Abstract
The aspartate-specific cysteine protease caspase-1 is activated by the inflammasomes and is responsible for the proteolytic maturation of the cytokines IL-1β and IL-18 during infection and inflammation. To discover new caspase-1 substrates, we made use of a proteome-wide gel-free differential peptide sorting methodology that allows unambiguous localization of the processing site in addition to identification of the substrate. Of the 1022 proteins that were identified, 20 were found to be specifically cleaved after Asp in the setup incubated with recombinant caspase-1. Interestingly, caspase-7 emerged as one of the identified caspase-1 substrates. Moreover half of the other identified cleavage events occurred at sites closely resembling the consensus caspase-7 recognition sequence DEVD, suggesting caspase-1-mediated activation of endogenous caspase-7 in this setup. Consistently recombinant caspase-1 cleaved caspase-7 at the canonical activation sites Asp23 and Asp198, and recombinant caspase-7 processed a subset of the identified substrates. In vivo, caspase-7 activation was observed in conditions known to induce activation of caspase-1, including Salmonella infection and microbial stimuli combined with ATP. Interestingly Salmonella- and lipopolysaccharide + ATP-induced activation of caspase-7 was abolished in macrophages deficient in caspase-1, the pattern recognition receptors Ipaf and Cryopyrin, and the inflammasome adaptor ASC, demonstrating an upstream role for the caspase-1 inflammasomes in caspase-7 activation in vivo. In contrast, caspase-1 and the inflammasomes were not required for caspase-3 activation. In conclusion, we identified 20 new substrates activated downstream of caspase-1 and validated caspase-1-mediated caspase-7 activation in vitro and in knock-out macrophages. These results demonstrate for the first time the existence of a nucleotide binding and oligomerization domain-like receptor/caspase-1/caspase-7 cascade and the existence of distinct activation mechanisms for caspase-3 and -7 in response to microbial stimuli and bacterial infection.
Cysteinyl aspartate-specific proteases (caspases)1 have essential roles in apoptosis and inflammation (1). They are synthesized as zymogens with a prodomain of variable length followed by a large and a small catalytic subunit. In humans, the caspase family consists of 11 members, which are classified into three phylogenetic groups correlating with their function (2).
Caspase-1 is the prototypical member of the inflammatory caspases and mediates the proteolytic maturation of the related cytokines IL-1β and IL-18 (3, 4) following its recruitment in large protein complexes termed “inflammasomes” (5–10). The molecular composition of the inflammasome depends on the identity of the nucleotide binding and oligomerization domain (NOD)-like receptor (NLR) family member serving as scaffold protein in the complex (6). The members of the cytosolic NLR family are believed to recognize conserved microbial and viral components called pathogen-associated molecular patterns (PAMPs) in intracellular compartments. In humans, the NLR family is composed of 23 members that share remarkable structural similarity to a subset of plant disease resistance genes (R genes) (11). The amino-terminal sequence of NLRs generally contains homotypic interaction motifs such as the caspase recruitment domain (CARD) and the pyrin domain. The central NOD is thought to be involved in self-oligomerization and activation, whereas the carboxyl-terminal leucine-rich repeat motifs sense specific PAMPs and autoregulate NLR activity. The bipartite adaptor protein apoptosis-associated specklike protein containing a CARD (ASC) bridges the interaction between NLR proteins and inflammatory caspases through homotypic interactions with its own amino-terminal pyrin and carboxyl-terminal CARD domains. As such, ASC plays a central role in the assembly of the inflammasomes and the activation of caspase-1 in response to a broad range of PAMPs and intracellular pathogens (7, 12). Whereas the Cryopyrin inflammasome is essential for caspase-1 activation in response to LPS, lipid A, lipoteichoic acid, lipoprotein, and double-stranded RNA in the presence of millimolar concentrations of ATP (8, 9, 13), intracellular pathogens such as Salmonella typhimurium (Salmonella) activate caspase-1 through the Ipaf inflammasome (5, 8, 14). Recently Salmonella flagellin was identified as the bacterial ligand that is sensed by Ipaf, although the mechanism remains obscure (5, 14). Interestingly Salmonella induces a rapid and specialized form of macrophage cell death, which is sometimes termed “pyroptosis” and requires activation of caspase-1 (15) through the Ipaf inflammasome (15).
The central roles of the executioner caspase-3 and -7 during apoptosis have been well established. Upon initiation of the cell death program, homotypic interaction motifs in the large prodomains of caspase-8 and -9 mediate their recruitment in the death-inducing signaling complex (DISC) and the apoptosome, respectively, where they undergo proximity-induced activation (16–18). Once activated, the initiator caspases induce an apoptotic caspase cascade by proteolytically removing the linker region between the large and small catalytic subunits of caspase-3 and -7, a step that is required for full proteolytic activity of these executioner caspases (19, 20). In turn, active caspase-3 and -7 cleave a large set of substrates, ultimately resulting in the morphological and biochemical hallmarks of apoptosis such as DNA fragmentation and mitochondrial damage (21, 22). As deficiency in caspase-3 induced a compensatory activation of caspase-7, the mild apoptotic phenotype of caspase-3 knock-out mice was suggested to be due to its functional redundancy with caspase-7 (23, 24). Consistently, caspase-7 knock-out mice have been recently reported to be born at normal Mendelian ratios and to display no gross abnormalities, whereas caspase-3/-7 double knock-out mice suffer from early perinatal lethality (25). Furthermore, caspase-7-deficient cells from adult mice exhibit normal activation of apoptosis in response to a wide variety of stimuli including death receptor activation, etoposide, and UV irradiation (25). These results indicate that caspase-3 and -7 perform redundant roles in the regulation of apoptosis during embryonic development and in response to a wide variety of “classical” apoptotic triggers (25). However, the molecular mechanisms that govern the activation of these executioner caspases during inflammation and infection remain unclear.
Here we identified caspase-7 as a caspase-1 substrate by a proteome-wide screen for caspase-1 targets using the amino-terminal combined fractional diagonal chromatography (COFRADIC) gel-free technology. By this technique, amino-terminal peptides, including those newly formed by protease processing, are isolated prior to LC-MS/MS analysis. Briefly S-alkylated and N-acetylated proteins are digested with trypsin, and the resulting peptide mixture is passed over an strong cation exchange column to enrich for α-N-acetylated amino-terminal peptides in the non-binding fraction (26). This peptide mixture is then fractionated by RP-HPLC, and contaminating, internal peptides carrying a free α-amino group are incubated with 2,4,6-trinitrobenzenesulfonic acid (TNBS), which renders such peptides more hydrophobic and thus segregates them from TNBS-unaffected (because they are already blocked) amino-terminal peptides during a series of identical, secondary RP-HPLC separations (27). Our data demonstrate that recombinant caspase-1 processes caspase-7 at the canonical activation sites Asp23 and Asp198. In vivo, activation of caspase-7 by microbial stimuli, including infection with Salmonella and stimulation with LPS, requires caspase-1 and components, respectively, of the Ipaf and Cryopyrin inflammasomes, whereas caspase-3 is activated independently of the caspase-1 inflammasomes. These results demonstrate the existence of a NOD-like receptor/caspase-1/caspase-7 cascade activated in response to microbial stimuli and bacterial infection and indicate for the first time the existence of differential activation mechanisms for caspase-3 and -7 during inflammation and infection.
EXPERIMENTAL PROCEDURES
COFRADIC Isolation of Amino-terminal Peptides—
108 Mf4/4 cells were grown for 10 days in SILAC (stable isotope labeling by amino acids in cell culture) RPMI 1640 medium containing [12C6] or [13C6]Arg (Invitrogen). Cells were collected, washed twice with cold PBS, and resuspended in 1 ml of ice-cold homogenization buffer (20 mm HEPES-KOH, pH 7.5, 10 mm KCl, 1.5 mm MgCl2, 1 mm EDTA, 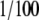 of a Complete protease inhibitor mixture tablet (Roche Applied Science), and 1 mm DTT). After three rounds of freeze-thawing in liquid N2 and cold ethanol, samples were cleared by centrifuging at 20,000 × g for 30 min at 4 °C, and the protein concentration was determined using the Bradford method (Bio-Rad). Subsequently 1 mg of protein extract was incubated with 400 nm recombinant mouse caspase-1 (12C-labeled setup) or left untreated (13C-labeled setup) for 1 h at 37 °C before 1 μm Ac-YVAD-cmk (Calbiochem) was added to stop the reaction. Subsequent steps for the sorting and identification of differentially generated amino-terminal peptides by COFRADIC were performed as described (full technical details are described elsewhere: see Ref. 26). Briefly the proteins in the lysates were reduced and S-alkylated by iodoacetamide. Subsequently free primary amino groups were blocked by trideuteroacetylation. Following trypsin digestion, the peptide mixtures were mixed in a 1:1 ratio, and amino-terminal peptides were enriched after passing them over a strong cation exchange cartridge at pH 3 and then fractionated by RP-HPLC (primary run). Peptides in each fraction were incubated with TNBS to block free α-amino termini of remaining internal (i.e. non-amino-terminal) peptides. Peptides carrying an amino-terminal protein part (i.e. amino-terminal peptides from either unprocessed proteins or protein fragments generated by processing) are not affected by TNBS because their α-amino group was already (in vitro or in vivo) blocked. During a series of replicate secondary RP-HPLC runs, the TNBS-altered internal peptides shift to later elution times because of their increased hydrophobicity. In this way, a set of unaltered, amino-terminal peptides that do not shift during the consecutive runs were collected for mass spectrometric analysis.
