Abstract
Radiofrequency ablation (RFA) is gaining popularity for treating colorectal liver metastases by inducing image guided tumor hyperthermia. In order to reduce tumor recurrence, adjuvant therapies have been administered post-RFA. We hypothesized that tumor cells escaping RFA cytotoxicity by being in the sublethal zones of tumor might develop differential behavior toward cytotoxic drugs. Here, we used cultured human colorectal cancer cells to evaluate the interaction between heat treatment and chemotherapeutic agents. Human colon cancer cell lines HT29 and HCT116 were subjected to temperatures of 42° to 50°C for 15 min, in combination with 5-fluorouracil, oxaliplatin, or irinotecan at different sequences. Cytotoxicity was determined by MTT assay. The cell cycle progression was analyzed by flow cytometry with propidium iodide staining. The expression of several genes associated with drug sensitivity was quantitated by real-time RT-PCR before and after heat treatment. Either heat treatment at 45°C by simultaneous or pre-treatment with three different chemotherapeutic agents didn't affect the cytotoxicity of the combined treatment to HT29 and HCT116 cells, except for irinotecan treatment in HCT116 cells. However, when pre-exposure to 45°C, HCT116 cells, but not HT29 cells, developed resistance to these three drugs. In an analysis of cell cycle profile after the drug followed heat treatment, a longer delay in cell cycle progression in HCT116 cells was observed in comparison to HT29 cells. Furthermore, HCT116 and HT29 cells exhibited different expression profiles of several drug-related genes in response to heat treatment at 45°C. An observation of a differential response to the drug and heat treatment sequences between two human colon cancer cell lines suggests that tumor heterogeneity and selection of chemotherapeutic agents need to be under consideration in the clinical setting.
Keywords: Hyperthermia, chemotherapy, colon cancer, drug resistance, cell cycle, gene expression
Introduction
Colorectal cancer strikes approximately 140,000 people and accounts for more than 55,000 deaths each year in the United States. For the vast majority of patients who die of colorectal cancer, liver is the most common site of metastases. Left untreated, patients with liver metastases have a poor prognosis with a median survival ranging from 4 to 21 months, and a 3-year survival below 3% [1]. Surgical resection is the only curative therapeutic option for liver metastases from colorectal cancer with 5-year survival rates of about 30%-35% [2]; however, only about 10% to 20% of these patients are candidates for liver surgery—the remainder have unresectable disease [3].
5-fluorouracil (5-FU) combined with leucovorin (LV) is the most commonly used chemotherapy regimen used in the treatment of advanced colorectal cancer. Objective response rates in two recently published large randomized trials were only 15.5% and 21% [4, 5]. In the 1990s, two additional agents, irinotecan and oxaliplatin, were found to have activity against advanced colorectal cancer. In a trial using irinotecan as the first-line agent, the irinotecan/5-FU/LV arm showed a significant improvement on median progression-free survival, overall survival and objective response rate in comparison to the 5-FU/LV arm [5]. Oxaliplatin in combination with 5-FU/LV has also significantly improved response rates and prolonged progression-free survival [6, 7]. However, 2-year survival in previously untreated patients receiving these two new drugs is still only 25% [5, 7] and data from 7 recently published phase III trials showed that the improvement in median survival did not exceed 3.5 months [8].
The mechanisms of cytotoxicity of 5-FU, oxaliplatin and irinotecan are different. When 5-FU is delivered by a short-duration bolus injection, it mainly inhibits RNA synthesis. When 5-FU is delivered by long-term infusion lasting days to weeks, it mainly inhibits DNA synthesis through inhibition of thymidylate synthesis [9]. Oxaliplatin, a platinum-based drug, is a DNA-damaging agent that blocks DNA replication and transcription [10]. Irinotecan inhibits topoisomerase I, thus impeding DNA uncoiling leading to double-stranded DNA break [11].
The sensitivity of cancer cells to chemotherapeutic agents is influenced by multiple molecular events. For example, thymidylate synthase (TS), a 5-FU target enzyme, is a key enzyme converting dUMP to dTMP, which is essential for DNA synthesis and repair; thymidine phosphorylase (TP) converts 5-FU to fluorodeoxyuridine (FUdR), which can then be converted to the active metabolite FdUMP by thymidine kinase (TK); dihydropyrimidine dehydrogenes (DPD) carries out the degradation of 5-FU into therapeutically inactive metabolites [12]. For oxaliplatin, xeroderma pigmentosum complementation group A (XPA) and excision repair cross complementing 1 (ERCC1) in the nucleotide excision repair (NER) system are required for repairing the platinum-DNA adducts [10]. Glutamylcysteine synthetase (GCS) catalyses the rate-limiting step in biosynthesis of glutathione which can inactivate oxaliplatin [13]. For irinotecan, carboxylesterase (CE) converts irinotecan to an active metabolite, SN38 [14]. Alteration in the expression of these enzymes/pathways is under scientific scrutiny to enable administration of rational chemotherapeutic agents.
In addition to systemic chemotherapy, several regional treatment strategies have been explored to improve the treatment of liver metastases, such as cryoablation, radiofrequency ablation (RFA), regional infusion therapy, and isolated liver perfusion. Among them, RFA has been shown to be safe and well tolerated and is gaining popularity in the treatment of unresectable primary and metastatic hepatic tumors [15]. RF thermal ablation works by converting RF waves into heat. A high-frequency alternating current (100 to 500 kHz) passes from an uninsulated electrode tip into the surrounding tissues and causes ionic vibration resulting in generation of frictional heating of the surrounding tissue [16]. The goal of RFA is to achieve local temperatures higher than 50°C such that tumor tissue is rendered nonviable [17]. After RFA, a local recurrence ranging from 1.8% to 34% has been reported, depending on the location and size of treated tumor [18]. In an effort to reduce recurrence, chemotherapy is increasing considered for the treatment of patients following RFA.
One potential mechanism of recurrence following RFA is the persistence of viable cancer cells in the tumor which have been subjected only to sublethal heat, in the range of 42°C-50°C. Potentially, perturbations induced by such sublethal heat may alter their sensitivity to subsequent chemotherapy. Alternatively, pretreatment of tumor by chemotherapy may alter their sensitivity to subsequent sublethal heat. In order to sift through these variables and the differential responses based on tumor heterogeneity, we evaluated the cytotoxicity of three chemotherapeutic agents, 5-FU, oxaliplatin and irinotecan, combined with heat treatment at different sequences in two human colon cancer cell lines HT29 and HCT116. We found that the effect of heat treatment at high temperature on drug sensitivity was different between these two cell lines. We further investigated the potential mechanisms contributing to this difference by comparing the cell cycle distribution of these two cell lines after exposure to heat and the drug. We also examined the effect of heat treatment on expression of several genes associated with the drug sensitivity in these two cell lines.
