Abstract
Radiation-induced injury may occur in various incidents as well as the terrorist radiation exposure scenario. The digestive tract is among the most radiosensitive organs in the body and its function, which is partly regulated by gastrointestinal (GI) peptides, can be affected by radiation exposure. However, very little is known about the effect of whole-body radiation on blood GI peptides. The aim of this study therefore was to determine the effect of whole-body radiation on circulating levels of GI peptides in the rat. To study this, rats were exposed to 5-Gy whole-body gamma radiation. They were then euthanized at 1, 2, 4, or 8 days after irradiation. Plasma levels of cholecystokinin (CCK), secretin, gastrin, and ghrelin were determined using specific enzyme immunoassays. Serum levels of alanine transaminase (ALT), alkaline phosphatase (ALP), total bilirubin, and lactate were also measured. Our results showed that whole-body irradiation significantly decreased plasma CCK levels by 57% and 54% at 1 and 2 days after irradiation (P<0.05), respectively. At 4 and 8 days after irradiation, plasma CCK levels returned to normal. Similarly, plasma levels of secretin decreased by 48% at 2 days after irradiation (P<0.05), and returned to normal at 8 days after irradiation. In contrast, there was no significant change in plasma levels of gastrin and ghrelin after irradiation. No significant differences were observed in ALT, ALP, total bilirubin, or lactate. In conclusion, whole-body radiation exposure alters blood GI peptides especially the ones that were produced in the small intestine, such as CCK and secretin. The diverse response of the GI peptides to irradiation could be due to a difference in radiosensitivity in different regions of the GI tract.
Keywords: Radiation injury, gastrin, cholecystokinin, secretin, ghrelin
Introduction
Radiation-induced injury may occur in various incidents including industrial and medical radiation accidents, nuclear reactor events, detonation of nuclear weapons, as well as potential terrorist attack involving radioactive materials. Ionizing radiation such as gamma rays, alpha and beta particles is released by unstable atoms that have an excess of energy, mass, or both. Whole-body exposure to gamma rays, x-rays, or neutrons, which can penetrate through the body, results in damage to multiple tissues and organ systems. Ionizing radiation damages human tissue in many ways [1]. It can interact directly with cellular macromolecules such as DNA, mRNA and proteins to break their covalent bonds, and irreversibly destroy their structure. Ionizing radiation can also indirectly interact with cells by causing hydrolysis reaction of cellular water resulting in hydrogen molecules and hydroxyl (free radical) molecules that disrupt adjacent cellular architecture and genomic information. These interactions may eventually lead to either mutation of the cell or cell death.
Radiosensitivity is defined as a susceptibility of cells to the detrimental effect of ionizing radiation which can be extended to tissues, organs and organisms. The gastrointestinal (GI) tract is among the most radiosensitive organ systems in the body. In addition to the intestinal epithelium crypt, radiation exposure damages supporting structures such as endocrine glands of the GI tract [2]. The GI tract has been considered as the largest endocrine organ because of its complement of endocrine cells which produce a variety of peptides that are involved in GI motility, secretion, absorption, growth, and development [3]. Alterations in either hormone secretion or action can have deleterious effects on the GI function. However, very little is known about the effect of radiation in the blood levels of GI peptides. The aim of this study therefore was to determine the effect of whole-body irradiation on circulating levels of GI peptides such as cholecystokinin (CCK), secretin, gastrin, and ghrelin in the rat.
Materials and Methods
Experimental animals
Male Sprague-Dawley rats (250–280g), purchased from Charles River Laboratories (Wilmington, MA), were used in this study. The rats were housed in a temperature-controlled room on a 12-h light/dark cycle and fed on a standard Purina rat chow diet. Animal experimentation was carried out in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources). This project was approved by the Institutional Animal Care and Use Committee of The Feinstein Institute for Medical Research.