of a Complete protease inhibitor mixture tablet (Roche Applied Science), and 1 mm DTT). After three rounds of freeze-thawing in liquid N2 and cold ethanol, samples were cleared by centrifuging at 20,000 × g for 30 min at 4 °C, and the protein concentration was determined using the Bradford method (Bio-Rad). Subsequently 1 mg of protein extract was incubated with 400 nm recombinant mouse caspase-1 (12C-labeled setup) or left untreated (13C-labeled setup) for 1 h at 37 °C before 1 μm Ac-YVAD-cmk (Calbiochem) was added to stop the reaction. Subsequent steps for the sorting and identification of differentially generated amino-terminal peptides by COFRADIC were performed as described (full technical details are described elsewhere: see Ref. 26). Briefly the proteins in the lysates were reduced and S-alkylated by iodoacetamide. Subsequently free primary amino groups were blocked by trideuteroacetylation. Following trypsin digestion, the peptide mixtures were mixed in a 1:1 ratio, and amino-terminal peptides were enriched after passing them over a strong cation exchange cartridge at pH 3 and then fractionated by RP-HPLC (primary run). Peptides in each fraction were incubated with TNBS to block free α-amino termini of remaining internal (i.e. non-amino-terminal) peptides. Peptides carrying an amino-terminal protein part (i.e. amino-terminal peptides from either unprocessed proteins or protein fragments generated by processing) are not affected by TNBS because their α-amino group was already (in vitro or in vivo) blocked. During a series of replicate secondary RP-HPLC runs, the TNBS-altered internal peptides shift to later elution times because of their increased hydrophobicity. In this way, a set of unaltered, amino-terminal peptides that do not shift during the consecutive runs were collected for mass spectrometric analysis.
LC-MS/MS Analysis and Peptide Identification by Mascot—
The collected peptide fractions were dried and redissolved in 20 μl of water/acetonitrile (98:2, v/v), and half of each analyte mixture was sampled by LC-MS/MS using a microfluidic interface (Agilent Chip Cube) mounted on an Agilent XCT-Ultra ion trap mass spectrometer that was operated as described previously (28). Mascot generic files were created as described previously (28) and used to search the mouse subset of the UniProtKB/Swiss-Prot release 51.0 (October 31, 2006 containing 241,242 sequence entries of which 11,897 entries originate from Mus musculus) using a locally installed version of Mascot (29) (version 2.1.04). The following search parameters were set. Both the peptide mass tolerance and the peptide fragment mass tolerance were set to ±0.5 Da, the “instrument setting” of Mascot was set to “ESI-TRAP,” and peptide charge was set to 1+, 2+, and 3+. Fixed modifications were trideutero-amino-acetylation of lysine and S-carbamidomethylation of cysteine (for identifying heavy labeled peptides; [13C6]arginine was also set as an additional fixed modification). Oxidation of methionine to its sulfoxide derivative, pyroglutamate formation (amino-terminal Gln), pyrocarbamidomethylcysteine formation (amino-terminal carbamidomethylated cysteines), acetylation and trideuteroacetylation of the α-amino terminus, and deamidation (Gln and Asn) were considered as variable modifications. Endoproteinase Arg-C/P was considered as the protease used with a maximum number of one missed cleavage.
Raw DAT result files of Mascot were further queried using in-house developed software. Only MS/MS spectra receiving a score exceeding the corresponding Mascot identity threshold score at the 95% confidence level were kept. As a rule of thumb, all such identified peptides holding six or fewer amino acids were discarded. In addition, spectra that received a low Mascot score (five or fewer points above the corresponding threshold) were further interrogated, and only spectra that contained a significant number of b and y peptide fragment ions typically covering a stretch of three consecutive amino acids were considered identified (see supplemental data with identified MS/MS spectra). Truncated peptide databases made by DBToolkit (30) were searched in parallel to pick up protein processing events more efficiently (e.g. see Refs. 30 and 31). Decoy databases (a shuffled version of the UniProtKB/Swiss-Prot database made by the DBToolkit algorithm (32)) were searched as suggested previously (33) to estimate the false discovery rate. At the Mascot identity threshold score (95%) used, the estimated false discovery rate was typically found to be between 2 and 4% on the spectrum level (26). When neo-amino-terminal peptides generated by the (downstream) action of caspase-1 were found to match multiple members of a protein family (i.e. redundancies found in the searched database), the corresponding proteins are listed in Tables I and II.
Table I.
Overview of targets and cleavage sites identified in a proteome-wide COFRADIC analysis with caspase-1
The complete list of internally located, trideuteroacetylated (AcD3) peptides with an Asp residue in P1 that were uniquely identified in the digested proteome of recombinant caspase-1-treated mouse Mf414 lysates is shown. Protein names are identified by using the Swiss Institute of Bioinformatics BLAST network service, and the accession numbers are according to the UniProtKB/Swiss-Prot database. Cleavage sites represent the amino acids preceding the identified peptide (i.e. P4–P1) as well as the P1′. The targets are divided into two groups based on the presence of an aliphatic (cluster I) or acidic (cluster II) residue at P4. The identified peptide sequences and modified sequences are presented. The predicted molecular mass of the full-length targets is indicated in integers. Mox, oxidized methionine; Dam, deamidation.
| Accession no. | Protein name | Molecular mass | Sequence | Modified sequence | Cleavage site |
|---|---|---|---|---|---|
| kDa | |||||
| Cluster I | |||||
| P97864 | Caspase-7 | 34 | SGPINDIDANPR | AcD3-SGPINDIDANPR-COOH | IQAD198↓S199 |
| P60710 | β-Actin | 42 | NGSGMCKAGFAGDDAPR | AcD3-NDamGSGMMoxCKAGFAGDDAPR-COOH | LVVD11↓N2 |
| P63260 | γ-Actin | LVID11↓N2 | |||
| P63038 | Hsp60 | 61 | AVAVTMGPKGR | AcD3-AVAVTMMoxGPKGR-COOH | LLAD49↓A50 |
| VANNTNEEAGDGTTTATVLAR | AcD3-VANNTNEEAGDGTTTATVLAR-COOH | LVQD100↓V101 | |||
| Q8R5A3 | PREL-1 | 74 | LVADISEAEQR | AcD3-LVADISEAEQR-COOH | LMAD70↓L71 |
| Q99KY4 | Cyclin G-associated kinase | 144 | GDGSEVSDEEEASFPSEER | AcD3-GDGSEVSDEEEASFPSEER-COOH | LIAD820↓G821 |
| Q62318 | TIF1b | 89 | GADSTGVVAKLSPANQR | AcD3-GADSTGVVAKLSPANQR-COOH | LSLD684↓G685 |
| Q5RJH6 | SMG7 | 127 | GSPGLKSVLSTGR | AcD3-GSPGLKSVLSTGR-COOH | LATD517↓G518 |
| Q7TMY8 | E3-histone | 483 | EAPSNLSQASTLQANR | AcD3-EAPSNLSQASTLQANR-COOH | VLMD2384↓E2385 |
| O35601 | FYN-binding protein | 90 | GTGNLEEEQESEGETYEDIDSSKER | AcD3-GTGNLEEEQDamESEGETYEDIDSSKER-COOH | MHSD434↓G435 |
| P25206 | MCM3 | 92 | DPDFTQDDQQDTR | AcD3-DPDFTQDDQQDTR-COOH | LATD530↓D531 |
| Cluster II | |||||
| P25206 | MCM3 | 92 | FTQDDQQDTR | AcD3-FTQDDQQDTR-COOH | DDPD533↓F534 |
| P08113 | Endoplasmin | 92 | VDGTVEEDLGKSR | AcD3-VDGTVEEDLGKSR-COOH | DEVD26↓V27 |
| Q91WR3 | Assc2 | 86 | GNQVGANDADSDDELISR | AcD3-GNQVGANDADSDDELISR-COOH | DTYD614↓G615 |
| Q9JLQ2 | GIT2 | 79 | YDSVASDEDTDVETR | AcD3-YDSVASDEDTDVETR-COOH | DQPD390↓Y391 |
| Q9WUK2 | eIF-4H | 27 | SLKEALTYDGALLGDR | AcD3-SLKEALTYDGALLGDR-COOH | DEVD92↓S93 |
| P47713 | cPLA2 | 85 | AAVADPDEFER | AcD3-AAVADPDEFER-COOH | DELD521↓A522 |
| Q61211 | Ligatin | 63 | GKPLQEQMDDLLLR | AcD3-GKPLQEQMMoxDDLLLR-COOH | DSLD243↓G244 |
| O88746 | Target of Myb protein 1 | 54 | MGPDPAATNNLSSQLAGMNLGSR | AcD3-MMoxGPDPAATNNDamLSSQLAGMMoxNLGSR-COOH | DLID324↓M325 |
| Q62481 | Vps72 | 41 | SDFDIDEGDEPSSDGEAEEPR | AcD3-SDFDIDEGDEPSSDGEAEEPR-COOH | DEVD58↓S59 |
| Q8K2H1 | Periphilin-1 | 44 | GTELYEDSQLSNR | AcD3-GTELYEDSQLSNR-COOH | NTVD291↓G292 |
| P60710 | β-Actin | 42 | GQVITIGNER | AcD3-GQVITIGNER-COOH | ELPD244↓G245 |
| P63260 | γ-Actin | ||||
| Q61152 | FLP-1 | 50 | GAQTGGLGFNLR | AcD3-GAQTGGLGFNLR-COOH | EVTD424↓G425 |
Table II.
