regulate expression of chemokines as well as chemokine receptors (Steele, Henderson, & Rogers 2003a). The opioid receptors and chemokine receptors are both members of the seven transmembrane G-protein coupled receptor (GPCR) super-family. Three primary types of opioid receptors have been cloned and are designated δ, κ, and µ (Chen et al. 1993;Evans et al. 1992;Kieffer et al. 1992;Li et al. 1993;Yasuda et al. 1993). Chemokines and chemokine receptors are involved in leukocyte recruitment to sites of inflammation, and are also involved in neuronal development (Stumm & Hollt 2007;Stumm et al. 2003). Classification of the chemokine receptors, designated C, CC, CXC, or CX3C, is based on the location of amino-terminal cysteine residue(s) (Murphy et al. 2000).
CXCR4 has been under intense study as a participant in a number of disease-related processes angiogenesis and human immunodeficiency virus (HIV) infection by virtue of its role as a major HIV-1 co-receptor (Gerard & Rollins 2001;Hesselgesser et al. 1997;Moore, Trkola, & Dragic 1997;Proudfoot, Wells, & Clapham 1999). CXCR4 is widely expressed in many tissues and cells, including the brain (Baggiolini, Dewald, & Moser 1997;Feil & Augustin 1998;Hesselgesser et al. 1997;Ohtani et al. 1998;Tanabe et al. 1997;Volin et al. 1998). At the same time, the opioid receptors have been found on neuronal cells of the brain, as well on certain leukocytes (Belkowski et al. 1995;Bidlack, Saripalli, & Lawrence 1992;Carr et al. 1989;Chuang et al. 1994;Sedqi et al. 1995).
The desensitization of one GPCR following agonist binding by another unrelated receptor is termed heterologous-desensitization. This is accomplished via an intracellular signaling process involving Gαi proteins with the participation of a secondary messenger-dependent kinase (protein kinase A or protein kinase C) (Ali et al. 1999;Zhang et al. 2003). As a part of this process, target receptor signaling is inhibited, and in some cases, the target receptor becomes internalized (Ali et al. 1999;Lefkowitz 1998;Olbrich, Proudfoot, & Oppermann 1999). Whether the receptor is internalized or not, the final result is loss of receptor function. Analysis of the heterologous desensitization between several GPCRs suggests a hierarchy exists with respect to the susceptibility to cross-desensitization (Zhang et al. 2003). Moreover, it appears that receptors that are resistant to cross-desensitization are typically strong desensitizers of other GPCRs and receptors that are easily desensitized are typically weak desensitizers of other GPCRs.
Our laboratories, and others, have shown that several cross-desensitization interactions occur between opioid and chemokine receptors, based on studies carried out both in vitro and in vivo (Chen et al. 2007;Grimm et al. 1998b;Rogers et al. 2000;Szabo et al. 2002d;Zhang et al. 2003). For example, it is apparent that both µ- and δ-opioid receptors (MOR and DOR) are able to induce heterologous desensitization of CCR1, CCR2, CCR5, CXCR1, and CXCR2, but not CXCR4. On the other hand, CCR1, CCR2, CCR5, CCR7, CX3CR1, and CXCR4, but not CXCR1 or CXCR2, cross-desensitize MOR and DOR (Grimm et al. 1998;Honczarenko et al. 2002;Szabo et al. 2002;Szabo et al. 2003;Zhang et al. 2003). Based on these results we have suggested that MOR and DOR can be classified as “intermediate” in the cross-desensitization hierarchy, while CXCR4 appears to be relatively resistant to cross-desensitization (Szabo et al. 2002). Up to this point, little attention has been given to the cross-talk function of KOR, an opioid receptor which is known to exert significant effects on the function of the immune system (Alicea et al. 1996). Moreover, KOR agonists have been shown in several studies to exhibit effects on the immune response which are in opposition to effects induced by either MOR or DOR agonists (Alicea et al. 1996). For example, we have found that MOR appears to exert opposing effects on the expression of the critical chemokine receptors CCR5 and CXCR4 (Rogers & Peterson 2003). In general, the function of KOR appears to be strongly anti-inflammatory, in contrast with MOR, and for this reason we chose to examine the capacity of KOR agonists to participate in cross-talk interactions with chemokine receptors.
In the present study, we examine the potential cross-talk between CXCR4 and KOR using both in vitro and in vivo analyses. We have found that there is profound desensitization between CXCR4 and KOR, and the cross-talk is bi-directional. Despite the fact that CXCR4 and KOR are relatively resistant to cross-desensitization, these receptors have the capacity to induce substantial cross-desensitization. Cross-desensitization of the opioid and chemokine receptors represents a level of regulatory control, which is only now becoming appreciated.
2. MATERIALS AND METHODS
Chemicals
The selective KOR agonist Trans-(-)-3,4-Dichloro-N-methyl-N-[2-(1-pyrrolidinyl)cyclohexyl]benzeneacetamide hydrochloride (U50,488H) was purchased from Tocris Cookson Inc. (Ellisville, MO, USA) or obtained as a gift from the Upjohn Company, Kalamazoo, MI. 17,17’-(Dicyclopropylmethyl)-6,6’,7,7’-6,6’-imino-7,7’- binorphinan-3,4’,14,14’-tetrol dihydrochloride (nor-Binaltrophimine dihydrochloride [nor-BNI]) was purchased from Tocris Cookson Inc. (Ellisville, MO, USA). Drugs were dissolved in 0.9% saline. The bicyclam 1,1’-[1,4-phenilenebis(methilene)]bis-1,4,8,11-tetraazacyclotetradecane octahydrochloride (AMD3100) was purchased from Sigma- Aldrich (Saint Louis, MO, USA) and is a highly selective CXCR4 antagonist (De 2003). Recombinant Human CXCL12 purchased from PreproTech Inc. (Rocky Hill, NJ, USA). Dynorphin A (H-Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Ile-Arg-Pro-Lys-Leu-Lys-Trp-Asp-Asn-Gln-OH) was made by Multiple Peptide Systems, San Diego, CA. For in vivo studies, the chemokines CCL5 and CXCL12 were obtained from Biosource International, Camarillo, CA. These drugs were dissolved in the 1% Bovine Fraction V albumin (Sigma).
Cell line and culture
Neutrophils were obtained from the normal human donors by Percoll-purification of buffy coat cells. All work with human subjects was reviewed and approved by the Temple University Institutional Review Board (IRB). Jurkat T cells were transfected with the human KOR cDNA in the vector pcDNA3.zeo and the clonal cell line stably expressing KOR (designated J-KOR 8.2) were established under selection with Zeocin (Invitrogen, Carlsbad, CA). The expression of the opioid receptor was verified by reverse transcriptase-polymerase chain reaction (RT-PCR) and radiolabeled-binding analysis using [H3]-diprenorphine (Szabo et al. 2003). J-KOR were cultured in RPMI 1640 (Mediatech Cellgro, VA) supplemented with 10% fetal bovine serum (FBS) (HyClone, Logan UT), 2mM glutamine, 1mM sodium pyruvate, 25mM HEPES, 1% gentamicin and 200µM Zeocin. Murine 300.19 pre-B cells were co-transfected with pcDNA3-Neo-human-KOR-FLAG and SRα-puro-human-CXCR4, under G418 sulfate or puromycin selection, respectively. Cells were sorted into single cell double-positive (CXCR4+ and KOR+) populations and clonal populations were developed. 300.19 pre-B CXCR4+ KOR+ were cultured in RPMI 1640 (Mediatech Cellgro, VA) supplemented with 10% FBS, 2mM glutamine, 1X MEM Nonessential amino acids, 1mM sodium pyruvate, 1µL/mL 2-Mercaptoethanol, 1% gentamycin, 1.5µg/mL puromycin and 1mg/mL G418 sulfate.
Flow Cytometry
The presence of CXCR4 on J-KOR was detected using PE-conjugated mouse anti-human CD184 (CXCR4) and the PE-conjugated mouse IgG2a, κ monoclonal isotype control antibodies (BD Biosciences, San Diego, CA). CXCR4 surface expression was measured in 300.19 Pre-B CXCR4+ KOR+ cells using APC-conjugated mouse antihuman CD184 (CXCR4) and the APC-conjugated mouse IgG2a, κ monoclonal isotype control antibodies (BD Biosciences). KOR surface expression was analyzed using a primary anti-FLAG-biotin M2 antibody followed by a secondary streptavidin-PE conjugated antibody (Sigma). Cells for internalization assay were kept on ice though out all steps and centrifuged at 2°C to prevent receptor trafficking. Harvested cells were washed and resuspended in Dulbecco’s phosphate buffered saline (DPBS) containing 1% BSA, 10% goat serum was added to block unspecific binding and the cells were incubated for 30 min at 4°C. After centrifugation and cell pellet was resuspended and stained with antibodies for 45 min at 4°C in the dark, washed and analyzed in a FACS Calibur (Becton-Dickinson, NJ).
Chemotaxis
Chemotaxis was performed in a 48-well chambers using 5µm pore- free polycarbonate membranes (Neuro Probe, Gaithersburg, MD) pre-coated with 20µg/ml fibronectin (Sigma). Untreated and treated cells were resuspended in chemotaxis buffer (RPMI 1640, 1% BSA, 10mM HEPES) at a concentration of 2×106/ml. Migration of cells was allowed to proceed for 1h at 37°C and 5% CO2. Membrane was fixed and stained with a Diff-Quick kit (Fisher Diagnostics, Middletown, VA, USA) and the cells migrated were counted in five high-power fields at 400×-magnification.
Calcium Mobilization
300.19 pre-B CXCR4+ KOR+ cells were washed twice with calcium mobilization medium (1X Hank’s balanced salt solution (HBSS) with Ca2+ and Mg2+ (Mediatech, Inc. Herndon, VA), 10mM HEPES (pH 7.4), and 0.5% w/v BSA and resuspended at a concentration of 106 cells/ml in calcium mobilization medium. Background autofluorescence was subtracted from all readings using unlabeled cells, while remaining cells were labeled with 1µM fura-2 AM (Molecular Probes Eugene, Oregon) for 30 minutes. Cells were washed twice with calcium mobilization medium and resuspended again at 106 cells per ml and kept on ice. Four million cells were loaded into a 4ml plastic cuvette in addition to 1.6ml calcium mobilization medium. Cells were brought to 37°C inside the Fluoromax-3 Spectrofluorometer (HORIBA Jobin Yvon, Edison, NJ, USA) and measured at 510nm emission with 340nm and 380nm excitation to create a ratio of emission.
