Abstract
Regulatory T cells (Tregs) have a key role in immune homeostasis and in suppressing unwanted inflammatory responses toward self-antigens. Tregs have been implicated in the control of initial activation events, and play roles in limiting proliferation, differentiation and effector functions of T helper cells. However, the activities of Tregs in the development and progression of kidney disease are not fully elucidated. We have demonstrated the potency of Tregs in animal models of kidney disease. In this review, we summarise mechanistic information from rodent models on the roles of Tregs in glomerular immunopathology and discuss the function of Tregs in diverse kidney diseases. Further studies of Tregs should provide important insights into designing of therapeutic strategies to prevent human kidney disease.
Keywords: Regulatory T cells, Foxp3, renal disease, immunotherapy
Introduction
The regulatory role of T cells has been apparent since their discovery in the early 1970s as suppressor T cells [1,2]. Numerous studies have suggested that Tregs have a key role in immune homeostasis and in suppressing unwanted inflammatory responses toward self-antigens. Defects in Treg numbers and functionality have been shown in human diseases including multiple sclerosis, rheumatoid arthritis, autoimmune polyglandular syndrome and myasthenia gravis [3–5]. Augmentation of Tregs may provide therapeutic opportunities to induce long-term tolerance, and to actively self regulate immune function. The best characterized subset of Tregs is the CD4+CD25+ T lymphocyte subset. Recent studies have indicated that the transcription factor gene Foxp3 is specifically expressed by CD4+CD25+ cells, and programs their development and function[6;7]. Foxp3+CD4+CD25+ T cells have been widely accepted as professional Tregs, by virtue of the stability of their suppressive function[8]. More recently, Tregs have been shown to limit immune responses to foreign antigen and to variously help or hinder transplantation and pathogen or tumour clearance [9–11].
Cell therapy using stem or progenitor cells has been used as a novel therapeutic strategy for kidney disease and effectively reduces injury in animal models [12]. We have recently shown that Tregs are able to protect against kidney injury in mouse models by mechanisms independent of other lymphocytes, apparently involving innate immune cells and renal parenchymal cells [13,14]. However, the roles and protective mechanisms of Tregs in human renal disease remain unclear. Inappropriate T cell responses can target the kidney, creating hypersensitivity reactions and causing glomerulonephritis. CD4+ T cells can mediate glomerular immunopathology by cytokine secretion, by activating effector cells or by inducing auto-antibodies or immune-complexes. Cytotoxic CD8+ T cell responses and failure of regulatory T cells may represent two additional types of anti-renal hypersensitivity.
From animal studies, it is clear that Tregs are potent modulators of tissue injury and repair in renal disease. Recent studies examining the therapeutic effect of these cells raise the exciting possibility that strategies targeting these cell types may be effective at treating and preventing kidney disease in humans. This review will describe the mechanistic information from rodent models on the roles of Tregs in glomerular immunopathology and their therapeutic potential in clinical nephrology.
Naturally-occurring CD4+ CD25+ Tregs
Naturally occurring CD4+CD25+ T cells were first described as a major regulatory subset in transplantation and auto-immune disease models. Several other cell types of Tregs have also been characterised, including NKT cells, γδ T cells [15], double-negative T cells and certain CD8 cells. CD25+CD4+ Tregs cells play an important role in establishing and maintaining immunological unresponsiveness to self constituents. They also negatively regulate various immune responses to non-self antigens. The majority of CD4+CD25+ Tregs are produced by the normal thymus as functionally distinct and mature subpopulation of T cells. They are derived from the thymus and appear to modulate a variety of immune functions, from initial T-cell and B-cell activation to effector function in the target tissue [6;16;17]. The possible mechanism of the suppression by CD25+CD4+ T cells may involve stimulation via CTLA-4. Additionally, the TCR of Tregs may physically interfere with the interaction of other T cells with APCs by competing for the costimulatory molecules on APCs, since Tregs express CTLA-4 and various adhesion molecules at high level [18]. The mechanisms of Treg suppression include cell–cell contact, direct secretion of suppressive factors such as interleukin-10 (IL-10), TGF-β, and IL-35; and the induction of secretion of such factors by APCs. Additionally, Tregs may compete for some cytokines that signal via receptors containing the common γ-chain (IL-2, IL-4 and IL-7) [17].
Adaptive Tregs
Tregs control self-reactivity and maintain immunologic homeostasis. Thymus-derived, CD4+CD25+ natural Tregs have been the centre of attention. However, T cells can be induced to develop regulatory activity during the course of a normal immune response including CD4+CD25- T cells are induced to develop regulatory activity [19–21]. It is now evident that conventional peripheral CD4+CD25- cells can be induced to become Foxp3+ Tregs [8]. Induction of Tregs depends on peripheral factors, such as the maturity or type of the stimulating APC and the availability of cytokines such as IL-2 and TGF-β. Many different subsets of induced Tregs have been reported [8,22,23]. These include CD4+ Tregs, which are induced from T helper cells by TGF-β in the absence of IL-6 [24,25]. Amongst these Tregs are T regulatory type 1 (Tr1) cells which have been shown to produce high levels of IL-10 [26]. Their suppressive function is through the secretion of immunosuppressive cytokines, such as IL-10 and TGF-β [27–29]. In TCR transgenic mouse models, in vitro culture with TGF-β has been shown to induce Foxp3 expression in CD4 T cells and the acquisition of regulatory function. Similar experiments using human CD4 T cells also show the ability of TGF-β to induce Tregs [30].
Recently, studies have shown that IL-17-producing T lymphocytes comprise a distinct lineage of pro-inflammatory T helper cells, termed Th17 cells [31–33]. There is a counterbalance between pathogenic (Th17) T cells that induce autoimmunity and regulatory (Foxp3+) T cells that inhibit autoimmune tissue injury. The generation of Th17 cells requires the addition of cytokine blockers, such as anti-IL-6 [32;34]. II-6 is an acute phase protein produced during inflammation. IL-6 and TGF-β together induce the differentiation of pathogenic Th17 cells from naïve T cells. To define whether the blockade of IL-6R may shift T cells from Th17 to Tregs, we have used anti-IL-6R for in the adriamycin nephropathy model. Our preliminary data suggest that antibody blockade of IL-6R can protect against renal damage in this murine model of chronic immune injury. The protective mechanism may involve an enhancement in the numbers of Tregs and down regulation of Th17 development.