Characteristics of peptides identified in the proteome-wide COFRADIC analysis with caspase-1
The complete list of internally located, trideuteroacetylated peptides with an Asp residue in P1 that were uniquely identified in the digested proteome of recombinant caspase-1-treated mouse Mf414 lysates is shown. Protein names are identified by using the Swiss Institute of Bioinformatics BLAST network service, and the accession numbers are according to the UniProtKB/Swiss-Prot database. The targets are divided into two groups based on the presence of an aliphatic (cluster I) or acidic (cluster II) residue at P4. The predicted molecular mass of the proteolytic fragments are indicated in integers. The number of identified spectra (Spectra no.), start/end, m/z, z, error (Da), threshold and Δ threshold of the identified spectra, the Mascot ion score of highest scoring spectra, and e value are given.
| Accession no. | Protein name | Molecular mass, fragments | Spectra no. | Start | End | m/z | z | Error | Threshold | Score of highest scoring spectra | Δ threshold | e value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| kDa | Da | |||||||||||
| Cluster I | ||||||||||||
| P97864 | Caspase-7 | 22–11 | 1 | 199 | 210 | 657.394 | 2 | 0.1284 | 31 | 39 | 8 | 0.007940871 |
| P60710 | β-Actin | 1–41 | 1 | 12 | 28 | 909.372 | 2 | −0.0332 | 42 | 46 | 4 | 0.019925952 |
| P63260 | γ-Actin | |||||||||||
| P63038 | Hsp60 | 6–56 | 2 | 50 | 60 | 596.878 | 2 | 0.0862 | 42 | 66 | 24 | 0.000200292 |
| 11–50 | 3 | 101 | 121 | 1075.46 | 2 | −0.1272 | 43 | 60 | 17 | 0.001002025 | ||
| Q8R5A3 | PREL-1 | 8–66 | 1 | 71 | 81 | 638.435 | 2 | 0.2009 | 42 | 56 | 14 | 0.001997753 |
| Q99KY4 | Cyclin G-associated kinase | 92–52 | 2 | 821 | 839 | 1050.923 | 2 | −0.0168 | 43 | 98 | 55 | 1.60378e−07 |
| Q62318 | TIF1b | 73–16 | 2 | 685 | 701 | 880.975 | 2 | 0.0019 | 42 | 62 | 20 | 0.000502592 |
| Q5RJH6 | SMG7 | 59–68 | 1 | 518 | 530 | 674.928 | 2 | 0.0786 | 42 | 64 | 22 | 0.000317278 |
| Q7TMY8 | E3-histone | 263–220 | 2 | 2385 | 2400 | 866.493 | 2 | 0.1088 | 42 | 63 | 21 | 0.000399326 |
| O35601 | FYN-binding protein | 46–44 | 1 | 435 | 459 | 974.771 | 3 | 0.0584 | 43 | 54 | 11 | 0.003982951 |
| P25206 | MCM3 | 59–33 | 1 | 531 | 543 | 813.364 | 2 | 0.0454 | 42 | 63 | 21 | 0.000399326 |
| Cluster II | ||||||||||||
| P25206 | MCM3 | 59–33 | 1 | 534 | 543 | 649.82 | 2 | 0.064 | 31 | 35 | 4 | 0.019925952 |
| P08113 | Endoplasmin | 3–89 | 1 | 27 | 39 | 747.911 | 2 | 0.0594 | 42 | 76 | 34 | 2.00811e−05 |
| Q91WR3 | Assc2 | 70–16 | 1 | 615 | 632 | 960.958 | 2 | 0.0479 | 42 | 131 | 89 | 6.44113e−11 |
| Q9JLQ2 | GIT2 | 44–35 | 1 | 391 | 405 | 873.885 | 2 | 0.0247 | 42 | 90 | 48 | 8.02341e−07 |
| Q9WUK2 | eIF-4H | 10–17 | 1 | 93 | 108 | 906.399 | 2 | −0.1749 | 47 | 72 | 25 | 0.000159139 |
| P47713 | cPLA2 | 59–26 | 2 | 522 | 532 | 632.848 | 2 | 0.1004 | 42 | 58 | 16 | 0.001261149 |
| Q61211 | Ligatin | 26–37 | 2 | 244 | 257 | 881.52 | 2 | 0.1005 | 42 | 66 | 24 | 0.000200292 |
| O88746 | Target of Myb protein 1 | 36–18 | 1 | 325 | 347 | 794.043 | 3 | 0.0199 | 43 | 45 | 2 | 0.031564182 |
| Q62481 | Vps72 | 6–35 | 1 | 59 | 79 | 780.951 | 3 | −0.0916 | 38 | 40 | 2 | 0.031564182 |
| Q8K2H1 | Periphilin-1 | 33–11 | 2 | 292 | 304 | 778.858 | 2 | −0.0179 | 47 | 71 | 24 | 0.000200292 |
| P60710 | β-Actin | 27–15 | 3 | 245 | 254 | 566.381 | 2 | 0.1352 | 41 | 87 | 46 | 1.27097e−06 |
| P63260 | γ-Actin | |||||||||||
| Q61152 | FLP-1 | 47–3 | 1 | 425 | 436 | 618.324 | 2 | −0.0163 | 42 | 81 | 39 | 6.35841e−06 |
In Vitro Caspase Cleavage Assays—
cDNAs encoding wild type murine caspase-7 or site-directed mutants harboring D23A, D198A, or D23A/D198A mutations were cloned in the pLT10 vector downstream of the T7 promoter. pGEM11-proIL-1β was constructed by inserting murine pro-IL-1β cDNA into the unique HindII site of the pGEM11Zf(+) vector (Promega). pCMV-Sport6-Ligatin, pCMV-Sport6-Vps72, pCMV-Sport6-eIF4H, pCMV-Sport6-MCM3, pCMV-Sport6-Ascc2, pCMV-Sport6-Hsp60, pYX-Asc-TIF1β, and pYX-Asc-GIT2 plasmids were purchased from ATCC. Plasmids were used as a template (250 ng each) for in vitro coupled transcription/translation in a rabbit reticulocyte lysate system according to the manufacturer's instructions (Promega). For detection of the translation products, [35S]methionine was added to the translation reactions. Translation reactions (2 μl each) were incubated with 30 nm purified recombinant caspase-1 or caspase-7 in 23 μl of cell-free system buffer (10 mm HEPES, pH 7.4, 220 mm mannitol, 68 mm sucrose, 2 mm NaCl, 2.5 mm KH2PO4, 0.5 mm EGTA, 2 mm MgCl2, 5 mm sodium pyruvate, and 1 mm DTT) for 1 h at 37 °C. In some experiments, recombinant caspase-1 was preincubated with 1 μm YVAD-cmk (Calbiochem). The resulting cleavage products were analyzed by SDS-PAGE and autoradiography.
