Abstract
Sepsis refers to a systemic inflammatory response syndrome resulting from a microbial infection, which kills > 225,000 people annually in the U.S. alone. The high mortality of sepsis is partly mediated by bacterial endotoxin, which stimulates macrophages/monocytes to sequentially release early (e.g., TNF) and late (e.g., HMGB1) pro-inflammatory cytokines. Although early proinflammatory cytokines may be protective against infection, excessive accumulation of late-acting proinflammatory mediators (such as HMGB1) may sustain a potentially injurious inflammatory response. Agents capable of inhibiting HMGB1 activities (e.g., neutralizing antibodies) or release [e.g., Chinese herbs, Danggui (Angelica sinensis), Danshen (Salvia miltiorrhiza) and Green tea (Camellia sinensis)] rescue mice from lethal sepsis even when given 24 hours after onset of the disease. Here we review emerging evidence that support a critical role for extracellular HMGB1 as a late mediator of lethal sepsis, and several commonly used Chinese herbs (Danggui, Danshen and Green tea) as potential HMGB1- targeting therapeutic agents in experimental sepsis.
Keywords: Inate immune cells, cytokines, sepsis, Chinese herbs, tanshinone, catechin
Introduction
In response to microbial infection, innate immune cells (such as macrophages, monocytes, and neutrophils) constitute a front line of defense by ingesting and killing invading pathogens. If the invading pathogens are efficiently eliminated, the inflammatory response resolves normally to restore immunologic homeostasis. In contrast, inefficient pathogen clearance can lead to a rigorous inflammatory response manifested by excessive production of various proinflammatory mediators. Sepsis refers to a systemic inflammatory response syndrome resulting from a microbial infection. As a continuum of increasing clinical severity, “severe sepsis” is defined as sepsis associated with one or more acute organ dysfunctions [1]. Despite recent advances in antibiotic therapy and intensive care, sepsis is still the most common cause of death in the intensive care units, claiming approximately 225,000 victims annually in the U.S. alone.
The high mortality of sepsis is partly mediated by bacterial endotoxins (e.g., Lipopolysaccharide, LPS) [2], which activate macrophages and monocytes to release various proinflammatory mediators such as nitric oxide [3], tumor necrosis factor (TNF) [4], interleukin (IL)- 1 [5], interferon (IFN)-γ 6], and macrophage migration inhibitory factor (MIF) [7]. These proinflammatory mediators, individually or in combination, contribute to the pathogenesis of lethal systemic inflammation. For instance, neutralizing antibodies to TNF, the first cytokine elaborated in inflammatory cascade, reduces lethality in an animal model of endotoxemic/bacteremic shock [4]. However, the early kinetics of TNF production makes it difficult to target in a clinical setting [4], forcing us to search for other late proinflammatory mediators that may offer a wider therapeutic window for the treatment of lethal systemic inflammatory diseases.
Several years ago, we made the seminal observation that a ubiquitous protein, high mobility group box 1 (HMGB1), was released by activated macrophages/monocytes [8,9,10], and functioned as a late mediator of lethal endotoxemia [8] and sepsis [11, 12, 13, 14]. Subsequently, we found that aqueous extracts and/or components of three Chinese herbs, Danggui (Angelica sinensis) [14], Danshen Salvia miltiorrhiza) [15] and Green tea (Camellia sinensis) [16] effectively inhibited bacterial endotoxin-induced HMGB1 release in vitro, and protected mice against lethal endotoxemia and sepsis (induced by CLP) in vivo. Here we review accumulating evidence that support a critical role for extracellular HMGB1 as a late mediator of lethal sepsis, and emerging data that suggest several Chinese medicinal herbs as potential theerapeutic agents for experimental sepsis.
Discovery of HMGB1 as a Late Mediator of Lethal Sepsis
In an effort to broaden the therapeutic window for sepsis, we initiated a search for other macrophage-derived mediators that are endotoxemia. Following stimulation of macrophage cultures with bacterial endotoxin, a 30 kDa protein accumulated late in the culture medium, and was identified as the HMGB1 by N-terminal amino acid sequencing analysis[8]
Nuclear HMGB1 as a DNA-binding protein
As a non-histone nucleosomal protein, HMGB1 was purified from nuclei > 30 years ago, and termed high mobility group box 1 (HMGB1) based on its apid mobility on electrophoresis gels [17]. It is constitutively expressed in many cell types, and a large “pool” of preformed HMGB1 is stored in the nucleus due to the presence of two lysine-rich nuclear localization sequences [18, 19]. As an evolutionarily conserved protein, HMGB1 shares 100% homology (in amino acid sequence) between mouse and rat, and a 99% homology between rodent and human. HMGB1 contains two internal repeats of positively charged domains (“HMG boxes” known as “A box” and “B box”) in the N-terminus, and a continuous stretch of negatively charged (aspartic and glutamic acid) residues in the C-terminus (Figure 1). These HMG boxes enable HMGB1 to bind chromosomal DNA and fulfill its nuclear functions including determination of nucleosomal structure and stability, and regulation of gene expression [13]. Intriguingly, HMGB1 contains consensus binding (LXCXE) motif for retinoblastoma (RB), and functions as a tumor suppressor through HMGB1/RB interaction in human breast cancer cells [20].
Figure 1.
Amino acid sequence of human HMGB1. The N-terminal portion of HMGB1 comprises two internal repeats of a positively charged domain of about 80 amino acids (termed “HMG boxes”) (shown by bold text). The cytokine-stimulating motif (“Cytokine Domain”) of HMGB1 does not overlap with its RAGE-binding site, supporting the potential involvement of other cell surface receptors (such as TLR2 and TLR4) for HMGB1- mediated inflammatory responses.
Extracellular release of HMGB1
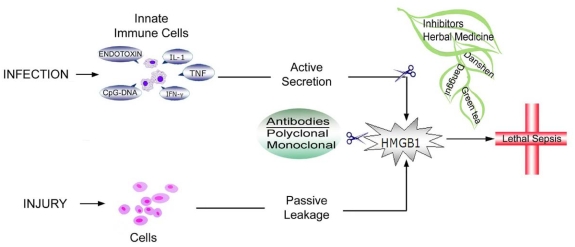
In response to exogenous bacterial products (such as endotoxin or CpG-DNA) [8, 21], or endogenous inflammatory stimuli (e.g., TNF, IFN-γ, or hydrogen peroxide) [8, 9, 22], macrophages and monocytes actively release HMGB1 in a dose- and time-dependent manner (Figure 2). In addition, HMGB1 can be released passively from necrotic or damaged cells [23], and similarly triggers an inflammatory response.