Animals
All work with experimental animals was reviewed and approved by the Temple University Institutional Animal Care and Use Committee (IACUC). Male Sprague-Dawley (SD) rats, weighing 175–200 g, were housed in groups of 3–4 for at least 1 week in an animal room maintained at 22±2°C and approximately 50% relative humidity. Lighting was on a 12/12 h light/dark cycle (lights on at 7:00 and off at 19:00). Rats were allowed free access to food and water. Rats were anesthetized with a mixture of ketamine hydrochloride (100–150 mg/kg) and acepromazine maleate (0.2 mg/kg). A cannula was implanted into the periaqueductal gray matter (PAG) using the following stereotaxic coordinates: AP: 0.6, R: 0.8, V:1, according to system A by Pellegrino and Cushman (Pellegrino & Cushman 1967), and fixed with dental cement. The animals were housed individually after surgery. Experiments began 1 week postoperatively. Each rat was used only once. At the end of the experiment, sites of injection were verified using microinjection of bromobenzene blue.
Surgery procedures
Rats were anesthetized with a mixture of ketamine hydrochloride (100–150 mg/kg) and acepromazine maleate (0.2 mg/kg). A sterilized stainless steel C313G cannula guide (22 gauge, Plastic One) was implanted into the lateral PAG and fixed with dental cement. The stereotaxic coordinates for PAG were as follows: bite bar 3.3 mm below 0, 7.8 mm posterior to bregma, 0.5 mm from midline and 5 mm ventral to the dura mater (Paxinos 1998). A C313DC cannula dummy (plastic one) of the identical length was inserted into the guide tube to prevent its occlusion. The animals were housed individually after surgery. Experiments began 1 week postoperatively. Each rat was used only once. At the end of the experiment, cannula placements were verified using microinjection of 1% bromobenzene blue according to the standard procedures in our laboratory (Xin et al. 1997).
Nociceptive test
The latency to flick the tail in cold water was used as the antinociceptive index, according to a standard procedure in our laboratory (Pizziketti et al. 1985). A 1:1 mix of ethylene glycol:water was maintained at −3°C with a circulating water bath (Model 9500, Fisher Scientific; Pittsburgh, PA). Rats were held over the bath with their tails submerged approximately half-way into the solution. All animals were tested at 60, 15 and 0 min before drug injection. For each animal, the first reading was discarded and the mean of the second and third readings was taken as the baseline value. Latencies to tail flick after injection were expressed as percentage change from baseline. The percent of maximal possible antinociception (MPA%) for each animal at each time was calculated using the formula: %MPA = [(test latency - baseline latency)/(60 - baseline latency)] × 100. A cut-off limit of 60 s was set to avoid damage to the tail.
Injections
Drugs injected into the PAG were given in a volume of 1.0 µl over a 30-second period. For subcutaneous injection, U50,488H or saline was given mid-dorsally in 10 seconds. Injection of AMD3100 was given 15 minutes before CXCL12 both at a concentration of 100ng/mL. Dynorphin was given 30 minutes after CXCL12.
Statistical analysis
The data are expressed as the mean and standard error. Statistical analysis of difference between groups was assessed with an analysis of variance (ANOVA) followed by Tukey's test. P ≤ 0.05 was taken as the significant level of difference.
3. RESULTS
Cross-desensitization of KOR- and CXCR4-induced chemotactic responses
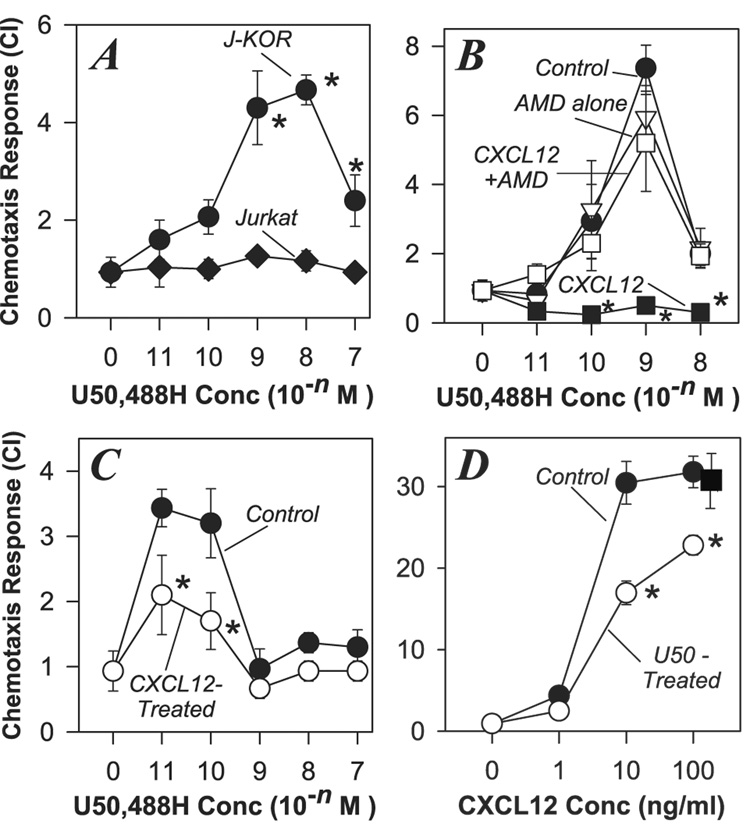
We have previously reported results which show that activation of CXCR4 cross-desensitizes both MOR and DOR, but neither MOR or DOR are able to desensitize CXCR4 (Grimm et al. 1998;Honczarenko et al. 2002;Rogers et al. 2000;Szabo et al. 2002;Szabo et al. 2003). We have also observed that MOR and KOR induce distinct influences on the immune system, and exert opposing effects on the expression of CXCR4 (Rogers & Peterson 2003). We exmined the capacity of KOR to participate in cross-talk interactions with CXCR4. In order to carry out these studies, we chose to examine the influence of CXCR4 activation with CXCL12 on the functional activity of KOR using Jurkat cells stably transfected to express KOR (J-KOR), in addition to the endogenous expression of CXCR4. Our results show that J-KOR cells possess fully functional KOR and CXCR4 receptors, based on the strong chemotactic responses of these cells to the highly selective KOR agonist U50,488H as well as CXCL12 (Figure 1A and 1B). In contrast, the parental (non-transfected) Jurkat cells failed to manifest a chemotactic response to U50,488H (Figure 1A). In an effort to assess potential cross-talk between these receptors, we carried out experiments in which the J-KOR cells were pretreated with CXCL12, and the chemotactic response of these cells to U50,488H was determined. The results show (Figure 1B) that CXCL12 pretreated cells fail to manifest a normal KOR chemotactic response. In contrast, pre-treatment of the J-KOR cells with the CXCR4 antagonist AMD3100, blocked the ability of CXCL12 to induce cross-desensitization of KOR (Figure 1B). We have verified these results in primary human neutrophils, an additional cell population that expresses both KOR and CXCR4 (Kulkarni-Narla, Walcheck, & Brown 2001;Loetscher et al. 1994). The results from these experiments (Figure 1C) show that pre-treatment of neutrophils with CXCL12 results in a significant reduction in the ability of these cells to mount a chemotaxis response to U50,488H. These results suggest that activation of CXCR4 leads to the inactivation or cross-desensitization of KOR.
FIGURE 1. Bi-directional desensitization of the chemotactic responses of CXCR4 and KOR.
(A). Parental Jurkat and KOR-transfected Jurkat (J-KOR) cells were subjected to chemotaxis analysis with the designated doses of U50,488H, and the chemotaxis response was determined. (B). J-KOR cells were either untreated (●) or pre-treated for 45 min with 5µM AMD3100 alone (Δ), or AMD3100 followed by 30 min with 10ng/ml CXCL12 (□), or 30 min with 10ng/ml CXCL12 alone (■). After the treatments J-KOR cells were washed and the chemotactic response to the designated concentrations of U50,488H was determined. (C). Fresh human neutrophils were either untreated or pretreated with with 10 ng/ml CXCL12 for 30 min, and the chemotactic response to U50,488H was determined. (D). J-KOR cells were either untreated (●), pre-treated with 100 nM U50,488H (○) for 30 min, or pre-treated with 100 nM norBNI for 30 min, followed by U50,488H for 30 min (■), and then the chemotactic response to CXCL12 was determined. In each case the migration was determined by assessing the number of cells per 5 high-power fields and the results are representative of 3 to 6 independent experiments (* = P < 0.05 for comparison with the control group).
We also used these cells to examine the influence of pre-treatment with U50,488H on the chemotactic response of the J-KOR cells to CXCL12. Our results showed that pre-treatment with U50,488H significantly reduced the ability of J-KOR cells to mount a CXCR4 chemotactic response (Figure 1D). Taken together, our results suggest that bi-directional cross-desensitization can be induced to regulate the functional activity of CXCR4 and KOR.