Mechanisms of injury in kidney disease and immune responses
Chronic kidney disease (CKD), especially end stage renal disease (ESRD) is a major health problem in Australia and worldwide [35,36]. The primary causes of ESRD are diabetic nephropathy, glomerulonephritis, hypertension and polycystic kidney disease. Interstitial infiltration of inflammatory cells is seen in all forms of CKD, with some differences in intensity and cellular composition. The immune system is involved to a variable extent, by either responding to tissue injury or to the ongoing inflammatory response. In most forms of renal disease, the inciting antigens are undefined. The progression of renal injury usual involves both non-immune and immune factors. Most natural immune responses involve a mixture of innate and cognate processes. Even in diseases incited by immune mechanisms, non-immune factors -such as hypertension and proteinuria - play a crucial role in the progression of renal injury. Cognate and innate immune responses are involved not only in kidney disease activation but also in disease regulation. Tregs comprise a small proportion of the total lymphocyte population and yet are able to regulate key immune responses across a variety of disease settings.
Foxp3+ Tregs and kidney injury
Studies have suggested that the forkhead/winged helix transcription factor, Foxp3 is the crucial molecule necessary for Treg development and function; this is best illustrated by the finding that forced expression of Foxp3 in naïve T cells can convert them to Tregs functionally and phenotypically [7,37]. Foxp3 was originally reported to be the causative gene for an X-linked multi-organ autoimmune/inflammatory disease in mice and humans [6,38]. In humans, mutation of the Foxp3 gene results in severe organ specific autoimmune disease [39,40]. Studies have demonstrated that expression of Foxp3 is highly restricted to the αβ subset of T cells and, irrespective of CD25 expression, correlates with suppressor activity. A Foxp3-mutant mouse strain known as the Scurfy model develops a similar X-linked multi-organ pathology involving uncontrolled activation of CD4+ T cells. Adaptive transfer of CD4+CD25+ Tregs can protect Foxp3 deficient mice from development of lymphoproliferative disorders [7]. Furthermore, Foxp3-mutant scurfy mice and Foxp3-deficient mice produced by gene targeting generate few CD25+CD4+ Tregs and inoculation of normal CD4CD25+ Tregs can prevent autoimmune/inflammatory diseases in these mice. These results indicate that Foxp3 expressing T cells may be more effective regulatory T cells than CD4+CD25+ T cells alone.
Forced expression of Foxp3 in naïve CD4+ T cells induces a regulatory T cell phenotype [7]. This has been applied directly in type 1 diabetes using Foxp3 transduction of TCRtransgenic T cells specific for pancreatic islet antigens in non-obese diabetic (NOD) mice [41]. We and other groups have used adriamycin nephropathy (AN) in mice as a model of chronic proteinuric renal disease [13,14]. AN is induced in either mice or rats by a single intravenous injection of adriamycin. After the initial toxic injury, an immune mediated chronic proteinuric renal disease develops, resembling human focal segmental glomerulosclerosis [42]. The pathologic features of AN are severe nephrotic syndrome, focal glomerular sclerosis, tubular injury, and interstitial compartment expansion with massive mononuclear cell infiltration composed largely of macrophages and T cells [42–45]. We have shown that adaptive transfer of CD8+ T cells worsen AN and that CD4+ T cells ameliorate disease [45,46]. We find that immune deficient SCID mice develop more severe AN which is reduced in severity by adaptively transferred CD4+ T cells, suggesting an influence on innate immune function (unpublished data).
In order to examine the effect of Foxp3 transduced Tregs on AN in a mouse model, we generated Tregs by retroviral transduction of Foxp3 to naïve T cells [14]. Our results showed that transduction of Foxp3 induced a regulatory phenotype and inhibited the proliferation of CD4+CD25− cells in vitro and also adaptive transfer of the Foxp3-transduced Tregs can protect against renal injury in vivo. There were a few studies suggested that Histone/protein Deacetylases inhibitor (HDACi) down regulated IL-12, IFN-gamma, IL-6 and IL-10 and have therapeutic benefit in the treatment of kidney diseases by increasing the numbers and function of Foxp3+ Tregs [47–49].
In transplantation, Foxp3-transduced naïve CD4+ expressed a Treg cell phenotype and induced transplantation tolerance in a minor mismatch skin graft model. These cells were also functionally anergic and suppressive in vitro [50]. Tregs have been shown to be induced after donor specific transfusions (DST) in both animal models and humans and protect against allograft rejection. In our laboratory, Foxp3-transduced polyclonal CD4+ T cells were also shown to prolong skin allograft rejection in a major MHC mismatched skin transplant model, while CD4+ T cells transduced with control vector rapidly rejected skin allografts (unpublished data). It appears that both IL-10 and TGF-β may play a role in tolerance induction [30,51]. Of particular interest, antigen-specific Tregs that express IL-10, can limit antigen-specific immune responses and appear important as mediators of transplant tolerance [52,53].
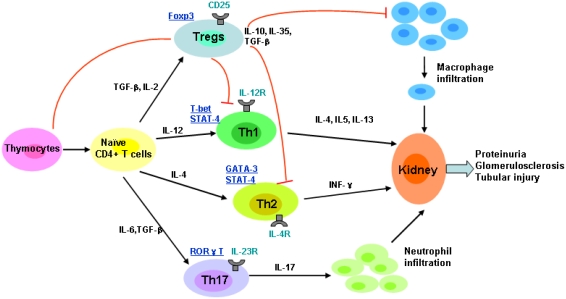
Therefore cellular therapy using Tregs to replace missing regulatory function is an attractive option for many immune-mediated kidney diseases and immune injury in transplantation. The suppressive function of Tregs for kidney disease on cognate and innate immune function was shown in Figure 1, which indicated that naïve CD4+ T cells can be skewed towards the functional phenotypes of Th1, Th2, Th17 and Treg on the basis of the cytokine environment.