Figure 2.
Schematic summary of recent development in pharmacological inhibition of HMGB1 release and action. In addition to HMGBl-specific antibodies, a number of commonly used Chinese herbs (e.g., Danggui (Angelica sinensis), Danshen (Salvia miltiorrhiza) and Green tea (Camellia sinensis)] are capable of inhibiting HMGB1 release or cytokine activities, thereby rescuing animals from lethal experimental sepsis.
In murine models of endotoxemia (induced by intraperitoneal administration of bacterial endotoxin, LPS) and sepsis (induced by cecal ligation and puncture, CLP), HMGB1 is first detectable in the circulation eight hours after the onset of lethal endotoxemia and sepsis, subsequently increasing to plateau levels from 16 to 32 hours [8, 11]. This late appearance of circulating HMGB1 parallels with the onset of animal lethality from endotoxemia or sepsis, and distinguishes itself from TNF and other early proinflammatory cytokines [24].
In critically ill patients with sepsis, HMGB1 levels in the < 100 kDa serum fraction were significantly elevated, and higher in patients who did not survive than those who survived sepsis [8]. Subsequent studies indicated that levels of HMGB1 in unfractionated crude serum were persistently elevated even in patients that are recovering from severe infections [25, 26]. Unfortunately, immunoblotting analysis of HMGB1 in crude human serum could be perplexed by a few potential problems. For instance, some HMGBl-targeted antibodies could also cross-react with the light chain of human immunoglobulin on Western blots (IgGl, 25 kDa, our unpublished observations), giving potential false positive results. In contrast, many serum/plasma components (such as human IgGl) can bind to HMGB1 [27], negatively interfering with HMGB1 detection by ELISA assays. Furthermore, these serum HMGBl-binding protein(s) may also potentially modulate or mask various proinflammatory activities of HMGB1 [25, 27], adding another layer of complexity to the regulation of already intricate extracellular HMGB1 functions.
Extracellular HMGB1 as an alarmin danger signal
Recently, a number of structurally diverse, multifunctional, ubiquitous host proteins [such as high mobility group box 1 (HMGBl), and heat shock protein 72 (Hsp72)] have been categorized as “alarmins” based on several common properties [28]. First, HMGBl is actively secreted by innate immune cells [8], and/or passively released by injured/damaged cells [23, 29]. Subsequently, extracellular HMGBl is capable of recruiting cells to sites of infection of injury [30, 31], and facilitates innate recognition of bacterial products (e.g., CpG-DNA) by innate immune cells [21, 32]. For instance, extracellular HMGBl can augment CpG-DNA-mediated cytokine production by innate immune cells (e.g., macrophages and dendritic cells) [21, 32], consequently facilitating innate recognition of bacterial/viral CpG-DNA to mount an effective inflammatory response.
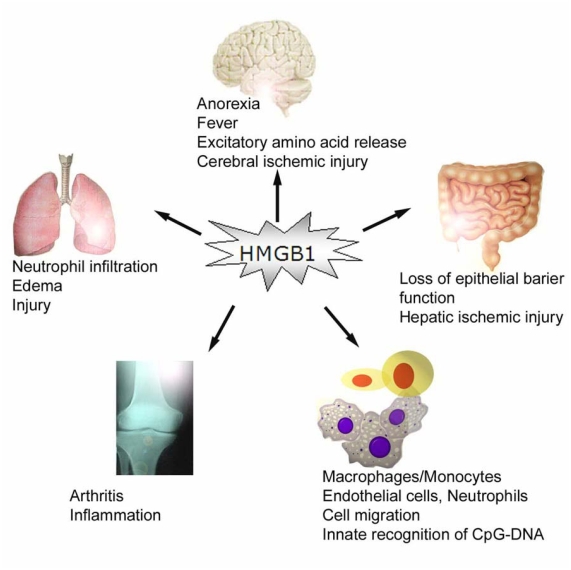
In addition, extracellular HMGBl binds to several cell surface receptors including the receptor for advanced glycation end products (RAGE), and the Toll-like receptor 2 (TLR2), and TLR4 [33, 34], and consequently activates innate immune cells to produce pro-inflammatory cytokines (Figure 3) [33-36]. Indeed, fluorescence resonance energy transfer (FRET) analysis has demonstrated a close physical interaction between HMGBl and TLR2 or TLR4 on macrophage cell surface within 5-15 minutes of HMGBl incubation [35]. Intriguingly, we observed a time-dependent accumulation of exogenous HMGBl clustering on macrophage cell surface within 4-6 hours of HMGBl incubation [16], which correlates with the kinetics of HMGB1-induced release of proinflammatory cytokines [37]. It is plausible that engagement of exogenous HMGBl to cell-surface receptors (such as TLR2, TLR4, and RAGE) induces clustering of ligand/receptor complexes at cell surface [16], thereby activating various innate immune cells.
Figure 3.
HMGBl mediates proinflammatory responses. In vivo, administration of HMGBl via intracerebro-ventricular, intratracheal, intraperitoneal, and intraarticular routes induces marked inflammatory responses. In vitro, HMGBl activate various innate immune cells, and facilitate innate recognition of microbial products (such as CpG-DNA).
Similarly, HMGBl stimulates endothelial cells to express intracellular adhesion molecule 1 (ICAM-1), vascular adhesion molecule 1 (VCAM-1), proinflammatory cytokines (e.g., TNF), and chemokines (e.g., IL-8) (Figure 3) [38, 39]. In the brain, exogenous HMGBl induces release of proinflammatory cytokines [40] and excitatory amino acids (such as glutamate) [41], fever [42], and exacerbates cerebral ischemic injury (Figure 3) [43]. In the lung, HMGBl induces lung neutrophil infiltration, and acute lung injury [44, 45, 46]. Focal administration of HMGBl near the sciatic nerve induces unilateral and bilateral low threshold mechanical allodynia [47]. Similarly, intraperitoneal injection of HMGBl increases ileal mucosal permeability, leading to bacterial translocation to mesenteric lymph nodes [48], and exacerbates hepatic ischemic injury [49].