Materials and methods
Cell culture
Human colon cancer cell lines HT29 and HCT116 obtained from the American Type Culture Collection (ATCC) were cultured in McCoy's 5A medium (Invitrogen, Carlsbad, CA) containing 10% FBS (Gemini Byproducts, Calabasas, CA) and supplemented with penicillin and streptomycin. Cells were maintained in a humidified incubator at 37°C and 5% CO2.
Heat and drug treatments and MTT assay
Cells grown on T25 flasks or 96-well plates were sealed with parafilm and exposed to heat treatment at 42°C, 45°C, 47°C and 50°C for 15 min by immersion into a water bath. Cells subjected to 37°C served as controls. All temperatures were maintained within ± 0.05 °C by testing for accuracy with a thermocouple (Fischer Scientific, Pittsburgh, PA). 5-FU, oxaliplatin (Sigma, St. Louis, MO) and irinotecan (Pharmacia, Kalamazoo, Ml) were added to medium at the indicated concentration either before, immediately, or after heat treatment for 24 h. Cell viability was measured by MTT assay 72 h after heat treatment. The medium containing 0.5 mg/ml MTT (Sigma) was added to each well and incubated at 37°C for 3 h. The formazan product was dissolved in isopropanol and the plates were read at 570 nm and 690 nm (background). All measurements were performed in quadruplicate and each experiment was repeated at least 3 times.
Flow cytometric analysis of cell cycle
The harvested cells were fixed in 75% cold ethanol. The fixed cells were washed with PBS, incubated in PBS containing RNase (100 μg/ml) and propidium iodide (10 μg/ml), and then subjected to a FACScan flow cytometer (Becton Dickinson, San Jose, CA). 10,000 events were collected per sample. Data acquisition and cell cycle analysis were performed using CellQuest software.
Reverse transcription-polymerase chain reaction (RT-PCR) assays
Total RNA was extracted from cells using the Qiagen RNeasy isolation kit (Valencia, CA). The RNA concentration was determined by reading the absorbance at 260 nm and was checked for purity at 280 nm in a spectrophotometer. Only samples with a 260/280 ratio higher than 1.8 were applied for analysis. The cDNA was synthesized from 1 ug of DNase-treated total RNA using TaqMan Multiscrible reverse transcriptase and random hexamers (Applied Biosystems, Foster City, CA). Real-time PCR analysis was carried out on an ABI Prism 7900HT Sequence Detection System (Applied Biosystems). The hot-start PCR was performed with the SYBR Green PCR Master Mix (Applied Biosystems). PCR primer sequences used in this study were listed in Table 1. The PCR mixtures were pre-heated at 95°C for 10 min to activate the AmpliTaq Gold DNA polymerase. PCR conditions used were denaturation at 95 °C for 15 sec, annealing/extension at 60°C for 1 min and continued for 40 cycles, followed by a final step at 60°C for 10 min. Each PCR run consisted of a five-point calibration curve and a negative control without template. GAPDH was used as an internal control. The triplicate was performed in each sample. The relative quantification of target gene was analyzed uusing the ABI Prism SDS2.1 software. The identity of the expected PCR products was confirmed by agarose gel electrophoresis.
Table 1.
Primer sequences used in the real-time RT-PCR
| Gene Name | Accession Number | Sense/Antisense (5′→3′) |
|---|---|---|
| Carboxylesterase 2 (CES2) | NM_003869 | AACCTGTCTGCCTGTGACCAAGT |
| ACATCAGCAGCGTTAACATTTTCTG | ||
| Dihydropyrimidine | NM_000110 | TTGGCTCGATTGGGGTACTCT |
| dehydrogenase (DPD) | NM 001983 | ACCAAGGTCCTTCATTAGCTCA |
| Excision repair cross | NM_001983 | GGGAATTTGGCGACGTAATTC |
| complementing 1 (ERCC1) | GCGGAGGCTGAGGAACAG | |
| G-3-P dehydrogenase (GAPDH) | NM_002046 | CATGAGAAGTATGACAACAGCCT |
| AGTCCTTCCACGATACCAAAGT | ||
| Y-Glutamylcysteine Synthetase | NM_001498 | GGCACAAGGACGTTCTCAAGT |
| (GCS) | CAAAGGGTAGGATGGTTTGGG | |
| Heat shock 70kDa protein | NM_005345 | TTCCGTTTCCAGCCCCCAATC |
| (HSP70) | CGTTGAGCCCCGCGATCACA | |
| Thymidine Kinase (TK) | NM_003258 | CCGGGAAGACCGTAATTGTGG |
| CGAGCCTCTTGGTATAGGCG | ||
| Thymidylate Synthase (TS) | NM_001071 | GGAAGGGTGTTTTGGAGGAGTT |
| AGATTTTCACTCCCTTGGAAGACA | ||
| Topoisomerase I (TOPO I) | NM_003286 | CCAGACGGAAGCTCGGAAAC |
| GTCCAGGAGGCTCTATCTTGAA | ||
| Topoisomerase II, α (TOPO II) | NM_001067 | TCATCAAGATTGTGGGTCTTCAG |
| CCTCCAGAAAACGATGTCGCA | ||
| Xeroderma pigmentosum, complementation group A (XPA) | NM_000380 | CCAGGACCTGTTATGGAATTTGA |
| GCTTCTTGACTACCCCAAACTTC |
Results
Cytotoxicity of chemotherapeutic agents in conjunction with heat Treatment
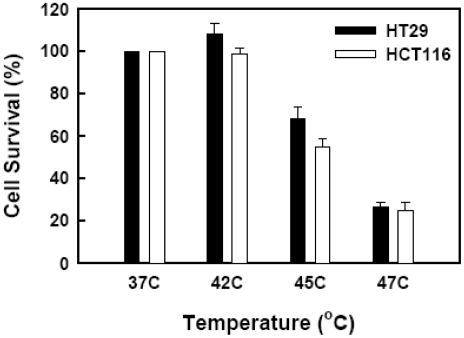
We first evaluated the survival of human colon cancer cells HT29 and HCT116 after short-term exposure to heat at various temperatures for 15 min. Cell viability was determined by MTT assay 72 h after heat shock. The survival rates of HT29 cells after heat treatment at 42°C, 45°C and 47°C were 108.4%, 68.4% and 26.7%, respectively, in comparison to 37°C controls (Figure 1). The survival rates of HCT116 cells after heat treatment at 42°C, 45°C and 47°C were 98.7%, 54.6% and 24.9%, respectively, in comparison to 37°C controls (Figure 1). Cells exposed to 50°C were all dead in both cell lines. The survival rates of both cell lines at 47°C were too low to further evaluate the combined effect on chemotherapy. Therefore, heat treatment at 42 °C and 45 °C was used in this study for 'sublethal' hypothermia effects.