Figure 1.
The suppressive function of Tregs for kidney disease on cognate and innate immune function; naïve CD4+ T cells can be skewed towards the functional phenotypes of Th1, Th2, Th17 and Treg on the basis of the cytokine environment. Key transcription factors for particular lineages are underlined.
Treg Immunotherapy in kidney disease and kidney transplantation
The adaptive transfer of CD4+CD25+ Tregs has been reported to protect against established macrophage-dependent, lymphocyte-independent injury in an innate murine model of chronic kidney disease. It has been demonstrated that transfer of CD4+CD25+ Treg can protect against anti–glomerular basement membrane disease in mice, decreasing proteinuria and infiltration of inflammatory cells, such as effector T cells, as well as macrophages [54,55].
Therapeutic applications involving Antigen Non-specific Clonal Expansion
Limited numbers of naturally occurring CD4+CD25+ Tregs exist in peripheral blood, and represent only 5–10% of the CD4+ T cell population. Therefore, several approaches have now been taken to expand the number of Treg cells in vitro, including various stimulation strategies, cytokines, and gene transfer approaches.
Magnetic beads coated with anti-CD3 and CD28 specific Abs plus high dose of IL-2 have been used in vitro to non-specifically expand naturally occurring Tregs isolated from both mice and humans [56–60]. These polyclonal Tregs can be expanded several hundred fold in 2–3 weeks under these conditions. The expanded Tregs maintain cell surface expression of multiple Treg markers, including CD25, CD62L, HLA-DR (human), CTLA4 and GITR, and most importantly, they maintain intracellular expression of Foxp3. The expanded Tregs produce less IL-2 and IFN-γ and more IL10 and TGF-β than conventional CD4+CD25− Tregs that are expanded in vitro in a similar manner. Ex vivo activated and expanded CD4+CD25+ Tregs have been shown to inhibit GVHD. Animal studies on transfer of freshly isolated Tregs and expanded CD4+CD25+ Tregs prevent GVHD in different models provided a compelling basis for clinical applications of expanded antigen non-specific expanded CD4+CD25+ Tregs in the setting of BM transplantation. One study showed adaptive transfer of Treg cells polyclonally expanded ex vivo with rapamycin, which blocks the proliferation of effector T cells while sparing Treg cells, promotes tolerance to allogeneic pancreatic islet grafts [61].
Several cytokines are used to expand Tregs. TGF-β plays an important role in Treg mediated immune suppression [62,63]. The combination of TGF-β and IL-2 has been shown to enhance growth, differentiation and survival of Tregs [64] and protect cells from activation induced cell death [56,65]. Tregs generated ex vivo using TGF-β and IL-2 have been shown to control autoimmune diseases such as systemic lupus erythematosus and other chronic inflammatory diseases [64,66]. In solid organ transplantation, the adaptive transfer of ex vivo generated Tregs demonstrated prolonged cardiac allograft survival in a rat model [67]. Tr1 cells generated ex vivo upon stimulation with alloantigens in the presence of IL-10 and TGFβ have been shown to be potent regulators of GVH responses after allogeneic BM transplantation [68]. Lauren et al reported that the inhibitory cytokine, IL-35 may be specifically produced by Tregs and contributes to Tregs function [69].
Therapeutic applications involving Antigen Specific Clonal Expansion
A number of studies in lymph-sufficient settings indicated that antigen-specific Tregs are more effective than polyclonal Tregs, especially for organ transplantation tolerance. In vitro experiments have shown that the suppressive functions of Tregs require their activation via the TCR [60,70], indicating that the in vivo activation and function of Tregs are also controlled by TCR specificity. Expanded islet-specific Tregs from TCR-transgenic mice using anti-CD3 and anti-CD28 specific antibodies have been shown to be markedly more effective at suppressing disease than polyclonal Tregs expanded using the same method in the NOD mouse model of type 1 diabetes [60]. More importantly, the function of these expanded antigen-specific Tregs was confined to the microenvironment where the cells are activated: the pancreatic LN [71]. Other approaches to expand antigen specific Treg cells include the use of peptide or antigen-pulsed DCs (or other APCs) and the use of peptide–MHC tetramers.
In transplantation, antigen specific Tregs expanded ex vivo by stimulation with allogeneic APCs prolonged survival in a GVH model [72]. Tr1 cells generated in vivo, in mice transplanted with allogeneic pancreatic islets and treated with rapamycin and IL-10, transfer antigen specific tolerance to secondary transplant recipients [73]. It has also been shown that chronic activation of human CD4+ cells by autologous APCs in the presence of IL-10 and type I interferons gives rise to CD4+ Tr1 clones that produce high levels of IL-10. These cells suppressed the in vitro alloantigen-specific proliferation of CD4+ [74].
Limitations of regulation
While the use of Tregs to induce and maintain tolerance is a potential therapeutic strategy to prevent solid organ allograft rejection, as well as GVHD in transplantation, and several autoimmune kidney diseases, there are several obstacles that need to be addressed prior to clinical application [75]. Firstly, the limitations of Treg-based immunotherapy at present are mainly technical and relate to cell manipulation. Limited numbers of Treg cells isolated from the donor's circulating pool of CD4+ T cells need to be further expanded in vitro. Tr1 cells are generated from CD4+ T cells, which need first to be isolated and primed in vitro. Safety of the infused ex vivo manipulated product is clearly a high priority. From cell collection to cell processing, expansion and differentiation, and final infusion into the patient, clinical good manufacturing practice (GMP) procedures need to be performed. Various quality controls are required in each manufacturing step before the final product can be released and infused into the patients. It is therefore unavoidable that only a few institutions can provide the entire necessary infrastructure to make Treg-based therapy a reality.
However, the transfer of Tregs is non-specific, in that the suppressive effects can be exerted on all T cells, not restricted to alloreactive T cells. Therefore, manipulation of Tregs may be detrimental in down-regulating an effective immune response to tumours [76] or infections because of an effect on CD8+ T cell immunity against tumours and viruses [77]. Furthermore, superior efficacy of Tregs immunotherapy over conventional therapy should be clearly demonstrated.