Proximal events in the bi-directonal cross-talk between CXCR4 and KOR
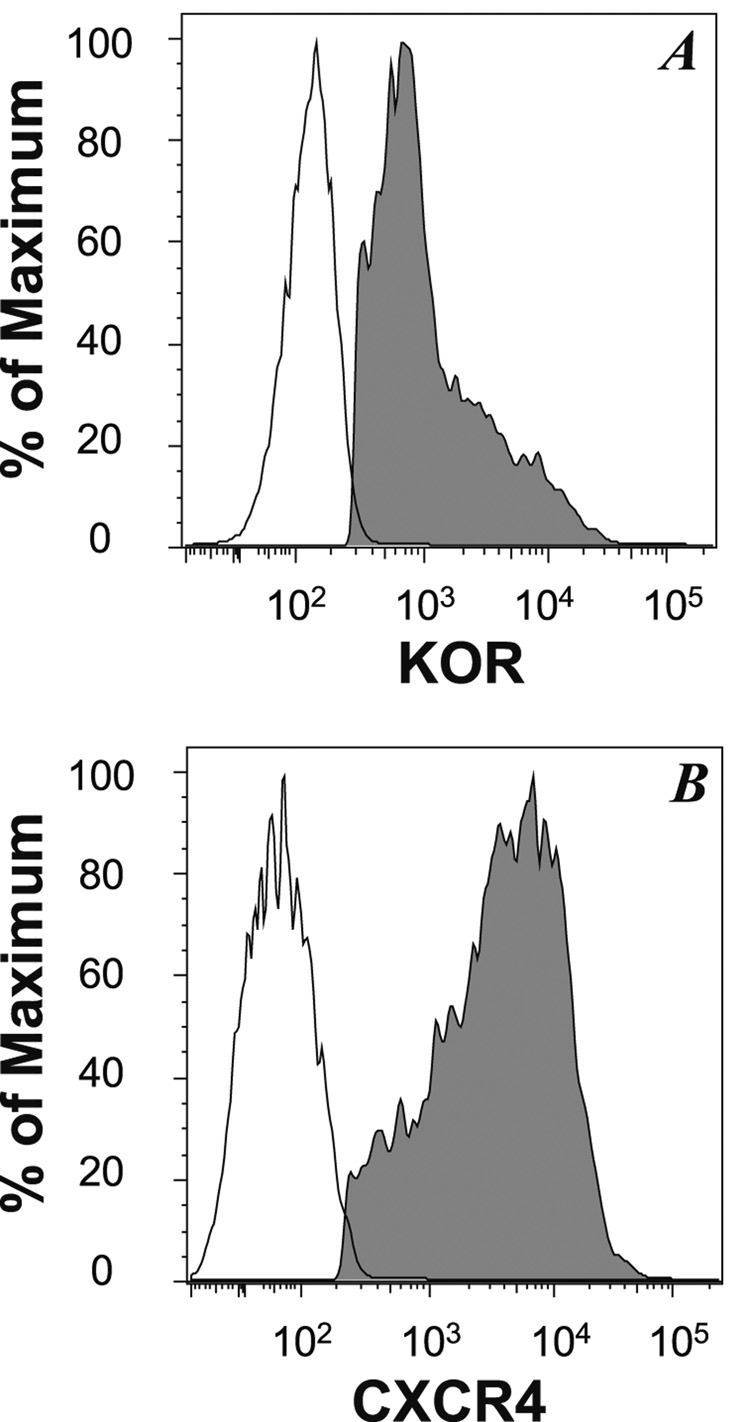
Because the chemotactic response is a process that requires a complex set of biochemical activities, including changes in adhesion molecule activity, cytoskeletal re-organization, and a number of other events, we wished to examine an early (proximal) event in the function of these receptors. We chose to determine the receptor-induced calcium mobilization for this purpose, because this is a relatively sensitive measure of receptor function. In order to carry out these studies, we chose to utilize 300.19 murine B cells stably transfected with human KOR and CXCR4 (designated 300.19.K2) because these receptors induce a relatively strong calcium response in this cell line. Flow cytometry analysis (Figure 2) of the 300.19.K2 cells confirms that both CXCR4 and KOR are expressed by virtually all of these cells.
FIGURE 2. 300.19.K2 cells express both human KOR and human CXCR4.
300.19.K2 cells were stained for the expression of human CXCR4 or human KOR (using anti-Flag monoclonal antibody), and the expression of these proteins was determined by flow cytometry. The fluorescent profile of unstained cells is also presented. The profile of cells stained with an isotype control antibody did not differ from the unstained control (data not shown). The data are presented as the % of maximum fluorescent intensity for each population of cells.
The 300.19.K2 cells, loaded with fura-2 AM, were stimulated with either the designated concentration of U50,488H or CXCL12, and following the first treatment to initiate cross-desensitization, cells were then re-stimulated with either CXCL12 or U50,488H. The magnitude of the calcium flux was calculated and compared to the control response to U50,488H alone or CXCL12 alone (Figure 3). Cells pre-treated with U50,488H at 1 µM, exhibited a very poor calcium response with subsequent CXCL12 administration, when compared to the calcium response to CXCL12 without the U50 pretreatment (Figure 3A). Similar results were observed with U50,488H at a 100 nM dose (Figure 3B). This effect is dose dependent in that pre-treatment with 10nM U50,488H exerted a much weaker inhibitory effect on the CXCL12-induced calcium response (Figure 3C). These results demonstrate that activation of KOR leads to desensitization of the calcium response activity of CXCR4.
FIGURE 3. Bi-directional cross-desensitization of the calcium mobilization response of 300.19.K2 cells.
(A–C). 300.19.K2 cells were either untreated (red tracing) or pre-stimulated (black tracing) with either 1 µM U50,488H (A), 100 nM U50,488H (B), or 10 nM U50,488H (C) followed by stimulation after 120 sec with 100 ng/ml CXCL12 was determined 120 sec later. (D–F). 300.19.K2 cells were either untreated (red tracing) or pre-treated (black tracing) with 100 ng/ml CXCL12 followed by stimulation after 120 sec with U50,488H at 1µM (D), or 100 nM (E), or 10 nM (F). (G). Data from panels A-C are presented as the maximal calcium mobilization versus baseline for the response to 100 ng/ml CXCL12 alone (●) or the 100 ng/ml CXCL12 response of cells pre-treated with the designated concentrations of U50,488H (○). (H). Data from panels D-E are presented as the maximal calcium mobilization versus baseline for the response to the designated U50,488H concentrations of non-pre-treated cells (●) or cells pre-treated with 100 ng/ml CXCL12 (○). In each case the calcium mobilization was measured by labeling cells with fura-2 AM and measuring the calcium response using a spectrofluorometer with oscillating excitation at 340nm and 380nm with the detector set at 510nm. Data are representative of 3 experiments.
Experiments were also carried out to test the effect of pre-treatment with CXCL12 on the calcium response of 300.19.K2 cells to U50,488H. The results (Figure 3D) show that pre-treatment with CXCL12 fails to alter the calcium response induced by subsequent U50,488H.at a relatively high dose (1 µM). However, the CXCL12 pretreatment exerted a much more substantial inhibitory effect on the response to lower doses of U50,488H (i.e., 100 nM [Figure 3E] and 10 nM [Figure 3F]). These results indicate that activation of CXCR4 leads to desensitization of the calcium response activity of KOR. A summary analysis of the bi-directional calcium mobilization data (Figure 3G) demonstrates the dose-dependent inhibitory effect of U50,488H on the CXCL12-induced calcium response. As the concentration of U50,488H pre-treatment dose increases, the inhibition of CXCR4 activity becomes more apparent. Conversely, the inhibition of KOR activity is most apparent at low doses of U50,488H, but is less apparent as the dose of the opioid increases (Figure 3H).
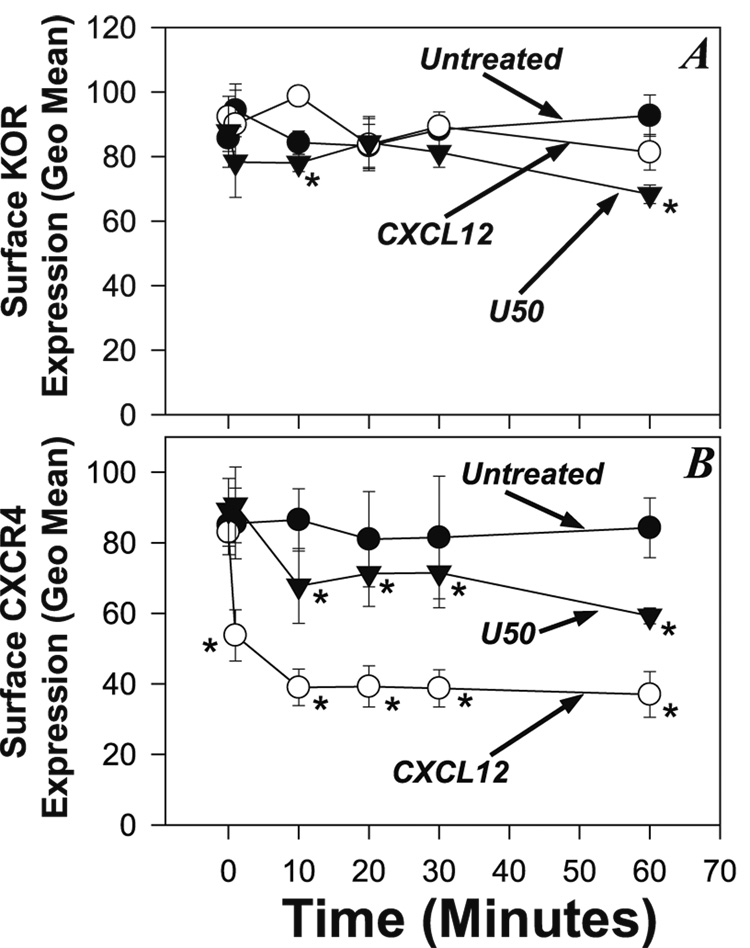
Receptor Internalization is not responsible for the decrease in calcium mobilization
We next considered the possibility that receptor internalization was responsible for bi-direction heterologous desensitization between CXCR4 and KOR. We used flow cytometry analysis to measure surface receptor levels following treatment of the 300.19.K2 cells. Cells were treated with either CXCL12 (100ng/ml) or U50,488H (1µM), and the expression of KOR (Figure 4A) and CXCR4 (Figure 4B) was determined over a period of 60 minutes. Our results show that treatment with CXCL12 induces a reduction of KOR expression at 60 min which is approximately 15% (Figure 4A). This reduction in surface expression is unlikely to provide the basis for the reduced responsiveness of these cells to U50,488H following the CXCL12 Pre-treatment. The control treatment in which the 300.19.K2 cells were treated with U50,488H exhibit a reduction in KOR surface expression which reached approximately 30% by 60 minutes.