Experimental protocol
Rats were anesthetized with intraperitoneal pentobarbital (40 mg/kg BW) and exposed to whole-body irradiation performed with Gammacell 1000 irradiator (Atomic Energy of Canada Ltd). The irradiator was set to deliver gamma-irradiation at a dose rate of approximately 360 rad/min for 1.4 min, a total of 5 Gy per animal. During irradiation, the rats were restrained with plastic wrap and placed in a fitted container in up-right position and during radiation the container rotated continuously in front of the radiation source for even exposure. The animals were returned to their cages after irradiation. They were euthanized at 1, 2, 4, or 8 days after irradiation. Blood samples were collected, and plasma as well as serum were separated. Sham animals underwent the same procedure as the irradiated rats but received no radiation.
Determination of plasma levels of cholecystokinin (CCK), secretin, and gastrin
The enzyme immunoassay kits for rat CCK and secretin were purchased from Phoenix Pharmaceuticals (Burlingame, CA). The gastrin enzyme immunoassay kit was purchased from Assay Designs (Ann Arbor, MI). The peptides in plasma samples were extracted by C18 SEP-Column (Code RK-SEPCOL-1) according to the manufacturer's instruction. Extracted peptides were reconstituted with assay buffer and then measured for CCK, secretin and gastrin levels using specific enzyme immunoassay kit. Procedures for the measurement of these peptides were performed in strict accordance with the manufacturers' instruction.
Determination of plasma levels of ghrelin
Plasma concentrations of ghrelin were measured using enzyme immunoassay kits according to the instructions provided by the manufacturer (Peninsula Laboratories, San Carlos, CA).
Determination of serum levels of alanine transaminase, alkaline phosphatase, total bilirubin, and lactate
Serum concentrations of alanine transaminase (ALT), alkaline phosphatase (ALP), total bilirubin, and lactate were determined by using assay kits according to the manufacturer's instructions (Pointe Scientific, Canton, MI).
Statistical analysis
All data are expressed as means ± standard error (SE) and compared by one-way analysis of variance (ANOVA) and Student-Newman-Keuls test for multiple group analysis. Differences in values were considered significant if P<0.05.
Results
Effects of whole-body irradiation on plasma levels of gastrointestinal peptides
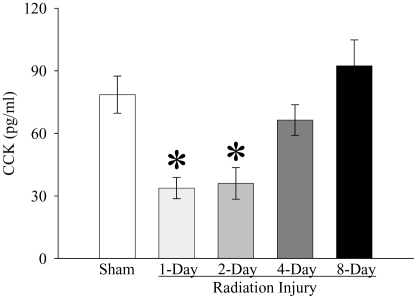
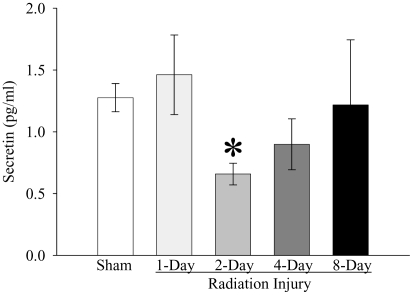
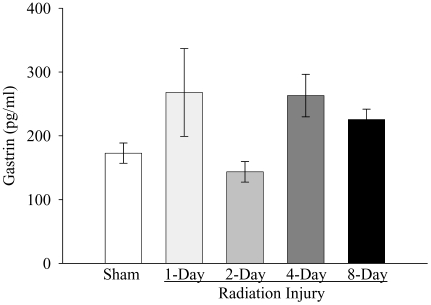
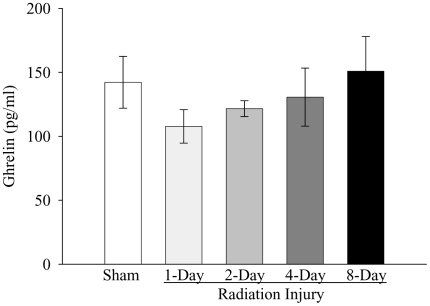
As shown in Figure 1, whole-body irradiation significantly decreased plasma CCK levels by 57% and 54% at 1 and 2 days post-irradiation, respectively, when compared with those in sham rats (P<0.05). The plasma level of CCK started to recover 4 days post-irradiation. At 8 days after irradiation, plasma CCK levels were even 17% higher than those in sham rats, but not statistically significant. As indicated in Figure 2, plasma secretin levels were reduced by 48% at 2 days after irradiation as compared with those in sham controls (P<0.05). At 4 days post irradiation, the secretin level was also 30% lower than that in sham rats, but no statistically significant differences were observed. The plasma secretin level returned to sham levels at 8 days after irradiation. As shown in Figure 3, plasma gastrin levels at 1, 2, 4, and 8 days after irradiation were 155%, 83%, 152%, and 130% of those in the sham animals, respectively. However, no statistically significant differences were observed in plasma gastrin levels between irradiated and sham animals. As displayed in Figure 4, plasma ghrelin levels decreased by 24%, 11% and 8% at 1, 2, and 4 days after irradiation, respectively. At 8 days post-irradiation, plasma levels of ghrelin were 6% higher than those in the sham group. Similar to plasma levels of gastrin, no statistically significant differences were observed in plasma ghrelin levels between irradiated and sham animals.
