Abstract
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) remain important sources of morbidity for patients in the ICUs in the developed world. However, imagine having as a therapeutic tool, the ability to regulate, in a tissue specific manner, the expression of a given gene. RNA interference, as potentially such a method of selectively suppressing protein expression, has evolved as an important tool in the study of gene specific function and targeted therapeutics. Significant progress has been made in identifying potential gene targets integral to the pathways leading to the development of inflammation-associated lung injury. This review will discuss the progress, thus far, in the application of in vivo RNA interference-based gene therapy in the investigation of inflammation-associated lung injury.
Keywords: Acute lung injury; inflammation; RNA interference; siRNA, gene silencing
Introduction
Imagine having as a therapeutic tool, the ability to regulate, in a tissue specific manner, the expression of a given gene. This appears to be the potential for RNA interference (RNAi) based therapeutics. As a treatment for inflammation related lung injury, the possible targets for gene silencing are numerous (Table 1). One such potential therapeutic target is acute lung injury (ALI); a common cause of mortality in critically ill patients. This progressive syndrome can arise from a number of different initial insults, including hemorrhagic shock, sepsis, trauma, aspiration and bacterial infections. However, irrespective of the origin, a similar pattern of clinical pathology is observed; loss of lung barrier function, accumulation of activated neutrophils, and pulmonary edema among others. Disrupting the pathways contributing to this pathology has been a focus of RNAi research since its discovery in 1998 [1]. This review will highlight some of the progress made in this undertaking, specifically in reference to its delivery in vivo into the lung.
Table 1.
siRNA used in the treatment of inflammation associated lung injury
| siRNA Target gene | Findings |
|---|---|
| Heme oxygenase-1 (HO-1) delivered intranasal (naked) | Suppression of HO-1 increased apoptosis in lung tissue, suggesting a protective role for HO-1 in response to lung injury [25] |
| Macrophage inflammatory protein-2 (MIP-2) delivered intratracheal (naked) | Suppression of MIP-2 following hemorrhagic shock decreased indices of acute lung injury subsequent to asecondary septic challenge [22] |
| Fas ligand (FasL) delivered intratracheal (naked) | Suppression of FasL following hemorrhagic shock reduced lung epithelial cell apoptosis, inflammation & neutrophil influx [18] |
| Angiogenic growth factor-2 (Ang-2) delivered intranasal (naked) | Suppression of Ang-2 reduced epithelial necrosis, pulmonary edema and BAL cellularity following hypoxia induced lung injury [29] |
| Discoidin domain receptor-1 (DDR-1) delivered transnasal (naked) | Suppression of DDR-1 in bleomycin infected mice reduced lung tissue levels of MIP-1a, MIP-2, TGF-b and MCP-1 mRNA and macrophage, neutrophils and lymphocytes in bronchoalveolar lavage fluid [31] |
| Epithelial sodium channel (ENaC) delivered intratrachel (naked) | Suppression of EnaC-dependent sodium transport reduced alveolar fluid clearance in rats with acid-induced lung injury [35] |
| Cavelin-1 delivered intravenous tailvein (liposomal) | Suppression of caveolin-1 increased microvascular permeability to albumin correlating with dilation of interendothelial junctions thus pointing to a role for caveolin-1 in the maintenance of fluid balance, important in lung inflammation [38] |
| C5A receptor (C5aR) delivered intratracheal (siRNA-expressing adenovirus) | C5aR was suppressed of in the lungs of septic mice following C5aR siRNA-expressing adenovirus administration reducing the presence of a potent pro-inflammatory protein and the injury associated with its presence in the lungs of septic mice [39] |
| Respiratory syncytial virus RNA-polymersase protein (RSV-P) delivered intranasal (naked | Suppression of RSV-P inhibited virus replication in the lungs of RSV infected mice. Currently in human clinical trials [50] |
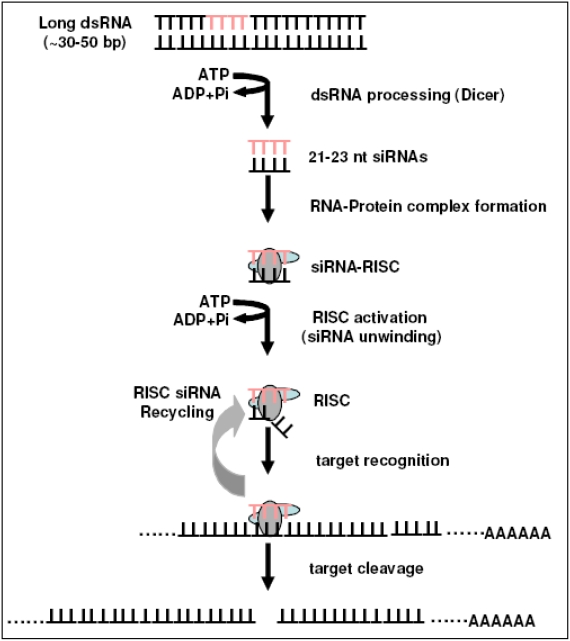