FIGURE 4. Analysis of internalization of CXCR4 and KOR during cross-desensitization.
(A) Analysis of surface expression of KOR at the designated times of untreated 300.19.K2 cells, or cells treated with either 1µM U50,488H or 100ng/ml CXCL12. KOR expression was determined by staining with anti-Flag monoclonal antibody and flow cytometry, and are expressed as the geometric mean of the stained cells. (B) Analysis of surface expression of CXCR4 at the designated times of untreated 300.19.K2 cells, or cells treated with either 1µM U50,488H or 100ng/ml CXCL12. CXCR4 expression was determined by flow cytometry and are expressed as the geometric mean of stained cells. (* = P < 0.05 for comparison with the control group.)
Conversely, our results (Figure 4B) show that treatment of the 300.19.K2 cells with U50,488H induced a decrease in CXCR4 expression of approximately 18% by 30 minutes, and approximately 30% by 60 minutes. Here again, this reduction in surface CXCR4 expression is unlikely to account for the magnitude of the cross-inhibition of CXCR4 function described above for both chemotactic activity and calcium responses. The control treatment, in which the cells were treated with CXCL12, show a much greater decrease in surface receptor expression, in which the receptor levels drop by over 50% by 60 minutes (Figure 4B). These results confirm that receptor internalization is unlikely to account for the heterologous desensitization between KOR and CXCR4.
Analgesia induced by in vivo administration of Dynorphin blocked by pretreatment with CXCL12
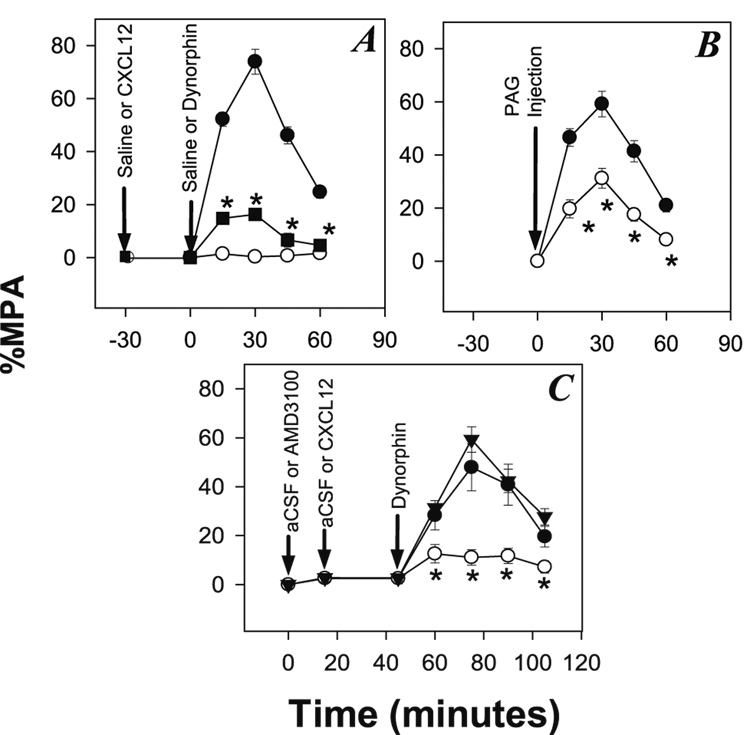
If the cross-desensitization of KOR function by CXCL12 demonstrated above is physiologically relevant, we anticipated that CXCL12 would cross-desensitize the analgesic function of KOR in vivo. We carried out experiments in which CXCL12 was administered into the brain, and the analgesic activity of KOR was determined. Rats were canulated in the PAG matter (an area of the brain that is important for opioid analgesic activity), and were given an injection of CXCL12 or saline 30 minutes before a subsequent injection in the PAG of dynorphin, and endogenous KOR agonist (20 µg). Animals given an initial injection of saline exhibited a substantial analgesic response to a subsequent injection of dynorphin (figure 5A). In contrast, intra-PAG injection of CXCL12, exhibited a significantly reduced the antinociceptive response to dynorphin. As a control, the administration of CXCL12 alone failed to induce any detectable analgesic activity.
FIGURE 5. Desensitization of KOR by administration of CXCL12.
(A) Animals were pre-treated with either CXCL12 (100 ng) or saline in the PAG followed at 30 min (t = 0) by treatment with either dynorphin (20 µg) or saline, and the tail flick response was measured at the designated times up to an additional 60 min. Results are shown for animals treated with saline followed by dynorphin (●), CXCL12 followed by saline (○), and CXCL12 followed by dynorphin (■). N=6–7. (B) Animals were treated either with 20 µg dynorphin (●) or the simultaneous treatment with 20 µg dynorphin and 100ng CXCL12 (○) and the tail flick response was measured at the designated times. N=11–12. (C) Animals were pre-treated with aCSF, CXCL12 (100ng), or AMD 3100 (100ng) in the PAG followed at 30 seconds (15 seconds for AMD 3100) with dynorphin (20ug) or CXCL12 (100ng) and dynorphin (20ug). Results are shown for animal treated with dynorphin (●), CXCL12 and dynorphin (○), and AMD 3100, CXCL12, and dynorphin (▼). N = 6–8, * = P < 0.05 for comparison with the control group.
We also carried out experiments to determine the effect of simultaneous administration of CXCL12 and dynorphin in the PAG, since this more closely represents the normal physiological situation in vivo. The results (Figure 5B) show that animals treated with both CXCL12 and dynorphin exhibit a significantly weaker analgesic response when compared to treatment with dynorphin alone. CXCL12 activation within 17 the PAG results in a greatly reduced KOR response to dynorphin. Furthermore, pretreatment of the PAG with AMD 3100 demonstrated that the effects of CXCL12 injection can be blocked by a competitive CXCR4 antagonist within the brain (Figure 5C). These results suggest that the activation of CXCR4 with CXCL12 induces desensitization of KOR, and this leads to an impairment of analgesic activity in vivo.
4. DISCUSSION
We report results here which show that the chemokine receptor, CXCR4, is regulated by cross-talk with KOR. This result is apparent using either primary neutrophils, or stably-transfected cell lines. This finding is somewhat surprising given previous findings from this laboratory, and others, which showed that both MOR and DOR are able to cross-desensitize CCR1, CCR2, CCR5, CXCR1, CXCR2, but not CXCR4 (Grimm et al. 1998;Szabo et al. 2003;Zhang et al. 2004). This set of results illustrate the selective nature of heterologous desensitization, and are consistent with the notion that a hierarchy among GPCRs exists with respect to the strength of cross-desensitization, and this appears to be inversely correlated with sensitivity to the heterologous desensitization (Ali et al. 1999). Given that KOR, but not MOR or DOR, can cross-desensitize CXCR4, it would appear that CXCR4 is a relatively strongdesensitizer, but is difficult to be cross-desensitized (Steele et al. 2002). The degree of inhibition of CXCR4 at the level of chemotaxis was found to be only partial, and would be consistent with the notion that CXCR4 is relatively resistant to cross-desensitization. However, it should be noted that CXCR4 has been shown to be susceptible to cross-desensitization by both the high-affinity formyl peptide receptor (FPR) and the formyl peptide receptor-like 1 receptor in studies with human monocyte-derived macrophages, CD4+ T cells, as well as stably-transfected cell lines (Deng et al. 1999;Li et al. 2001). Furthermore, CXCL8 induces cross-desensitization of both CCR5 and CXCR4, and the cross-talk in this case is mediated selectively by CXCR1 but not CXCR2 (Richardson et al. 2003), And finally, bi-directional heterologous desensitization between CCR5 and CXCR4 has also been observed in human T cells, and leads to the inhibition of cellular signaling, cellular adhesion, and chemotaxis driven by these receptors (Hecht et al. 2003).
Our current results are the first to show that CXCR4 is able to cross-desensitize KOR, and this effect is apparent at the level of chemotaxis, the calcium mobilization response, and in vivo, at the level of analgesia. These results are not entirely unexpected since our previous studies demonstrated that both MOR and DOR are desensitized by CXCR4, and these effects were apparent both in vitro and in vivo (Szabo et al. 2002). Given the well documented observation that CXCR4 is widely expressed by neurons in the brain, and that KOR is abundantly expressed in the PAG, there would appear to be ample opportunity for these receptors to interact on neurons at this anatomical site. Indeed, the juxtaposition of these receptors in the brain, has significant implications for potential cross-regulation between these receptors given the well established expression of the endogenous ligands for these receptors in this tissue. For example, CXCL12 is produced in the adult and developing brain, and is abundant in the cortex, hypothalamus, and hippocampus (Tham et al. 2001), and prominently by granule neurons of both the dentate gyrus and entorhinal cortex (Tran et al. 2007;Tran & Miller 2003). Conversely, the endogenous KOR agonist is expressed most abundantly in the brain in the nucleus acccumbens and caudate nucleus, but also at relatively high levels in the amygdale and hypothalamus.
The cross-desensitization of CXCR4 by KOR was accompanied by the internalization of approximately 40% of the target receptor from the cell surface. This result is consistent with data showing that FPR-induced cross-desensitizion of CXCR4 was accompanied by significant internalization in monocytes (Deng et al. 1999). In contrast, administration of CXCL12 itself induced internalization of over 60% of the CXCR4 from the surface, a finding which is in agreement with the degree of internalization induced by homologous desensitization of this receptor (Richardson et al. 2003). The internalization of CXCR4 by KOR is almost certainly a part of the mechanism of down-regulation of the functional activity of this receptor. However, the KOR-induced reduction in surface expression of CXCR4 is unlikely to account for all of the loss of CXCR4 function, since the calcium mobilization response to CXCL12 is almost entirely lost following activation of KOR with as little as 100 nM U50,488H. This would suggest that a substantial portion of the CXCR4 which remains on the cell surface has lost functional activity. These results also suggest that the activation of KOR targets CXCR4 in two distinct ways; i.e., first, through the activation of an undefined internalization pathway, and second, through a process in which target receptor is retained on the outer membrane but in a dysfunctional state.