Although highly purified eukaryotic, or bacterially produced recombinant HMGBl has a weak proinflammatory activity by itself [21, 32, 50], it can bind to various bacterial substances (such as CpG-DNA), thereby strengthening such proinflammatory activities [21, 32]. Considered together, these studies indicate that extracellular HMGBl can function as an alarmin signal, which alerts, recruits, and activates various innate immune cells, and consequently sustains a potentially injurious inflammatory response. Even though excessive HMGBl may be pathogenic, low levels of HMGBl might still be beneficial. For instance, HMGBl is capable of attracting stem cells [31], and may be needed for tissue repair and regeneration. Therefore, like other cytokines, there may be protective advantages of extracellular HMGBl when released at low amounts [51]. It is thus important to pharmacologically modulate, rather than abrogate, systemic HMGBl accumulation to conquer various inflammatory diseases.
Extracellular HMGBl as a later mediator of lethal endotoxemia and sepsis
The patho-genic role of HMGBl as a late mediator of lethal endotoxemia was originally examined using HMGBl-specific neutralizing antibodies, which conferred significant protection against lethal endotoxemia [8], and endotoxin-induced acute lung injury (Figure 2) [44]. In a more clinically relevant animal model of sepsis (induced by CLP), delayed administration of HMGBl-neutralizing antibodies beginning 24 h after the onset of sepsis, dose-dependently rescued mice from lethal sepsis (Figure 2) [11, 52]. An increasing number of agents (anti-HMGBl antibodies, ethyl pyruvate, stearoyl lysophosphatidyl-choline, nicotine, anti-IFN-y antibodies) have shown efficacy in inhibiting bacterial endotoxin-induced HMGBl release in vitro, and protecting animals against lethal endotoxemia [8] and sepsis [11,12, 53], even when the first doses are administered 24 hours after onset of diseases [11, 12]. Notably, the first dose of the HMGBl inhibitors were given 24 h after CLP, a time point at which mice developed clear signs of sepsis including lethargy, diarrhea, piloerection. Together, these experimental data establish HMGBl as a late mediator of lethal endotoxemia and sepsis with a wider therapeutic window for the treatment of lethal systemic inflammatory diseases [13, 24].
Regulation of HMGBl Release
To ensure a timely response to endotoxin, mammals have evolved an effective innate recognition system consisting of LPS-binding protein (LBP), CD14, and Toll-like receptor 4 (TLR4). When presented to CD14 by LBP, LPS is delivered to high affinity transmembrane receptors such as TLR4 [54], leading to activation of MAP kinase (e.g., p38, ERK1/2, and JNK) and NF-κB pathways, and sequential release of early (e.g., TNF) and late (e.g., HMGBl) proinflammatory cytokines. TNF is produced in vanishingly small amounts (if any at all) in quiescent macrophages/monocytes, but its transcription and translation are rapidly up-regulated by endotoxin (LPS), leading to TNF synthesis and secretion within 1-2 hours [55]. LPS fails to induce TNF secretion in CD14-deficient macrophages [10, 56], indicating that the innate recognition system is critically important for endotoxin-induced rapid TNF release [56]. As many other cytokines, TNF contains a leader signal sequence, and is secreted via a classical endoplasmic reticulum (ER)-Golgi secretory pathway.
In contrast, HMGBl is constitutively expressed in quiescent macrophages/monocytes, and a large “pool” of preformed HMGBl is stored in the nucleus [9, 10]. Lacking a leader signal sequence, HMGBl cannot be released via the classical ER-Golgi secretory pathway in response to endotoxin stimulation [8]. Instead, activated macrophages/monocytes acetylated HMGBl at its nuclear localization sequences, leading to sequestration of HMGBl within cytoplasmic vesicles and subsequent release into the extracellular milieu [9, 19, 57]. The LPS-stimulated HMGBl release was only partially (by 30-50%) reduced in CD14-deficient macrophages, suggesting that innate recognition system is somewhat less critical for endotoxin-induced HMGBl release.
In parallel, mammals have also evolved multiple negative regulatory mechanisms to counter-regulate potentially injurious inflammatory response. For instance, the central nervous system can directly and rapidly attenuate endotoxin-induced release of early (e.g., TNF) and late (e.g., HMGBl) proinflam-matory cytokines through acetylcholine, the principal neurotransmitter of the vagus nerve via nicotinic cholinergic receptors [12, 58]. Another local feedback mechanism regulates inflammatory response through spermine, a ubiquitous molecule that accumulates at sites of infection or injury to function as a negative regulator of innate immune response [10, 59-62].
Exploration of Chinese Medicinal Herbs for HMGBl-lnhibiting Agents
Currently, there are two new therapies available for patients with sepsis, including the “Early Goal Directed Therapy” (EGDT) and the use of activated protein C (APC). EGDT employs extremely tight control of a number of physiological parameters (such as central venous pressure, mean arterial blood pressure, central venous oxygen saturation, blood glucose and hematocrit), which reduces septic mortality by 16% (from 46.5% to 30.5%) in a single site clinical trial [63]. On the other hand, APC marginally reduces the 28-day mortality (from 30.8% to 24.7%) [64], but is associated with a 1.5% increase in hemorrhagic complication risk. Therefore, other agents capable of inhibiting late-acting, clinically accessible mediators are still needed for the clinical management of lethal systemic inflammatory diseases.
Traditional herbal medicine has formed the basis of folk remedies for various inflammatory ailments. For instance, Danggui (Angelica sinensis) has been traditionally used to treat gynecological disorders (such as abnormal menstruation), and recently tested for efficacy in animal models of bacteria-induced pneumonia [65], carrageenan-induced edema [66], and ethanol-induced hemorrhagic tissue damage [67]. Another Chinese herb, Danshen (Saliva miltorrhiza) has been widely used in China for patients with cardiovascular disorders [68]. Similarly, green tea brewed from the leaves of the plant, Camellia sinensis, has been associated with many important health benefits, such as reduction of risk of oxidative stress and damage [69], atherosclerosis [69], cancer [70], and cardiovascular diseases [71]. After screening more than two dozen commonly used Chinese herbs, we found that aqueous extracts of Danggui, Danshen and Green tea efficiently inhibited endotoxin-induced HMGBl release in vitro [14-16], and protected animals against lethal endotoxemia and sepsis in vivo [14].