Figure 1.
Effect of different temperatures on cell viability. HT29 (•) and HCT116 (□) cells were subjected to water bath at an indicated temperature for 15 min and then returned to a 37 °C incubator. Cell viability was determined by MTT assay 72 h after heat treatment.
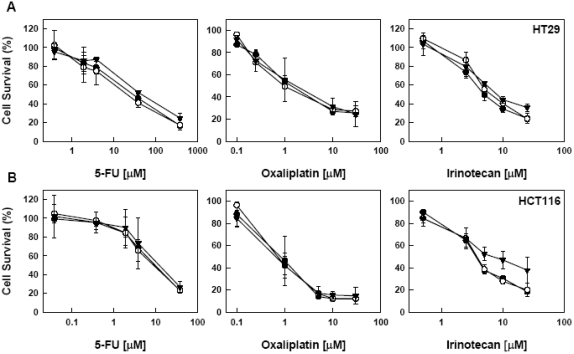
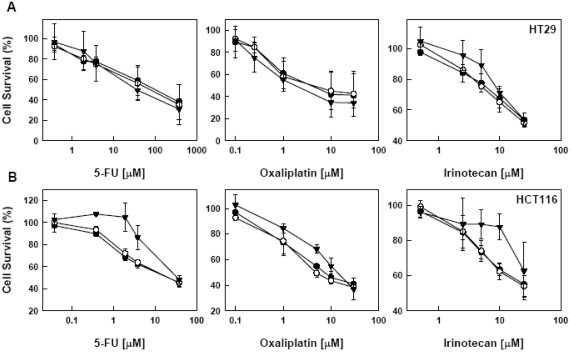
We then examined the effect of heat treatment on the drug sensitivity of these two cell lines. Cells were either exposed to heat treatment at 42°C and 45°C immediately after drug addition or incubated with a drug at 24 h post heat treatment. In HT29 cells, we did not observe any significant change in sensitivity to 5-FU, oxaliplatin and irinotecan either immediately or 24 h followed by heat treatment (Figure 2 and 3). In contrast, in HCT116 cells, the responses were variable. When treated with drugs immediately followed by heat treatment, there was no change in the sensitivity of HCT116 cells to 5-FU and oxaliplatin; however, the survival rate at 45°C was higher than at 42°C and 37°C when the concentration of irinotecan was higher than 5 uM (Figure 2). When the drugs were added 24 h after heat treatment, the survival rate of HCT116 cells at 45°C was higher than at 42°C and 37°C in the middle range of concentrations of these three drugs (Figure 3).
Figure 2.
Effect of immediate heart treatment on the sensitivity to 5-FU, oxalipalatin and irinotecan. HT29 (A) and HCT116 (B) cells were subjected to various concentrations of a drug, immediately followed by heat treatment at 37° (○) 42° (•) and 45°C (▾) for 15, min, Drugs were removed from medium after 24 h. Cell viability was determined by MTT assay 72 h after heat treatment. Data are shown as mean ± SD. Cell survival for each temperature was corrected for heat cytotoxicity.
Figure 3.
Effect of pre-exposure to heat on the sensitivity to 5-FU, oxaliplatin and irinotecan. HT29 (A) and HCT116 (B) cells were exposed to heat at 37° (○), 42° (•) and 45°C (▾) for 15 min. Twenty-four hours after heat treatment, cells were subjected to various concentrations of a drug for 24 h. Cell viability was determined by MTT assay 72 h after heat treatment. Data are shown as mean ± SD. Cell survival for each temperature was corrected for heat cytotoxicity.
Effect of pre-exposure to chemothera-peutic agents on heat sensitivity
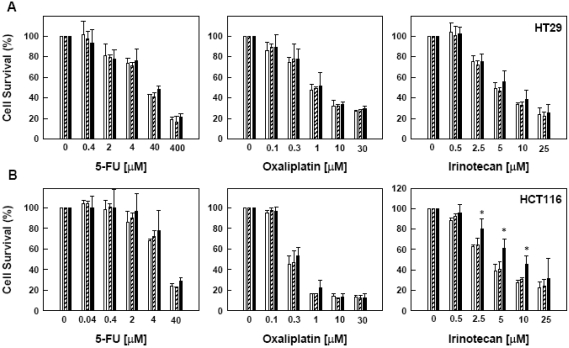
We next examined whether pre-exposure to 5-FU, oxaliplatin or irinotecan would change the survival rate of these two cell lines subsequently exposed to heat treatment. Cells were first incubated with various concentrations of a drug for 24 h and then subjected to heat treatment at 42 °C or 45°C for 15 min. Pre-exposure to 5-FU and oxaliplatin resulted in no significant change in cell survival after heat treatment in both HT29 and HCT116 cells (Figure 4). However, after exposure to high concentration irinotecan, HCT116 cells became more resistant to heat-induced cell death at 45°C in comparison to 42°C and 37°C controls (Figure 4).
Figure 4.
Effect of pre-treatment of 5-FU, oxaliplatin and irinotecan on heat-sensitivity at different temperatures, HT29 (A) and HCT116 (B) cells were pretreated with a drug for 24 h before heat treatment at 37° (□), 42°( ) and 45°C (▪) for 15 min. Drugs were removed form medium before heat treatment. Cell viability was determined by MTT assay 72 h after heat treatment. Data are shown as mean ± SD. *p < 0.05, compared to cells at 37°C by the Student's test. Cell survival for each termperature was corrected for heat cytotoxicity.
) and 45°C (▪) for 15 min. Drugs were removed form medium before heat treatment. Cell viability was determined by MTT assay 72 h after heat treatment. Data are shown as mean ± SD. *p < 0.05, compared to cells at 37°C by the Student's test. Cell survival for each termperature was corrected for heat cytotoxicity.