Mice and Macrophages—
Cryopyrin−/−, Ipaf−/−, ASC−/−, caspase-1−/−, and caspase-3−/− mice have been described previously (4, 5, 10, 23). caspase-7−/− mice in a C57BL/6 background have been described previously (25) and were purchased from The Jackson Laboratory. Mice were housed in a pathogen-free facility. Bone-marrow derived macrophages (BMDMs) were prepared as described before (34). The animal studies were conducted under protocols approved by the University of Michigan Committee on Use and Care of Animals.
Bacteria and Ligands—
Salmonella enterica serovar typhimurium strain SL1344 was kindly provided by D. Monack (Stanford University). The fliB−/fljC− Salmonella strain was a generous gift of A. Aderem (University of Washington). Single colonies were inoculated into 3 ml of brain-heart infusion medium and grown overnight at 30 °C with shaking. All bacterial ligands and heat-killed bacteria were purchased from Invivogen and used at a concentration of 10 μg/ml. ATP (Roche Applied Science) was used at 5 mm throughout all experiments. Infection and stimulation of BMDMs with LPS and ATP was performed as described previously (34).
Western Blotting—
Extracts were prepared, transferred to nitrocellulose membranes, and immunoblotted with primary antibodies, and then proteins were detected by enhanced chemiluminescence as described previously (6). Anti-caspase-1 antibody was described before (35). Anti-caspase-3 and anti-caspase-7 were purchased from Cell Signaling Technology.
Precipitation of Biotin-bound Caspase-3 and -7—
108 BMDMs were left untreated, stimulated with LPS + ATP, or infected with Salmonella. Cells were collected by centrifugation, and the cell pellet was resuspended in 1 ml of Buffer A (1% Nonidet P-40, 200 mm NaCl, 20 mm Tris-HCl, pH 7.4, and Roche Applied Science Complete protease inhibitor mixture tablet) containing 100 μm biotin-DEVD-fmk (Kamiya Biomedical Co.). Cells were incubated at room temperature for 15 min and lysed with three rounds of freeze-thawing in liquid N2. Supernatants were collected by centrifugation (14,000 rpm at 4 °C) and incubated overnight with high capacity streptavidin-agarose beads (Thermo Scientific) or stored at −70 °C for direct immunoblotting of cell lysates. Streptavidin-bound complexes were washed four times with cold Buffer A and analyzed by immunoblotting after elution in SDS-PAGE sample buffer.
Measurements of Cytokines—
Mouse cytokines were measured in serum and culture supernatants with ELISA kits (R&D Systems).
RESULTS
Identification of Caspase-7 in a Proteome-wide Screen for in Vitro Caspase-1 Substrates—
We performed a proteome-wide screen to identify novel caspase-1 substrates using the gel-free COFRADIC peptide sorting methodology used on a differential setup (30). In addition to the identification of the substrate, this technology provides the exact position of the processing site by specifically identifying the neo-amino terminus generated by protein processing. In this study, we prepared proteins from untreated and caspase-1-treated macrophage extracts for differential analysis of amino-terminal peptides. Following LC-MS/MS analysis, 2663 peptides were identified by Mascot (29), and these converged into 1022 different proteins. On the basis of the differential 12C6/13C6 isotope labeling of l-arginine used (36), peptides that display as couples of “light” and “heavy” forms spaced by 6 Da are present in both the control and caspase-1-treated samples and thus do not hint at protein processing. Such peptides were therefore not considered for further data interpretation. On the other hand, peptides that were (a) exclusively found in the proteome digest of caspase-1-treated cell lysate (thus represented by a single isotopic peptide envelope) and (b) were generated by cleavage after Asp residues were here regarded as candidate caspase-1 targets. Using this approach, 20 proteins cleaved after Asp were identified upon incubation with recombinant caspase-1 (Tables I and II). In addition, 29 proteins were processed after residues other than Asp (supplemental Table 1) and were hence not further considered. Eleven of the 23 (48%) identified Asp-specific cleavage sites resembled the caspase-1 cleavage sites in pro-IL-1β and pro-IL-18, suggesting that this subgroup of substrates was directly processed by caspase-1 (Tables I and II, cluster I): next to the requirement for Asp in P1 that is typical for all caspases, this cluster was characterized by a unique specificity for the aliphatic residues Leu, Ile, Met, and Val in the P4 position as evident from an analysis of the identified cleavage sites on the WebLogo (37) server (Fig. 1A, left). Notably the executioner caspase-7 was identified as a member of this substrate cluster with cleavage occurring at its canonical activation site IQAD198 between the p20 and p10 subunits (Tables I and II). This result suggested that recombinant caspase-1 induced activation of endogenous caspase-7 in the treated lysate. In line with this notion, the remaining 12 of 23 (52%) processing events occurred at cleavage sites that perfectly matched or closely resembled the optimal caspase-7 recognition sequence DEVD (Fig. 1A, right) identified in combinatorial peptide library screenings (38).
Fig. 1.
Caspase-1 process procaspase-7 at canonical Asp23 and Asp198 sites. A, WebLogo analysis of the cleavage sites of cluster I (left) and cluster II (right) targets identified in a proteome-wide screening for caspase-1 targets. B and C, 35S-labeled procaspase-7 and pro-IL-1β (B) or procaspase-7 and the indicated site-directed mutants (C) were incubated with buffer or 30 nm caspase-1 for 1 h, and cleavage fragments were analyzed by SDS-PAGE and autoradiography. D and E, 35S-labeled cluster I (D) and cluster II (E) targets were incubated with 30 nm caspase-7, and reaction products were analyzed by SDS-PAGE and autoradiography. Black arrows indicate full-length proteins, and white arrows mark cleavage fragments. CASP1, caspase-1; CASP7, caspase-7; CTRL, control; WT, wild type.
Caspase-1 Processes Procaspase-7 at Canonical Asp23 and Asp198 Sites—
To confirm that caspase-1 can cleave caspase-7, in vitro translated 35S-labeled procaspase-7 was incubated with 30 nm recombinant caspase-1 for 30 min, 1 h, or 2 h at 37 °C, and the resulting cleavage fragments were analyzed by SDS-PAGE and autoradiography. Similar to caspase-1-mediated cleavage of pro-IL-1β, the 33-kDa full-length procaspase-7 band was completely processed in less than 30 min, giving rise to 19- and 11-kDa fragments that are characteristic for the p20 and p10 subunits of active caspase-7, respectively (Fig. 1B). As expected, caspase-1-mediated processing of pro-IL-1β and procaspase-7 was abrogated by the caspase-1 inhibitor YVAD-cmk (Fig. 1B). We made use of site-directed procaspase-7 mutants to investigate whether caspase-1-mediated cleavage of procaspase-7 occurred at the IQAD198 site and to verify whether caspase-1 also removes the amino-terminal procaspase-7 peptide after Asp23 (39). Wild type procaspase-7 was found to be cleaved by caspase-1, generating the characteristic p19 and p11 fragments (Fig. 1C). Mutation of the canonical Asp23 activation site between the prodomain and the large catalytic subunit did not interfere with the generation of the p11 activation fragment, although a slightly larger p19 subunit was apparent. These results are consistent with caspase-1-mediated removal of the procaspase-7 prodomain by cleavage at Asp23. The prototypical p19 and p11 subunits were not detected when Asp198 was mutated to Ala, although some procaspase-7 was processed to a fragment of 30 kDa (Fig. 1C). The latter fragment is likely generated by caspase-1-mediated removal of the amino-terminal peptide at Asp23 as its formation was prevented in the D23A/D198A double caspase-7 mutant (Fig. 1C). Together these results show that caspase-1 can cleave procaspase-7 at its canonical activation sites Asp23 and Asp198. We next analyzed whether caspase-7 activated downstream of caspase-1 could be responsible for the cleavage events grouped under cluster II (Tables I and II). The cluster I members TIF1β and Hsp60 were incorporated as negative controls for caspase-7-mediated cleavage. The latter two proteins were not cleaved by recombinant caspase-7 even at enzyme concentrations 10 times higher than the 30 nm used to cleave the cluster II substrates (Fig. 1D and data not shown). In contrast, active caspase-7 processed all tested cluster II members (Assc2, GIT2, Ligatin, eIF4h, MCM3, and Vps72), generating cleavage fragments that were in line with those predicted from the cleavage sites identified by COFRADIC analysis (Fig. 1E).