Potential applications in human renal diseases – prospects and challenges
Human glomerulonephritis is characterized by inflammation within the glomerulus. Current treatment involves the use of drugs (e.g. cyclophosphamide and corticosteroids) with pan-immunosuppressive effects, which increase the risk of malignancy and infection. Treg immunotherapy could provide a potential opportunity for specific immune regulation and long-term tolerance induction. A large amount of research and pre-clinical or clinical trials aimed at augmenting Treg activities have been conducted in several human disease settings including graft versus host disease (GVHD) [78,79], multiple sclerosis (MS) [80,81], rheumatoid arthritis (RA) [82,83], T1D [84,85] and others,, which indicates the great potential of Tregs in preventing or treating CKD. Adaptive transfer of Tregs into mice with glomerulonephritis has also shown some promising results [14,86].
However there are several issues concerning the use of Treg immunotherapy in autoimmune diseases in general and CKD in particular, which are yet to be answered before successful clinical application is possible.
Firstly, ex-vivo expansion of Tregs remains one of the challenges in the development of Treg immunotherapy. Since Treg comprise only a small population of the circulating T cells, extensive research has been devoted to optimizing in vitro conditions for expanding Tregs without loss of their function. To date, several groups have managed to expand human Foxp3+ Tregs 100–13000-fold [58,59,87–90] by using anti-CD3/28 coated Dynal beads in the presence of high dose of exogenous IL-2. A limitation of these methods is the expansion of contaminating Foxp3- cells at the same time which decreases the percentage of Foxp3+ Tregs and compromises clinical applications. One solution to this problem relies on the identification of more specific surface markers of Tregs to enable isolation of purer Tregs. One possible protocol for selectively expanding Foxp3+ Tregs involves rapamycin, which has been shown to promote expansion of Foxp3+ Tregs in vivo[91].
The second issue that is important to the success of Treg immunotherapy is the choice between antigen-specific Tregs and polyclonal Tregs. There is clear evidence that antigen-specific Tregs are more effective than polyclonal Tregs in models of T1D [60], bone marrow transplantation [92] and organ transplantation [93]. Antigen-specific Tregs seem to be most crucial for organ-specific autoimmune diseases [94]. However, the decision to use antigen-specific or polyclonal Tregs will also depend on the disease in question. In the case of CKD, some of these diseases are caused by single antigen (e.g. Goodpasture's syndrome and Alport's syndrome), whereas others are as a result of systemic immune disease (e.g. lupus nephritis) or caused by a mixture of antigens. In the latter case, the use of polyclonal Tregs may be beneficial to target a myriad of antigens and cell types.
The third issue needing to be addressed is that isolation of pure Tregs represents a major challenge to the potential application of Treg immunotherapy in human diseases. Purification of Tregs relies on the identification of Treg-specific surface markers. Currently CD25 has been widely used for the purification of Foxp3+CD25+ Tregs, but this technique has limitations due to contaminating Foxp3-CD25+ cells. Recently, the IL-7 receptor (CD127) has been identified as an additional marker for distinguishing the Foxp3+CD25+ Treg subset, providing a promising tool for use in human Treg isolation protocols [95;96].
Finally, a major concern in the application of Treg immunotherapy is the effect of immunosuppressive drugs on these cells. It is currently thought that calcineurin inhibitors, such as cyclosporin A and FK506, are detrimental to CD4+CD25+ Foxp3+ Treg development whereas rapamycin and myophenolate mofetil (MMF) are beneficial [97–100]. Since most CKD patients will undergo treatment with either the abovementioned drugs or other immunosuppressive drugs, precautions need to be taken to ensure the compatibility of Treg immunotherapy with each patient's drug regimen.
In spite of all the above mentioned challenges lying ahead, Treg immunotherapy shows great potential as a treatment for CKD and other autoimmune diseases, acting directly to restore immune homeostasis and promote long term immune tolerance. Further enhancement of efficacy of Treg therapy will rely heavily on the discovery of better assays for predicting Treg function in vivo and mechanisms to increase Treg homing to sites of inflammation, to increase stability in vivo and to determine the best timing for Treg therapy.
Conclusions
The natural presence of Foxp3+ Tregs in the immune system presents a potentially suitable therapeutic target for a variety of kidney diseases. In vivo expansion of antigen-specific Tregs or transduced Foxp3+ Tregs may be able to inhibit the development of kidney diseases. These cells have been shown in animal models by us and others to effectively reduce injury in kidney diseases. Future study will hopefully enable better control of immune responses via molecular and cellular manipulation of natural and induced Tregs to facilitate therapeutic strategies which can protect against human kidney disease and induce transplantation tolerance.