In contrast to the results just described, the cross-desensitization of KOR by CXCR4 is not accompanied by detectable internalization. Control experiments in which cells were treated with U50,488H, show that approximately 30% of the receptors are internalized by this homologous desensitization process, a result which is very consistent with published results (Li et al. 2003). While the chemotaxis data suggest that activation of CXCR4 induces a very high degree of desensitization of KOR, the calcium mobilization analysis showed that the loss of KOR function was most apparent at lower doses of U50,488H. This suggests that the degree of cross-desensitization of KOR is “partial,” and would be in agreement with our hypothesis that KOR is a receptor which is actually relatively resistant to cross-desensitization. However, our in vivo studies demonstrate that KOR activity in the brain is substantially down-regulated following activation of CXCR4. Perhaps most importantly, we would suggest that the loss of functional activity of KOR in response to concentrations of U50,488H in the range of 10 nM is more important since this is more likely to represent a physiological environment.
The cross-desensitization of KOR by CXCR4 would be expected to occur in conditions where the levels of expression of either CXCL12 or CXCR4 occur. Both CXCL12 and CXCR4 are upregulated in certain inflammatory states, including rheumatoid arthritis and allergic airway disease (Buckley et al. 2000;Gonzalo et al. 2000;Nanki et al. 2000). Transforming growth factor-β1 (TGFβ1) is a potent inducer of CXCR4, and IL-1 is an effective inducer of both CXCR4 and CXCL12 (Buckley et al. 2000;Eddleston, Christiansen, & Zuraw 2002). Moreover, we have previously shown that MOR agonists induce the expression of CXCR4 by both monocytes and T cells (Steele, Henderson, & Rogers 2003), suggesting the possibility of a circuit in which the expression of MOR peptides may induce CXCR4, and in turn, promote cross-desensitization of KOR. However, it is most likely that inflammatory states in which CXCL12 and/or CXCR4 are up-regulated are most likely to favor the cross-desensitization of KOR. The desensitization of KOR would increase the sensitivity to pain, and contribute to the association of pain with the inflammatory response.
We believe that our results have several important implications for developmental and homeostatic processes in the brain. Targeted disruption in mice of either CXCL12 or CXCR4 is lethal at a relatively late stage of embryogenesis, and these animals display a number of developmental defects which affect cardiovascular, gastrointestinal, central nervous system, and hematopoietic systems (Ma et al. 1998;Zou et al. 1998). The observation that both CXCL12- and CXCR4-deficient mice exhibit essentially identical phenotypes has been considered evidence that the sole receptor for CXCL12 is CXCR4, and conversely, that CXCR4 is only activated by this chemokine. However, recent reports show that both CXCR2 and CXCR4 are functional receptors for the cytokine macrophage migration inhibitory factor (Bernhagen et al. 2007), and conversely the orphan GPCR designated RDC1 (now tentatively designated CXCR7) is an alternative receptor for CXCL12 (Balabanian et al. 2005). Nevertheless, there appears to be little doubt that the primary receptor and ligand pair is CXCR4 and CXCL12. In animals deficient in either gene, the nervous system, the development of the cerebellum, hippocampal dentate gyrus, cortex, and dorsal root ganglia are all substantially abnormal, and trafficking and proliferation of oligodendrocyte progenitors are significantly depressed (Bagri et al. 2002;Dziembowska et al. 2005;Stumm et al. 2003;Zou et al. 1998). During the first two post-natal weeks the cerebellar granule precursor cells proliferate, and then migrate to form the internal granule cell layer composed of mature granule neurons. This process is dependent on the Purkinje neuron secreted protein Sonic hedgehog, and requires the participation of CXCL12 and CXCR4 (Klein et al. 2001). The expression of KOR by neurons in this region provides the opportunity for cross-regulation with CXCR4 during this process. Those conditions which lead to elevated dynorphin expression would be expected to have the potential to down-regulate the proliferation and migration of the granule cells during this critical step. While current evidence suggests that dynorphin expression is constitutive in the nervous sytem, there is evidence that preprodynorphin mRNA expression is inducible through cAMP responsive elements in the preprodynorphin promoter (Messersmith et al. 1994). Furthermore, there is evidence inflammatory responses in the periphery are accompanied by an accumulation of dynorphin A at both the inflammatory site and in the spinal cord (Hassan et al. 1992;Ruda et al. 1988), and interleukin 1 induces the production of dynorphin A at sites of inflammation (Schafer, Carter, & Stein 1994). Neuroinflammatory conditions would create an environment in which elevated dynorphin would be capable of exerting greater cross-desensitization of CXCR4, potentially disrupting normal neuronal development.
The cross-desensitization of CXCR4 by KOR has a number of implications for the function of the acquired immune and inflammatory response. For example, it is well established that CXCR4 plays a central role in the accumulation of T cells in the synovium during rheumatoid arthritis (Buckley et al. 2000;Nanki et al. 2000). We would suggest that activation of KOR at the site of an inflammatory response would act as a dampening influence to reduce the intensity of the inflammation directed by CXCR4. It is likely that the overall influence of KOR agonist expression would be both at the localized site of inflammation (where dynorphin levels are likely to be elevated), but also potentially at a more systemic level in which there would be the prospect of inhibition of normal immune cell development and homeostasis. The overall potential effect of KOR activation would be to provide a broadly activating anti-inflammatory stimulus to the immune system. Of course, the therapeutic implications of these effects are very intriguing.
ACKNOWLEDGEMENTS
We thank Dr. Jodene Moore for assistance with flow cytometry and cell sorting.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
The authors have no financial conflict of interest.
This work was supported by the National Institutes of Health grants DA06650 (To M.W.A.), DA16544 (To T.J.R.), DA13429 (To M.W.A.), and DA14230 (To T.J.R.).
Abbreviations used in this paper: FPR, formyl peptide receptor; GPCR, G proteincoupled receptor; KOR, κ-opioid receptor; MPA%, percent maximal possible antinociception; nor-BNI, nor-binaltrophimine dihydrochloride; PAG, periaqueductal gray matter; U50,488H, trans-3,4-dichloro-N-methyl-N-[7-(1-pyrroliidinyl) cyclohexyl] benzene-acetamide methanesulfonate.
References
- Ali H, Richardson RM, Haribabu B, Snyderman R. Chemoattractant receptor cross-desensitization. Journal of Biological Chemistry. 1999a;vol. 274(no 10):6027–6030. doi: 10.1074/jbc.274.10.6027. [Review] [52 refs] [DOI] [PubMed] [Google Scholar]
- Ali H, Richardson RM, Haribabu B, Snyderman R. Chemoattractant receptor cross-desensitization. Journal of Biological Chemistry. 1999c;vol. 274(no 10):6027–6030. doi: 10.1074/jbc.274.10.6027. [Review] [52 refs] [DOI] [PubMed] [Google Scholar]
- Ali H, Richardson RM, Haribabu B, Snyderman R. Chemoattractant receptor cross-desensitization. Journal of Biological Chemistry. 1999b;vol. 274(no 10):6027–6030. doi: 10.1074/jbc.274.10.6027. [Review] [52 refs] [DOI] [PubMed] [Google Scholar]
- Alicea C, Belkowski S, Eisenstein TK, Adler MW, Rogers TJ. Inhibition of primary murine macrophage cytokine production in vitro following treatment with the kappa-opioid agonist U50,488H. Journal of Neuroimmunology. 1996a;vol. 64(no 1):83–90. doi: 10.1016/0165-5728(95)00159-x. [DOI] [PubMed] [Google Scholar]
- Alicea C, Belkowski S, Eisenstein TK, Adler MW, Rogers TJ. Inhibition of primary murine macrophage cytokine production in vitro following treatment with the kappa-opioid agonist U50,488H. Journal of Neuroimmunology. 1996b;vol. 64(no 1):83–90. doi: 10.1016/0165-5728(95)00159-x. [DOI] [PubMed] [Google Scholar]
- Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annual Review of Immunology. 1997;vol. 15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [Review] [181 refs] [DOI] [PubMed] [Google Scholar]
- Bagri A, Gurney T, He X, Zou YR, Littman DR, Tessier-Lavigne M, Pleasure SJ. The chemokine SDF1 regulates migration of dentate granule cells. Development. 2002;vol. 129(no 18):4249–4260. doi: 10.1242/dev.129.18.4249. [DOI] [PubMed] [Google Scholar]