RNAi is an evolutionary conserved mechanism utilized by many organisms in the maintenance of genomic stability and in the regulation of endogenous gene expression, viral double stranded RNA, for example [2-4]. Andrew Fire and colleagues, in experiments performed in the nematode, C. elegans [1] described the mechanism and potential for RNAi in 1998. The phenomenon they reported, anti-sense RNA associated interference of a gene's expression, has now been investigated on both the molecular and cellular levels [5-11]. Small interference (si) RNAs are the products of enzymatic cleavage of long dsRNAs by a RNase III ribonuclease, Dicer [12,13]. The anti-sense strand of the siRNA incorporates into a RNAinduced silencing complex (RISC). The complex then initiates the targeting of complementary mRNA resulting in its cleavage and degradation and the functional silencing of the gene [14,15]. This mechanism (see Figure 1) presents a ready system for geneticists and medical researchers for the study of specific gene function and ultimately treatment for, through gene therapy, many life threatening diseases. However, numerous issues need to be resolved before siRNA represents a viable clinical therapy. These issues, sequence stability, dosing, experimental controls and routes of administration will be touched on briefly in this review.
Figure 1.
Overview of the primary steps in the RNA interference pathway. Initially Dicer-RNase III-like enzyme cleaves dsRNA into siRNA. siRNAs are then incorporated into RNA-Induced Silencing Complexes (RISCs) and unwinds. The RISC probes subsequently target RNAmediated cleavage of complementary mRNA inducing gene specific silencing.
Initial gene silencing experiments used immortalized cell lines and cultured primary cells to explore the efficacy of siRNA. Exploring a potential therapy for atherosclerotic diseases, focusing on the inhibition of leukocyte adhesion, Nishiwaki et al. used siRNA to suppress Eselectin in human umbilical vein endothelial cells [16]. As a tool to investigate the mechanism of inflammatory response in the lung epithelium, Puttagunta et al. silenced the protein tyrosine kinase, Syk, in cultured respiratory epithelial cells [17].
As research has moved beyond cell culture experimentation, into in vivo models of siRNA directed gene silencing, issues such as tissue and cell specific delivery and sequence stability have confronted scientists. The lung has presented a unique specimen in this respect. The airway epithelia are exposed to inhaled pathogens, irritants, and trauma that serve to initiate an inflammatory response. In the regulation of lung inflammation, these epithelial cells produce pro-inflammatory mediators and up-regulate adhesion molecules, potentiating inflammation related lung injury [18,19]. In addition, the lung endothelium is stimulated by systemic inflammatory mediators and in response release cytokines that serve to modulate adhesion molecules that direct the migration of leukocytes from the blood into the lung tissue [20-22].
Reports of systemic in vivo use of siRNA to silence gene expression were published as early as 2002. McCaffrey et al. showed that the systemic administration of hepatitis B virus siRNA inhibited virus replication in mouse liver [23]. In early 2003, Song et al. targeted Fas protein in mouse hepatocytes and effectively prevented hepatitis induced liver injury in these mice [24].
In late 2003, Zhang and colleagues published the first organ specific siRNA experiments in the lung [25]. In this study mice were given naked siRNA targeting heme oxygenase-1 (HO-1) (an enzyme that catalyzes the breakdown of heme) via intranasal administration prior to ischemia reperfusion (I-R) injury. HO-1 is induced in response to injury and is believed to be anti-apoptotic [26, 27]. Zhang et al. were able to demonstrate that, counter to the anti-apoptotic effects observed with HO-1 over expression, HO-1 gene silencing with siRNA significantly increased apoptosis in mouse lung tissue. These findings highlighted the anti-apoptotic properties of HO-1 during I-R injury and demonstrated that siRNA could be effectively delivered by inhalation [25]. In 2006, using siRNA, this group also demonstrated a protective role for HO-1 in oxidant-induced lung injury [28]. However, these studies did not address the extent to which siRNA delivery was restricted to the lungs or the cell population(s) taking up the siRNA.