Cell cycle profile in combination of heat treatment and chemotherapeutic agents
By examining the effect of combined heat and drug treatments in three different treatment sequences on cell survival, we found that HT29 and HCT116 cells responded differently after exposure to heat at 45°C. We further investigated whether the perturbation of cell cycle progression from heat treatment would contribute to this difference. Figure 5 shows the results of the flow cytometric analyses of these two cell lines 24 h after exposure to heat alone, drug alone, or the combination of heat and drug. In heat treatment, HCT116 cells showed a decrease in Gl phase and a concomitant increase G2/M phase, and HT29 cells showed a decrease in S phase and a concomitant increase in G2/M phase. Such an accumulation of cells in the G2 compartment after hyperthermia has also been observed in other cell lines [19, 21].
Figure 5.
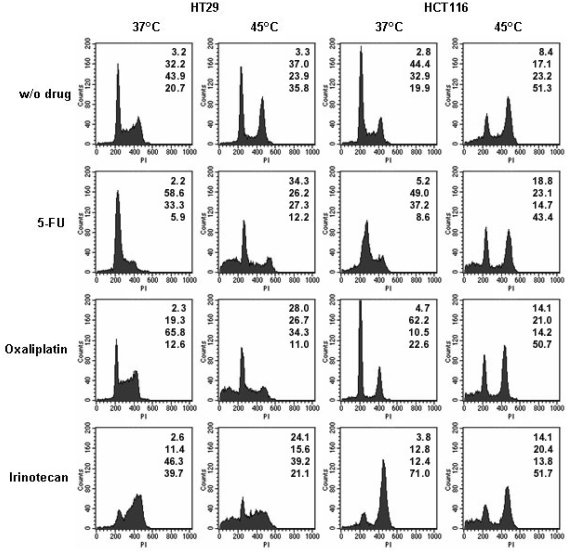
Effect of heat treatment and chemotherapeutic agents on cell cycle distribution. HT29 and HCT116 cells were subjected to 5-FU (5 uM), oxaliplatin (1 uM), or irinotecan (5 uM) with or without heat treatment at 45°C. In the single treatment, cells were harvested 24 h after treatment and subjected to cell cycle analysis. In the combined treatment, cells were pre-exposed to heat treatment at 45°C for 15 min. Twenty-four hours after heat treatment, cells were subjected to a drug for another 24 h. Flow cytometry with propidium iodide staining was used for cell cycle analysis. The numbers at the right top corner of each histogram indicate the percentage of cell phase distribution in the order of sub-Gl, Gl, S, and G2/M from top to bottom. The data represent three independent experiments.
For 5-FU, an accumulation in Gl/S phase was observed in both cell lines, which is in agreement with previous studies [22, 23]. For oxaliplatin, HCT116 cells exhibited a decrease in S phase and a concomitant increase in Gl phase, while HT29 cells exhibited a decrease in Gl phase and a concomitant increase in S phase. It has been demonstrated that treatment by oxaliplatin leads to inhibition of cell cycle progression in S and/or G2/M phases [24, 25]. For irinotecan, a decrease in Gl phase and an accumulation in G2/M phase were observed in both cell lines. The induction of the G2/M arrest by irinotecan has also been reported in other studies [26].
When exposed to drug after heat treatment, all heat/drug-treated HCT116 cells still had a high percentage of cells in G2/M phase, which was very similar to the profile in heat treatment alone, except for an increase in sub-Gl phase (Figure 5). In contrast, the effectiveness of heat treatment on cell cycle progression had been diminished following drug treatments in HT29 cells and exhibited a similar cell cycle profile as drug alone in 37°C (Figure 5). An increased sub-Gl phase was also observed in HT29 cells treated with heat and drug (Figure 5). This result suggests that the development of drug resistance in heat-treated HCT116 cells might be due to a prolongation of arrest in cell cycle progression induced by heat in comparison with that in HT29 cells.
Expression of genes associated with drug sensitivity after heat treatment
The expression levels of several genes involved in drug metabolism, damage repair, and drug-target proteins have been linked to the development of drug resistance. We then examined the effect of heat treatment at 45°C on the expression levels of several genes in HT29 and HCT116 cells using real time RT-PCR analysis. Figure 6 shows the change in expression levels of genes associated with drug sensitivity in HT29 and HCT116 cells at 6 h and 24 h after heat treatment in comparison to 37°C controls. In general, most genes are down-regulated at 6 h after heat treatment; in contrast, the expression of HSP70 was increased to 106.56- and 42.91-fold in HT29 and HCT116 cells, respectively.
Figure 6.
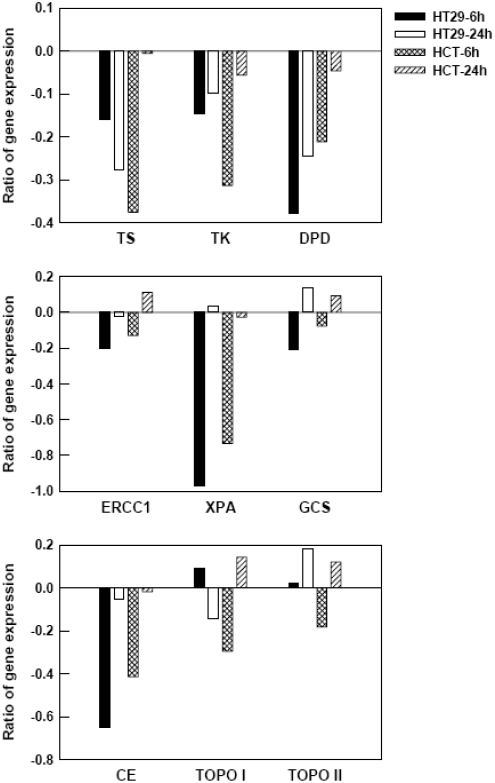
Effect of heat treatment on the mRNA levels of several genes associated with drug sensitivity. Total RNAs were isolated from HT29 and HCT116 cells at 6 h and 24 h after heat treatment (45°C for 15 min). Cells subjected to 37°C were used as controls. The mRNA level of each gene was measured by real-time RT-PCR and its expression level was normalized to GAPDH expression. Ratio of gene expression is given as log of normalized target gene expression after heat treatment versus controls. For clarity, SD is not shown and all are less than 10% of each value.
