Abstract
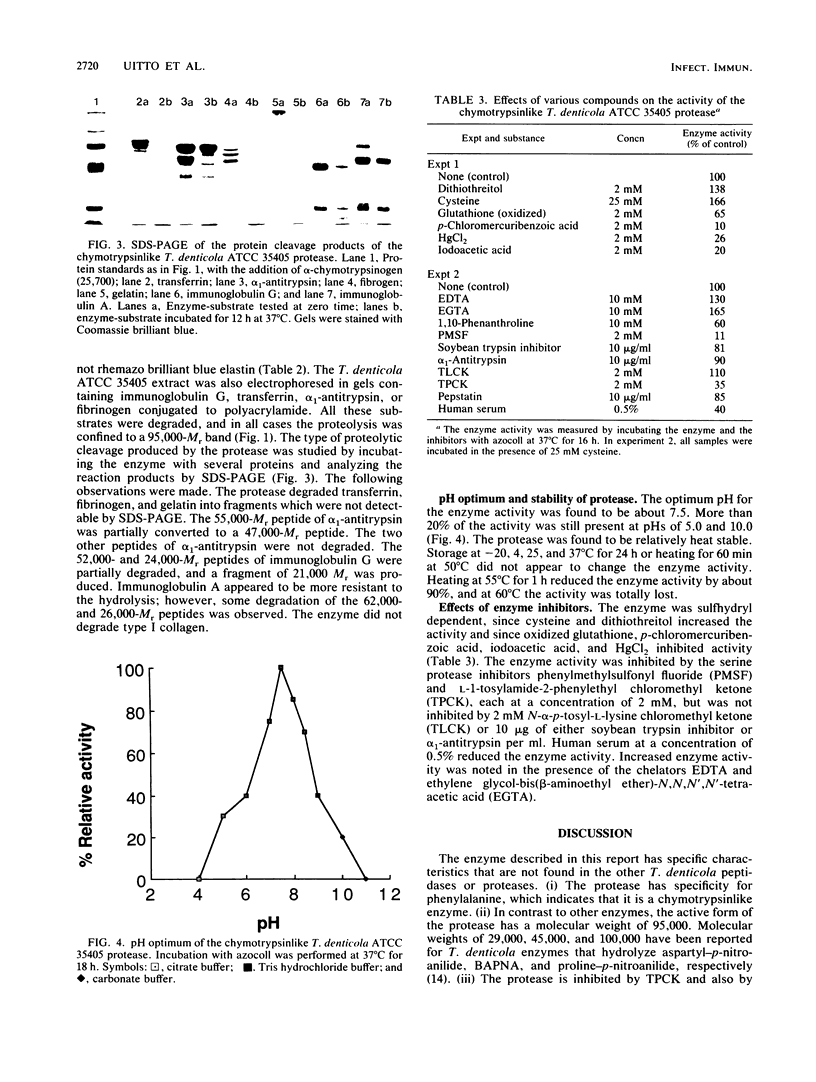
A chymotrypsinlike protease with an Mr of 95,000 was extracted from Treponema denticola ATCC 35405 and was partially purified by preparative sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The proteolytic activity was detected in an electrophoretogram containing polyacrylamide that was conjugated to bovine serum albumin. A single band of activity was detected when the T. denticola extract was solubilized and electrophoresed in the presence of sodium dodecyl sulfate. No activity was found in extracts of Treponema vincentii. The enzyme hydrolyzed transferrin, fibrinogen, alpha 1-antitrypsin, immunoglobulin A, immunoglobulin G, gelatin, bovine serum albumin, and a synthetic peptide containing phenylalanine. It did not degrade collagen or synthetic substrates containing arginine or proline. For the hydrolysis of azocoll, the pH optimum of the enzyme was 7.5. Heating at temperatures above 50 degrees C destroyed the activity. Reducing agents and the chelators EDTA and ethylene glycol-bis(beta-aminoethyl ether)-N,N,N',N'-tetraacetic acid increased the enzyme activity, while phenylmethylsulfonyl fluoride, L-1-tosylamide-2-phenylethyl chloromethyl ketone, sulfhydryl reagents, and human serum reduced activity. The ability of the enzyme to hydrolyze a number of humoral proteins suggests that it may be involved in spirochete invasiveness and tissue destruction.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armitage G. C., Dickinson W. R., Jenderseck R. S., Levine S. M., Chambers D. W. Relationship between the percentage of subgingival spirochetes and the severity of periodontal disease. J Periodontol. 1982 Sep;53(9):550–556. doi: 10.1902/jop.1982.53.9.550. [DOI] [PubMed] [Google Scholar]
- Boehringer H., Taichman N. S., Shenker B. J. Suppression of fibroblast proliferation by oral spirochetes. Infect Immun. 1984 Jul;45(1):155–159. doi: 10.1128/iai.45.1.155-159.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S. L., Chan E. C. The routine isolation, growth, and maintenance of the intermediate-size anaerobic oral spirochetes from periodontal pockets. J Periodontal Res. 1983 Jul;18(4):362–368. doi: 10.1111/j.1600-0765.1983.tb00371.x. [DOI] [PubMed] [Google Scholar]
- Fiehn N. E. Enzyme activities from eight small-sized oral spirochetes. Scand J Dent Res. 1986 Apr;94(2):132–140. doi: 10.1111/j.1600-0722.1986.tb01377.x. [DOI] [PubMed] [Google Scholar]
- Foltmann B., Szecsi P. B., Tarasova N. I. Detection of proteases by clotting of casein after gel electrophoresis. Anal Biochem. 1985 May 1;146(2):353–360. doi: 10.1016/0003-2697(85)90551-2. [DOI] [PubMed] [Google Scholar]
- Frank R. M. Bacterial penetration in the apical pocket wall of advanced human periodontitis. J Periodontal Res. 1980 Nov;15(6):563–573. doi: 10.1111/j.1600-0765.1980.tb00315.x. [DOI] [PubMed] [Google Scholar]
- Kelleher P. J., Juliano R. L. Detection of proteases in polyacrylamide gels containing covalently bound substrates. Anal Biochem. 1984 Feb;136(2):470–475. doi: 10.1016/0003-2697(84)90246-x. [DOI] [PubMed] [Google Scholar]