Suppression of endotoxin-induced HMGBl release
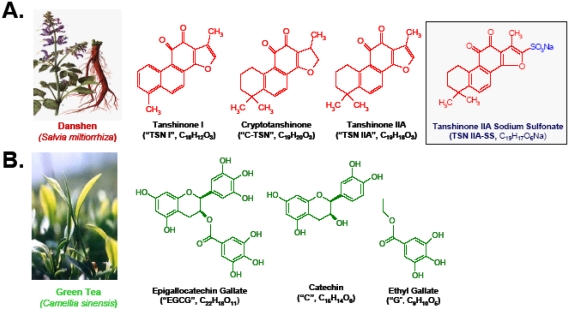
As the first step to elucidate immunemodula-tory mechanisms of the above Chinese herbs, we examined their major anti-inflammatory components for HMGBl-inhibiting activities. Danshen contains abundant red pigments (termed tanshinone I, tanshinone IIA, and cryptotanshinone), a group of substance with medicinal value for patients with cardiovascular abnormalities [68]. Interestingly, all these tanshinones (I, IIA, and cryptotanshinone) effectively attenuated LPS-induced HMGBl release, with estimated IC50 < 25 μM (Figure 4). Despite a structural resemblance (i.e., the presence of a four-fused-ring structure) between tanshinones and steroidal anti-inflammatory drugs (such as dexamethasone and cortisone), these steroids failed to attenuate LPS-induced HMGB1 release [15], indicating that tanshinones and steroidal drugs exert anti-inflammatory action through distinct mechanisms.
Figure 4.
Structures of major components of Danshen (A) and Green tea (B). Danshen contains abundant red pigments termed tanshinone I, tanshinone IIA, and cryptotanshinone. Green tea contains catechins which harbor two or more aromatic rings (each carrying at least one aromatic hydroxyl) connected with a carbon bridge (consisting of five carbons and one oxygen).
Green tea contains a class of biologically active polyphenols called catechins, which harbor two or more aromatic rings (each carrying at least one aromatic hydroxyl) connected with a carbon bridge (consisting of five carbons and one oxygen) (Figure 4). Among them, EGCG accounts for 50-80% of the total catechin, representing approximately 50 mg in a single cup (100 ml) of green tea [72]. Interestingly, EGCG effectively attenuated endotoxin-induced HMGB1 release in a dose-dependent fashion, with an estimated IC50 < 1.0 μM [16]. In contrast, two relevant molecules, catechin and ethyl gallate, did not affect LPS-induced HMGB1 release, even at concentrations up to 10 μM, indicating that functional groups of both catechin and gallate are needed for EGCG's HMGB1- inhibiting properties [16].
To investigate the mechanisms by which Danggui extract and Danshen components (e.g., tanshinone IIA) inhibit HMGB1 release, we determined their effects on endotoxin-induced HMGB1 translocation – an essential step for HMGB1 release [9, 10, 57]. Danggui extract or Danshen component (TSN IIA) almost completely abrogated LPS-induced HMGB1 cytoplasmic translocation in most endotoxin-stimulated cells [14, 16], indicating that Danggui extract and Danshen component attenuate HMGB1 release by interfering with its cytoplasmic translocation.
Suppression of endotoxin-induced release of other cytokines
To better understand Danshen and Green tea's anti-inflammatory properties, we also examined their effects on LPS-induced release of other cytokines. At concentrations (15 μM) that completely abrogated LPS-induced HMGB1 release, EGCG similarly inhibited LPS-induced release of many other cytokines including IL-6, MIP-lα, MIP-lγ, MIP-2, RANTES, KC, MCP1, and CXCL16 [16]. In sharp contrast, a watersoluble derivative (sodium sulfonate) of tanshinone IIA, TSN IIA-SS (Figure 4), at concentrations (100 uM) that completely abrogated LPS-induced HMGBl release, did not suppress LPS-induced release of most cytokines, and only partially attenuated LPS-induced release of IL-12p70, IL-lα, platelet factor 4 (PF-4), and MCP-5 [15]. Taken together, these data indicate that Danshen and Green tea components inhibit several common mediators (e.g., HMGBl), and at the same time exhibit distinct specificities with respect to other cytokines.
Protection against lethal endotoxemia and sepsis
In light of the capacity of aqueous extracts and components of Danggui, Danshen and Green tea in attenuating LPS-induced HMGBl release, we explored their efficacy in an animal model of lethal endotoxemia. Repeated administration of Danggui extract, TSN IIA-SS and EGCG conferred a dose-dependent protection against lethal endo-toxemia [14–16]. More importantly, in animal models of experimental sepsis induced by cecal ligation and puncture, repeated administration of the above agents beginning at +24 h, followed by additional doses at +48, +72 and + 96 h after the onset of sepsis, dose-dependently rescued mice from lethal sepsis (Figure 2) [14-16].
To gain insight into the mechanisms underlying herbal extract or component-mediated protection against lethal sepsis, we evaluated their effects on systemic accumulation of various cytokines. Delayed administration of Danggui extract, TNS IIA-SS, or EGCG did not attenuate circulating levels of TNF or nitric oxide at 52 h after the onset of sepsis (data not shown), but dose-dependently attenuated circulating HMGBl levels in septic mice [14-16]. Furthermore, delayed administration of EGCG markedly attenuated circulating levels of IL-6 and KC [16] – two most reliable surrogate markers of experimental sepsis that can predict outcome [73]. Considered together, these experimental data indicate that these herbal extracts and/or components protect mice against lethal sepsis partly by attenuating systemic accumulation of a late proinflammatory mediator, HMGBl. At present, our experimental data can not exclude the possibility that herbal extracts and/or components confer protection against lethal sepsis through additional unknown mechanisms. Therefore, future studies are needed to better understand the protective mechanisms underlying Chinese herbal medicinal herb-mediated protective effects.