SiRNA specific investigations into the cells and mediators contributing to All appeared in early 2005. Our laboratory sought to decrease neutrophil migration to the lung in order to reduce neutrophil associated lung tissue injury. We were able to demonstrate that targeting neutrophil chemotactic protein, macrophage inflammatory protein-2 (MIP-2), with intratracheally (i.t.) delivered naked siRNA, following hemorrhagic shock, significantly reduced neutrophil influx into the lung and decreased indices of All subsequent to a secondary septic challenge [22]. Control experiments for this study using mice that express green fluorescent protein (GFP) in all dividing cells, verified the lung-restricted uptake of i.t. administration using GFP siRNA [22]. Perl et al., in the same model of hemorrhagic shock and sepsis, showed a significant reduction in pulmonary cell apoptosis, inflammation and neutrophil influx when Fas ligand, but not caspase-8 siRNA was administered i.t. following hemorrhage [18]. In addition, the cells targeted by the fluorescently labeled siRNA were demonstrated, by co-localization with fluorescently labeled cytokeratin-18 staining, to be pulmonary epithelial cells [18].
The role of angiogenic growth factor, angiopoietin (Ang)2, in the pathogenesis of hyperoxia induced acute lung injury in mice, was explored by Bhandari et al. [29]. Ang2 plays a role in angiogenesis and vascular remodeling by inducing vascular destabilization to promote vascular matrix formation [30]. However, Bhandari et al. found significant decrease in hypoxia induced inflammation and lung tissue injury in Ang2 deficient mice compared to wild type [29]. To further assess this finding and to rule out confounding abnormalities associated with the Ang2 mutant, naked siRNA against Ang2 was administered intranasally prior to and 48 hours following exposure to very high concentrations of oxygen. In conjunction with a significant suppression of Ang2 expression in lung tissue, a reduction in epithelial necrosis, pulmonary edema and broncheoalveolar lavage fluid cellularity was observed [29]. These findings implicate Ang2 in the pathogenesis of All in response to hyperoxia while also presenting evidence supporting the use of siRNA as a potential therapeutic.
In a bleomycin-induced model of lung inflammation, Matsuyama and colleagues describe a role for the receptor tyrosine kinase, discoidin domain receptor (DDR)l in the upregulation of pro-inflammatory cytokines and chemokines in the lung [31]. Previously described by Matsuyama et al., DDR1 is constitutively expressed in organ tissue cells and is activated in the lung by binding to collagen in the extracellular matrix [32]. To investigate a role for DDR1 in the development of pulmonary inflammation, DDR1 siRNA was administered transnasally every 3 days for 12 days, following intratracheal administration of bleomycin. Western analysis showed DDR1 expression to be lung specific and significantly suppressed following the first administration of siRNA, continuing for at least 10 days after last administration [31]. MlP-la, MIP-2, TGF-_ and MCP-1 mRNA in lung tissue was decreased as were the number of macrophages, neutrophils and lymphocytes in broncheoalveolar lavage fluid [31], thus demonstrating the capacity of transnasal administration of DDR1 to reduce bleomycin associated lung inflammation in mice.
The failure to maintain fluid balance in the lungs of patients with All can result in flooding of the alveolar air spaces, impaired gas exchange and mortality [33,34]. Guidot et al., in 2006, investigated the role of epithelial sodium channel (ENaC)-dependent sodium transport in alveolar fluid clearance in rats with acid-induced lung injury [35]. Intratracheal administration of ENaC siRNA attenuated the sensitivity of these channels to clear alveolar fluid, thus pointing to their involvement in the maintenance of fluid balance in All [35].