References
- 1.Janeway C. A., Jr., Sharrow S. O., Simpson E. T-cell populations with different functions. Nature. 1975;253:544–6. doi: 10.1038/253544a0. [DOI] [PubMed] [Google Scholar]
- 2.Cantor H., Boyse E. A. Functional subclasses of T lymphocytes bearing different Ly antigens. II. Cooperation between subclasses of Ly+ cells in the generation of killer activity. J.Exp.Med. 1975;141:1390–9. doi: 10.1084/jem.141.6.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balandina A., Lecart S., Dartevelle P., Saoudi A., Berrih-Aknin S. Functional defect of regulatory CD4(+)CD25+ T cells in the thymus of patients with autoimmune myasthenia gravis. Blood. 2005;105:735–41. doi: 10.1182/blood-2003-11-3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Viglietta V., Baecher-Allan C., Weiner H. L., Hafler D. A. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J.Exp.Med. 2004;199:971–9. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kriegel M. A., Lohmann T., Gabler C., Blank N., Kalden J. R., Lorenz H. M. Defective suppressor function of human CD4+ CD25+ regulatory T cells in autoimmune polyglandular syndrome type II. J.Exp.Med. 2004;199:1285–91. doi: 10.1084/jem.20032158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakaguchi S., Ono M., Setoguchi R., Yagi H., Hori S., Fehervari Z., et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol.Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 7.Hori S., Nomura T., Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 8.Sakaguchi S. Regulatory T cells in the past and for the future. Eur.J.Immunol. 2008;38:901–37. doi: 10.1002/eji.200890012. [DOI] [PubMed] [Google Scholar]
- 9.Belkaid Y. Regulatory T cells and infection: a dangerous necessity. Nat.Rev.Immunol. 2007;7:875–88. doi: 10.1038/nri2189. [DOI] [PubMed] [Google Scholar]
- 10.Wang H. Y., Wang R. F. Regulatory T cells and cancer. Curr.Opin.Immunol. 2007;19:217–23. doi: 10.1016/j.coi.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Cobbold S. P., Adams E., Graca L., Daley S., Yates S., Paterson A., et al. Immune privilege induced by regulatory T cells in transplantation tolerance. Immunol.Rev. 2006;213:239–55. doi: 10.1111/j.1600-065X.2006.00428.x. [DOI] [PubMed] [Google Scholar]
- 12.Zenovich A. G., Taylor D. A. Cell therapy in kidney disease: cautious optimism… but optimism nonetheless. Perit.Dial.Int. 2007;27(2):S94–103. [PubMed] [Google Scholar]
- 13.Mahajan D., Wang Y., Qin X., Wang Y., Zheng G., Wang Y. M., et al. CD4+CD25+ regulatory T cells protect against injury in an innate murine model of chronic kidney disease. J.Am.Soc.Nephrol. 2006;17:2731–41. doi: 10.1681/ASN.2005080842. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y. M., Zhang G. Y., Wang Y., Hu M., Wu H., Watson D., et al. Foxp3-transduced polyclonal regulatory T cells protect against chronic renal injury from adriamycin. J.Am.Soc.Nephrol. 2006;17:697–706. doi: 10.1681/ASN.2005090978. [DOI] [PubMed] [Google Scholar]
- 15.Wu H., Knight J. F., Alexander S. I. Regulatory gamma delta T cells in Heymann nephritis express an invariant Vgamma6/Vdelta1 with a canonical CDR3 sequence. Eur.J.Immunol. 2004;34:2322–30. doi: 10.1002/eji.200324780. [DOI] [PubMed] [Google Scholar]
- 16.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu.Rev.Immunol. 2004;22:531–62. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 17.Sojka D. K., Huang Y. H., Fowell D. J. Mechanisms of regulatory T-cell suppression - a diverse arsenal for a moving target. Immunology. 2008;124:13–22. doi: 10.1111/j.1365-2567.2008.02813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahashi T., Tagami T., Yamazaki S., Uede T., Shimizu J., Sakaguchi N., et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J.Exp.Med. 2000;192:303–10. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groux H., O'Garra A., Bigler M., Rouleau M., Antonenko S., De Vries J. E., et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–42. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 20.Levings M. K., Sangregorio R., Galbiati F., Squadrone S., De Waal Malefyt R., Roncarolo M. G. IFN-alpha and IL-10 induce the differentiation of human type 1 T regulatory cells. J.Immunol. 2001;166:5530–9. doi: 10.4049/jimmunol.166.9.5530. [DOI] [PubMed] [Google Scholar]
- 21.Sundstedt A., O'Neill E. J., Nicolson K. S., Wraith D. C. Role for IL-10 in suppression mediated by peptide-induced regulatory T cells in vivo. J.Immunol. 2003;170:1240–8. doi: 10.4049/jimmunol.170.3.1240. [DOI] [PubMed] [Google Scholar]
- 22.Walker M. R., Kasprowicz D. J., Gersuk V. H., Benard A., Landeghen M., Buckner J. H., et al. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+ J.Clin.Invest. 2003;112:1437–43. doi: 10.1172/JCI19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roncarolo M. G., Battaglia M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat.Rev.Immunol. 2007;7:585–98. doi: 10.1038/nri2138. [DOI] [PubMed] [Google Scholar]
- 24.Bettelli E., Carrier Y., Gao W., Korn T., Strom T. B., Oukka M., et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 25.Tato C. M., O'Shea J. J. Immunology: what does it mean to be just 17? Nature. 2006;441:166–8. doi: 10.1038/441166a. [DOI] [PubMed] [Google Scholar]
- 26.Groux H., O'Garra A., Bigler M., Rouleau M., Antonenko S., De Vries J. E., et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–42. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 27.Groux H., O'Garra A., Bigler M., Rouleau M., Antonenko S., De Vries J. E., et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–42. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 28.Levings M. K., Sangregorio R., Galbiati F., Squadrone S., De Waal Malefyt R., Roncarolo M. G. IFN-alpha and IL-10 induce the differentiation of human type 1 T regulatory cells. J.Immunol. 2001;166:5530–9. doi: 10.4049/jimmunol.166.9.5530. [DOI] [PubMed] [Google Scholar]