In light of the clinical use of TSN IIA-SS in China for patients with cardiovascular disorders [68], we also determined whether it improves cardiovascular function in septic animals. Administration of TSN IIA-SS did not significantly affect the mean arterial blood pressure, but slightly reduced the heart rate (from 378.3 ± 25.1 to 334.1 ± 25.8 beats/minutes, P < 0.05). More importantly, it dosedependently reduced total peripheral vascular resistance, and yet significantly increased cardiac stroke volume, and cardiac output [15]. As an important organ frequently compromised by sepsis and septic shock, poor cardiac output as a consequence of depressed myocardial function may contribute to the pathogenesis of lethal sepsis or septic shock [74]. The dual effects of TSN IIA-SS in attenuating late inflammatory response and improving cardiovascular function make it a promising therapeutic agent for patients with sepsis.
Suppression of HMGBl cytokine activities
To elucidate additional mechanisms underlying EGCG-mediated protection, we determined whether Green tea component (EGCG) inhibits HMGBl-mediated inflame-matory response. Indeed, EGCG dose-dependently inhibited HMGBl-induced release of TNF, IL-6, and nitric oxide in macrophage cultures [16]. Furthermore, EGCG effectively inhibited HMGBl-induced release of IL-6 release, even when it was given 2-4 hours after HMGBl stimulation. Despite the fact that EGCG failed to inhibit LPS-induced nitric oxide, it dose-dependently suppressed HMGBl-induced release of nitric oxide in macrophage cultures, supporting the notion that LPS and HMGBl use distinct mechanisms to activate innate immune cells [37, 75]. Taken together, these data suggest that EGCG confers protection against lethal sepsis partly by inhibiting HMGBl cytokine activities.
To elucidate the mechanism by which EGCG attenuates HMGBl-mediated cytokine production, we determined its effect on macrophage cell surface accumulation/clustering of exogenous HMGBl. Cell surface accumulation/clustering of exogenous (biotin-labeled) HMGBl, as indicated by streptavidin-conjugated Alexa 594 fluorescence, was noted at 4-6 h post HMGBl treatment [16]. In the presence of EGCG (10 μM), the cell surface accumulation/clustering of exogenous HMGB1 was almost completely eliminated [16], suggesting that EGCG inhibits HMGB1 cytokine activities by preventing its cell surface accumulation/clustering.
Conclusion
A ubiquitous nuclear protein, HMGB1, is released by activated macrophages/monocytes, and functions as a late mediator of lethal endotoxemia and sepsis. First, circulating HMGB1 levels are elevated in a delayed fashion in endotoxemic and septic animals. Second, administration of exogenous HMGB1 to mice induces fever, derangement of intestinal barrier function, and tissue injury. Third, administration of anti-HMGBl antibodies or inhibitors (e.g., Chinese medicinal herbs, Danggui, Danshen, and Green tea) protects mice against lethal endotoxemia, and rescues mice from lethal experimental sepsis even when the first doses are given 24 hours after onset of sepsis. Taken together, these experimental data establish HMGB1 as a late mediator of lethal endotoxemia and sepsis with a wider therapeutic window, and support several commonly used Chinese herbs (e.g., Danggui, Danshen and Green tea) as potential therapeutic agents for experimental sepsis. The downstream or “late” action of HMGB1 is a marked departure from the early activities of TNF and other classical proinflammatory cytokines, and has significant implications for understanding and manipulating innate immune responses. Thus, future studies are needed to further explore the therapeutic potential of Chinese herbal medicine in the clinical management of human sepsis.
Acknowledgments
The studies summarized in this review were supported in part by NIH grants R01-GM063075 and R01GM070817 (to HW).
References
- 1.Riedemann NC, Quo RF, Ward PA. The enigma of sepsis. J Clin Invest. 2003;112:460–467. doi: 10.1172/JCI19523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayala A, Song GY, Chung CS, Redmond KM, Chaudry IH. Immune depression in polymicrobial sepsis: the role of necrotic (injured) tissue and endotoxin. Crit Care Med. 2000;28:2949–2955. doi: 10.1097/00003246-200008000-00044. [DOI] [PubMed] [Google Scholar]
- 3.Dinapoli MR, Calderon CL, Lopez DM. The altered tumoricidal capacity of macrophages isolated from tumor-bearing mice is related to reduce expression of the inducible nitric oxide synthase gene. J Exp Med. 1996;183:1323–1329. doi: 10.1084/jem.183.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tracey KJ, Fong Y, Hesse DG, Manogue KR, Lee AT, Kuo GC, Lowry SF, Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330:662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- 5.Dinarello CA, Thompson RC. Blocking IL-1: interleukin 1 receptor antagonist in vivo and in vitro. Immunol Today. 1991;12:404–410. doi: 10.1016/0167-5699(91)90142-G. [DOI] [PubMed] [Google Scholar]
- 6.Heinzel FP. The role of IFN-gamma in the pathology of experimental endotoxemia. J Immunol. 1990;145:2920–2924. [PubMed] [Google Scholar]
- 7.Calandra T, Echtenacher B, Roy DL, Pugin J, Metz CN, Hultner L, Heumann D, Mannel D, Bucala R, Glauser MP. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nat Med. 2000;6:164–170. doi: 10.1038/72262. [DOI] [PubMed] [Google Scholar]