- LISTGARTEN M. A. ELECTRON MICROSCOPIC OBSERVATIONS ON THE BACTERIAL FLORA OF ACUTE NECROTIZING ULCERATIVE GINGIVITIS. J Periodontol. 1965 Jul-Aug;36:328–339. doi: 10.1902/jop.1965.36.4.328. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laughon B. E., Syed S. A., Loesche W. J. API ZYM system for identification of Bacteroides spp., Capnocytophaga spp., and spirochetes of oral origin. J Clin Microbiol. 1982 Jan;15(1):97–102. doi: 10.1128/jcm.15.1.97-102.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listgarten M. A., Helldén L. Relative distribution of bacteria at clinically healthy and periodontally diseased sites in humans. J Clin Periodontol. 1978 May;5(2):115–132. doi: 10.1111/j.1600-051x.1978.tb01913.x. [DOI] [PubMed] [Google Scholar]
- Listgarten M. A. Structure of the microbial flora associated with periodontal health and disease in man. A light and electron microscopic study. J Periodontol. 1976 Jan;47(1):1–18. doi: 10.1902/jop.1976.47.1.1. [DOI] [PubMed] [Google Scholar]
- Loesche W. J., Syed S. A., Schmidt E., Morrison E. C. Bacterial profiles of subgingival plaques in periodontitis. J Periodontol. 1985 Aug;56(8):447–456. doi: 10.1902/jop.1985.56.8.447. [DOI] [PubMed] [Google Scholar]
- Mikx F. H., Ngassapa D. N., Reijntjens F. M., Maltha J. C. Effect of splint placement on black-pigmented Bacteroides and spirochetes in the dental plaque of beagle dogs. J Dent Res. 1984 Nov;63(11):1284–1288. doi: 10.1177/00220345840630110601. [DOI] [PubMed] [Google Scholar]
- Mikx F. H., de Jong M. H. Keratinolytic activity of cutaneous and oral bacteria. Infect Immun. 1987 Mar;55(3):621–625. doi: 10.1128/iai.55.3.621-625.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V., Cato E. P., Smibert R. M., Burmeister J. A., Palcanis K. G., Ranney R. R. Comparative bacteriology of juvenile periodontitis. Infect Immun. 1985 May;48(2):507–519. doi: 10.1128/iai.48.2.507-519.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitzan D., Sperry J. F., Wilkins T. D. Fibrinolytic activity of oral anaerobic bacteria. Arch Oral Biol. 1978;23(6):465–470. doi: 10.1016/0003-9969(78)90078-x. [DOI] [PubMed] [Google Scholar]
- Ohta K., Makinen K. K., Loesche W. J. Purification and characterization of an enzyme produced by Treponema denticola capable of hydrolyzing synthetic trypsin substrates. Infect Immun. 1986 Jul;53(1):213–220. doi: 10.1128/iai.53.1.213-220.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen I. Attachment of Treponema denticola to cultured human epithelial cells. Scand J Dent Res. 1984 Feb;92(1):55–63. doi: 10.1111/j.1600-0722.1984.tb00860.x. [DOI] [PubMed] [Google Scholar]
- Reijntjens F. M., Mikx F. H., Wolters-Lutgerhorst J. M., Maltha J. C. Adherence of oral treponemes and their effect on morphological damage and detachment of epithelial cells in vitro. Infect Immun. 1986 Feb;51(2):642–647. doi: 10.1128/iai.51.2.642-647.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saglie R., Newman M. G., Carranza F. A., Jr, Pattison G. L. Bacterial invasion of gingiva in advanced periodontitis in humans. J Periodontol. 1982 Apr;53(4):217–222. doi: 10.1902/jop.1982.53.4.217. [DOI] [PubMed] [Google Scholar]
- Shenker B. J., Listgarten M. A., Taichman N. S. Suppression of human lymphocyte responses by oral spirochetes: a monocyte-dependent phenomenon. J Immunol. 1984 Apr;132(4):2039–2045. [PubMed] [Google Scholar]
- Uitto V. J., Chan E. C., Quee T. C. Initial characterization of neutral proteinases from oral spirochetes. J Periodontal Res. 1986 Mar;21(2):95–100. doi: 10.1111/j.1600-0765.1986.tb01442.x. [DOI] [PubMed] [Google Scholar]
- Uitto V. J. Degradation of basement membrane collagen by proteinases from human gingiva, leukocytes and bacterial plaque. J Periodontol. 1983 Dec;54(12):740–745. doi: 10.1902/jop.1983.54.12.740. [DOI] [PubMed] [Google Scholar]