Abstract
Experimental evidence indicates that the mammalian cell membrane is compartmentalized. A structural feature that supports membrane segmentation implicates assemblies of selected lipids broadly referred to as lipid rafts. In T-lymphocytes, lipid rafts are implicated in signalling from the T-cell antigen receptor (TCR) and in localization and function of proteins residing proximal to the receptor. This review summarizes the current literature that deals with lipid raft involvement in T-cell activation and places particular emphasis in recent studies investigating lipid rafts in autoimmunity. The potential of lipid rafts as targets for the development of a new class of immune-modulating compounds is discussed.
Keywords: Lipid raft, T-cell receptor, Autoimmunity
1. Introduction
The original lipid raft hypothesis proposes the existence of assemblies of specific lipids that compartmentalise the plasma membrane into functionally distinct areas [1–4]. Studies with model membranes have shown that sphingolipids aggregate to form microdomains which are stabilized by addition of cholesterol. These self-assembled domains are in liquid ordered phase and are resistant to solubilisation with non-ionic detergents [5,6]. Likewise, mammalian cells extracted with similar detergents produce a detergent resistant membrane (DRM) fraction. DRMs are enriched in sphingomyelin, glycosphingolipids and cholesterol, however, it is debatable as to whether they reliably correspond to lipid raft domains as they exist in living cells [7,8]. Microscopy and the ability to observe differential membrane condensation using specific fluorescent probes provide additional methods which have significantly improved detection of specialized areas in the plasma membrane either before or after cell stimulation [9,10].
The latest results suggest that lipid rafts in non-stimulated cells are dynamic and of a size too small to detect with conventional microscopy. Various studies have suggested a size as small as 20 nm and up to 200 nm [11–14]. Despite their small size, molecules that are thought to partition into rafts show restricted lateral mobility, while proteins that are excluded diffuse freely [15,16]. Upon receptor stimulation, however, small lipid rafts may coalesce into bigger structures or become more rigid possibly by associating to the cytoskeleton [17]. Stimulation-induced protein–protein interactions are important as they most likely promote microdomain aggregation [18–20]. However, the prototypical raft model which proposes that self assembly of certain lipids creates platforms capable of regulating protein topology and function, is intensely debated [21–23]. Thus, far the evidence for their existence is suggestive rather than direct. The publication, in some instances, of opposing results and skepticism regarding the validity of certain methods used to describe lipid rafts (summarized in [22]) have tempered initial enthusiasm. Nevertheless, as more refined technologies are developed and new data are incorporated into existing ones, the intricacies of the lipid raft model will evolve accordingly. Any alternative theory that is put forward, however, will have to explain the large volume of data that show the critical role of protein acylation, and of cholesterol and glycosphingolipid manipulation on cell function.
Biological functions attributed to lipid rafts include endocytosis, pinocytosis, sorting and transport of proteins, and signal transduction [1,24]. The presence of many key signalling proteins and receptors including Src-family kinases, GTP-binding proteins and glycosylphosphatidyl-inositol (GPI)-linked receptors into lipid rafts and the potential of these domains to support protein–protein interactions at the plasma membrane has attracted many researchers into studying their involvement in signal transduction across many biological disciplines [1,25–27]. It is important to note here that for many proteins thought to localize to lipid rafts, this assignment has often been made because of their copurification with DRMs. Also, following fractionation, many of these proteins are detected in both DRM and detergent-soluble fractions (see discussion below for T-cell lipid rafts). In the text that follows we only concentrate on lipid raft function in TCR signalling and activation.