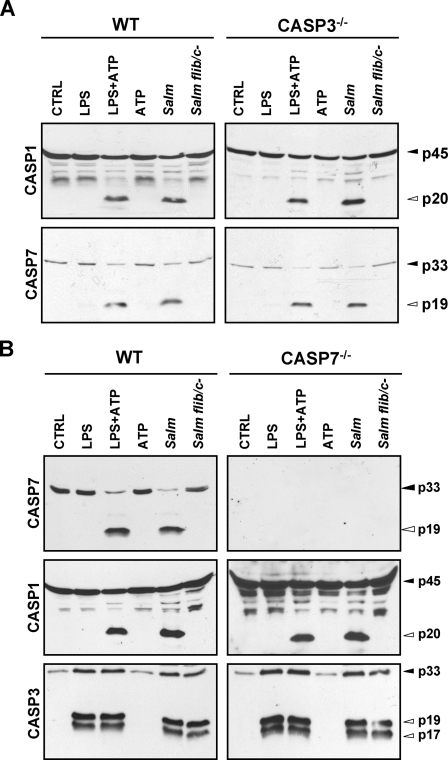
Caspase-1 Is Essential for Activation of Caspase-7, but Not Caspase-3, in Response to Microbial Stimuli—
To assess whether caspase-1 acts upstream of caspase-7 in vivo, BMDMs from wild type and caspase-1−/− macrophages were stimulated with LPS or LPS combined with ATP or pulsed with ATP alone for 30 min, and proteolytic activation of endogenous caspase-1, caspase-3, and caspase-7 was assessed by immunoblotting. As a control and in accord with previous reports (8, 9, 34), stimulation of LPS-treated macrophages with ATP induced activation of caspase-1 in wild type BMDMs, whereas LPS or ATP alone did not (Fig. 2A). Similar to caspase-1, activation of caspase-7 occurred only by combined stimulation with LPS and ATP (Fig. 2A). In contrast, the related caspase-3 was activated in macrophages stimulated with LPS alone (Fig. 2A), suggesting a differential activation mechanism for caspase-3 and -7. Indeed whereas activation of caspase-7 triggered by LPS and ATP was abolished in caspase-1-deficient macrophages, that of caspase-3 was not affected (Fig. 2A). To determine whether caspase-7 is activated by bacterial infection, macrophages were incubated with wild type S. typhimurium or a flagellin-deficient mutant. As a control and consistent with published studies (5, 14), Salmonella-induced caspase-1 activation required flagellin as flagellin-deficient bacteria failed to induce caspase-1 activation (Fig. 2A). As seen with caspase-1, caspase-7 was activated only when macrophages were infected with flagellated bacteria, whereas caspase-3 activation occurred independently of flagellin (Fig. 2A). Importantly Salmonella-induced caspase-7 activation was abolished in caspase-1−/− macrophages in line with an essential requirement for caspase-1 in the activation of caspase-7. In contrast, caspase-1 deficiency did not impair caspase-3 activation in response to wild type or flagellin-deficient Salmonella (Fig. 2A). To further confirm activation of caspase-3 and -7 in LPS + ATP-stimulated and Salmonella-infected BMDMs, we made use of biotin-DEVD-fmk to pull down the active forms of caspase-3 and -7 (40). The amount of active caspase-3 and -7 recovered from streptavidin beads was then determined by immunoblotting for these caspases. As expected, full-length caspase-3 and -7 were detected in the cell lysates but were not recovered from the streptavidin beads (Fig. 2, B and C). In contrast, the large catalytic subunits of cleaved caspase-3 (Fig. 2B, p17 and p19) and cleaved caspase-7 (Fig. 2C, p19) were efficiently pulled down from the setups stimulated with LPS + ATP or infected with Salmonella, confirming the activation status of caspase-3 and -7 under these conditions.
Fig. 2.
Caspase-1 is essential for activation of caspase-7, but not caspase-3, in response to microbial stimuli. A, macrophages from wild type and caspase-1−/− mice were stimulated with LPS for 3 h and then pulsed with ATP for 30 min, stimulated with LPS alone, pulsed with ATP alone, or infected with wild type S. typhimurium (Salm) or a flagellin-deficient mutant (Salm flib/c−) for 1 h. Extracellular bacteria were washed away, and macrophages were further incubated for 2 h in medium containing gentamycin. Cell extracts were immunoblotted with antibodies against caspase-1 (CASP1), caspase-3 (CASP3), and caspase-7 (CASP7). Black arrows indicate full-length caspases, and white arrows mark the large subunits of activated caspase-1, -3, and -7. Results are representative of at least three independent experiments. B and C, macrophages were left untreated, stimulated with LPS for 3 h and then pulsed with ATP, or infected with S. typhimurium (Salm) for 1 h. Cell lysates were incubated with biotin-DEVD-fmk, and streptavidin-bound complexes were precipitated as described under “Experimental Procedures.” Cell lysates and streptavidin-purified complexes were immunoblotted for caspase-3 (B) and caspase-7 (C). Results are representative of three experiments. CTRL, control; WT, wild type; IP, immunoprecipitation.
Caspase-3 and -7 Are Activated Independently in Response to Inflammatory Stimuli—
As caspase-3 was activated in LPS-stimulated and Salmonella-infected macrophages, it was possible that caspase-3 was also required for caspase-7 activation under these conditions. To examine this possibility, wild type and caspase-3−/− macrophages were stimulated as described above, and activation of caspase-1 and -7 was assessed by immunoblotting. We found that activation of caspase-1 and caspase-7 in response to LPS + ATP and Salmonella infection proceeded normally in macrophages deficient in caspase-3 (Fig. 3A), demonstrating that caspase-3 is not required for activation of caspase-1 and -7. Similarly caspase-7 deficiency did not affect activation of caspase-1 and -3 (Fig. 3B), indicating that these caspases do not require caspase-7 for their activation. As expected, the caspase-7 antibody failed to detect immunoreactive bands in lysates of caspase-7-deficient macrophages, thus confirming its specificity (Fig. 3B). These results indicate that the regulation of caspase-7 activation triggered by LPS and ATP or Salmonella is highly specific and relies on caspase-1 but not caspase-3. Reciprocally the activation of caspase-3 by microbial stimuli is independent of caspase-1 and -7. These results demonstrate the existence of non-redundant molecular mechanisms governing the activation of the executioner caspase-3 and -7 in response to microbial stimuli.
Fig. 3.
Caspase-3 and -7 are activated independently in response to microbial stimuli. A and B, macrophages from wild type and caspase-3−/− (A) or caspase-7−/− (B) mice were stimulated with LPS for 3 h and then pulsed with ATP for 30 min, stimulated with LPS alone, pulsed with ATP alone, or infected with wild type S. typhimurium (Salm) or a flagellin-deficient mutant (Salm flib/c−) for 1 h. Cell extracts were immunoblotted with antibodies against caspase-1 (CASP1), caspase-3 (CASP3), and caspase-7 (CASP7). Black arrows indicate full-length caspases, and white arrows mark the large subunits of activated caspase-1, -3, and -7. Results are representative of at least three independent experiments. CTRL, control; WT, wild type.
The Inflammasomes Control Activation of Caspase-7 but Not Caspase-3—
ATP induces the activation of caspase-1 in LPS-stimulated macrophages through the Cryopyrin inflammasome (8–10, 34), whereas Salmonella and flagellin require components of the Ipaf inflammasome to activate caspase-1 (5, 7, 14). We made use of BMDMs lacking the NLR proteins Cryopyrin or Ipaf as well as macrophages deficient in the essential inflammasome adaptor ASC to examine whether the Cryopyrin and Ipaf inflammasomes are required for caspase-7 activation in response to microbial stimuli. As a control, the activation of caspase-1 induced by stimulation with LPS and ATP was abolished in ASC−/− and Cryopyrin−/− macrophages but proceeded normally in Ipaf−/− macrophages (Fig. 4A). Notably processing of caspase-7 induced by LPS and ATP was prevented in BMDMs lacking ASC or Cryopyrin but not in Ipaf−/− cells (Fig. 4B) consistent with an essential role for the Cryopyrin inflammasome in the activation of caspase-7. In contrast, caspase-3 activation in response to LPS alone or in combination with ATP proceeded normally in ASC−/−, Cryopyrin−/−, and Ipaf−/− macrophages (Fig. 4C), demonstrating that the Cryopyrin and Ipaf inflammasomes are not required for caspase-3 activation. As reported previously (5, 14, 34), wild type and Cryopyrin−/− macrophages activated caspase-1 through the cytosolic recognition of Salmonella flagellin, which was abolished in ASC−/− and Ipaf−/− cells (Fig. 4A). Caspase-7 processing in response to Salmonella required flagellin, Ipaf, and ASC but not Cryopyrin (Fig. 4B), demonstrating that the Ipaf inflammasome functions upstream of caspase-7 activation in Salmonella-infected macrophages. In contrast, caspase-3 activation occurred in all tested knock-out macrophages (Fig. 4C), indicating that activation of this executioner caspase proceeds independently of the Ipaf and Cryopyrin inflammasomes in response to Salmonella infection. The latter results confirm our previous findings in caspase-1-deficient cells (Fig. 2A). Thus, activation of caspase-7, but not caspase-3, in response to LPS and ATP requires the assembly of a functional Cryopyrin inflammasome, whereas the Ipaf inflammasome is required for caspase-7 activation in Salmonella-infected macrophages. In addition, activation of caspase-1 (Fig. 5A) and caspase-7 (Fig. 5B) was abolished in ASC-deficient BMDMs stimulated with an array of microbial ligands and ATP and in LPS + nigericin-treated macrophages, demonstrating that the inflammasome is also required for activation of caspase-7 in response to these proinflammatory stimuli. Collectively these results demonstrate the activation of an NLR-specific caspase-1/caspase-7 cascade in response to microbial stimuli.