- 29.Sundstedt A., O'Neill E. J., Nicolson K. S., Wraith D. C. Role for IL-10 in suppression mediated by peptide-induced regulatory T cells in vivo. J.Immunol. 2003;170:1240–8. doi: 10.4049/jimmunol.170.3.1240. [DOI] [PubMed] [Google Scholar]
- 30.Chen T. C., Cobbold S. P., Fairchild P. J., Waldmann H. Generation of anergic and regulatory T cells following prolonged exposure to a harmless antigen. J.Immunol. 2004;172:5900–7. doi: 10.4049/jimmunol.172.10.5900. [DOI] [PubMed] [Google Scholar]
- 31.Iwakura Y., Ishigame H. The IL-23/IL-17 axis in inflammation. J.Clin.Invest. 2006;116:1218–22. doi: 10.1172/JCI28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bettelli E., Carrier Y., Gao W., Korn T., Strom T. B., Oukka M., et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 33.Park H., Li Z., Yang X. O., Chang S. H., Nurieva R., Wang Y. H., et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat.Immunol. 2005;6:1133–41. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ivanov I. I., McKenzie B. S., Zhou L., Tadokoro C. E., Lepelley A., Lafaille J. J., et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 35.Disney A. P. Demography and survival of patients receiving treatment for chronic renal failure in Australia and New Zealand: report on dialysis and renal transplantation treatment from the Australia and New Zealand Dialysis and Transplant Registry. Am.J.Kidney Dis. 1995;25:165–75. doi: 10.1016/0272-6386(95)90641-x. [DOI] [PubMed] [Google Scholar]
- 36.Lee G. End-stage renal disease in the Asian-Pacific region. Semin.Nephrol. 2003;23:107–14. doi: 10.1053/snep.2003.50009. [DOI] [PubMed] [Google Scholar]
- 37.Sakaguchi S., Sakaguchi N., Shimizu J., Yamazaki S., Sakihama T., Itoh M., et al. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol.Rev. 2001;182:18–32. doi: 10.1034/j.1600-065x.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- 38.Yagi H., Nomura T., Nakamura K., Yamazaki S., Kitawaki T., Hori S., et al. Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int.Immunol. 2004;16:1643–56. doi: 10.1093/intimm/dxh165. [DOI] [PubMed] [Google Scholar]
- 39.Chatila T. A., Blaeser F., Ho N., Lederman H. M., Voulgaropoulos C., Helms C., et al. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J.Clin.Invest. 2000;106:R75–R81. doi: 10.1172/JCI11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wildin R. S., Ramsdell F., Peake J., Faravelli F., Casanova J. L., Buist N., et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat.Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 41.Jaeckel E., Von Boehmer H., Manns M. P. Antigen-Specific FoxP3-Transduced T-Cells Can Control Established Type 1 Diabetes. Diabetes. 2005;54:306–10. doi: 10.2337/diabetes.54.2.306. [DOI] [PubMed] [Google Scholar]
- 42.Rangan G. K., Wang Y., Tay Y. C., Harris D. C. Inhibition of nuclear factor-kappaB activation reduces cortical tubulointerstitial injury in proteinuric rats. Kidney Int. 1999;56:118–34. doi: 10.1046/j.1523-1755.1999.00529.x. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y., Wang Y. P., Tay Y. C., Harris D. C. Progressive adriamycin nephropathy in mice: sequence of histologic and immunohistochemical events. Kidney Int. 2000;58:1797–804. doi: 10.1046/j.1523-1755.2000.00342.x. [DOI] [PubMed] [Google Scholar]
- 44.Rangan G. K., Wang Y., Harris D. C. Induction of proteinuric chronic glomerular disease in the rat (Rattus norvegicus) by intracardiac injection of doxorubicin hydrochloride. Contemp.Top.Lab Anim Sci. 2001;40:44–9. [PubMed] [Google Scholar]
- 45.Wang Y., Wang Y. P., Tay Y. C., Harris D. C. Role of CD8(+) cells in the progression of murine adriamycin nephropathy. Kidney Int. 2001;59:941–9. doi: 10.1046/j.1523-1755.2001.059003941.x. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y., Wang Y., Feng X., Bao S., Yi S., Kairaitis L., et al. Depletion of CD4(+) T cells aggravates glomerular and interstitial injury in murine adriamycin nephropathy. Kidney Int. 2001;59:975–84. doi: 10.1046/j.1523-1755.2001.059003975.x. [DOI] [PubMed] [Google Scholar]
- 47.Mishra N., Reilly C. M., Brown D. R., Ruiz P., Gilkeson G. S. Histone deacetylase inhibitors modulate renal disease in the MRL-lpr/lpr mouse. J.Clin.Invest. 2003;111:539–52. doi: 10.1172/JCI16153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reilly C. M., Thomas M., Gogal R., Jr., Olgun S., Santo A., Sodhi R., et al. The histone deacetylase inhibitor trichostatin A upregulates regulatory T cells and modulates autoimmunity in NZB/W F1 mice. J.Autoimmun. 2008 doi: 10.1016/j.jaut.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 49.Tao R., De Zoeten E. F., Ozkaynak E., Chen C., Wang L., Porrett P. M., et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat.Med. 2007;13:1299–307. doi: 10.1038/nm1652. [DOI] [PubMed] [Google Scholar]
- 50.Chai J. G., Xue S. A., Coe D., Addey C., Bartok I., Scott D., et al. Regulatory T cells, derived from naive CD4+ Transplantation. 2005;79:1310–6. doi: 10.1097/01.tp.0000159147.56408.9c. [DOI] [PubMed] [Google Scholar]
- 51.Lin C. Y., Graca L., Cobbold S. P., Waldmann H. Dominant transplantation tolerance impairs CD8+ T cell function but not expansion. Nat.Immunol. 2002;3:1208–13. doi: 10.1038/ni853. [DOI] [PubMed] [Google Scholar]
- 52.Davies J. D., Leong L. Y., Mellor A., Cobbold S. P., Waldmann H. T cell suppression in transplantation tolerance through linked recognition. J.Immunol. 1996;156:3602–7. [PubMed] [Google Scholar]
- 53.Nishimura E., Sakihama T., Setoguchi R., Tanaka K., Sakaguchi S. Induction of antigen-specific immunologic tolerance by in vivo and in vitro antigen-specific expansion of naturally arising Foxp3+CD25+CD4+ regulatory T cells. Int.Immunol. 2004;16:1189–201. doi: 10.1093/intimm/dxh122. [DOI] [PubMed] [Google Scholar]