2. Structure and signalling by the T-cell antigen receptor
The TCR identifies antigenic peptides presented in the context of cognate Major Histocompatibility Complex (MHC) proteins expressed on the surface of antigen presenting cells (APCs). The part of the receptor that recognizes the large variety of antigens is a highly polymorphic heterodimer of α and β chains. For a subset of T-cells with limited tissue distribution this dimer is composed of the related γ and δ chains. The polymorphic dimer closely associates with a cluster of signal transducing, non-polymorphic proteins named the CD3 complex [28]. The polypeptides that comprise the CD3 complex are γ, δ, ε and ζ in a stoichiometry of 2ε, one of each γ and δ and a ζ homodimer. All CD3 chains contain a signal transducing motif called ITAM (immunoreceptor tyrosine-based activation motif) [29,30]. The γ, δ and ε chains contain one ITAM while ζ has 3. Therefore, a single TCR is equipped with 10 ITAMs altogether. The large number of ITAMs is needed to augment the strength of the signal generated by the receptor even if only a very small number of stimulatory antigen/MHC complexes are presented by the APC [31]. Direct evidence for this premise comes from a recent study. By generating animals with TCRs that contained variable number of functional ITAMs, it was shown that reduced number (7 or less) of functional ITAMs resulted in breakdown of central tolerance and development of autoimmune disease. This was due to incomplete deletion of autoreactive T-cells during development, a process that requires intact TCR signaling [32].
Signal transduction by the TCR is initiated by the phosphorylation of the tyrosine residues (Y) within the ITAMs primarily by the Src-family tyrosine kinase Lck [33–35] (Fig. 1). The 2 phospho-Y in each ITAM recruit from the cytosol the ZAP-70 tyrosine kinase which is phosphorylated and activated by Lck [36]. Activated ZAP-70 propagates transmission of the signal by phosphorylating the adapter molecule linker for activation of T-cells (LAT) [37]. LAT is phosphorylated on multiple Y residues, which then form docking sites for other adapter proteins like SLP-76, Grb-2 and Gads, and enzymes such as PI3K and PLCγ1 [37]. Thus, the central role of LAT in TCR signalling is its capacity when phosphorylated, to form complexes at the plasma membrane from which a number of signalling cascades originate. Lack of LAT expression uncouples TCR proximal tyrosine phosphorylation from these down-stream signalling cascades [38].
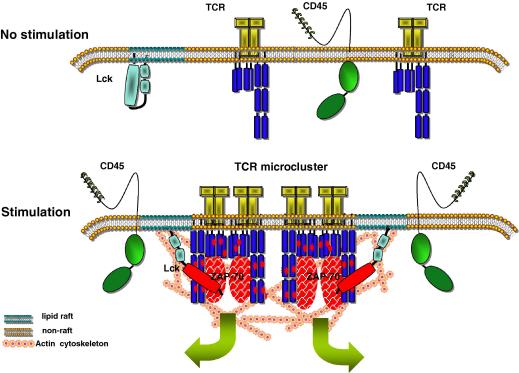
Fig. 1.
A model that correlates changes in the plasma membrane with TCR stimulation. In non-stimulated T-cells, the TCR is in monomeric form and its interaction with lipid rafts is transitory and unable to initiate signalling. Upon antigenic challenge, the reorganization of actin cytoskeleton results in formation of TCR microclusters and reduces the mobility of lipid rafts thus creating an environment conducive to long-lasting interactions. Concomitantly, the activity of Lck in lipid rafts increases owing to the action of a pool of CD45 molecules that moves to close proximity. These changes favour phosphorylation of ITAMs and initiation of signalling.
Like all the members of the Src-family kinases, Lck is regulated by the phosphorylation/dephosphorylation cycle of a C-terminal Y (Y-505). This Y when phosphorylated by the C-terminal kinase (Csk) associates with the Src homology 2 (SH2) domain of the molecule inducing a ‘closed’ conformation, which has low catalytic activity. Y-505 is dephosphorylated by the receptor-type tyrosine phosphatase CD45 [39]. Dephosphorylation converts Lck into an ‘open’ form [40]. At this stage autophosphorylation of another Y within the kinase domain (Y-394) renders the enzyme fully active [39]. Therefore, the primary role of CD45 is to prevent deactivation of Lck. Studies of CD45-null mice and cell lines which show hyperphosphorylation of Y-505 and impaired TCR signaling confirm the positive role of CD45 in T-cells [41,42]. Paradoxically, CD45 can also play a negative role by dephosphorylating Y-394 [43,44]. A recent report addressing this dichotomy has shown that while low levels of CD45 expression are sufficient for Y-505 dephosphorylation, high wild type levels of expression were necessary for Y-394 dephosphorylation and suppression of unwanted signalling [45].