TS, TK, and DPD for 5-FU
The expression of these three genes was down-regulated in both cell lines after heat treatment. Twenty-four hours after heat treatment, expression of these three genes in HCT116 cells was observed to be back to baseline. In contrast, the expression levels of TS, TK, and DPD in HT29 cells were still down to 0.53-, 0.80-, and 0.57-fold relative to control levels, respectively, at 24 h after heat treatment. Such a quick recovery of gene expression in HCT116 cells may be one of the factors contributing to a decrease of drug sensitivity after heat treatment, when compared to HT29 cells.
ERCC1, XPA, and GCS for oxaliplatin
ERCC1 expression increased 1.29-fold in HCT116 cells at 24 h after heat treatment, while its expression was close to control levels in HT29 cells. The effect of heat treatment on XPA and GCS expression in HT29 cells is similar to that in HCT116 cells. XPA expression decreased at 6 h and returned to control levels at 24 h, while GCS expression increased to 1.37- and 1.24-fold in HT29 and HCT116 cells, respectively, at 24 h. By comparing the expression of these three genes between HT29 and HCT116 cells, we summarize that the elevated expression of ERCC1 after heat treatment may contribute the development of oxaliplatin resistance in HCT116 cells.
CE, TOPO I, and TOPO II for irinotecan
CE expression decreased at 6 h and returned to control levels at 24 h in both cell lines. The expression pattern of TOPO I in HT29 cells was opposite to that in HCT116 cells. At 6 h, TOPO I expression increased to 1.24-fold in HT29 cells and decreased to 0.66-fold in HCT116 cells, whereas, at 24 h, its expression decreased to 0.72-fold in HT29 cells and increased to 1.39-fold in HCT116 cells. TOPO II expression remained unchanged at 6 h and increased to 1.52-fold at 24 h in HT29 cells, while its expression decreased to 0.66-fold at 6 h and increased to 1.32-fold at 24 h in HCT116 cells. The analysis of expression profiles of these three genes in HT29 and HCT116 cells does not provide a clear correlation to the development of irinotecan resistance in HCT116 cells after heat treatment.
Discussion
There are several factors that hinder successful performance of RFA in the clinical setting and result in recurrence at the treated tumor site: cooling effect from blood vessels, thermal gradient formation at the treated margin, tumor size and irregular tumor shape [27]. Furthermore, systemic recurrence often occurs in patients with colorectal liver metastases after RFA [28]. Therefore, adjuvant therapy is needed to reduce the rate of recurrence following RFA treatment. Indeed, combination of RFA and chemotherapy to treat patients with unresectable liver metastases from primary colorectal tumors has been under evaluation in an EORTC Phase II clinical trial (EORTC-40004).
In the evaluation of the interaction between chemotherapeutic agents and heat treatment in vitro, most studies focus on the potential enhancement of cytotoxicity of drugs by applying simultaneously with hyperthermia. In this case, cancer cells are subjected to moderate hyperthermia (no higher than 43°C) with long exposure time (≥ 1 h). In the clinical setting of applying RFA, tumors are subjected to a high temperature for a short period of time. In order to investigate the cellular responses of cancer cells to chemotherapeutic agents under sublethal RFA conditions, we examined the cytotoxicity of heat treatment (at temperature of 42°C and 45°C for 15 min) with 5-FU, oxaliplatin, or irinotecan, in different sequences in human colon cancer HT29 and HCT116 cells. Thus, our intent is to mimic the clinical scenario of RFA followed by systemic and/or regional chemotherapy.
When the drug was added right before heat treatment, we did not observe any significant change in drug sensitivity in these two cell lines, except for irinotecan in HCT116 cells at 45°C. In a study of human colorectal cancer WiDr cells, no synergistic cytotoxic effect was observed when irinotecan was combined with 43°C heat for 1 h [29]. Similarly, breast cancer cells did not show a change in sensitivity to 5-FU following heat shock at 42°C for 2 h [30]. A recent study demonstrated that the efficacy of oxaliplatin could be markedly enhanced by concurrent exposure to heat at 42°C for 1 h in HT29 cells only at concentrations higher than 25 uM [31]. However, a sensitizing effect of simultaneous hyperthermia (43°C for 1 h) to 5-FU was observed in prostate cancer cells [32]. Hyperthermia also enhanced the cytotoxicity of oxaliplatin at 41° and 43°C for 1 h in human lung carcinoma cells [33]. For irinotecan at low concentration (7.38 uM), an additive effect on inhibition of DNA synthesis was observed with a combination of heat at 44°C for 1 h at; whereas a protective effect was observed at high concentration (73.8 uM) in mouse mammary carcinoma FM3A cells [34]. Thus, the different outcomes of these studies could be attributed to the duration and concentrations of drug, temperature and time of heat exposure, or difference in cell types.
In evaluating the cytotoxicity of heat and chemotherapy in different sequences, we found that pre-exposed colon cancer cells to these three drugs did not hinder the efficacy of heat treatment. Only irinotecan treated HCT116 cells became less sensitive to heat at 45°C. The potential clinical implication of this result is that cancer patients who have been previously treated with some chemotherapeutic agents can still have an effective RFA treatment to eradicate tumors with perhaps a marginally higher recurrence rate if the prior treatment agent was irinotecan. However, in the reverse order, we found that HCT116 cells exposed to heat developed resistance to all three drugs, which was not observed in HT29 cells. By comparing the effect of heat treatment and drug on cell cycle distribution, we observed a longer delay of cell cycle progression in HCT116 cells than in HT29 cells. Such prolongation of arrest in cell cycle progression in HCT116 cells could be one of factors contributing to the development of drug resistance. A delay in cell cycle progression has also been observed in 5-FU resistant cells, compared with parental colon and breast cancer cell lines in other studies [35].
It is well known that p53 is involved in the regulation of Gl/S and G2/M arrest [36]. Therefore, a difference in heat-induced cell cycle delay between HCT116 and HT29 cells could be influenced by p53 since HCT116 cells carry wild-type p53, whereas HT29 cells have mutant p53. The prolonged cell cycle arrest induced by heat shock in HCT116 cells can provide extra time for damage repair and reduce the cytotoxicity of the drug. The requirement of p53 in delaying cell cycle transition after hyperthermia has also been demonstrated in several studies by comparing cell lines carrying either wild-type or mutant p53 [21] with isogenic cell lines established by stable transfection [20]. Furthermore, wild-type p53 can increase the thermosensitivity and stimulate apoptosis induced by heat shock, as demonstrated in mouse fibroblasts and human head and neck squamous carcinoma cells [19, 37]. Indeed, we also observed that HCT116 cells had a lower survival rate with more apoptotic cells than HT29 cells after heat shock at 45°C.