- 8.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama AE, Tracey KJ. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 9.Rendon-Mitchell B, Ochani M, Li J, Han J, Wang H, Yang H, Susarla S, Czura C, Mitchell RA, Chen G, Sama AE, Tracey KJ, Wang H. IFN-gamma Induces High Mobility Group Box 1 Protein Release Partly Through a TNF-Dependent Mechanism. J Immunol. 2003;170:3890–3897. doi: 10.4049/jimmunol.170.7.3890. [DOI] [PubMed] [Google Scholar]
- 10.Chen G, Li J, Ochani M, Rendon-Mitchell B, Qiang X, Susarla S, Ulloa L, Yang H, Fan S, Goyert SM, Wang P, Tracey KJ, Sama AE, Wang H. Bacterial endotoxin stimulates macrophages to release HMGB1 partly through CD14- and TNF-dependent mechanisms. J Leukoc Biol. 2004;76:994–1001. doi: 10.1189/jlb.0404242. [DOI] [PubMed] [Google Scholar]
- 11.Yang H, Ochani M, Li J, Qiang X, Tanovic M, Harris HE, Susarla SM, Ulloa L, Wang H, Diraimo R, Czura CJ, Wang H, Roth J, Warren HS, Fink MP, Fenton MJ, Andersson U, Tracey KJ. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci USA. 2004;101:296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H, Liao H, Ochani M, Justiniani M, Lin X, Yang L, Al Abed Y, Wang H, Metz C, Miller EJ, Tracey KJ, Ulloa L. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med. 2004;10:1216–1221. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Yang H, Tracey KJ. Extracellula role of HMGB1 in inflammation and sepsis. J Intern Med. 2004;255:320–331. doi: 10.1111/j.1365-2796.2003.01302.x. [DOI] [PubMed] [Google Scholar]
- 14.Wang H, Li W, Li J, Rendon-Mitchell B, Ochani M, Ashok M, Yang L, Yang H, Tracey KJ, Wang P, Sama AE. The Aqueous Extract of a Popular Herbal Nutrient Supplement, Angelica sinensis, Protects Mice against Lethal Endotoxemia and Sepsis. J Nutr. 2006;136:360–365. doi: 10.1093/jn/136.2.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li W, Li J, Ashok M, Wu R, Chen D, Yang L, Yang H, Tracey KJ, Wang P, Sama AE, Wang H. A cardiovascular drug rescues mice from lethal sepsis by selectively attenuating a late-acting proinflammatory mediator, high mobility group box 1. J Immunol. 2007;178:3856–3864. doi: 10.4049/jimmunol.178.6.3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li W, Ashok M, Li J, Yang H, Sama AE, Wang H. A Major Ingredient of Green Tea Rescues Mice from Lethal Sepsis Partly by Inhibiting HMGB1. PLoS ONE. 2007;2:ell53. doi: 10.1371/journal.pone.0001153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johns EW, Johns EW. The HMG Chromosomal Proteins. London: Academic Press Inc London Ltd; 1982. History, Definitions and Problems; pp. 1–8. [Google Scholar]
- 18.Chen G, Li J, Qiang X, Czura CJ, Ochani M, Ochani K, Ulloa L, Yang H, Tracey KJ, Wang P, Sama AE, Wang H. Suppression of HMGB1 release by stearoyl lysophosphatidylcholine:an additional mechanism for its therapeutic effects in experimental sepsis. J Lipid Res. 2005;46:623–627. doi: 10.1194/jlr.C400018-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, Bachi A, Rubartelli A, Agresti A, Bianchi ME. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 2003;22:5551–5560. doi: 10.1093/emboj/cdg516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiao Y, Wang H, Fan SJ. Growth suppression and radiosensitivity increase by HMGB1 in breast cancer. Acta Pharmacol Sin. 2007;28:1957–1967. doi: 10.1111/j.1745-7254.2007.00669.x. [DOI] [PubMed] [Google Scholar]
- 21.Ivanov S, Dragoi AM, Wang X, Dallacosta C, Louten J, Musco G, Sitia G, Yap GS, Wan Y, Biron CA, Bianchi ME, Wang H, Chu WM. A novel role for HMGB1 in TLR9-mediated inflammatory responses to CpG-DNA. Blood. 2007;10(6):1970–1981. doi: 10.1182/blood-2006-09-044776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang D, Shi Y, Kang R, Li T, Xiao W, Wang H, Xiao X. Hydrogen peroxide stimulates macrophages and monocytes to actively release HMGB1. J Leukoc Biol. 2007;81:741–747. doi: 10.1189/jlb.0806540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 24.Wang H, Yang H, Czura CJ, Sama AE, Tracey KJ. HMGB1 as a Late Mediator of Lethal Systemic Inflammation. Am J Respir Crit Care Med. 2001;164:1768–1773. doi: 10.1164/ajrccm.164.10.2106117. [DOI] [PubMed] [Google Scholar]
- 25.Sunden-Cullberg J, Norrby-Teglund A, Rouhiainen A, Rauvala H, Herman G, Tracey KJ, Lee ML, Andersson J, Tokics L, Treutiger CJ. Persistent elevation of high mobility group box-1 protein (HMGB1) in patients with severe sepsis and septic shock. Crit Care Med. 2005;33:564–573. doi: 10.1097/01.ccm.0000155991.88802.4d. [DOI] [PubMed] [Google Scholar]
- 26.Angus DC, Yang L, Kong L, Kellum JA, Delude RL, Tracey KJ, Weissfeld L. Circulating high-mobility group box 1 (HMGB1) concentrations are elevated in both uncomplicated pneumonia and pneumonia with severe sepsis. Crit Care Med. 2007;35:1061–1067. doi: 10.1097/01.CCM.0000259534.68873.2A. [DOI] [PubMed] [Google Scholar]
- 27.Urbonaviciute V, Furnrohr BG, Weber C, Haslbeck M, Wilhelm S, Herrmann M, Voll RE. Factors masking HMGB1 in human serum an plasma. J Leukoc Biol. 2007;81:67–74. doi: 10.1189/jlb.0306196. [DOI] [PubMed] [Google Scholar]