Upon its stimulation the TCR changes topology forming signal transducing microclusters. Formation of TCR-rich microclusters, which also contain activated Lck and ZAP-70, is the earliest event measured following TCR stimulation [46,47] (Fig. 1). Pharmacological disruption of actin polymerization inhibited microcluster formation. Interestingly, TCR microclusters were observed in the presence of a Src-family kinase inhibitor indicating that their formation precedes Lck activation [46]. It will be important to dissect how the TCR induces actin polymerization independent of Lck activity. The TCR microclusters subsequently gather in an area in the membrane which is commonly referred to as central supramolecular activation cluster (cSMAC) [47,48]. This area is part of the immunological synapse formed between a T-cell and the APC [49]. Coreceptors and signalling molecules also concentrate in the cSMAC, while large glycosylated molecules such as CD45 and CD43 are excluded [50–52]. It is thought that the TCR molecules accumulating into the cSMAC do not transduce signals but are rather destined for endocytosis. However, newly formed TCR microclusters in the periphery maintain signal transmission by recruiting ZAP-70 and SLP-76 [47,53]. Staining of cells with cholera toxin B subunit (CTB), which binds to raft associated glycosphingolipid GM1, has shown that lipid rafts accumulate at the IS during T-cell activation [20,54,55]. Furthermore, the use of membrane intercalating fluorescent dyes such as Laurdan and di-4-ANEPPDHQ has provided evidence for increased condensation of the membrane at the site of TCR activation [19,56]. However, other reports argue against the preferential accumulation of lipid rafts to the IS [57,58].
3. Lipid rafts in TCR signalling and activation
Appropriate spatio-temporal localization of proteins is a key factor determining signalling activity. Dual acylation is required for Lck to transmit signals by the TCR [59] and LAT palmitoylation is important for its function [60]. Since acylation of proteins frequently determines their localization to cholesterol-sensitive lipid raft domains, these results have been interpreted as evidence that lipid rafts play a critical role in TCR signalling by means of the proteins they accrue. This premise was strengthened by experiments showing that extraction of cholesterol with chelating agents or treatment of cells with statins destabilize lipid rafts and modulate signalling [61–64].
The mechanistic model of how lipid rafts could regulate TCR signalling proposes that signalling starts when the TCR comes into close proximity with Lck- and LAT-containing lipid rafts (the size of which is yet to be defined). However, initiation of signalling should be controlled by receptor engagement. Such regulation could take place at two levels. First, at the formation of actin-dependent microclusters as mentioned earlier [46,47], and second at the upregulation of the enzymatic activity of a pool of Lck that is in the vicinity of the TCR. Regarding the first level of regulation, Lck-containing lipid rafts should become part of the TCR-rich microclusters, whose initial formation does not require Src-family kinase activity [46] (Fig. 1). Although this has not been shown directly, a recent report provides some evidence that shows clustering of membrane rafts is dependent on actin cytoskeleton and that the actin cytoskeleton and cholesterol levels determine the status of Lck activation [65,66]. Furthermore, single particle tracking technology revealed that the actin cytoskeleton slows down the lateral diffusion of acylated Lck in the plasma membrane and thus facilitates accumulation of the kinase to areas of stimulated TCR clusters [67].