We further examined whether the molecular changes induced by heat shock could correlate with the development of drug resistance in HCT116 cells. The expression of several molecules involved in regulating drug sensitivity was analyzed by real-time RT-PCR. HSP70 is well known to be induced by heat stress and used as positive control in this assay [38]. As expected, expression of HSP70 was increased dramatically in both HT29 and HCT116 cells after heat treatment at 45 °C. HSP70 functions as a chaperon protein and has been recognized for its role in protecting cells from apoptosis induced by several chemotherapeutic drugs [30, 39, 40]. Thus, the increased expression of HSP70 in HCT116 cells may contribute to the development of drug resistance in cells surviving heat shock. However, heat-treated HT29 cells with an increase of HSP70 expression didn't change their sensitivities to these three drugs, compared to cells at 37 °C. Such no correlation of HSP70 expression to drug sensitivity has also been observed in human squamous carcinoma cells treated with cisplatin [41] and prostate carcinoma cells subjected to cisplatin and 5-FU [32].
Several enzymes involved in the action and metabolism of FU and other fluoropyrimidines have been demonstrated to affect the sensitivity of cancer cells to 5-FU [12]. Overexpression of TS is the major mechanism of acquired resistance to 5-FU in cancer cells and its expression correlates with clinical responses in patients treated with 5-FU based therapy [12]. There is an inverse correlation between the DPD mRNA expression and the 5-FU response in human cancer cell lines [42]. It has been reported that TK is overexpressed in HCT116 cells resistant to 5-FU in comparison to parental cells [43]. In this study, a decrease of TS, TK, and DPD expression was observed in early heat treated-HCT116 cells which develop a resistance to 5-FU. Therefore, the change of expression of these three genes may not be the major factor in regulating 5-FU resistance in HCT116 cells after heat treatment. Intriguingly, we observed that the time needed for the repression of these three genes after heat shock in HCT116 cells was shorter than that in HT29 cells. Whether this phenomenon contributes to the differential response to 5-FU between heat treated-HT29 and HCT116 cells needs to be further investigated.
ERCC1 and XPA are involved in the repair of DNA lesions caused by platinum-based chemotherapy agents [10]. We only observed an increase of ERCC1 expression in HCT116 cells at 24 h after heat treatment. Therefore, the development of oxaliplatin resistance in HCT116 cells after heat shock could be due to the stimulation of their NER ability. There is a similar pattern of expression of GCS after heat treatment between HT29 and HCT116 cells, indicating that GCS may not be involved in the development of oxaliplatin resistance in HCT116 cells. Such non-correlation between glutathione and oxaliplatin sensitivity has also been reported in colon cancer cells [44], whereas a study demonstrated glutathione and glutathione-related enzymes are involved in the sensitivity of ovarian cancer cells to platinum compounds [13].
The current understanding is that cells with low CE expression, which are unable to convert irinotecan to its active metabolite SN-38, or with the reduced cellular level of TOPO I, a target of SN-38, will confer resistance to irinotecan [45, 46]. However, we did not observe a clear correlation between the development of irinotecan resistance in heat treated-HCT116 cells and the change of expression patterns of CE and TOPO I after heat treatment. Although CE expression decreased at 6 h after heat treatment in HCT116 cells, it also occurred in HT29 cells. In contrast, TOPO I expression increased in HCT116 cells at 24 h after heat treatment, whereas its expression decreased in H29 cells. We also examined the expression of another topoisomerase enzyme, TOPO II, whose expression increased in both cell lines at 24 h after heat treatment.
In summary, we demonstrated that there was no change in the cytotoxicity of heat treatment at high temperature in conjunction with chemotherapeutic agents in HT29 cells. In contrast, cross resistance to cytotoxic heat was observed in HCT116 cells when irinotecan, but not 5-FU or oxaliplatin, was added immediately or 24 h before heat treatment. In addition, pre-exposure to heat at 45°C for 15 min decreased the sensitivity of HCT116 cells to 5-FU, oxaliplatin and irinotecan. A prolonged delay of cell cycle transition induced by heat in HCT116 cells, compared to HT29 cells, could be one of the factors contributing to the development of drug resistance in HCT116 cells. Furthermore, a difference in expression profiles of several genes associated with drug sensitivity between HCT116 and HT29 cells in response to heat stress could be an additional factor for acquiring drug resistance in HCT116 cells. Taken together, tumor heterogeneity and cross-interaction between a selected chemotherapeutic agent and hypothermia may need to be considered when patients are treated with the combination of RFA and chemotherapy.
Acknowledgments
This work was supported by Faculty Practice Plan Research Fund of North Shore-Long Island Jewish Health System. Randall P. Owen was partially supported by New York State Department of Health Empire Clinical Investigator Program (ECRIP). We thank Dr. Missak Haigentz for providing us irinotecan and Huali Dong for assisting in real-time RT-PCR analysis.