Fig. 4.
Specific activation of caspase-7 by NLR inflammasomes. A–C, macrophages from wild type, ASC−/−, Ipaf−/−, and Cryopyrin−/− mice were stimulated with LPS for 3 h and then pulsed with ATP for 30 min, stimulated with LPS alone, pulsed with ATP alone, or infected with wild type S. typhimurium (Salm) or a flagellin-deficient mutant (Salm flib/c−) for 1 h. Extracellular bacteria were washed away, and macrophages were further incubated for 2 h in medium containing gentamycin. Cell extracts were immunoblotted with antibodies against caspase-1 (CASP1) (A), caspase-7 (CASP7) (B), and caspase-3 (CASP3) (C). Black arrows indicate full-length caspases, and white arrows mark the large subunits of activated caspase-1, -3, and -7. Results are representative of at least three independent experiments. CTRL, control; WT, wild type.
Fig. 5.
Inflammasomes mediate caspase-7 activation in response to LPS + nigericin and bacterial ligands with ATP. A and B, macrophages from wild type and ASC−/− mice were stimulated with the indicated bacterial ligands and heat-killed bacteria for 3 h and then pulsed with 5 mm ATP or 20 μm nigericin for 30 min. Cell extracts were immunoblotted with antibodies against caspase-1 (A) and caspase-7 (B). Black arrows indicate full-length caspases, and white arrows mark the large subunits of activated caspase-1 and -7. Results are representative of three independent experiments. CTRL, control; WT, wild type; Pam3, Pam3-GSK4; PGN, peptidoglycan; LTA, lipoteichoic acid; HKLM, heat-killed Listeria monocytogenes; HKSA, heat-killed Staphylococcus aureus; HKLP, heat-killed Legionella pneumophila; HKPG, heat-killed Porphyromonas gingivalis.
Caspase-7 Deficiency Does Not Prevent Cytokine Production or Salmonella-induced Macrophage Cell Death—
Caspase-1 is best known for its role in the proteolytic maturation of the proinflammatory cytokines IL-1β and IL-18 (3, 4, 41–43). However, the mechanism by which the mature cytokines are secreted from the cytosol is unknown. To test whether caspase-7 is essential for the secretion of IL-1β and IL-18 in response to LPS + ATP or Salmonella, we measured the levels of these cytokines in the culture supernatants of stimulated macrophages. As expected, the combination of LPS and ATP or Salmonella infection triggered the release of significant levels of IL-1β and IL-18 in the culture supernatants of wild type macrophages, whereas LPS or ATP alone and flagellin-deficient Salmonella failed to do so (Fig. 6, A and B). The secretion of IL-1β and IL-18 triggered by LPS and ATP or Salmonella infection was abolished in caspase-1−/−, whereas cytokine levels comparable to those released by wild type cells were detected in the culture supernatants of caspase-7−/− macrophages (Fig. 6, A and B). Similarly secretion of IL-1β (Fig. 6C) and tumor necrosis factor-α (Fig. 6D) was not affected in stimulated caspase-3-deficient macrophages. These results suggest that although caspase-7 is activated downstream of caspase-1 it is not required in the secretion of the caspase-1-dependent cytokines IL-1β and IL-18.
Fig. 6.
Caspase-3 and -7 are dispensable for LPS- and Salmonella-induced cytokine secretion. A–D, macrophages from wild type, caspase-1−/−, caspase-3−/−, and caspase-7−/− mice were stimulated with LPS for 3 h and then pulsed with ATP for 30 min, stimulated with LPS alone, pulsed with ATP alone, or infected with wild type S. typhimurium (Salm) or a flagellin-deficient mutant (Salm Flib/c−) for 1 h. Cell culture medium was analyzed for release of IL-1β (A and C), IL-18 (B), or tumor necrosis factor-α (TNF-α) (D) by ELISA. CASP1, caspase-1; CASP3, caspase-3; CASP7, caspase-7; CTRL, control; WT, wild type. Results represent mean ± standard deviation of triplicates and are representative of these three independent experiments.
Caspase-1 is not directly implicated in apoptosis (3) except for the specialized form of macrophage cell death induced by pathogenic bacteria such as Salmonella (15). As both caspase-3 and -7 were activated in response to Salmonella infection (Fig. 2), we assessed the respective contribution of the executioner caspase-3 and -7 in Salmonella-induced macrophage cell death. As expected, substantial cell death of wild type macrophages infected with wild type Salmonella, but not with the flagellin-deficient Salmonella mutant, was evident 2 h after infection. In contrast to caspase-1-deficient cells, caspase-3−/− and caspase-7−/− macrophages were not protected from Salmonella-induced membrane damage and DNA fragmentation (Fig. 7, A and B). Therefore, the lack of protection from Salmonella-induced macrophage death in caspase-7-deficient cells might be due to functional redundancy with caspase-3 as occurs during developmental cell death (23–25) and in response to classical apoptotic triggers (25).
Fig. 7.
Neither caspase-3 nor caspase-7 deficiency protects from Salmonella-induced macrophage cell death. A and B, macrophages from wild type, caspase-1−/−, caspase-3−/−, and caspase-7−/− mice were infected with wild type S. typhimurium (Salm) or a flagellin-deficient mutant (Salm flib/c−) for 2 h at multiplicity of infection 5. Extracellular bacteria were washed away, and cell death was studied by measuring membrane damage (Live/Dead assay, Invitrogen) (A) and DNA fragmentation using ELISA (Roche Applied Science) (B) according to the manufacturers’ instructions. Results represent mean ± standard deviation of triplicates and are representative of three independent experiments. CASP1, caspase-1; CASP3, caspase-3; CASP7, caspase-7; CTRL, control; WT, wild type.
DISCUSSION
Using a proteomics approach to search for novel caspase-1 targets, we identified 11 cleavage events that occurred at sites closely matching the caspase-1 cleavage sites in pro-IL-1β and pro-IL-18. In addition to cytoskeletal proteins, chaperones, and transcription factors, the executioner caspase-7 was identified as a member of this substrate cluster with cleavage occurring at its canonical activation site IQAD198 between the p20 and p10 subunits. These results suggest that this subgroup of substrates was directly processed by caspase-1 and that recombinant caspase-1 induced activation of endogenous caspase-7 in the treated lysate. Biochemical analysis confirmed that the caspase-7 precursor was processed at the canonical activation sites Asp23 and Asp198 in the linker region, a step that is required for full activation (19, 20). In line with this notion, the remaining 12 of 23 (52%) processing events identified in the proteomics analysis occurred at cleavage sites that perfectly matched or closely resembled the optimal caspase-7 recognition sequence DEVD identified in combinatorial peptide library screenings (38). Indeed recombinant caspase-7 cleaved several members of the second substrate cluster but failed to process members containing caspase-1-like cleavage sites.