During the second step of control, the activity of the pool of Lck that gathers to the TCR-microclusters may need to be transiently increased. This is because Lck was found to be hyperphosphorylated on Y-505 and to have reduced catalytic activity in DRMs [68,69]. Csk was present in these preparations and most likely is responsible for Lck inactivation in these domains [70]. Inactivation of Lck in lipid rafts might be required to preserve homeostatic control and to avoid inappropriate signalling in the absence of antigenic challenge [27]. Two proteins that bind to Csk were identified in T-cell DRMs. These are the Csk-binding protein (Cbp) [71], also known as PAG [72], and the Lck-interacting molecule (LIME) [73,74], which are palmitoylated adapter molecules and substrates for Src-family tyrosine kinases. Cbp is constitutively phosphorylated in DRMs [75,76]. Significantly, stimulation of the TCR induced its dephosphorylation and release of Csk [70,72]. These observations led to the hypothesis that Cbp could be the protein anchor that recruits Csk to the plasma membrane to inactivate Lck. However, the generation of mutant mice deficient in Cbp expression has shown that T-cell development and function remain normal in the absence of Cbp [77], although this adapter may have an important role as a tumor–supressor protein [78]. Similarly, LIME knock-out animals show no overt signs of abnormal T-cell development and function [79]. Therefore, the search for the plasma membrane Csk-anchor in T-cells is still ongoing, although generation of Cbp/LIME double knock-out animals may show that these two adapters have a redundant function in T-cells (Fig. 2).
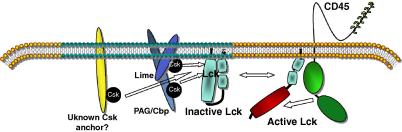
Fig. 2.
A schematic model for the regulation of Lck activity in T-cells. The activity of Lck in lipid rafts is low owing to the recruitment of Csk by PAG/Cbp, LIME and possibly an additional, as yet unknown, adaptor to these domains. Movement of Lck out of lipid rafts or transient association of lipid rafts with CD45 could result in Y-505 dephosphorylation and increase in Lck activity.
Transient activation of Lck in microdomains that move adjacent to TCR-microclusters could be initiated by CD45-mediated dephosphorylation of Y-505 (Fig. 1). Although CD45 is not an integral part of lipid rafts [9,50], upon TCR triggering, a small fraction may affix to lipid raft domains [80,81]. This pool of CD45 although small, could be sufficient for Y-505 dephosphorylation [45]. Such movement of CD45 to lipid rafts could be mediated by binding to raft associated molecules including CD26 or influenced by actin polymerization [82].
Following the initial steps of TCR stimulation many signalling proteins translocate to DRMs and colocalise with lipid rafts as shown by CTB staining [20,65,83–87]. Furthermore increased condensation of the plasma membrane at the site of TCR activation [19,56] and on the other hand decreased TCR signaling in cells loaded with a cholesterol derivative that inhibits membrane condensation [88], point towards a role for lipid raft membranes in TCR signalling. However, the exact size of these microdomains and their detailed mechanism of action remain undefined.
4. Lipid rafts in autoimmunity: potential drug targets?
An important question arising from the work summarized above is whether the status/function of lipid rafts is altered in autoimmune diseases. Elevated cholesterol and GM1 have been noted in activated T-cells [89,90] and in cells from older donors [91,92]. Interestingly, peripheral blood T-cells from patients with the autoimmune disease systemic lupus erythematosus (SLE), contain higher levels of GM1 and cholesterol [93]. These levels gradually revert to normal when the cells are ‘rested’ in vitro. This may indicate that T-cells in these patients are continuously activated by autoantigens. A pending question is whether higher levels of cholesterol and GM1, and possibly of other lipids, signify only a higher metabolic rate or whether they induce changes in membrane density and segmentation. If the latter is true then changes in protein mobility and function may occur potentially impacting on signalling homeostasis. Defects in various signalling cascades and in expression of key molecules have been noted in SLE T-cells [94–96]. For example, T-cells from patients with active disease expressed less Lck owing to increased consumption of the protein [94]. This is reminiscent of what was seen after sustained activation of normal T-cells [83]. In addition, we have observed higher levels of CD45 in lipid rafts in autoimmune lymphocytes and stimulation of cells with anti-CD3/CD28-coated beads revealed faster kinetics of CD45 reorientation in relation to the cell:bead contact area [93,97]. It will be interesting to investigate the topology and kinetics of CD45 in autoimmune T-cells in relation to TCR microclusters. These results suggest that the size of a pool of CD45 within or in close proximity to lipid rafts could be important for converting Lck into an active form to support signalling by the TCR (Fig. 3).