- Balabanian K, Lagane B, Infantino S, Chow KY, Harriague J, Moepps B, renzana-Seisdedos F, Thelen M, Bachelerie F. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. Journal of Biological Chemistry. 2005;vol. 280(no 42):35760–35766. doi: 10.1074/jbc.M508234200. [DOI] [PubMed] [Google Scholar]
- Belkowski SM, Zhu J, Liu-Chen LY, Eisenstein TK, Adler MW, Rogers TJ. Sequence of kappa-opioid receptor cDNA in the R1.1 thymoma cell line. Journal of Neuroimmunology. 1995;vol. 62(no 1):113–117. doi: 10.1016/0165-5728(95)00116-j. [DOI] [PubMed] [Google Scholar]
- Bernhagen J, Krohn R, Lue H, Gregory JL, Zernecke A, Koenen RR, Dewor M, Georgiev I, Schober A, Leng L, Kooistra T, Fingerle-Rowson G, Ghezzi P, Kleemann R, McColl SR, Bucala R, Hickey MJ, Weber C. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat.Med. 2007;vol. 13(no 5):587–596. doi: 10.1038/nm1567. [DOI] [PubMed] [Google Scholar]
- Bidlack JM, Saripalli LD, Lawrence DM. kappa-Opioid binding sites on a murine lymphoma cell line. European Journal of Pharmacology. 1992;vol. 227(no 3):257–265. doi: 10.1016/0922-4106(92)90003-e. [DOI] [PubMed] [Google Scholar]
- Buckley CD, Amft N, Bradfield PF, Pilling D, Ross E, Arenzana-Seisdedos F, Amara A, Curnow SJ, Lord JM, Scheel-Toellner D, Salmon M. Persistent induction of the chemokine receptor CXCR4 by TGF-beta 1 on synovial T cells contributes to their accumulation within the rheumatoid synovium. Journal of Immunology. 2000c;vol. 165(no 6):3423–3429. doi: 10.4049/jimmunol.165.6.3423. [DOI] [PubMed] [Google Scholar]
- Buckley CD, Amft N, Bradfield PF, Pilling D, Ross E, Arenzana-Seisdedos F, Amara A, Curnow SJ, Lord JM, Scheel-Toellner D, Salmon M. Persistent induction of the chemokine receptor CXCR4 by TGF-beta 1 on synovial T cells contributes to their accumulation within the rheumatoid synovium. Journal of Immunology. 2000b;vol. 165(no 6):3423–3429. doi: 10.4049/jimmunol.165.6.3423. [DOI] [PubMed] [Google Scholar]
- Buckley CD, Amft N, Bradfield PF, Ross E, Arenzana-Seisdedos F, Amara A, Curnow SJ, Lord JM, Scheel-Toellner D, Salmon M. Persistent induction of the chemokine receptor CXCR4 by TGF-beta 1 on synovial T cells contributes to their accumulation within the rheumatoid synovium. Journal of Immunology. 2000a;vol. 165(no 6):3423–3429. doi: 10.4049/jimmunol.165.6.3423. [DOI] [PubMed] [Google Scholar]
- Carr DJ, DeCosta BR, Kim CH, Jacobson AE, Guarcello V, Rice KC, Blalock JE. Opioid receptors on cells of the immune system: evidence for delta- and kappa-classes. Journal of Endocrinology. 1989;vol. 122(no 1):161–168. doi: 10.1677/joe.0.1220161. [DOI] [PubMed] [Google Scholar]
- Carr DJ, Rogers TJ, Weber RJ. The relevance of opioids and opioid receptor on immunocompetence and immune homeostasis. Proceedings of the Society for Experimental Biology & Medicine. 1996;vol. 213(no 3):248–257. doi: 10.3181/00379727-213-44056. [Review] [97 refs] [DOI] [PubMed] [Google Scholar]
- Chen X, Geller EB, Rogers TJ, Adler MW. Rapid heterologous desensitization of antinociceptive activity between mu or delta opioid receptors and chemokine receptors in rats. Drug Alcohol Depend. 2007;vol. 88(no 1):36–41. doi: 10.1016/j.drugalcdep.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Mestek A, Liu J, Hurley JA, Yu L. Molecular cloning and functional expression of a mu-opioid receptor from rat brain. Molecular Pharmacology. 1993;vol. 44(no 1):8–12. [PubMed] [Google Scholar]
- Chuang LF, Chuang TK, Killam KF, Jr, Chuang AJ, Kung HF, Yu L, Chuang RY. Delta opioid receptor gene expression in lymphocytes. Biochemical & Biophysical Research Communications. 1994;vol. 202(no 3):1291–1299. doi: 10.1006/bbrc.1994.2071. [DOI] [PubMed] [Google Scholar]
- De CE. The bicyclam AMD3100 story. Nature Reviews. 2003;vol. Drug Discovery. 2(no 7):581–587. doi: 10.1038/nrd1134. [Review] [50 refs] [DOI] [PubMed] [Google Scholar]
- Deng X, Ueda H, Su SB, Gong W, Dunlop NM, Gao JL, Murphy PM, Wang JM. A synthetic peptide derived from human immunodeficiency virus type 1 gp120 downregulates the expression and function of chemokine receptors CCR5 and CXCR4 in monocytes by activating the 7-transmembrane G-protein-coupled receptor FPRL1/LXA4R. Blood. 1999b;vol. 94(no 4):1165–1173. [PubMed] [Google Scholar]
- Deng X, Ueda H, Su SB, Gong W, Dunlop NM, Gao JL, Murphy PM, Wang JM. A synthetic peptide derived from human immunodeficiency virus type 1 gp120 downregulates the expression and function of chemokine receptors CCR5 and CXCR4 in monocytes by activating the 7-transmembrane G-protein-coupled receptor FPRL1/LXA4R. Blood. 1999a;vol. 94(no 4):1165–1173. [PubMed] [Google Scholar]
- Dziembowska M, Tham TN, Lau P, Vitry S, Lazarini F, Dubois-Dalcq M. A role for CXCR4 signaling in survival and migration of neural and oligodendrocyte precursors. Glia. 2005;vol. 50(no 3):258–269. doi: 10.1002/glia.20170. [DOI] [PubMed] [Google Scholar]
- Eddleston J, Christiansen SC, Zuraw BL. Functional expression of the C-X-C chemokine receptor CXCR4 by human bronchial epithelial cells: regulation by proinflammatory mediators. Journal of Immunology. 2002;vol. 169(no 11):6445–6451. doi: 10.4049/jimmunol.169.11.6445. [DOI] [PubMed] [Google Scholar]
- Evans CJ, Keith D, Jr, Morrison H, Magendzo K, Edwards RH. Cloning of a delta opioid receptor by functional expression. Science. 1992;vol. 258(no 5090):1952–1955. doi: 10.1126/science.1335167. [see comments] [DOI] [PubMed] [Google Scholar]
- Feil C, Augustin HG. Endothelial cells differentially express functional CXC-chemokine receptor-4 (CXCR-4/fusin) under the control of autocrine activity and exogenous cytokines. Biochemical & Biophysical Research Communications. 1998;vol. 247(no 1):38–45. doi: 10.1006/bbrc.1998.8499. [DOI] [PubMed] [Google Scholar]
- Gerard C, Rollins BJ. Chemokines and disease. Nature Immunology. 2001;vol. 2(no 2):108–115. doi: 10.1038/84209. [Review] [118 refs] [DOI] [PubMed] [Google Scholar]
- Gonzalo JA, Lloyd CM, Peled A, Delaney T, Coyle AJ, Gutierrez-Ramos JC. Critical involvement of the chemotactic axis CXCR4/stromal cell-derived factor-1 alpha in the inflammatory component of allergic airway disease. Journal of Immunology. 2000;vol. 165(no 1):499–508. doi: 10.4049/jimmunol.165.1.499. [DOI] [PubMed] [Google Scholar]
- Grimm MC, Ben Baruch A, Taub DD, Howard OM, Resau JH, Wang JM, Ali H, Richardson R, Snyderman R, Oppenheim JJ. Opiates transdeactivate chemokine receptors: delta and mu opiate receptor-mediated heterologous desensitization. Journal of Experimental Medicine. 1998c;vol. 188(no 2):317–325. doi: 10.1084/jem.188.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm MC, Ben Baruch A, Taub DD, Howard OM, Resau JH, Wang JM, Ali H, Richardson R, Snyderman R, Oppenheim JJ. Opiates transdeactivate chemokine receptors: delta and mu opiate receptor-mediated heterologous. Journal of Experimental Medicine. 1998d;vol. 188(no 2):317–325. doi: 10.1084/jem.188.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm MC, Ben Baruch A, Taub DD, Howard OM, Resau JH, Wang JM, Ali H, Richardson R, Snyderman R, Oppenheim JJ. Opiates transdeactivate chemokine receptors: delta and mu opiate receptor-mediated heterologous desensitization. Journal of Experimental Medicine. 1998a;vol. 188(no 2):317–325. doi: 10.1084/jem.188.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm MC, Ben Baruch A, Taub DD, Howard OM, Resau JH, Wang JM, Ali H, Richardson R, Snyderman R, Oppenheim JJ. Opiates transdeactivate chemokine receptors: delta and mu opiate receptor-mediated heterologous Desensitization. Journal of Experimental Medicine. 1998b;vol. 188(no 2):317–325. doi: 10.1084/jem.188.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan AH, Pzewlocki R, Herz A, Stein C. Dynorphin, a preferential ligand for kappa-opioid receptors, is present in nerve fibers and immune cells within inflamed tissue of the rat. Neuroscience Letters. 1992;vol. 140(no 1):85–88. doi: 10.1016/0304-3940(92)90688-4. [DOI] [PubMed] [Google Scholar]
- Hecht I, Cahalon L, Hershkoviz R, Lahat A, Franitza S, Lider O. Heterologous desensitization of T cell functions by CCR5 and CXCR4 ligands: inhibition of cellular signaling, adhesion and chemotaxis. International Immunology. 2003;vol. 15(no 1):29–38. doi: 10.1093/intimm/dxg002. [DOI] [PubMed] [Google Scholar]
- Hesselgesser J, Halks-Miller M, DelVecchio V, Peiper SC, Hoxie J, Kolson DL, Taub D, Horuk R. CD4-independent association between HIV-1 gp120 and CXCR4: functional chemokine receptors are expressed in human neurons. Current Biology. 1997b;vol. 7(no 2):112–121. doi: 10.1016/s0960-9822(06)00055-8. [DOI] [PubMed] [Google Scholar]
- Hesselgesser J, Halks-Miller M, DelVecchio V, Peiper SC, Hoxie J, Kolson DL, Taub D, Horuk R. CD4-independent association between HIV-1 gp120 and CXCR4: functional chemokine receptors are expressed in human neurons. Curren Biology. 1997a;vol. 7(no 2):112–121. doi: 10.1016/s0960-9822(06)00055-8. [DOI] [PubMed] [Google Scholar]