Importantly, genetics studies demonstrated that caspase-1 was required for proteolytic activation of caspase-7 in response to Salmonella infection and stimulation with LPS and ATP. These results provide evidence for a critical role for the inflammasome in the induction of caspase-7 activation in response to microbial stimuli. Previous reports revealed that caspase-1 activation is mediated by NLR family members that link recognition of specific bacterial molecules to caspase-1 activation (5, 7–10, 44, 45). Consistently, we report here that caspase-7 processing induced by Salmonella infection required Ipaf and flagellin, whereas Cryopyrin was essential for that triggered by LPS and ATP. These results delineate an NLR/caspase-1/caspase-7 pathway activated in response to specific microbial stimuli. In contrast, activation of caspase-3 was independent of inflammasome components including caspase-1, thus revealing an important difference with the classical apoptotic pathways where both caspase-3 and -7 are believed to be activated by the DISC and apoptosome complexes. Thus, although caspase-3 and caspase-7 can be activated in concert by the initiator proteases caspase-8 and caspase-9 in response to classical apoptotic triggers such as death receptor engagement and UV irradiation (1, 2), the executioner caspases differ in their upstream activation mechanisms in response to inflammatory stimuli. Furthermore the absence of caspase-3 did not have an impact on caspase-1 or caspase-7 activation in response to bacterial stimuli. Nevertheless neither caspase-3−/− nor caspase-7−/− macrophages were protected from Salmonella-induced membrane damage and DNA fragmentation. This finding is in agreement with what has been reported during developmental cell death (23–25) and in response to classical apoptotic triggers such as death receptor activation and UV irradiation (25) and suggests that the lack of protection from Salmonella-induced macrophage death in caspase-3- and -7-deficient cells might be due to their functional redundancy. Unfortunately this interesting possibility is hard to address experimentally in doubly deficient macrophages because of the early perinatal lethality of caspase-3/-7-deficient mice (25) and awaits the generation of conditionally targeted mice. Altogether we report here the identification of caspase-7 as a caspase-1 substrate, and we demonstrated that caspase-7 processing occurs at its canonical activation sites. In addition, we found that activation of caspase-7, but not caspase-3, is controlled by the inflammasomes in LPS + ATP-stimulated and in Salmonella-infected macrophages. These results establish for the first time the existence of an inflammatory NLR/caspase-1/caspase-7 cascade in macrophages and demonstrate the existence of differential activation mechanisms for the executioner caspase-3 and -7 in response to bacterial stimuli.
Supplementary Material
Acknowledgments
We thank Anthony Coyle, Ethan Grant, and John Bertin (Millennium Pharmaceuticals) for generous supply of Cryopyrin−/−, Ipaf−/− and ASC−/− mice; Richard Flavell (Yale School of Medicine) for caspase-1−/− mice; and Kevin Roth (University of Alabama at Birmingham) for supplying femurs of caspase-3−/− mice. We also thank Joel Whitfield from the Cellular Immunology Core Facility of the University of Michigan Cancer Center and Niklaas Colaert at the Department of Medical Protein Research of the Flanders Institute for Biotechnology and Ghent University for technical support.
Footnotes
Published, MCP Papers in Press, July 30, 2008, DOI 10.1074/mcp.M800132-MCP200
The abbreviations used are: caspase, cysteinyl aspartate-specific protease; CARD, caspase recruitment domain; ASC, apoptosis-associated specklike protein containing a CARD; ATP, adenosine triphosphate; BMDM, bone marrow-derived macrophage; COFRADIC, combined fractional diagonal chromatography; DISC, death-inducing signaling complex; IL-1β, interleukin-1β; IL-18, interleukin-18; LPS, lipopolysaccharide; NLR, NOD-like receptor; PAMP, pathogen-associated molecular pattern; NOD, nucleotide binding and oligomerization domain; RP, reverse phase; TNBS, 2,4,6-trinitrobenzenesulfonic acid; cmk, chloromethyl ketone; fmk, fluoromethyl ketone.
This work was supported, in whole or in part, by National Institutes of Health Grants AI063331 and AI064748 (to G. N.). This work was also supported by a grant of the Inter University Attraction Poles (IAP-Phase VI) (to J. V.), by grants from the European Union (Marie Curie, DeathTrain, Framework Program 6, Grant MRTN-CT-035624), Inter University Attraction Poles Grant IAP6/18, the Research Fund of Ghent University (Geconcerteerde Onderzoeksacties 12.0505.02), the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen (Grant 2G.0218.06), and the Belgian Foundation against Cancer (SCIE2003-48 Research) (to P. V.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.mcponline.org) contains supplemental material.
REFERENCES
- 1.Nicholson, D. W. ( 1999) Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ. 6, 1028–1042 [DOI] [PubMed] [Google Scholar]
- 2.Lamkanfi, M., Declercq, W., Kalai, M., Saelens, X., and Vandenabeele, P. ( 2002) A phylogenetic analysis of caspases from worm to man. Cell Death Differ. 9, 358–361 [DOI] [PubMed] [Google Scholar]
- 3.Li, P., Allen, H., Banerjee, S., Franklin, S., Herzog, L., Johnston, C., McDowell, J., Paskind, M., Rodman, L., Salfeld, J., Towne, E., Tracey, D., Wardwell, S., Wei, F.-Y., Wong, W., Kamen, R., and Seshadriand, T. ( 1995) Mice deficient in IL-1β-converting enzyme are defective in production of mature IL-1β and resistant to endotoxic shock. Cell 80, 401–411 [DOI] [PubMed] [Google Scholar]
- 4.Kuida, K., Lippke, J. A., Ku, G., Harding, M. W., Livingston, D. J., Su, M. S., and Flavell, R. A. ( 1995) Altered cytokine export and apoptosis in mice deficient in interleukin-1β converting enzyme. Science 267, 2000–2003 [DOI] [PubMed] [Google Scholar]
- 5.Franchi, L., Amer, A., Body-Malapel, M., Kanneganti, T. D., Ozoren, N., Jagirdar, R., Inohara, N., Vandenabeele, P., Bertin, J., Coyle, A., Grant, E. P., and Nunez, G. ( 2006) Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1β in Salmonella-infected macrophages. Nat. Immunol. 7, 576–582 [DOI] [PubMed] [Google Scholar]
- 6.Lamkanfi, M., Kanneganti, T. D., Franchi, L., and Nunez, G. ( 2007) Caspase-1 inflammasomes in infection and inflammation. J. Leukoc. Biol. 82, 220–225 [DOI] [PubMed] [Google Scholar]
- 7.Mariathasan, S., Newton, K., Monack, D. M., Vucic, D., French, D. M., Lee, W. P., Roose-Girma, M., Erickson, S., and Dixit, V. M. ( 2004) Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 430, 213–218 [DOI] [PubMed] [Google Scholar]
- 8.Mariathasan, S., Weiss, D. S., Newton, K., McBride, J., O'Rourke, K., Roose-Girma, M., Lee, W. P., Weinrauch, Y., Monack, D. M., and Dixit, V. M. ( 2006) Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440, 228–232 [DOI] [PubMed] [Google Scholar]
- 9.Sutterwala, F. S., Ogura, Y., Szczepanik, M., Lara-Tejero, M., Lichtenberger, G. S., Grant, E. P., Bertin, J., Coyle, A. J., Galan, J. E., Askenase, P. W., and Flavell, R. A. ( 2006) Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity 24, 317–327 [DOI] [PubMed] [Google Scholar]
- 10.Kanneganti, T. D., Ozoren, N., Body-Malapel, M., Amer, A., Park, J. H., Franchi, L., Whitfield, J., Barchet, W., Colonna, M., Vandenabeele, P., Bertin, J., Coyle, A., Grant, E. P., Akira, S., and Nunez, G. ( 2006) Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature 440, 233–236 [DOI] [PubMed] [Google Scholar]
- 11.Inohara, N., Chamaillard, M., McDonald, C., and Nunez, G. ( 2005) NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Annu. Rev. Biochem. 74, 355–383 [DOI] [PubMed] [Google Scholar]
- 12.Ozoren, N., Masumoto, J., Franchi, L., Kanneganti, T. D., Body-Malapel, M., Erturk, I., Jagirdar, R., Zhu, L., Inohara, N., Bertin, J., Coyle, A., Grant, E. P., and Nunez, G. ( 2006) Distinct roles of TLR2 and the adaptor ASC in IL-1β/IL-18 secretion in response to Listeria monocytogenes. J. Immunol. 176, 4337–4342 [DOI] [PubMed] [Google Scholar]