Related to fluid balance regulation, Miyawaki-Shimizu et al. investigated the role of caveolin-1 (a membrane protein that makes up caveolae, subcellular organelles found in lung vascular endothelial cells) in the regulation of vascular permeability [36,37]. Liposomal encapsulated caveolin-1 siRNA was delivered via intravenous (i.v.) tailvein injection in mice. Lung microvascular permeability to albumin was found to increase 48 hours post siRNA treatment, this correlated with a decrease in expression of caveolin-1 in mouse lung lysates [38]. In addition, electron micrographs from caveolin-1 suppressed mouse lung showed a decrease in the number of caveolae and dilation of inter-endothelial junctions; vascular permeability was restored to normal levels 8 days post siRNA treatment [38].
While most of the published studies to date, assessing siRNA as a potential therapeutic or to highlight a specific gene's function, administer either naked (lung specific) or liposomal (systemic delivery) siRNA preparations, Sun and colleagues designed a siRNA-expressing adenovirus to express siRNA against complement protein anaphylatoxin, C5a receptor, C5aR [39]. The interaction of C5a with its receptor induces a potent inflammatory response that has been implicated in the pathogenesis of inflammation related injury and disease [40-46]. Sun et al. found that intratracheal administration of adenovirus-mediated C5aR siRNA for four days, prior to cecal ligation and puncture (CLP) induced sepsis, suppressed expression of C5aR in the lungs of treated mice 24 hours following CLP [39].
Presently, one of the most commercially successful studies exploring siRNA gene therapy in the lung was conducted by Bitko et al. Respiratory Syncytial Virus (RSV) is a viral infection of the lung most commonly diagnosed in infants. Earlier attempts at prophylaxis were complicated when RSV denatured-protein vaccines were found to potentiate the disease [47] and inhibitors of the viral fusion protein failed in part due to rapid mutations in the RSV RNA genome [48, 49]. Bitko and colleagues designed a siRNA-sequence targeting an essential RSV RNA-polymerase protein (RSV-P) [50]. Intranasal administration of naked RSV-P siRNA as a post treatment following RSV infection in mice was demonstrated by Bitko et al. to be capable of inhibiting the replication of RSV [50]. Currently, based on the work of Bitko and colleagues, human clinical trials are underway testing the efficacy of inhaled siRNA targeting RSV infection.
Summary
Since first described by Fire et al. in the late 1990s, significant progress has been made in elucidating the mechanisms and exploring the therapeutic potential of RNA interference [1]. Despite the frenetic pace of research by both academic and commercial laboratories, and just as with antisense and ribozymes therapies, translation of siRNA experimental successes from animal models to drugs for the treatment of diseases in humans, remains illusive. Many challenges related to the safe and effective administration of siRNA in vivo stand in the way of this realization. The studies described in this review are examples of the expanding scope in the use of in vivo siRNA in the lung to probe disease pathways for potential therapeutic targets.
Important for the success of siRNA therapeutics is an understanding of the signaling pathways and proteins involved in mediating them. This requires identifying the cellular targets and developing a delivery system that will facilitate, in a tissue specific fashion, sufficient uptake of siRNA into target cells. Ensuring tissue specific targeting appears to be less problematic in the lung compared with other organs. Delivery of naked siRNA by inhalation or intratracheal administration has been shown to be restricted to the lung [18,28,29], however, only recently have studies begun to identify the cellular targets taking up the siRNA [18,51]. This information will be of significant importance in determining realistic dosing of siRNA since effective silencing with siRNA is dependent upon the rate of target cell division.
Other considerations for potential therapeutics include disease pathology. Is the disease being treated inflammation driven as in ALI, a chronic disease like COPD, of an infectious origin such RSV or even a loss-of-function mutation as in lung cancer? These answers will define not only the genes to be targeted by siRNA silencing, but the time course for treatment. While viral vector delivery systems are still used with caution due to safely concerns, this system currently offers a practical treatment option for prolonged siRNA gene silencing [39].
Translation of this RNA interference based technology into human therapeutics represents a major advance in the treatment of diseases. The specificity of siRNA gene silencing; the ability to chemically synthesize (or generate the synthesis of) a tool to transiently silence the expression of a desired target gene has the potential to give surgical precision to the treatment of not only lung inflammatory diseases, but a host of other pulmonary disease pathologies.
Acknowledgments
This work was supported in part by funds from NIH-ROls GM53209 and HL73525 (to A.A.).