- 28.Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol. 2005;17:359–365. doi: 10.1016/j.coi.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Tsung A, Klune JR, Zhang X, Jeyabalan G, Cao Z, Peng X, Stolz DB, Geller DA, Rosengart MR, Billiar TR. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J Exp Med. 2007;204:2913–2923. doi: 10.1084/jem.20070247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Degryse B, Bonaldi T, Scaffidi P, Muller S, Resnati M, Sanvito F, Arrigoni G, Bianchi ME. The high mobility group (HMG) boxes of the nuclear protein HMG1 induce chemotaxis and cytoskeleton reorganization in rat smooth muscle cells. J Cell Biol. 2001;152:1197–1206. doi: 10.1083/jcb.152.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palumbo R, Sampaolesi M, De Marchis F, Tonlorenzi R, Colombetti S, Mondino A, Cossu G, Bianchi ME. Extracellular HMGB1, a signal of tissue damage, induces mesoangioblast migration and proliferation. J Cell Biol. 2004;164:441–449. doi: 10.1083/jcb.200304135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, Parroche P, Drabic S, Golenbock D, Sirois C, Hua J, An LL, Audoly L, La Rosa G, Bierhaus A, Naworth P, Marshak-Rothstein A, Crow MK, Fitzgerald KA, Latz E, Kiener PA, Coyle AJ. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–496. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 33.Park JS, Svetkauskaite D, He, Kim JY, Strassheim D, Ishizaka A, Abraham E. Involvement of TLR 2 and TLR 4 in cellular activation by high mobility group box 1 protein (HMGB1) J Biol Chem. 2004;279:7370–7377. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 34.Yu M, Wang H, Ding A, Golenbock DT, Latz E, Czura CJ, Fenton MJ, Tracey KJ, Yang H. HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock. 2006;26:174–179. doi: 10.1097/01.shk.0000225404.51320.82. [DOI] [PubMed] [Google Scholar]
- 35.Park JS, Gamboni-Robertson F, He Q, Svetkauskaite D, Kim JY, Strassheim D, Sohn JW, Yamada S, Maruyama I, Banerjee A, Ishizaka A, Abraham E. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol. 2006;290:C917–C924. doi: 10.1152/ajpcell.00401.2005. [DOI] [PubMed] [Google Scholar]
- 36.Kokkola R, Andersson A, Mullins G, Ostberg T, Treutiger CJ, Arnold B, Nawroth P, Andersson U, Harris RA, Harris HE. RAGE is the Major Receptor for the Proinflammatory Activity of HMGB1 in Rodent Macrophages. Scand J Immunol. 2005;61:1–9. doi: 10.1111/j.0300-9475.2005.01534.x. [DOI] [PubMed] [Google Scholar]
- 37.Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M, Yang H, Tracey KJ. High Mobility Group 1 Protein (HMG-1) Stimulates Proinflammatory Cytokine Synthesis in Human Monocytes. J Exp Med. 2000;192:565–570. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fiuza C, Bustin M, Talwar S, Tropea M, Gerstenberger E, Shelhamer JH, Suffredini AF. Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells. Blood. 2003;101:2652–2660. doi: 10.1182/blood-2002-05-1300. [DOI] [PubMed] [Google Scholar]
- 39.Treutiger CJ, Mullins GE, Johansson AS, Rouhiainen A, Rauvala HM, Erlandsson-Harris H, Andersson U, Yang H, Tracey KJ, Andersson J, Palmblad JE. High mobility group 1 Bbox mediates activation of human endothelium. J Intern Med. 2003;254:375–385. doi: 10.1046/j.1365-2796.2003.01204.x. [DOI] [PubMed] [Google Scholar]
- 40.Agnello D, Wang H, Yang H, Tracey KJ, Ghezzi P. HMGB1, a DNA-binding protein with cytokine activity, induces brain TNF and IL-6 production, and mediates anorexia and taste aversion. Cytokine. 2002;18:231–236. doi: 10.1006/cyto.2002.0890. [DOI] [PubMed] [Google Scholar]
- 41.Pedrazzi M, Raiteri L, Bonanno G, Patrone M, Ledda S, Passalacqua M, Milanese M, Melloni E, Raiteri M, Pontremoli S, Sparatore B. Stimulation of excitatory amino acid release from adult mouse brain glia subcellular particles by high mobility group box 1 protein. J Neurochem. 2006;99:827–838. doi: 10.1111/j.1471-4159.2006.04120.x. [DOI] [PubMed] [Google Scholar]
- 42.O'Connor KA, Hansen MK, Rachal PC, Deak MM, Biedenkapp JC, Milligan ED, Johnson JD, Wang H, Maier SF, Tracey KJ, Watkins LR. Further characterization of high mobility group box 1 (HMGB1) as a proinflammatory cytokine: central nervous system effects. Cytokine. 2003;24:254–265. doi: 10.1016/j.cyto.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 43.Liu K, Mori S, Takahashi HK, Tomono Y, Wak H, Kanke T, Sato Y, Hiraga N, Adachi N, Yoshino T, Nishibori M. Anti-high mobility group box 1 monoclonal antibody ameliorates brain infarction induced by transient ischemia in rats. FASEB J. 2007 doi: 10.1096/fj.07-8770com. in press. [DOI] [PubMed] [Google Scholar]
- 44.Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. HMG-1 as a mediator of acute lun inflammation. J Immunol. 2000;165:2950–2954. doi: 10.4049/jimmunol.165.6.2950. [DOI] [PubMed] [Google Scholar]
- 45.Ueno H, Matsuda T, Hashimoto S, Amaya F, Kitamura Y, Tanaka M, Kobayashi A, Maruyama I, Yamada S, Hasegawa N, Soejima J, Koh H, Ishizaka A. Contributions of high mobility group box protein in experimental and clinical acute lung injury. Am J Respir Crit Care Med. 2004;170:1310–1316. doi: 10.1164/rccm.200402-188OC. [DOI] [PubMed] [Google Scholar]
- 46.Lin X, Yang H, Sakuragi T, Hu M, Mantell Ll, Hayashi S, Al Abed Y, Tracey KJ, Ulloa L, Miller EJ. {alpha}-Chemokine receptor blockade reduces high mobility group box 1 protein-induced lung inflammation and injury and improves survival in sepsis. Am J Physiol Lung Cell Mol Physiol. 2005;289:L583–L590. doi: 10.1152/ajplung.00091.2005. [DOI] [PubMed] [Google Scholar]
- 47.Chacur M, Milligan ED, Gazda LS, Armstrong C, Wang H, Tracey KJ, Maier SF, Watkins LR. A new model of sciatic inflammatory neuritis (SIN): induction of unilateral and bilateral mechanical allodynia following acute unilateral peri-sciatic immune activation in rats. Pain. 2001;94:231–244. doi: 10.1016/S0304-3959(01)00354-2. [DOI] [PubMed] [Google Scholar]
- 48.Sappington PL, Yang R, Yang H, Tracey KJ, Delude RL, Fink MP. HMGB1 B box increases the permeability of Caco-2 enterocytic monolayers and impairs intestinal barrier function in mice. Gastroenterology. 2002;123:790–802. doi: 10.1053/gast.2002.35391. [DOI] [PubMed] [Google Scholar]