- 13.Kanneganti, T. D., Body-Malapel, M., Amer, A., Park, J. H., Whitfield, J., Franchi, L., Taraporewala, Z. F., Miller, D., Patton, J. T., Inohara, N., and Nunez, G. ( 2006) Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J. Biol. Chem. 281, 36560–36568 [DOI] [PubMed] [Google Scholar]
- 14.Miao, E. A., Alpuche-Aranda, C. M., Dors, M., Clark, A. E., Bader, M. W., Miller, S. I., and Aderem, A. ( 2006) Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1β via Ipaf. Nat. Immunol. 7, 569–575 [DOI] [PubMed] [Google Scholar]
- 15.Hersh, D., Monack, D. M., Smith, M. R., Ghori, N., Falkow, S., and Zychlinsky, A. ( 1999) The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc. Natl. Acad. Sci. U. S. A. 96, 2396–2401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boatright, K. M., Renatus, M., Scott, F. L., Sperandio, S., Shin, H., Pedersen, I. M., Ricci, J. E., Edris, W. A., Sutherlin, D. P., Green, D. R., and Salvesen, G. S. ( 2003) A unified model for apical caspase activation. Mol. Cell 11, 529–541 [DOI] [PubMed] [Google Scholar]
- 17.Salvesen, G. S., and Dixit, V. M. ( 1999) Caspase activation: the induced-proximity model. Proc. Natl. Acad. Sci. U. S. A. 96, 10964–10967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi, Y. ( 2004) Caspase activation: revisiting the induced proximity model. Cell 117, 855–858 [DOI] [PubMed] [Google Scholar]
- 19.Chai, J., Wu, Q., Shiozaki, E., Srinivasula, S. M., Alnemri, E. S., and Shi, Y. ( 2001) Crystal structure of a procaspase-7 zymogen: mechanisms of activation and substrate binding. Cell 107, 399–407 [DOI] [PubMed] [Google Scholar]
- 20.Stennicke, H. R., Jurgensmeier, J. M., Shin, H., Deveraux, Q., Wolf, B. B., Yang, X., Zhou, Q., Ellerby, H. M., Ellerby, L. M., Bredesen, D., Green, D. R., Reed, J. C., Froelich, C. J., and Salvesen, G. S. ( 1998) Pro-caspase-3 is a major physiologic target of caspase-8. J. Biol. Chem. 273, 27084–27090 [DOI] [PubMed] [Google Scholar]
- 21.Fischer, U., Janicke, R. U., and Schulze-Osthoff, K. ( 2003) Many cuts to ruin: a comprehensive update of caspase substrates. Cell Death Differ. 10, 76–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Timmer, J. C., and Salvesen, G. S. ( 2007) Caspase substrates. Cell Death Differ. 14, 66–72 [DOI] [PubMed] [Google Scholar]
- 23.Zheng, T. S., Hunot, S., Kuida, K., Momoi, T., Srinivasan, A., Nicholson, D. W., Lazebnik, Y., and Flavell, R. A. ( 2000) Deficiency in caspase-9 or caspase-3 induces compensatory caspase activation. Nat. Med. 6, 1241–1247 [DOI] [PubMed] [Google Scholar]
- 24.Houde, C., Banks, K. G., Coulombe, N., Rasper, D., Grimm, E., Roy, S., Simpson, E. M., and Nicholson, D. W. ( 2004) Caspase-7 expanded function and intrinsic expression level underlies strain-specific brain phenotype of caspase-3-null mice. J. Neurosci. 24, 9977–9984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lakhani, S. A., Masud, A., Kuida, K., Porter, G. A., Jr., Booth, C. J., Mehal, W. Z., Inayat, I., and Flavell, R. A. ( 2006) Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science 311, 847–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Staes, A., Van Damme, P., Helsens, K., Demol, H., Vandekerckhove, J., and Gevaert, K. ( 2008) Improved recovery of proteome-informative, protein N-terminal peptides by combined fractional diagonal chromatography (COFRADIC). Proteomics 8, 1362–1370 [DOI] [PubMed] [Google Scholar]
- 27.Gevaert, K., Goethals, M., Martens, L., Van Damme, J., Staes, A., Thomas, G. R., and Vandekerckhove, J. ( 2003) Exploring proteomes and analyzing protein processing by mass spectrometric identification of sorted N-terminal peptides. Nat. Biotechnol. 21, 566–569 [DOI] [PubMed] [Google Scholar]
- 28.Staes, A., Timmerman, E., Van Damme, J., Helsens, K., Vandekerckhove, J., Vollmer, M., and Gevaert, K. ( 2007) Assessing a novel microfluidic interface for shotgun proteome analyses. J. Sep. Sci. 30, 1468–1476 [DOI] [PubMed] [Google Scholar]
- 29.Perkins, D. N., Pappin, D. J., Creasy, D. M., and Cottrell, J. S. ( 1999) Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20, 3551–3567 [DOI] [PubMed] [Google Scholar]
- 30.Van Damme, P., Martens, L., Van Damme, J., Hugelier, K., Staes, A., Vandekerckhove, J., and Gevaert, K. ( 2005) Caspase-specific and nonspecific in vivo protein processing during Fas-induced apoptosis. Nat. Methods 2, 771–777 [DOI] [PubMed] [Google Scholar]
- 31.Vande Walle, L., Van Damme, P., Lamkanfi, M., Saelens, X., Vandekerckhove, J., Gevaert, K., and Vandenabeele, P. ( 2007) Proteome-wide identification of HtrA2/Omi substrates. J. Proteome Res. 6, 1006–1015 [DOI] [PubMed] [Google Scholar]
- 32.Martens, L., Vandekerckhove, J., and Gevaert, K. ( 2005) DBToolkit: processing protein databases for peptide-centric proteomics. Bioinformatics 21, 3584–3585 [DOI] [PubMed] [Google Scholar]
- 33.Elias, J. E., and Gygi, S. P. ( 2007) Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat. Methods 4, 207–214 [DOI] [PubMed] [Google Scholar]
- 34.Kanneganti, T. D., Lamkanfi, M., Kim, Y. G., Chen, G., Park, J. H., Franchi, L., Vandenabeele, P., and Nunez, G. ( 2007) Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity 26, 433–443 [DOI] [PubMed] [Google Scholar]
- 35.Lamkanfi, M., Kalai, M., Saelens, X., Declercq, W., and Vandenabeele, P. ( 2004) Caspase-1 activates nuclear factor of the κ-enhancer in B cells independently of its enzymatic activity. J. Biol. Chem. 279, 24785–24793 [DOI] [PubMed] [Google Scholar]
- 36.Ong, S. E., Blagoev, B., Kratchmarova, I., Kristensen, D. B., Steen, H., Pandey, A., and Mann, M. ( 2002) Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol. Cell. Proteomics 1, 376–386 [DOI] [PubMed] [Google Scholar]
- 37.Crooks, G. E., Hon, G., Chandonia, J. M., and Brenner, S. E. ( 2004) WebLogo: a sequence logo generator. Genome Res. 14, 1188–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thornberry, N. A., Rano, T. A., Peterson, E. P., Rasper, D. M., Timkey, T., Garcia-Calvo, M., Houtzager, V. M., Nordstrom, P. A., Roy, S., Vaillancourt, J. P., Chapman, K. T., and Nicholson, D. W. ( 1997) A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J. Biol. Chem. 272, 17907–17911 [DOI] [PubMed] [Google Scholar]
- 39.Denault, J. B., Bekes, M., Scott, F. L., Sexton, K. M., Bogyo, M., and Salvesen, G. S. ( 2006) Engineered hybrid dimers: tracking the activation pathway of caspase-7. Mol. Cell 23, 523–533 [DOI] [PubMed] [Google Scholar]
- 40.Grabarek, J., Amstad, P., and Darzynkiewicz, Z. ( 2002) Use of fluorescently labeled caspase inhibitors as affinity labels to detect activated caspases. Hum. Cell 15, 1–12 [DOI] [PubMed] [Google Scholar]
- 41.Ghayur, T., Banerjee, S., Hugunin, M., Butler, D., Herzog, L., Carter, A., Quintal, L., Sekut, L., Talanian, R., Paskind, M., Wong, W., Kamen, R., Tracey, D., and Allen, H. ( 1997) Caspase-1 processes IFN-γ-inducing factor and regulates LPS-induced IFN-γ production. Nature 386, 619–623 [DOI] [PubMed] [Google Scholar]
- 42.Thornberry, N. A., Bull, H. G., Calaycay, J. R., Chapman, K. T., Howard, A. D., Kostura, M. J., Miller, D. K., Molineaux, S. M., Weidner, J. R., Aunins, J., Elliston, K. O., Ayala, J. M., Casanoparalle, F. J., Chin, J., Ding, G. J.-F., Egger, L. A., Gaffney, E. P., Limjuco, G., Palyha, O. C., Raju, S. M., Rolandoparallel, A. M., Salley, J. P., Yamin, T.-T., Lee, T. D., Shively, J. E., MacCross, M., Mumford, R. A., Schmidt, J. A., and Tocciparallel, M. J. ( 1992) A novel heterodimeric cysteine protease is required for interleukin-1β processing in monocytes. Nature 356, 768–774 [DOI] [PubMed] [Google Scholar]
- 43.Cerretti, D. P., Kozlosky, C. J., Mosley, B., Nelson, N., Van Ness, K., Greenstreet, T. A., March, C. J., Kronheim, S. R., Druck, T., Cannizzaro, L. A., Huebner, K., and Black, R. A. ( 1992) Molecular cloning of the interleukin-1β converting enzyme. Science 256, 97–100 [DOI] [PubMed] [Google Scholar]
- 44.Agostini, L., Martinon, F., Burns, K., McDermott, M. F., Hawkins, P. N., and Tschopp, J. ( 2004) NALP3 forms an IL-1β-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity 20, 319–325 [DOI] [PubMed] [Google Scholar]
- 45.Martinon, F., Burns, K., and Tschopp, J. ( 2002) The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol. Cell 10, 417–426 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.