References
- 1.Fire A, Xu SQ, Montgomery MK, et al. Potent and specific genetic interference by doublestranded RNA in caenorhabditis elegans. Nature. 1998;391:806–11. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 2.Li WX, Ding SW. Viral suppressors of RNA silencing. Curr Opin Biotechnol. 2001;12:150–4. doi: 10.1016/s0958-1669(00)00190-7. [DOI] [PubMed] [Google Scholar]
- 3.Li H. Induction and suppression of RNA silencing by an animal virus. Science. 2002;296:1319–21. doi: 10.1126/science.1070948. [DOI] [PubMed] [Google Scholar]
- 4.Saksela K. Human viruses under attack by small inhibitory RNA. Trends in Microbiology. 2003;11:345–7. doi: 10.1016/s0966-842x(03)00158-6. [DOI] [PubMed] [Google Scholar]
- 5.Gampel A, Mellor H. Small interfering RNAs as a tool to assign Rho GTPase exchangefactor function in vivo. Biochem J. 2002;366:393–8. doi: 10.1042/BJ20020844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Platz J, Pinkenburg O, Beisswenger C, et al. Application of small interfering RNA (siRNA) for modulation of airway epithelial gene expression. Oligonucleotides. 2005;15:132–8. doi: 10.1089/oli.2005.15.132. [DOI] [PubMed] [Google Scholar]
- 7.Mura M, Han B, Andrade CF, et al. The early responses of VEGF and its receptors during acute lung injury: implication of VEGF in alveolar epithelial cell survival. Critical Care. 2006:10. doi: 10.1186/cc5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trian T, Girodet PO, Ousova O, et al. RNA interference decreases PAR-2 expression and function in human airway smooth muscle cells. Am J Respir Cell Mol Biol. 2006;34:49–55. doi: 10.1165/rcmb.2005-0187OC. [DOI] [PubMed] [Google Scholar]
- 9.Birukova AA, Malyukova I, Poroyko V, Birukov K. Paxillin-beta-catenin interactions are involved in Rac/Cdc42-mediated endothelial barrier-protective reponse to oxidized phospholipids. Am J Physiol Lung Cell Mol Physiol. 2007;293:L199–L211. doi: 10.1152/ajplung.00020.2007. [DOI] [PubMed] [Google Scholar]
- 10.Bell EL, Klimova TA, Eisenbart J, Schumacker PT, Chandel NS. Mitochondrial reactive oxygen species trigger hypoxia-inducible factor-dependent extension of the replicative life span during hypoxia. Mol Cell Biol. 2006;27:5737–45. doi: 10.1128/MCB.02265-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moschos SA, Williams AE, Lindsay MA. Cell-penetrating-peptide-mediated siRNA lung delivery. Biochemical Society Transactions. 2007;35:807–10. doi: 10.1042/BST0350807. [DOI] [PubMed] [Google Scholar]
- 12.Elbashir SM, Harborth J, Lendeckel W, et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–8. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 13.Dykxhoorn DM, Novina CD, Sharp PA. Killing the messenger: short RNAs that silence gene expression. Molecular Cell Biology. 2003;4:457–67. doi: 10.1038/nrm1129. [DOI] [PubMed] [Google Scholar]
- 14.Nykanen A, Haley B, Zamore PD. ATP requirements and small interferring RNA structure in the RNA interference pathway. Cell. 2001;107:309–21. doi: 10.1016/s0092-8674(01)00547-5. [DOI] [PubMed] [Google Scholar]
- 15.Elbashir SM, Martinez J, Patkaniowski A, Lendeckel W, Tuschi T. Functional anatomy of siRNAs for mediating efficient RNAi in Drosophilia melanogaster embryo lysate. EMBO J. 2001;20:6877–88. doi: 10.1093/emboj/20.23.6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishiwaki Y, Yokota T, Hiraoka M, et al. Introduction of short interfering RNA to silence endogenous E-selectin in vascular endothelium leads to successful inhibition of leukocyte adhesion. Biochem Biophys Res Comm. 2003;310:1062–6. doi: 10.1016/j.bbrc.2003.09.125. [DOI] [PubMed] [Google Scholar]
- 17.Ulanova M, Puttagunta L, Marcet-Palacios M, et al. Syk tyrosine kinase participates in betal-integrin signaling and inflammatory responses in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2005;288:L497–L507. doi: 10.1152/ajplung.00246.2004. [DOI] [PubMed] [Google Scholar]