References
- 1.Stangl R, Altendorf-Hofmann Hofmann, Charnley RM, Scheele J. Factors influencing the natural history of colorectal liver metastases. Lancet. 1994;343:1405–1410. doi: 10.1016/s0140-6736(94)92529-1. [DOI] [PubMed] [Google Scholar]
- 2.Fong Y, Cohen AM, Fortner JG, Enker WE, Turnbull AD, Coit DG, Marrero AM, Prasad M, Blumgart LH, Brennan MF. Liver resection for colorectal metastases. J Clin Oncol. 1997;15:938–946. doi: 10.1200/JCO.1997.15.3.938. [DOI] [PubMed] [Google Scholar]
- 3.Cady B, Jenkins RL, Steele GD, Lewis WD, Stone MD, McDermott WV, Jessup JM, Bothe A, Lalor P, Lovett EJ, Lavin P, Linehan DC. Surgical margin in hepatic resection for colorectal metastasis: a critical and improvable determinant of outcome. Ann Surg. 1998;227:566–571. doi: 10.1097/00000658-199804000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoff PM, Ansari R, Batist G, Cox J, Kocha W, Kuperminc M, Maroun J, Walde D, Weaver C, Harrison E, Burger HU, Osterwalder B, Wong AO, Wong R. Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: results of a randomized phase III study. J Clin Oncol. 2001;19:2282–2292. doi: 10.1200/JCO.2001.19.8.2282. [DOI] [PubMed] [Google Scholar]
- 5.Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta N, Elfring GL, Miller LL. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343:905–914. doi: 10.1056/NEJM200009283431302. [DOI] [PubMed] [Google Scholar]
- 6.Giacchetti S, Perpoint B, Zidani R, Le Bail N, Faggiuolo R, Focan C, Chollet P, Llory JF, Letourneau Y, Coudert B, Bertheaut-Cvitkovic Cvitkovic, Larregain-Fournier Fournier, Le Rol A, Walter S, Adam R, Misset JL, Levi F. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2000;18:136–147. doi: 10.1200/JCO.2000.18.1.136. [DOI] [PubMed] [Google Scholar]
- 7.De Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes Funes, Cervantes A, Freyer G, Papamichael D, Le Bail N, Louvet C, Hendler D, De Braud F, Wilson C, Morvan F, Bonetti A. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 8.Grothey A, Sargent D, Goldberg RM, Schmoll HJ. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol. 2004;22:1209–1214. doi: 10.1200/JCO.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 9.Sobrero AF, Aschele C, Bertino JR. Fluorouracil in colorectal cancer-a tale of two drugs: implications for biochemical modulation. J Clin Oncol. 1997;15:368–381. doi: 10.1200/JCO.1997.15.1.368. [DOI] [PubMed] [Google Scholar]
- 10.Reed E. Platinum-DNA adduct, nucleotide excision repair and platinum based anti-cancer chemotherapy. Cancer Treat Rev. 1998;24:331–344. doi: 10.1016/s0305-7372(98)90056-1. [DOI] [PubMed] [Google Scholar]
- 11.Hsiang YH, Liu LF. Identification of mammalian DNA topoisomerase I as an intracellular target of the anticancer drug camptothecin. Cancer Res. 1988;48:1722–1726. [PubMed] [Google Scholar]
- 12.Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 13.el-akawi Z, Abu-Hadid M, Perez R, Glavy J, Zdanowicz J, Creaven PJ, Pendyala L. Altered glutathione metabolism in oxaliplatin resistant ovarian carcinoma cells. Cancer Lett. 1996;105:5–14. doi: 10.1016/0304-3835(96)04245-0. [DOI] [PubMed] [Google Scholar]
- 14.Kawato Y, Aonuma M, Hirota Y, Kuga H, Sato K. Intracellular roles of SN-38, a metabolite of the camptothecin derivative CPT-11, in the antitumor effect of CPT-11. Cancer Res. 1991;51:4187–4191. [PubMed] [Google Scholar]
- 15.Ravikumar TS, Kaleya R, Devita VT, Hellman S, Rosenberg SA. Progress in onclogy-2001. Boston, Jones Bartlett; 2001. Radiofrequency ablation for the treatment of liver metastases; pp. 25–277. [Google Scholar]
- 16.Goldberg SN. Radiofrequency tumor ablation: Principles and techniques. Eur J Ultrasound. 2001;13:129–147. doi: 10.1016/s0929-8266(01)00126-4. [DOI] [PubMed] [Google Scholar]
- 17.Dickson JA, Calderwood SK. Temperature range and selective sensitivity of tumors to hyperthermia: a critical review. Ann NY Acad Sci. 1980;335:180–205. doi: 10.1111/j.1749-6632.1980.tb50749.x. [DOI] [PubMed] [Google Scholar]
- 18.Decadt B, Siriwardena AK. Radiofrequency ablation of liver tumours: systematic review. Lancet Oncol. 2004;5:550–560. doi: 10.1016/S1470-2045(04)01567-0. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto H, Takahashi A, Wang X, Ohnishi K, Ohnishi T. Transfection of p53-knockout mouse fibroblasts with wild-type p53 increases the thermosensitivity and stimulates apoptosis induced by heat stress. Int J Radiat Oncol Biol Phys. 1997;39:197–203. doi: 10.1016/s0360-3016(97)00149-1. [DOI] [PubMed] [Google Scholar]
- 20.van Bree C, van der Maat B, Ceha HM, Franken NA, Haveman J, Bakker PJ. Inactivation of p53 and of pRb protects human colorectal carcinoma cells against hyperthermia-induced cytotoxicity and apoptosis. J Cancer Res Clin Oncol. 1999;125:549–555. doi: 10.1007/s004320050315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zolzer F, Streffer C. Quiescence in S-phase and Gl arrest induced by irradiation and/or hyperthermia in six human tumour cell lines of different p53 status. Int J Radiat Biol. 2000;76:717–725. doi: 10.1080/095530000138394. [DOI] [PubMed] [Google Scholar]
- 22.Pizzorno G, Sun Z, Handschumacher RE. Aberrant cell cycle inhibition pattern in human colon carcinoma cell lines after exposure to 5-fluorouracil. Biochem Pharmacol. 1995;49:553–557. doi: 10.1016/0006-2952(94)00444-q. [DOI] [PubMed] [Google Scholar]
- 23.Yoshikawa R, Kusunoki M, Yanagi H, Noda M, Furuyama JL, Yamamura T, Hashimoto-Tamaoki T. Dual antitumor effects of 5-fluorouracil on the cell cycle in colorectal carcinoma cells: a novel target mechanism concept for pharmacokinetic modulating chemotherapy. Cancer Res. 2001;61:1029–1037. [PubMed] [Google Scholar]
- 24.Mastbergen SC, Duivenvoorden I, Versteegh RT, Geldof AA. Cell cycle arrest and clonogenic tumor cell kill by divergent chemotherapeutic drugs. Anticancer Res. 2000;20:1833–1838. [PubMed] [Google Scholar]