- Honczarenko M, Le Y, Glodek AM, Majka M, Campbell JJ, Ratajczak MZ, Silberstein LE. CCR5-binding chemokines modulate CXCL12 (SDF-1)-induced responses of progenitor B cells in human bone marrow through heterologous desensitization of the CXCR4 chemokine receptor. Blood. 2002;vol. 100(no 7):2321–2329. doi: 10.1182/blood-2002-01-0248. [DOI] [PubMed] [Google Scholar]
- Honczarenko M, Le Y, Glodek AM, Majka M, Campbell JJ, Ratajczak MZ, Silberstein LE. CCR5-binding chemokines modulate CXCL12 (SDF-1)-induced responses of progenitor B cells in human bone marrow through heterologous desensitization of the CXCR4 chemokine receptor. Blood. 2002b;vol. 100(no 7):2321–2329. doi: 10.1182/blood-2002-01-0248. [DOI] [PubMed] [Google Scholar]
- Kieffer BL, Befort K, Gaveriaux-Ruff C, Hirth CG. The delta-opioid receptor: isolation of a cDNA by expression cloning and pharmacological characterization. Proceedings of the National Academy of Sciences of the United States of America. 1992;vol. 89(no 24):12048–12052. doi: 10.1073/pnas.89.24.12048. [published erratum appears in Proc Natl Acad Sci U S A 1994 Feb 1;91(3):1193] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RS, Rubin JB, Gibson HD, DeHaan EN, Alvarez-Hernandez X, Segal RA, Luster AD. SDF-1 alpha induces chemotaxis and enhances Sonic hedgehog-induced proliferation of cerebellar granule cells. Development - Supplement. 2001;vol. 128(no 11):1971–1981. doi: 10.1242/dev.128.11.1971. [DOI] [PubMed] [Google Scholar]
- Kulkarni-Narla A, Walcheck B, Brown DR. Opioid receptors on bone marrow neutrophils modulate chemotaxis and CD11b/CD18 expression. European Journal of Pharmacology. 2001;vol. 414(no 2–3):289–294. doi: 10.1016/s0014-2999(01)00727-0. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ. G protein-coupled receptors. III. New roles for receptor kinases and beta-arrestins in receptor signaling and desensitization. Journal of Biological Chemistry. 1998;vol. 273(no 30):18677–18680. doi: 10.1074/jbc.273.30.18677. [Review] [47 refs] [DOI] [PubMed] [Google Scholar]
- Li BQ, Wetzel MA, Mikovits JA, Henderson EE, Rogers TJ, Gong W, Le Y, Ruscetti FW, Wang JM. The synthetic peptide WKYMVm attenuates the function of the chemokine receptors CCR5 and CXCR4 through activation of formyl peptide receptor-like 1. Blood. 2001;vol. 97(no 10):2941–2947. doi: 10.1182/blood.v97.10.2941. [DOI] [PubMed] [Google Scholar]
- Li JG, Zhang F, Jin XL, Liu-Chen LY. Differential regulation of the human kappa opioid receptor by agonists: etorphine and levorphanol reduced dynorphin A- and U50,488H-induced internalization and phosphorylation. J.Pharmacol.Exp.Ther. 2003;vol. 305(no 2):531–540. doi: 10.1124/jpet.102.045559. [DOI] [PubMed] [Google Scholar]
- Li S, Zhu J, Chen C, Chen YW, Deriel JK, Ashby B, Liu-Chen LY. Molecular cloning and expression of a rat kappa opioid receptor. Biochemical Journal. 1993;vol. 295(no Pt 3):629–633. doi: 10.1042/bj2950629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loetscher M, Geiser T, O'Reilly T, Zwahlen R, Baggiolini M, Moser B. Cloning of a human seven-transmembrane domain receptor, LESTR, that is highly expressed in leukocytes. Journal of Biological Chemistry. 1994;vol. 269(no 1):232–237. [PubMed] [Google Scholar]
- Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 1998;vol. 95(no 16):9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy L, Wetzel M, Sliker JK, Eisenstein TK, Rogers TJ. Opioids, opioid receptors, and the immune response. Drug & Alcohol Dependence. 2001;vol. 62(no 2):111–123. doi: 10.1016/s0376-8716(00)00181-2. [Review] [128 refs] [DOI] [PubMed] [Google Scholar]
- Messersmith DJ, Gu J, Dubner R, Douglass J, Iadarola MJ. Basal and inducible transcriptional activity of an upstream AP-1/CRE element (DYNCRE3) in the prodynorphin promoter. Mol.Cell Neurosci. 1994;vol. 5(no 3):238–245. doi: 10.1006/mcne.1994.1028. [DOI] [PubMed] [Google Scholar]
- Moore JP, Trkola A, Dragic T. Co-receptors for HIV-1 entry. Current Opinion in Immunology. 1997;vol. 9(no 4):551–562. doi: 10.1016/s0952-7915(97)80110-0. [see comments]. [Review] [93 refs] [DOI] [PubMed] [Google Scholar]
- Murphy PM, Baggiolini M, Charo IF, Hebert CA, Horuk R, Matsushima K, Miller LH, Oppenheim JJ, Power CA. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacological Reviews. 2000;vol. 52(no 1):145–176. [Review] [520 refs] [PubMed] [Google Scholar]
- Nanki T, Hayashida K, El Gabalawy HS, Suson S, Shi K, Girschick HJ, Yavuz S, Lipsky PE. Stromal cell-derived factor-1-CXC chemokine receptor 4 interactions play a central role in CD4+ T cell accumulation in rheumatoid arthritis synovium. Journal of Immunology. 2000a;vol. 165(no 11):6590–6598. doi: 10.4049/jimmunol.165.11.6590. [DOI] [PubMed] [Google Scholar]
- Nanki T, Hayashida K, El Gabalawy HS, Suson S, Shi K, Girschick HJ, Yavuz S, Lipsky PE. Stromal cell-derived factor-1-CXC chemokine receptor 4 interactions play a central role in CD4+ T cell accumulation in rheumatoid arthritis synovium. Journal of Immunology. 2000b;vol. 165(no 11):6590–6598. doi: 10.4049/jimmunol.165.11.6590. [DOI] [PubMed] [Google Scholar]
- Ohtani Y, Minami M, Kawaguchi N, Nishiyori A, Yamamoto J, Takami S, Satoh M. Expression of stromal cell-derived factor-1 and CXCR4 chemokine receptor mRNAs in cultured rat glial and neuronal cells. Neuroscience Letters. 1998;vol. 249(no 2–3):163–166. doi: 10.1016/s0304-3940(98)00425-x. [DOI] [PubMed] [Google Scholar]
- Olbrich H, Proudfoot AE, Oppermann M. Chemokine-induced phosphorylation of CC chemokine receptor 5 (CCR5) Journal of Leukocyte Biology. 1999;vol. 65(no 3):281–285. doi: 10.1002/jlb.65.3.281. [Review] [36 refs] [DOI] [PubMed] [Google Scholar]
- Paxinos GaWC. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1998. Ref Type: Generic. [Google Scholar]
- Pellegrino LJ, Cushman AJ. A stereotaxic atlas of the rat brain. New York: Appleton-Century-Crofts; 1967. Ref Type: Generic. [Google Scholar]
- Pizziketti RJ, Pressman NS, Geller EB, Cowan A, Adler MW. Rat cold water tail-flick: a novel analgesic test that distinguishes opioid agonists from mixed agonist-antagonists. European Journal of Pharmacology. 1985;vol. 119(no 1–2):23–29. doi: 10.1016/0014-2999(85)90317-6. [DOI] [PubMed] [Google Scholar]
- Proudfoot AE, Wells TN, Clapham PR. Chemokine receptors-future therapeutic targets for HIV? Biochemical Pharmacology. 1999;vol. 57(no 5):451–463. doi: 10.1016/s0006-2952(98)00339-6. [Review] [123 refs] [DOI] [PubMed] [Google Scholar]
- Richardson RM, Tokunaga K, Marjoram R, Sata T, Snyderman R. Interleukin-8-mediated heterologous receptor internalization provides resistance to HIV-1 infectivity. Role of signal strength and receptor desensitization. Journal of Biological Chemistry. 2003a;vol. 278(no 18):15867–15873. doi: 10.1074/jbc.M211745200. [DOI] [PubMed] [Google Scholar]
- Richardson RM, Tokunaga K, Marjoram R, Sata T, Snyderman R. Interleukin-8-mediated heterologous receptor internalization provides resistance to HIV-1 infectivity. Role of signal strength and receptor desensitization. Journal of Biological Chemistry. 2003b;vol. 278(no 18):15867–15873. doi: 10.1074/jbc.M211745200. [DOI] [PubMed] [Google Scholar]
- Rogers TJ, Peterson PK. Opioid G protein-coupled receptors: signals at the crossroads of inflammation. Trends in Immunology. 2003b;vol. 24(no 3):116–121. doi: 10.1016/s1471-4906(03)00003-6. [Review] [43 refs] [DOI] [PubMed] [Google Scholar]
- Rogers TJ, Peterson PK. Opioid G protein-coupled receptors: signals at the crossroads of inflammation. Trends in Immunology. 2003a;vol. 24(no 3):116–121. doi: 10.1016/s1471-4906(03)00003-6. [Review] [43 refs] [DOI] [PubMed] [Google Scholar]
- Rogers TJ, Steele AD, Howard OM, Oppenheim JJ. Bidirectional heterologous desensitization of opioid and chemokine receptors. Annals of the New York Academy of Sciences. 2000;vol. 917:19–28. doi: 10.1111/j.1749-6632.2000.tb05369.x. [Review] [51 refs] [DOI] [PubMed] [Google Scholar]
- Ruda MA, Iadarola MJ, Cohen LV, Young WS., III In situ hybridization histochemistry and immunocytochemistry reveal an increase in spinal dynorphin biosynthesis in a rat model of peripheral inflammation and hyperalgesia. Proc.Natl.Acad.Sci.U.S.A. 1988;vol. 85(no 2):622–626. doi: 10.1073/pnas.85.2.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer M, Carter L, Stein C. Interleukin 1 beta and corticotropin-releasing factor inhibit pain by releasing opioids from immune cells in inflamed tissue. Proc.Natl.Acad.Sci.U.S.A. 1994;vol. 91(no 10):4219–4223. doi: 10.1073/pnas.91.10.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedqi M, Roy S, Ramakrishnan S, Elde R, Loh HH. Complementary DNA cloning of a mu-opioid receptor from rat peritoneal macrophages. Biochemical & Biophysical Research Communications. 1995;vol. 209(no 2):563–574. doi: 10.1006/bbrc.1995.1538. [DOI] [PubMed] [Google Scholar]