- 49.Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, Li J, Tracey KJ, Geller DA, Billiar TR. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rouhiainen A, Tumova S, Valmu L, Kalkkinen N, Rauvala H. Pivotal advance: analysis of proinflammatory activity of highly purified eukaryotic recombinant HMGB1 (amphoterin) J Leukoc Biol. 2007;81:49–58. doi: 10.1189/jlb.0306200. [DOI] [PubMed] [Google Scholar]
- 51.Li W, Sama AE, Wang H. Role of HMGB1 in cardiovascular diseases. Curr Opin Pharmacol. 2006;6:130–135. doi: 10.1016/j.coph.2005.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qin S, Wang H, Yuan R, Li H, Ochani M, Ochani K, Rosas-Ballina M, Czura CJ, Huston JM, Miller E, Lin X, Sherry B, Kumar A, Larosa G, Newman W, Tracey KJ, Yang H. Role of HMGB1 in apoptosis-mediated sepsis lethality. J Exp Med. 2006;203:1637–1642. doi: 10.1084/jem.20052203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pahuja M, Tran C, Wang H, Yin K. Alveolar macrophage suppression in sepsis is associated with high mobility group box 1 transmigration. Shock. 2007 doi: 10.1097/shk.0b013e31815d0c8f. in press. [DOI] [PubMed] [Google Scholar]
- 54.Poltorak A, He X, Smirnova I, Liu MY, Huffel CV, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 55.Wang H, Czura CJ, Tracey K, Thomson A, Lotze MT. The Cytokine Handbook. Oxford: Academic Press; 2003. J. TNF. pp. 837–860. [Google Scholar]
- 56.Haziot A, Ferrero E, Kontgen F, Hijiya N, Yamamoto S, Silver J, Stewart CL, Goyert SM. Resistance to endotoxin shock and reduced dissemination of gram-negative bacteria in CD14- deficient mice. Immunity. 1996;4:407–414. doi: 10.1016/s1074-7613(00)80254-x. [DOI] [PubMed] [Google Scholar]
- 57.Gardella S, Andrei C, Ferrera D, Lotti LV, Torrisi MR, Bianchi ME, Rubartelli A. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 2002;3:955–1001. doi: 10.1093/embo-reports/kvf198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GL, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 59.Zhang M, Caragine T, Wang H, Cohen PS, Botchkina G, Soda K, Bianchi M, Ulrich P, Cerami A, Sherry B, Tracey KJ. Spermine inhibits proinflammatory cytokine synthesis in human mononuclear cells: a counterregulatory mechanism that restrains the immune response. J Exp Med. 1997;185:1759–1768. doi: 10.1084/jem.185.10.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang M, Borovikova LV, Wang H, Metz C, Tracey KJ. Spermine Inhibition Of Monocyte Activation And Inflammation. Mol Med. 1999;5:595–605. [PMC free article] [PubMed] [Google Scholar]
- 61.Wang H, Zhang M, Bianchi M, Sherry B, Sama AE, Tracey KJ. Fetuin (alpha2-HSglycoprotein) opsonizes cationic macrophagedeactivating molecules. Proc Natl Acad Sci USA. 1998;95:14429–14434. doi: 10.1073/pnas.95.24.14429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang M, Wang H, Tracey KJ. Regulation of macrophage activation and inflammation by spermine: a new chapter in an old story. Crit Care Med. 2000;28:N60–N66. doi: 10.1097/00003246-200004001-00007. [DOI] [PubMed] [Google Scholar]
- 63.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 64.Bernard GR, Vincent JL, Laterre PF, Larosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJJ. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 65.Song ZJ, Johansen HK, Moser C, Faber V, Kharazmi A, Rygaard J, Hoiby N. Effects of Radix Angelicae sinensis and shuanghuanglian on a rat model of chronic Pseudomonas aeruginosa pneumonia. Chin Med Sci J. 2000;15:83–88. [PubMed] [Google Scholar]
- 66.Hu H, Hang B, Wang P. Anti-inflammatory effect of radix Angelicae sinensis. Zhongguo Zhong Yao Za Zhi. 1991;16(684-6):704. [PubMed] [Google Scholar]
- 67.Cho CH, Mei QB, Shang P, Lee SS, So HL, Guo X, Li Y. Study of the gastrointestinal protective effects of polysaccharides from Angelica sinensis in rats. Planta Med. 2000;66(4):348–351. doi: 10.1055/s-2000-8552. [DOI] [PubMed] [Google Scholar]
- 68.Ji XY, Tan BK, Zhu YZ. Salvia miltiorrhiza and ischemic diseases. Acta Pharmacol Sin. 2000;21:1089–1094. [PubMed] [Google Scholar]
- 69.Frei B, Higdon JV. Antioxidant activity of tea polyphenols in vivo: evidence from animal studies. J Nutr. 2003;133:32753–32843S. doi: 10.1093/jn/133.10.3275S. [DOI] [PubMed] [Google Scholar]
- 70.Crespy V, Williamson G. A review of the health effects of green tea catechins in in vivo animal models. J Nutr. 2004;134:3431S–3440S. doi: 10.1093/jn/134.12.3431S. [DOI] [PubMed] [Google Scholar]
- 71.Vita JA. Tea consumption and cardiovascular disease: effects on endothelial function. J Nutr. 2003;133:3293S–3297S. doi: 10.1093/jn/133.10.3293S. [DOI] [PubMed] [Google Scholar]
- 72.Graham HN. Green tea composition, consumption, and polyphenol chemistry. Prev Med. 1992;21:334–350. doi: 10.1016/0091-7435(92)90041-f. [DOI] [PubMed] [Google Scholar]
- 73.Osuchowski MF, Welch K, Siddiqui J, Remick DG. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J Immunol. 2006;177:1967–1974. doi: 10.4049/jimmunol.177.3.1967. [DOI] [PubMed] [Google Scholar]
- 74.Makwana N, Baines PB. Myocardial dysfunction in meningococcal septic shock. Curr Opin Crit Care. 2005;11:418–423. doi: 10.1097/01.ccx.0000176699.51456.13. [DOI] [PubMed] [Google Scholar]
- 75.Park JS, Arcaroli J, Yum HK, Yang H, Wang H, Yang KY, Choe KH, Strassheim D, Pitts TM, Tracey KJ, Abraham E. Activation of gene expression in human neutrophils by high mobility group box 1 protein. Am J Physiol Cell Physiol. 2003;284:C870–C879. doi: 10.1152/ajpcell.00322.2002. [DOI] [PubMed] [Google Scholar]