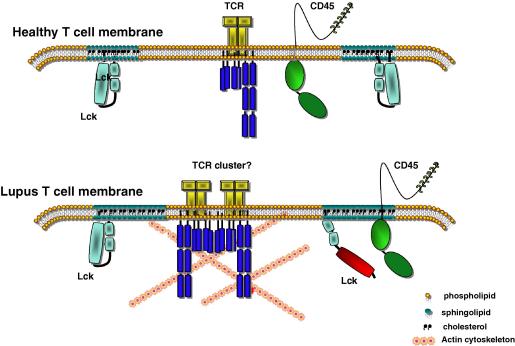
Fig. 3.
Changes in the membrane of lupus T-cells may associate with their autoimmune phenotype. The higher cholesterol and GM1 content of lupus T-cell membrane may result in larger and/or less mobile lipid rafts. This in combination with the increased association of CD45 with lipid rafts, higher Lck activity, and changes in the actin cytoskeleton seen in these cells could reduce the threshold for activation.
There is evidence for a role for lipid rafts in other autoimmune diseases as well. For example, CD4loCD40+ is an autoreactive T-cell subpopulation recently identified to be expanded in autoimmunity [98]. Survival and expansion of these cells is dependent on the distribution of the CD40 receptor and its associated signalling complex into lipid rafts [99]. In neuroinflammatory diseases including multiple sclerosis (MS), there is breach of the blood–brain barrier by inflammatory leukocytes [100]. Transmigration of inflammatory cells into the central nervous system requires expression of adhesion molecules. Proteomic analysis of lipid raft microdomains from human blood–brain barrier endothelium identified CD166 the expression of which was elevated during neuroinflammation [101]. CD166 was found to have an important role in the transmigration of leukocytes from blood to the CNS [101].
Recent studies where levels of raft-forming lipids were altered by pharmacological or other means have shown that there is a correlation between their level of expression and regulation of the immune system. For example, it has been shown recently that sialidases, a group of plasma-membrane-associated enzymes, play a role in modulating microdomain-associated glycosphingolipid content and influencing activation in T-lymphocytes [102,103]. Interestingly, inhibitors of sialidase, such as oseltamivir (Tamiflu), down-regulated GM1 expression on the surface of T-cells and reduced cytokine production in activated T-cells [104].
Interestingly, B-cell depleting anti-CD20 antibodies, such as rituximab, which are used for the treatment of rheumatoid arthritis (RA), were shown to induce translocation of CD20 to the lipid raft fraction [105]. This event was crucial for induction of cell apoptosis and was prevented by cholesterol extraction and disruption of lipid rafts [106]. Therefore, certain biologic agents used in the clinic may require intact lipid rafts to exert their therapeutic function.
The development of statins (inhibitors of 3-hydroxy-3-methylgluttaryl coenzyme A reductase), which are blockers of cholesterol biosynthesis, have provided an additional tool to address how reduction of cholesterol affects subcellular targeting of proteins and signalling. The use of statins in various autoimmune conditions has started to provide some interesting results [107,108]. In experimental autoimmune encephalomyelitis (EAE), an animal model of MS, statin treatment reduced pathogenesis by interfering with leukocyte recruitment, T-cell activation and cell adhesion (reviewed in [109]). Furthermore, statin treatment was found to reduce N-methyl-d-aspartate (NMDA)-induced neuronal damage most likely because it reduced NMDA receptor movement to lipid rafts [110]. Also, statins reduce human blood–brain barrier permeability and downregulate transmigration of leukocytes [111]. Therefore, statins may prove to be beneficial in the early stages of MS. It is unclear however, how statins affect the immune system. In addition to reducing cholesterol, they interfere with post-translation modification and membrane targeting of signalling proteins such as several small GTP-binding proteins [112]. These proteins become isoprenylated, a process that depends on the action of HMG-CoA reductase and mevalonate production, and is required for lipid raft targeting. Another action of statins could be their ability to modify lipid raft structure and function. Ex vivo treatment of T-lymphocytes from SLE patients with atorvastatin reduced colocalisation of Lck and CD45 resulting in less active Lck and reduced production of the pathogenic cytokines IL10 and IL6 upon stimulation [63]. In an animal model of acute colitis, simvastatin reduced the severity of the disease and downmodulated the activity of the NF-κB pathway in stimulated intestinal epithelial cells [113]. Furthermore, production of pathogenic chemokines from intestinal epithelial cells was dependent on cholesterol suggesting possible involvement of lipid rafts in this process [114]. Therefore, statins mediate their effects on the immune system via multiple mechanisms. In certain cases their effect may be mediated by inducing alterations in lipid rafts and/or inhibiting targeting of key signalling molecules to these domains. Nevertheless, additional roles for cholesterol and its metabolites are becoming apparent. A recent report makes some progress in unraveling how cholesterol levels affect T-cell proliferation. Activation of T-cells induced cholesterol synthesis and suppression of its transport, which is mediated by the LXR (liver X receptor) pathway [115], suggesting that a specific pool of cellular cholesterol is important for T-cell proliferation, although its location is unclear.