- 18.Perl M, Chung CS, Lomas-Neira J, et al. Silencing of Fas- but not caspase-8 in lung epithelial cells ameliorates pulmonary apoptosis, inflammation, and neutrophil influx after hemorrhagic shock and sepsis. Am J Pathol. 2005;167:1545–59. doi: 10.1016/S0002-9440(10)61240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muir A, Soong G, Sokol S, et al. Toll-like receptors in normal and cystic fibrosis airway epithelial cells. Am J Respir Cell Mol Biol. 2004;30:777–83. doi: 10.1165/rcmb.2003-0329OC. [DOI] [PubMed] [Google Scholar]
- 20.Simon RH, Paine III R. Participation of pulmonary alveolar epithelial cells in lung inflammation. J Lab Clin Med. 2007;126:108–18. [PubMed] [Google Scholar]
- 21.Kagnoff MF, Eckmann L. Epithelial cells as sensors for microbial infection. The Journal of Clinical Investigation. 1997;100:6–10. doi: 10.1172/JCI119522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lomas-Neira JL, Chung C-S, Wesche DE, Perl M, Ayala A. In vivo gene silencing (with siRNA) of pulmonary expression of MIP-2 versus KC results in divergent effects on hemorrhage-induced, neutrophil-mediated septic acute lung injury. J Leukocyte Biol. 2005;77:1–8. doi: 10.1189/jlb.1004617. [DOI] [PubMed] [Google Scholar]
- 23.McCaffrey AP, Meuse L, Pham T-TT, et al. RNA interference in adult mice. Nature. 2002;418:39. doi: 10.1038/418038a. [DOI] [PubMed] [Google Scholar]
- 24.Song E, Lee S-K, Wang J, et al. RNA interference targeting Fas protects mice from fulminant hepatitis. Nature Medicine. 2003;9:347–51. doi: 10.1038/nm828. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Shan P, Jiang D, et al. Small interfering RNA targeting heme oxygenase-1 enhances ischemia-reperfusion-induced lung apoptosis. Journal of Biological Chemistry. 2004;279:10677–84. doi: 10.1074/jbc.M312941200. [DOI] [PubMed] [Google Scholar]
- 26.Abraham NG, Lavrovsky Y, Schwartzman ML, et al. Proc Natl Acad Sci U S A. 1995;92:6798–802. doi: 10.1073/pnas.92.15.6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Otterbein LE, Choi AM. Am J Physiol. 2000;279:L1029–L1037. doi: 10.1152/ajplung.2000.279.6.L1029. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Shan P, Jiang G, et al. Endothelial STATS is essential for the protective effects of HO-1 in oxidant-induced lung injury. FASEB J. 2006;20:E1528–E1538. doi: 10.1096/fj.06-5668fje. [DOI] [PubMed] [Google Scholar]
- 29.Bhandari V, Choo-Wing R, Lee CG, et al. Hyperoxia causes angiopoietin 2-mediated acute lung injury and necrotic cell death. Nature Medicine. 2006;12:1286–93. doi: 10.1038/nm1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–93. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 31.Matsuyama W, Watanabe M, Shirahama Y, et al. Suppression of discoidin domain receptor 1 by RNA interference attenuates lung inflammation. J Immunol. 2006;176:1928–36. doi: 10.4049/jimmunol.176.3.1928. [DOI] [PubMed] [Google Scholar]
- 32.Matsuyama W, Wang L, Farrar WL, Faure M, Yoshimura T. Activation of discoidin domain receptor 1 isoform b with collagen up-regulates chemokine production in human macrophages: role of p38 mitogen-activated protein kinase and NF-KB. J Immunol. 2004;172:2332–40. doi: 10.4049/jimmunol.172.4.2332. [DOI] [PubMed] [Google Scholar]
- 33.Johnson MD, Widdicombe JH, Allen L, Barbry P, Dobbs LG. Alveolar epitelial type 1 cells contain transport proteins and transport sodium, supporting an active role for type 1 cells in regulation of lung liquid homeostasis. Proc Natl Acad Sci. 2002;99:1966–71. doi: 10.1073/pnas.042689399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mutlu GM, Sznajder JL. Mechanisms of pulmonary edema clearance. Am J Physiol Lung Cell Mol Physiol. 2005;289:L685–L695. doi: 10.1152/ajplung.00247.2005. [DOI] [PubMed] [Google Scholar]