Figure 1.
Alterations in plasma levels of cholecystokinin (CCK) in non-irradiated animals (Sham) and irradiated animals at 1, 2, 4, or 8 days after radiation injury. Data are presented as means ± SE (n=6) and compared by one-way ANOVA and student-Newman-Keuls method: * P<0.05 versus Sham group.
Figure 2.
Alterations in plasma levels of secretin in non-irradiated animals (Sham) and irradiated animals at 1, 2, 4, or 8 days after radiation injury. Data are presented as means ± SE (n=6) and compared by one-way ANOVA and student-Newman-Keuls method: * P<0.05 versus Sham group.
Figure 3.
Alterations in plasma levels of gastrin in non-irradiated animals (Sham) and irradiated animals at 1, 2, 4, or 8 days after radiation injury. Data are presented as means ± SE (n=6) and compared by one-way ANOVA and student-Newman-Keuls method.
Figure 4.
Alterations in plasma levels of ghrelin in non-irradiated animals (Sham) and irradiated animals at 1, 2, 4, or 8 days after radiation injury. Data are presented as means ± SE (n=6) and compared by one-way ANOVA and student-Newman-Keuls method.
Effects of whole-body irradiation on serum levels of ALT, ALP, total bilirubin, and lactat
Serum ALT, ALP, and total bilirubin were determined to evaluate hepatic injury, while serum lactate concentration was studied to assess oxygen deprivation and general tissue damage. As demonstrated in Table 1, the serum levels of ALT, ALP, total bilirubin, and lactate were within normal range and there was no significant differences found between the irradiated animals and the sham group.
Table 1.
Alterations in serum levels of ALT, ALP, total bilirubin, and lactate in non-irradiated animals (Sham) and irradiated animals at 1, 2, 4, or 8 days after radiation injury.
| Sham | Radiation Injury | ||||
|---|---|---|---|---|---|
| 1-Day | 2-Day | 4-Day | 8-Day | ||
| ALT (IU/L) | 12.8±2.3 | 11.1±1.6 | 12.1±4.6 | 15.3±5.4 | 11.3±2.0 |
| ALP (IU/L) | 80.8±9.2 | 95.8±18.6 | 70.9±17.6 | 89.5±8.5 | 88.4±13.1 |
| Total bilirubin (mg/dl) | 0.5±0.1 | 0.4±0.1 | 0.4±0.1 | 0.4±0.1 | 0.5±0.1 |
| Lactate (mg/dl) | 10.9±2.0 | 7.8±1.5 | 8.8±1.9 | 9.7±1.4 | 7.6±1.2 |
Data are presented as means ± SE (n=6) and compared by one-way ANOVA and student- Newman-Keuls method, which showed no statistical differences.
Discussion
It is known that radiation can damage human cells which may involve every major organ system. Clinical syndromes and symptoms arising from radiation exposures are referred to as radiation sickness or acute radiation syndrome (ARS). The ARS occurs after whole-body or significant partial-body irradiation of greater than 1 Gy delivered at a relatively high-dose rate [4]. Clinical manifestations of GI syndrome, one of clinical components of the ARS, include nausea, vomiting, gastric atony, abdominal pain, diarrhea, GI bleeding, and fluid and electrolyte imbalance [2,5]. The mechanisms of radiation-induced digestive tract injury are complicated, multi-factorial, and still incompletely defined.