- Steele AD, Henderson EE, Rogers TJ. Mu-opioid modulation of HIV-1 coreceptor expression and HIV-1 replication. Virology. 2003b;vol. 309(no 1):99–107. doi: 10.1016/s0042-6822(03)00015-1. [DOI] [PubMed] [Google Scholar]
- Steele AD, Henderson EE, Rogers TJ. Mu-opioid modulation of HIV-1 coreceptor expression and HIV-1 replication. Virology. 2003a;vol. 309(no 1):99–107. doi: 10.1016/s0042-6822(03)00015-1. [DOI] [PubMed] [Google Scholar]
- Steele AD, Szabo I, Bednar F, Rogers TJ. Interactions between opioid and chemokine receptors: heterologous desensitization. Cytokine & Growth Factor Reviews. 2002;vol. 13(no 3):209–222. doi: 10.1016/s1359-6101(02)00007-2. [Review] [142 refs] [DOI] [PubMed] [Google Scholar]
- Stumm R, Hollt V. CXC chemokine receptor 4 regulates neuronal migration and axonal pathfinding in the developing nervous system: implications for neuronal regeneration in the adult brain. J.Mol.Endocrinol. 2007;vol. 38(no 3):377–382. doi: 10.1677/JME-06-0032. [DOI] [PubMed] [Google Scholar]
- Stumm RK, Zhou C, Ara T, Lazarini F, Dubois-Dalcq M, Nagasawa T, Hollt V, Schulz S. CXCR4 regulates interneuron migration in the developing neocortex. Journal of Neuroscience. 2003a;vol. 23(no 12):5123–5130. doi: 10.1523/JNEUROSCI.23-12-05123.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumm RK, Zhou C, Ara T, Lazarini F, Dubois-Dalcq M, Nagasawa T, Hollt V, Schulz S. CXCR4 regulates interneuron migration in the developing neocortex. Journal of Neuroscience. 2003b;vol. 23(no 12):5123–5130. doi: 10.1523/JNEUROSCI.23-12-05123.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo I, Chen XH, Xin L, Adler MW, Howard OM, Oppenheim JJ, Rogers TJ. Heterologous desensitization of opioid receptors by chemokines inhibits chemotaxis and enhances the perception of pain. Proceedings of the National Academy of Sciences of the United States of America. 2002e;vol. 99(no 16):10276–10281. doi: 10.1073/pnas.102327699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo I, Chen XH, Xin L, Adler MW, Howard OM, Oppenheim JJ, Rogers TJ. Heterologous desensitization of opioid receptors by chemokines inhibits chemotaxis and enhances the perception of pain. Proceedings of the National Academy of Sciences of the United States of America. 2002a;vol. 99(no 16):10276–10281. doi: 10.1073/pnas.102327699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo I, Chen XH, Xin L, Adler MW, Howard OM, Oppenheim JJ, Rogers TJ. Heterologous desensitization of opioid receptors by chemokines inhibits chemotaxis and enhances the perception of pain. Proceedings of the National Academy of Sciences of the United States of America. 2002b;vol. 99(no 16):10276–10281. doi: 10.1073/pnas.102327699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo I, Chen XH, Xin L, Adler MW, Howard OM, Oppenheim JJ, Rogers TJ. Heterologous desensitization of opioid receptors by chemokines inhibits chemotaxis and enhances the perception of pain. Proceedings of the National Academy of Sciences of the United States of America. 2002c;vol. 99(no 16):10276–10281. doi: 10.1073/pnas.102327699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo I, Chen XH, Xin L, Adler MW, Howard OM, Oppenheim JJ, Rogers TJ. Heterologous desensitization of opioid receptors by chemokines inhibits chemotaxis and enhances the perception of pain. Proceedings of the National Academy of Sciences of the United States of America. 2002d;vol. 99(no 16):10276–10281. doi: 10.1073/pnas.102327699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo I, Wetzel MA, Zhang N, Steele AD, Kaminsky DE, Chen C, Liu-Chen LY, Bednar F, Henderson EE, Howard OM, Oppenheim JJ, Rogers TJ. Selective inactivation of CCR5 and decreased infectivity of R5 HIV-1 strains mediated by opioid-induced heterologous desensitization. Journal of Leukocyte Biology. 2003d;vol. 74(no 6):1074–1082. doi: 10.1189/jlb.0203067. [DOI] [PubMed] [Google Scholar]
- Szabo I, Wetzel MA, Zhang N, Steele AD, Kaminsky DE, Chen C, Liu-Chen LY, Bednar F, Henderson EE, Howard OM, Oppenheim JJ, Rogers TJ. Selective inactivation of CCR5 and decreased infectivity of R5 HIV-1 strains mediated by opioid-induced heterologous desensitization. Journal of Leukocyte Biology. 2003a;vol. 74(no 6):1074–1082. doi: 10.1189/jlb.0203067. [DOI] [PubMed] [Google Scholar]
- Szabo I, Wetzel MA, Zhang N, Steele AD, Kaminsky DE, Chen C, Liu-Chen LY, Bednar F, Henderson EE, Howard OM, Oppenheim JJ, Rogers TJ. Selective inactivation of CCR5 and decreased infectivity of R5 HIV-1 strains mediated by opioid-induced heterologous desensitization. Journal of Leukocyte Biology. 2003b;vol. 74(no 6):1074–1082. doi: 10.1189/jlb.0203067. [DOI] [PubMed] [Google Scholar]
- Szabo I, Wetzel MA, Zhang N, Steele AD, Kaminsky DE, Chen C, Liu-Chen LY, Bednar F, Henderson EE, Howard OM, Oppenheim JJ, Rogers TJ. Selective inactivation of CCR5 and decreased infectivity of R5 HIV-1 strains mediated by opioid-induced heterologous desensitization. Journal of Leukocyte Biology. 2003c;vol. 74(no 6):1074–1082. doi: 10.1189/jlb.0203067. [DOI] [PubMed] [Google Scholar]
- Tanabe S, Heesen M, Yoshizawa I, Berman MA, Luo Y, Bleul CC, Springer TA, Okuda K, Gerard N, Dorf ME. Functional expression of the CXC-chemokine receptor-4/fusin on mouse microglial cells and astrocytes. Journal of Immunology. 1997;vol. 159(no 2):905–911. [PubMed] [Google Scholar]
- Tham TN, Lazarini F, Franceschini IA, Lachapelle F, Amara A, Dubois-Dalcq M. Developmental pattern of expression of the alpha chemokine stromal cell-derived factor 1 in the rat central nervous system. European Journal of Neuroscience. 2001;vol. 13(no 5):845–856. doi: 10.1046/j.0953-816x.2000.01451.x. [DOI] [PubMed] [Google Scholar]
- Tran PB, Banisadr G, Ren D, Chenn A, Miller RJ. Chemokine receptor expression by neural progenitor cells in neurogenic regions of mouse brain. J.Comp Neurol. 2007;vol. 500(no 6):1007–1033. doi: 10.1002/cne.21229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran PB, Miller RJ. Chemokine receptors: signposts to brain development and disease. Nature Reviews Neuroscience. 2003;vol. 4(no 6):444–455. doi: 10.1038/nrn1116. [Review] [126 refs] [DOI] [PubMed] [Google Scholar]
- Volin MV, Joseph L, Shockley MS, Davies PF. Chemokine receptor CXCR4 expression in endothelium. Biochemical & Biophysical Research Communications. 1998;vol. 242(no 1):46–53. doi: 10.1006/bbrc.1997.7890. [DOI] [PubMed] [Google Scholar]
- Xin L, Geller EB, Liu-Chen LY, Chen C, Adler MW. Substance P release in the rat periaqueductal gray and preoptic anterior hypothalamus after noxious cold stimulation: effect of selective mu and kappa opioid agonists. Journal of Pharmacology & Experimental Therapeutics. 1997;vol. 282(no 2):1055–1063. [PubMed] [Google Scholar]
- Yasuda K, Raynor K, Kong H, Breder CD, Takeda J, Reisine T, Bell GI. Cloning and functional comparison of kappa and delta opioid receptors from mouse brain. Proceedings of the National Academy of Sciences of the United States of America. 1993;vol. 90(no 14):6736–6740. doi: 10.1073/pnas.90.14.6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Hodge D, Rogers TJ, Oppenheim JJ. Ca2+-independent protein kinase Cs mediate heterologous desensitization of leukocyte chemokine receptors by opioid receptors. Journal of Biological Chemistry. 2003a;vol. 278(no 15):12729–12736. doi: 10.1074/jbc.M300430200. [DOI] [PubMed] [Google Scholar]
- Zhang N, Hodge D, Rogers TJ, Oppenheim JJ. Ca2+-independent protein kinase Cs mediate heterologous desensitization of leukocyte chemokine receptors by opioid receptors. Journal of Biological Chemistry. 2003b;vol. 278(no 15):12729–12736. doi: 10.1074/jbc.M300430200. [DOI] [PubMed] [Google Scholar]
- Zhang N, Hodge D, Rogers TJ, Oppenheim JJ. Ca2+-independent protein kinase Cs mediate heterologous desensitization of leukocyte chemokine receptors by opioid receptors. Journal of Biological Chemistry. 2003c;vol. 278(no 15):12729–12736. doi: 10.1074/jbc.M300430200. [DOI] [PubMed] [Google Scholar]
- Zhang N, Hodge D, Rogers TJ, Oppenheim JJ. Ca2+-independent protein kinase Cs mediate heterologous desensitization of leukocyte chemokine receptors by opioid receptors. Journal of Biological Chemistry. 2003d;vol. 278(no 15):12729–12736. doi: 10.1074/jbc.M300430200. [DOI] [PubMed] [Google Scholar]
- Zhang N, Rogers TJ, Caterina M, Oppenheim JJ. Proinflammatory chemokines, such as C-C chemokine ligand 3, desensitize mu-opioid receptors on dorsal root ganglia neurons. Journal of Immunology. 2004;vol. 173(no 1):594–599. doi: 10.4049/jimmunol.173.1.594. [DOI] [PubMed] [Google Scholar]
- Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998b;vol. 393(no 6685):595–599. doi: 10.1038/31269. [see comments] [DOI] [PubMed] [Google Scholar]
- Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998a;vol. 393(no 6685):595–599. doi: 10.1038/31269. [see comments] [DOI] [PubMed] [Google Scholar]