- 54.Wolf D., Hochegger K., Wolf A. M., Rumpold H. F., Gastl G., Tilg H., et al. CD4+CD25+ regulatory T cells inhibit experimental anti-glomerular basement membrane glomerulonephritis in mice. J.Am.Soc.Nephrol. 2005;16:1360–70. doi: 10.1681/ASN.2004100837. [DOI] [PubMed] [Google Scholar]
- 55.Hochegger K., Wolf D., Rosenkranz A. R. CD4CD25 regulatory T cells: a new treatment option in glomerulonephritis. Kidney Int. 2005;68:1898–9. doi: 10.1038/ki.2005.4496299. [DOI] [PubMed] [Google Scholar]
- 56.Taylor P. A., Lees C. J., Blazar B. R. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99:3493–9. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- 57.Bluestone J. A. Regulatory T-cell therapy: is it ready for the clinic? Nat.Rev.Immunol. 2005;5:343–9. doi: 10.1038/nri1574. [DOI] [PubMed] [Google Scholar]
- 58.Hoffmann P., Eder R., Kunz-Schughart L. A., Andreesen R., Edinger M. Large-scale in vitro expansion of polyclonal human CD4(+)CD25high regulatory T cells. Blood. 2004;104:895–903. doi: 10.1182/blood-2004-01-0086. [DOI] [PubMed] [Google Scholar]
- 59.Godfrey W. R., Ge Y. G., Spoden D. J., Levine B. L., June C. H., Blazar B. R., et al. In vitro-expanded human CD4(+)CD25(+) T-regulatory cells can markedly inhibit allogeneic dendritic cell-stimulated MLR cultures. Blood. 2004;104:453–61. doi: 10.1182/blood-2004-01-0151. [DOI] [PubMed] [Google Scholar]
- 60.Tang Q., Henriksen K. J., Bi M., Finger E. B., Szot G., Ye J., et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J.Exp.Med. 2004;199:1455–65. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Battaglia M., Stabilini A., Roncarolo M. G. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105:4743–8. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 62.Nakamura K., Kitani A., Fuss I., Pedersen A., Harada N., Nawata H., et al. TGF-beta 1 plays an important role in the mechanism of CD4+CD25+ regulatory T cell activity in both humans and mice. J.Immunol. 2004;172:834–42. doi: 10.4049/jimmunol.172.2.834. [DOI] [PubMed] [Google Scholar]
- 63.Chen W., Wahl S. M. TGF-beta: the missing link in CD4+CD25+ regulatory T cell-mediated immunosuppression. Cytokine Growth Factor Rev. 2003;14:85–9. doi: 10.1016/s1359-6101(03)00003-0. [DOI] [PubMed] [Google Scholar]
- 64.Horwitz D. A., Gray J. D., Zheng S. G. The potential of human regulatory T cells generated ex vivo as a treatment for lupus and other chronic inflammatory diseases. Arthritis Res. 2002;4:241–6. doi: 10.1186/ar414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang X., Giangreco L., Broome H. E., Dargan C. M., Swain S. L. Control of CD4 effector fate: transforming growth factor beta 1 and interleukin 2 synergize to prevent apoptosis and promote effector expansion. J.Exp.Med. 1995;182:699–709. doi: 10.1084/jem.182.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Horwitz D. A., Zheng S. G., Gray J. D., Wang J. H., Ohtsuka K., Yamagiwa S. Regulatory T cells generated ex vivo as an approach for the therapy of autoimmune disease. Semin.Immunol. 2004;16:135–43. doi: 10.1016/j.smim.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 67.Watanabe M., Mencel R. L., Cramer D. V., Starnes V. A., Barr M. L. Transforming growth factor-beta/interleukin-2-induced regulatory CD4+ T cells prolong cardiac allograft survival in rats. J.Heart Lung Transplant. 2005;24:2153–9. doi: 10.1016/j.healun.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 68.Zeller J. C., Panoskaltsis-Mortari A., Murphy W. J., Ruscetti F. W., Narula S., Roncarolo M. G., et al. Induction of CD4+ T cell alloantigen-specific hyporesponsiveness by IL-10 and TGF-beta. J.Immunol. 1999;163:3684–91. [PubMed] [Google Scholar]
- 69.Collison L. W., Workman C. J., Kuo T. T., Boyd K., Wang Y., Vignali K. M., et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–9. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 70.Thornton A. M., Shevach E. M. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J.Exp.Med. 1998;188:287–96. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bluestone J. A. Regulatory T-cell therapy: is it ready for the clinic? Nat.Rev.Immunol. 2005;5:343–9. doi: 10.1038/nri1574. [DOI] [PubMed] [Google Scholar]
- 72.Trenado A., Charlotte F., Fisson S., Yagello M., Klatzmann D., Salomon B. L., et al. Recipient-type specific CD4+CD25+ regulatory T cells favor immune reconstitution and control graft-versus-host disease while maintaining graft-versus-leukemia. J.Clin.Invest. 2003;112:1688–96. doi: 10.1172/JCI17702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Battaglia M., Stabilini A., Draghici E., Gregori S., Mocchetti C., Bonifacio E., et al. Rapamycin and interleukin-10 treatment induces T regulatory type 1 cells that mediate antigen-specific transplantation tolerance. Diabetes. 2006;55:40–9. [PubMed] [Google Scholar]
- 74.Roncarolo M. G., Bacchetta R., Bordignon C., Narula S., Levings M. K. Type 1 T regulatory cells. Immunol.Rev. 2001;182:68–79. doi: 10.1034/j.1600-065x.2001.1820105.x. [DOI] [PubMed] [Google Scholar]
- 75.Roncarolo M. G., Battaglia M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat.Rev.Immunol. 2007;7:585–98. doi: 10.1038/nri2138. [DOI] [PubMed] [Google Scholar]
- 76.Antony P. A., Piccirillo C. A., Akpinarli A., Finkelstein S. E., Speiss P. J., Surman D. R., et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J.Immunol. 2005;174:2591–601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Toka F. N., Suvas S., Rouse B. T. CD4+ CD25+ T cells regulate vaccine-generated primary and memory CD8+ T-cell responses against herpes simplex virus type 1. J.Virol. 2004;78:13082–9. doi: 10.1128/JVI.78.23.13082-13089.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Roncarolo M. G., Battaglia M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat.Rev.Immunol. 2007;7:585–98. doi: 10.1038/nri2138. [DOI] [PubMed] [Google Scholar]