- 25.Arnould S, Guichard S, Hennebelle I, Cassar G, Bugat R, Canal P. Contribution of apoptosis in the cytotoxicity of the oxaliplatin-irinotecan combination in the HT29 human colon adenocarcinoma cell line. Biochem Pharmacol. 2002;64:1215–1226. doi: 10.1016/s0006-2952(02)01291-1. [DOI] [PubMed] [Google Scholar]
- 26.Magrini R, Bhonde MR, Hanski ML, Notter M, Scherubl H, Boland CR, Zeitz M, Hanski C. Cellular effects of CPT-11 on colon carcinoma cells: dependence on p53 and hMLHl status. Int J Cancer. 2002;101:23–31. doi: 10.1002/ijc.10565. [DOI] [PubMed] [Google Scholar]
- 27.Stippel DL, Brochhagen HG, Arenja M, Hunkemoller J, Holscher AH, Beckurts KT. Variability of size and shape of necrosis induced by radiofrequency ablation in human livers: a volumetric evaluation. Ann Surg Oncol. 2004;11:420–425. doi: 10.1245/ASO.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 28.Parikh AA, Curley SA, Fornage BD, Ellis LM. Radiofrequency ablation of hepatic metastases. Semin Oncol. 2002;29:168–182. doi: 10.1053/sonc.2002.31673. [DOI] [PubMed] [Google Scholar]
- 29.Hirohashi Y, Hidaka K, Sato S, Kuwano M, Kohno K, Hisatsugu T. Biomodulation by hyperthermia of topoisomerase ll-targeting drugs in human colorectal cancer cells. Jpn J Cancer Res. 1995;86:1097–1105. doi: 10.1111/j.1349-7006.1995.tb03026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ciocca DR, Fuqua SA, Lock-Lim Lim, Toft DO, Welch WJ, McGuire WL. Response of human breast cancer cells to heat shock and chemotherapeutic drugs. Cancer Res. 1992;52:3648–3654. [PubMed] [Google Scholar]
- 31.Atallah D, Marsaud V, Radanyi C, Kornprobst M, Rouzier R, Elias D, Renoir JM. Thermal enhancement of oxaliplatin-induced inhibition of cell proliferation and cell cycle progression in human carcinoma cell lines. Int J Hyperthermia. 2004;20:405–419. doi: 10.1080/02656730310001637325. [DOI] [PubMed] [Google Scholar]
- 32.Roigas J, Wallen ES, Loaning SA, Moseley PL. Effects of combined treatment of chemotherapeutics and hyperthermia on survival and the regulation of heat shock proteins in Dunning R3327 prostate carcinoma cells. Prostate. 1998;34:195–202. doi: 10.1002/(sici)1097-0045(19980215)34:3<195::aid-pros7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 33.Rietbroek RC, Van de Vaart PJ, Haveman J, Blommaert FA, Geerdink A, Bakker PJ, Veenhof CH. Hyperthermia enhances the cytotoxicity and platinum-DNA adduct formation of lobaplatin and oxaliplatin in cultured SW 1573 cells. J Cancer Res Clin Oncol. 1997;123:6–12. doi: 10.1007/BF01212608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kondo T, Ueda K, Kano E. Combined effects of hyperthermia and CPT-11 on DNA strand breaks in mouse mammary carcinoma FM3A cells. Anticancer Res. 1995;15:83–86. [PubMed] [Google Scholar]
- 35.Wang W, Cassidy J, O'brien V, Ryan KM, Collie-Duguid E. Mechanistic and predictive profiling of 5-Fluorouracil resistance in human cancer cells. Cancer Res. 2004;64:8167–8176. doi: 10.1158/0008-5472.CAN-04-0970. [DOI] [PubMed] [Google Scholar]
- 36.Agarwal ML, Agarwal A, Taylor WR, Stark GR. p53 controls both the G2/M and the Gl cell cycle checkpoints and mediates reversible growth arrest in human fibroblasts. Proc Natl Acad Sci U S A. 1995;92:8493–8497. doi: 10.1073/pnas.92.18.8493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ota I, Ohnishi K, Takahashi A, Yane K, Kanata H, Miyahara H, Ohnishi T, Hosoi H. Transfection with mutant p53 gene inhibits heat-induced apoptosis in a head and neck cell line of human squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2000;47:495–501. doi: 10.1016/s0360-3016(00)00437-5. [DOI] [PubMed] [Google Scholar]
- 38.Lindquist S, Craig EA. The heat shock protein. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- 39.Arrigo AP. Small stress proteins: chaperones that act as regulators of intracellular redox state and programmed cell death. Biol Chem. 1998;379:19–26. [PubMed] [Google Scholar]
- 40.Samali A, Cotter TG. Heat shock proteins increase resistance to apoptosis. Exp Cell Res. 1996;223:163–170. doi: 10.1006/excr.1996.0070. [DOI] [PubMed] [Google Scholar]
- 41.Yang WL, Yang-Biggs G, Wu Y, Ye X, Gallos G, Owen RP, Ravikumar TS. Development of cross-resistance between heat and cisplatin or hydroxyurea treatments in FaDu squamous carcinoma cells. J Surg Res. 2003;111:143–151. doi: 10.1016/s0022-4804(03)00105-7. [DOI] [PubMed] [Google Scholar]
- 42.Scherf U, Ross DT, Waltham M, Smith LH, Lee JK, Tanabe L, Kohn KW, Reinhold WC, Myers TG, Andrews DT, Scudiero DA, Eisen MB, Sausville EA, Pommier Y, Botstein D, Brown PO, Weinstein JN. A gene expression database for the molecular pharmacology of cancer. Nat Genet. 2000;24:236–244. doi: 10.1038/73439. [DOI] [PubMed] [Google Scholar]
- 43.Boyer J, McLean EG, Aroori S, Wilson P, McCulla A, Carey PD, Longley DB, Johnston PG. Characterization of p53 wild-type and null isogenic colorectal cancer cell lines resistant to 5-fluorouracil, oxaliplatin, and irinotecan. Clin Caner Res. 2004;10:2158–2167. doi: 10.1158/1078-0432.ccr-03-0362. [DOI] [PubMed] [Google Scholar]
- 44.Arnould S, Hennebelle I, Canal P, Bugat R, Guichard S. Cellular determinants of oxaliplatin sensitivity in colon cancer cell lines. Eur J Cancer. 2003;39:112–119. doi: 10.1016/s0959-8049(02)00411-2. [DOI] [PubMed] [Google Scholar]
- 45.Kojima A, Hackett NR, Crystal RG. Reversal of CPT-11 resistance of lung cancer cells by adenovirus-mediated gene transfer of the human carboxylesterase cDNA. Cancer Res. 1998;58:4368–4374. [PubMed] [Google Scholar]
- 46.Giovanella BC, Stehlin JS, Wall ME, Wani MC, Nicholas AW, Liu LF, Silber R, Potmesil M. DNA topoisomerase l-targeted chemotherapy of human colon cancer in xenografts. Science. 1989;246:1046–1048. doi: 10.1126/science.2555920. [DOI] [PubMed] [Google Scholar]