GI hormones play an important role in regulating GI function [3,6]. CCK stimulates gallbladder contraction and pancreatic secretion, regulate gastric emptying and bowel motility, and induce satiety [3]. The primary action of secretin is to regulate pancreatic fluid and bicarbonate secretion [7]. In physiological concentrations, secretin inhibits gastric acid release, gastric motility, and gastrin release. Secretin also increases bile flow, gastrointestinal motility, and lower esophageal sphincter pressure. Gastrin is the major hormone that stimulates gastric acid secretion and has growth promoting effects on gastric mucosa [8]. Ghrelin stimulates growth hormone (GH) secretion, increases food intake, and produces weight gain [9]. Ghrelin also can stimulate gastric contraction and enhances stomach emptying. In the current study, we exposed rats to whole-body irradiation in an effort to study effects of irradiation on circulating GI peptides (i.e., CCK, secretin, gastrin, and ghrelin), which regulate GI function.
Our results demonstrated that plasma levels of GI peptides, especially CCK and secretin, were affected by whole-body irradiation. The reason for the various responses of the GI peptides to radiation exposure could be due to a difference of radiosensitivity in each region of the GI tract. The duodenum is the most radiosensitive region of the digestive tract, followed by the jejunum, ileum, esophagus, stomach, colon, and rectum [2]. These are consistent with our findings showing that the plasma level of CCK, which are produced by “I cells” that are concentrated in proximal small intestine particularly in duodenum, were decreased significantly earlier (observed at 1 day after radiation exposure) than other studied GI peptides. The level of plasma CCK continued to be declined at 2 days post-irradiation with a tendency to decline in 4-day post-irradiation. Secretin-producing cells (S cells) are found along the small intestine with increasing in concentration distally [10]. We demonstrated that the plasma level of secretin was reduced significantly when compared with the control group after irradiation. However, its decline was found at 2 days after radiation exposure which occurred later than the decline in the level of plasma CCK (at 1 day after irradiation). This delay in decline of secretin levels could be due to the difference in radiosensitivity from proximal to the distal region. Thus, it can be speculated that higher radiation exposure can irreversibly damage the GI functions. Further studies are warranted for such conclusion.
Ghrelin and gastrin are GI peptides that are mainly produced in the stomach. The gastric fundus is the most abundant source of ghrelin [11] and most gastrin is produced in endocrine cells (G cells) of the gastric antrum [6]. Since the gastric mucosa is relatively more radioresistant than the duodenum and other parts of small bowels such as the jejunum and ileum, the lack of significant changes in plasma levels of ghrelin and gastrin after irradiation were not surprising.
The levels of all GI peptides measured in this study returned to normal at 8 days post irradiation. This is probably due to the recovery of GI peptide-producing cells and mucosa from radiation-induced injury which was consistent with a previous published data by Carr et al [12]. They reported the changes in epithelial and non-epithelial cells in small intestines after ionizing irradiation and found a drop in number of visible enterocytes and endocrine cells at 1 and 3 days post irradiation. By 7 days after irradiation, the number of enterocytes and endocrine cells returned to control levels [12].
The hepatic cells are relatively radioresistant and there is a marked capacity for regeneration following destruction of a large portion of the liver [13]. Radiation-induced hepatic injury represents clinical findings ranging from asymptomatic biochemical changes including elevations of serum transaminases and ALP to fulminant hepatic failure [13]. We found that the serum levels of ALT, ALP, total bilirubin, and lactate were within normal range, suggesting no measurable radiation-induced hepatic injury or general tissue hypoxia at this dose of irradiation in the rat.
The mechanisms of radiation-induced GI tract injury are complicated. The relationship between alterations of plasma GI peptides and symptoms and signs of the radiation sickness has not been previously well described. Our observations provide the evidence that radiation exposure alters blood levels of GI peptides especially the ones that produced in the small intestine, such as CCK and secretin. The various responses of GI peptides to whole-body irradiation are possibly because of the differences in radiosensitivity in each part of the GI tract. Further studies are needed for better understanding of the relationship between radiation-induced injury and radiation sickness syndrome. At least, the observation in this study may apply and partly explain GI manifestations after radiation-induced injury.