- 35.Guidot DM, Folkesson HG, Jain L, et al. Integrating acute lung injury and regulation of alveolar fluid clearance. Am J Physiol Lung Cell Mol Physiol. 2006;291:L301–L306. doi: 10.1152/ajplung.00153.2006. [DOI] [PubMed] [Google Scholar]
- 36.Predescu SA, Predescu DN, Palade GE. Plasmalemmal vesicles function as transcyotic carriers for small proteins in the continuous endothelium. Am J Physiol Heart Circ Physiol. 1997;272:H937–H949. doi: 10.1152/ajpheart.1997.272.2.H937. [DOI] [PubMed] [Google Scholar]
- 37.Cohen AW, Hnasko R, Schubert W. Lisanti MP. Role of caveolae and caveolins in health and disease. Physiol Rev. 2004;84:1341–79. doi: 10.1152/physrev.00046.2003. [DOI] [PubMed] [Google Scholar]
- 38.Miyawaki-Shimizu K, Predescu D, Shimizu J, et al. siRNA-induced caveolin-1 knockdown in mice increases lung vascular permeability via the junctional pathway. Am J Physiol Lung Cell Mol Physiol. 2005;290:L405–L413. doi: 10.1152/ajplung.00292.2005. [DOI] [PubMed] [Google Scholar]
- 39.Sun L, Gao H, Sarma VJ, Quo R-F, Ward PA. Adenovirus-mediated in vivo silencing of anaphylatoxin receptor C5aR. Journal of Biomedicine and Biotechnology. 2006:1–9. doi: 10.1155/JBB/2006/28945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ward PA. The dark side of C5a in sepsis. Nature Reviews in Immunology. 2004;4:133–42. doi: 10.1038/nri1269. [DOI] [PubMed] [Google Scholar]
- 41.Robbins RA, Russ WD, Rasmussen JK, Clayton MM. Activation of the complement system in the adult respiratory distress syndrome. Am Rev Respir Dis. 1987;135:651–8. doi: 10.1164/arrd.1987.135.3.651. [DOI] [PubMed] [Google Scholar]
- 42.Linton SM. Complement activation and inhibition in experimental models of arthritis. Mol Immunol. 1999;36:905–14. doi: 10.1016/s0161-5890(99)00113-3. [DOI] [PubMed] [Google Scholar]
- 43.Welch TR. Complement in glomerulonephritis. Nature Genetics. 2002;31:333–4. doi: 10.1038/ng933. [DOI] [PubMed] [Google Scholar]
- 44.Ffrench-Constant C. Pathogenesis of multiple sclerosis. Lancet. 1994;343:271–5. doi: 10.1016/s0140-6736(94)91118-5. [DOI] [PubMed] [Google Scholar]
- 45.Arumugam TV, Shiels IA, Woodruff TM, Granger DN, Taylor SM. The role of the complement system in ischemia-reperfusion injury. Shock. 2004;21:401–9. doi: 10.1097/00024382-200405000-00002. [DOI] [PubMed] [Google Scholar]
- 46.Hawlisch H, Wills-Karp M, Karp CL, Kohl J. The anaphylatoxins bridge innate and adaptive immune responses in allergic asthma. Mol Immunol. 2004;41:123–31. doi: 10.1016/j.molimm.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 47.Polack FP. A role for immune complexes in enhanced respiratory syncytial virus disease. J Exp Med. 2002;196:859–65. doi: 10.1084/jem.20020781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sullender WM. Respiratory syncytial virus genetic and antigenic diversity. Clin Microbial Rev. 2000;13:1–15. doi: 10.1128/cmr.13.1.1-15.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morton CJ. Structural characterization of respiratory syncytial virus fusion inhibitor escape mutants: homology model of the F protein and a syncytium formation assay. Virology. 2003;311:275–88. doi: 10.1016/s0042-6822(03)00115-6. [DOI] [PubMed] [Google Scholar]
- 50.Bitko V, Musiyenko A, Shulyayeva O, Barik S. Inhibition of respiratory viruses by nasally administered siRNA. Nature Medicine. 2005;11:50–5. doi: 10.1038/nm1164. [DOI] [PubMed] [Google Scholar]
- 51.Larson SD, Jackson LN, Chen LA, Rychahou PG, Evers BM. Effectiveness of siRNA uptake in target issues by various delivery methods. Surgery. 2007;142:262–9. doi: 10.1016/j.surg.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]