- 79.Hoffmann P., Boeld T. J., Eder R., Albrecht J., Doser K., Piseshka B., et al. Isolation of CD4+CD25+ regulatory T cells for clinical trials. Biol.Blood Marrow Transplant. 2006;12:267–74. doi: 10.1016/j.bbmt.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 80.De Andres C., Aristimuno C., De Las Heras V., Martinez-Gines M. L., Bartolome M., Arroyo R., et al. Interferon beta-1a therapy enhances CD4+ regulatory T-cell function: an ex vivo and in vitro longitudinal study in relapsing-remitting multiple sclerosis. J.Neuroimmunol. 2007;182:204–11. doi: 10.1016/j.jneuroim.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 81.Vandenbark A. A., Culbertson N. E., Bartholomew R. M., Huan J., Agotsch M., Latocha D., et al. Therapeutic vaccination with a trivalent T-cell receptor (TCR) peptide vaccine restores deficient FoxP3 expression and TCR recognition in subjects with multiple sclerosis. Immunology. 2008;123:66–78. doi: 10.1111/j.1365-2567.2007.02703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ehrenstein M. R., Evans J. G., Singh A., Moore S., Warnes G., Isenberg D. A., et al. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J.Exp.Med. 2004;200:277–85. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nadkarni S., Mauri C., Ehrenstein M. R. Anti-TNF-alpha therapy induces a distinct regulatory T cell population in patients with rheumatoid arthritis via TGF-beta. J.Exp.Med. 2007;204:33–9. doi: 10.1084/jem.20061531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chatenoud L., Bluestone J. A. CD3-specific antibodies: a portal to the treatment of autoimmunity. Nat.Rev.Immunol. 2007;7:622–32. doi: 10.1038/nri2134. [DOI] [PubMed] [Google Scholar]
- 85.Chatenoud L. CD3-specific antibody-induced active tolerance: from bench to bedside. Nat.Rev.Immunol. 2003;3:123–32. doi: 10.1038/nri1000. [DOI] [PubMed] [Google Scholar]
- 86.Wolf D., Hochegger K., Wolf A. M., Rumpold H. F., Gastl G., Tilg H., et al. CD4+CD25+ regulatory T cells inhibit experimental anti-glomerular basement membrane glomerulonephritis in mice. J.Am.Soc.Nephrol. 2005;16:1360–70. doi: 10.1681/ASN.2004100837. [DOI] [PubMed] [Google Scholar]
- 87.Earle K. E., Tang Q., Zhou X., Liu W., Zhu S., Bonyhadi M. L., et al. In vitro expanded human CD4+CD25+ regulatory T cells suppress effector T cell proliferation. Clin.Immunol. 2005;115:3–9. doi: 10.1016/j.clim.2005.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jiang S., Camara N., Lombardi G., Lechler R. I. Induction of allopeptide-specific human CD4+CD25+ regulatory T cells ex vivo. Blood. 2003;102:2180–6. doi: 10.1182/blood-2003-04-1164. [DOI] [PubMed] [Google Scholar]
- 89.Jiang S., Lechler R. I., He X. S., Huang J. F. Regulatory T cells and transplantation tolerance. Hum.Immunol. 2006;67:765–76. doi: 10.1016/j.humimm.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 90.Allan S. E., Alstad A. N., Merindol N., Crellin N. K., Amendola M., Bacchetta R., et al. Generation of potent and stable human CD4+ T regulatory cells by activation-independent expression of FOXP3. Mol.Ther. 2008;16:194–202. doi: 10.1038/sj.mt.6300341. [DOI] [PubMed] [Google Scholar]
- 91.Battaglia M., Stabilini A., Migliavacca B., Horejs-Hoeck J., Kaupper T., Roncarolo M. G. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J.Immunol. 2006;177:8338–47. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]
- 92.Trenado A., Charlotte F., Fisson S., Yagello M., Klatzmann D., Salomon B. L., et al. Recipient-type specific CD4+CD25+ regulatory T cells favor immune reconstitution and control graft-versus-host disease while maintaining graft-versus-leukemia. J.Clin.Invest. 2003;112:1688–96. doi: 10.1172/JCI17702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sanchez-Fueyo A., Sandner S., Habicht A., Mariat C., Kenny J., Degauque N., et al. Specificity of CD4+CD25+ regulatory T cell function in alloimmunity. J.Immunol. 2006;176:329–34. doi: 10.4049/jimmunol.176.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tang Q., Bluestone J. A. Regulatory T-cell physiology and application to treat autoimmunity. Immunol.Rev. 2006;212:217–37. doi: 10.1111/j.0105-2896.2006.00421.x. [DOI] [PubMed] [Google Scholar]
- 95.Liu W., Putnam A. L., Xu-Yu Z., Szot G. L., Lee M. R., Zhu S., et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J.Exp.Med. 2006;203:1701–11. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Seddiki N., Santner-Nanan B., Martinson J., Zaunders J., Sasson S., Landay A., et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J.Exp.Med. 2006;203:1693–700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Allan S. E., Broady R., Gregori S., Himmel M. E., Locke N., Roncarolo M. G., et al. CD4+ T-regulatory cells: toward therapy for human diseases. Immunol.Rev. 2008;223:391–421. doi: 10.1111/j.1600-065X.2008.00634.x. [DOI] [PubMed] [Google Scholar]
- 98.Tao R., De Zoeten E. F., Ozkaynak E., Wang L., Li B., Greene M. I., et al. Histone deacetylase inhibitors and transplantation. Curr.Opin.Immunol. 2007;19:589–95. doi: 10.1016/j.coi.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Segundo D. S., Ruiz J. C., Izquierdo M., Fernandez-Fresnedo G., Gomez-Alamillo C., Merino R., et al. Calcineurin inhibitors, but not rapamycin, reduce percentages of CD4+CD25+FOXP3+ regulatory T cells in renal transplant recipients. Transplantation. 2006;82:550–7. doi: 10.1097/01.tp.0000229473.95202.50. [DOI] [PubMed] [Google Scholar]
- 100.Pascual J., Bloom D., Torrealba J., Brahmbhatt R., Chang Z., Sollinger H. W., et al. Calcineurin Inhibitor Withdrawal After Renal Transplantation with Alemtuzumab: Clinical Outcomes and Effect on T-Regulatory Cells. Am.J.Transplant. 2008 doi: 10.1111/j.1600-6143.2008.02260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]