Acknowledgments
We sincerely appreciate the superb technical assistances of Youxin Ji and Weifeng Dong at the Laboratory of Surgical Research, The Feinstein Institute for Medical Research. We also thank Dr. Asha Jacob for critical review of the manuscript. This study was supported by National Institutes of Health grants R21 AI080536, R01 GM053008, and R01 AG028352 (PW).
References
- 1.Reeves GI. Radiation injuries. Crit Care Clin. 1999;15:457–473. doi: 10.1016/s0749-0704(05)70063-4. [DOI] [PubMed] [Google Scholar]
- 2.Hauer-Jensen M, Wang J, Boerma M, Fu Q, Denham JW. Radiation damage to the gastrointestinal tract: mechanisms, diagnosis, and management. Curr Opin Support Palliat Care. 2007;1:23–29. doi: 10.1097/SPC.0b013e3281108014. [DOI] [PubMed] [Google Scholar]
- 3.Rozengurt E, Guha S, Sinnett-Smith J. Gastrointestinal peptide signalling in health and disease. Eur J Surg Suppl. 2002:23–38. [PubMed] [Google Scholar]
- 4.Waselenko JK, MacVittie TJ, Blakely WF, Pesik N, Wiley AL, Dickerson WE, Tsu H, Confer DL, Coleman CN, Seed T, Lowry P, Armitage JO, Dainiak N. Medical management of the acute radiation syndrome: recommendations of the Strategic National Stockpile Radiation Working Group. Ann Intern Med. 2004;140:1037–1051. doi: 10.7326/0003-4819-140-12-200406150-00015. [DOI] [PubMed] [Google Scholar]
- 5.Bismar MM, Sinicrope FA. Radiation enteritis. Curr Gastroenterol Rep. 2002;4:361–365. doi: 10.1007/s11894-002-0005-3. [DOI] [PubMed] [Google Scholar]
- 6.Rehfeld JF. The new biology of gastrointestinal hormones. Physiol Rev. 1998;78:1087–1108. doi: 10.1152/physrev.1998.78.4.1087. [DOI] [PubMed] [Google Scholar]
- 7.Lam IP, Siu FK, Chu JY, Chow BK. Multiple actions of secretin in the human body. Int Rev Cytol. 2008;265:159–190. doi: 10.1016/S0074-7696(07)65004-9. [DOI] [PubMed] [Google Scholar]
- 8.Dockray G, Dimaline R, Varro A. Gastrin: old hormone, new functions. Pflugers Arch. 2005;449:344–355. doi: 10.1007/s00424-004-1347-5. [DOI] [PubMed] [Google Scholar]
- 9.Kojima M, Kangawa K. Structure and function of ghrelin. Results Probl Cell Differ. 2008;46:89–115. doi: 10.1007/400_2007_049. [DOI] [PubMed] [Google Scholar]
- 10.Mutoh H, Ratineau C, Ray S, Leiter AB. Review article: transcriptional events controlling the terminal differentiation of intestinal endocrine cells. Aliment Pharmacol Ther. 2000;14(Suppl 1):170–175. doi: 10.1046/j.1365-2036.2000.014s1170.x. [DOI] [PubMed] [Google Scholar]
- 11.Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, Suda M, Koh T, Natsui K, Toyooka S, Shirakami G, Usui T, Shimatsu A, Doi K, Hosoda H, Kojima M, Kangawa K, Nakao K. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab. 2001;86:4753–4758. doi: 10.1210/jcem.86.10.7885. [DOI] [PubMed] [Google Scholar]
- 12.Carr KE, Hume SP, Nelson AC, O'Shea O, Hazzard RA, McCullough JS. Morphological profiles of neutron and X-irradiated small intestine. J Radiat Res (Tokyo) 1996;37:38–48. doi: 10.1269/jrr.37.38. [DOI] [PubMed] [Google Scholar]
- 13.Cromheecke M, Konings AW, Szabo BG, Hoekstra HJ. Liver tissue tolerance for irradiation: experimental and clinical investigations. Hepatogastroenterology. 2000;47:1732–1740. [PubMed] [Google Scholar]