Polyunsaturated fatty acids (PUFAs) are shown to intercalate and remodel lipid raft domains [116]. Treatment of T-lymphocytes with PUFAs resulted in the delocalisation of raft-anchored signalling proteins such as LAT [117]. LAT delocalisation was accompanied by its reduced tyrosine phosphorylation and IL2 production following TCR stimulation [118]. PUFAs were also shown to interfere with the formation of the IS [119]. In addition, cell treatment with dietary fish oils altered the structure and size of membrane domains [120] and reduced signalling by the TCR [121,122]. In an experimental model of colitis, PUFA administration prevented inflammation-induced exit of tight junction proteins from lipid rafts and diminished disruption of tight junctions in the intestinal mucosa [123]. Collectively, these results lay the case for closer investigation of PUFAs and dietary fish oils as lipid raft modifiers and as beneficial agents in autoimmunity and inflammation.
5. Perspectives
Suggestive evidence argues for the involvement of lipid rafts in autoimmunity. Some existing agents used in the clinic, such as anti-CD20 antibodies and statins, may exert their therapeutic effect, at least in part, via lipid rafts. In our view, there is sufficient evidence suggesting that lipid rafts are valid targets for the development of new pharmaceuticals capable of modifying their function in autoimmunity. These can be agents that are either general lipid raft modulators acting on all cells or specific for a raft-associated protein with a restricted pattern of expression. More specific agents will also serve as tools to better elucidate the role of lipid raft domains in the development and pathophysiology of autoimmune diseases.
Acknowledgements
The work conducted in our laboratories has been supported by the Arthritis Research Campaign (ARC) UK project grants (16018, 17319, 17428, I0536), a University College London Hospital (CRDC) project grant (GCT/2008/EJ), an ARC Career Development award to E.J. (18106) and a Wellcome Trust Career Development Fellowship to P.K. (58408). We are grateful to our colleagues David Isenberg, Fabian Flores-Borja and Fulvio D’Acquisto for discussions and input, and to Giovanna Leoni for assistance with the figures.
Contributor Information
Panagiotis S. Kabouridis, Email: p.s.kabouridis@qmul.ac.uk.
Elizabeth C. Jury, Email: e.jury@ucl.ac.uk.
References
- 1.Simons K., Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 2.Edidin M. Lipid microdomains in cell surface membranes. Curr. Opin. Struct. Biol. 1997;7:528–532. doi: 10.1016/s0959-440x(97)80117-0. [DOI] [PubMed] [Google Scholar]
- 3.Brown D.A., London E. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- 4.Simons K., Toomre D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell. Biol. 2000;1:31–34. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 5.Brown R.E. Sphingolipid organization in biomembranes: what physical studies of model membranes reveal. J. Cell Sci. 1998;111:1–9. doi: 10.1242/jcs.111.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dietrich C., Bagatolli L.A., Volovyk Z.N., Thompson N.L., Levi M., Jacobson K., Gratton E. Lipid rafts reconstituted in model membranes. Biophys. J. 2001;80:1417–1428. doi: 10.1016/S0006-3495(01)76114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heerklotz H. Triton promotes domain formation in lipid raft mixtures. Biophys. J. 2002;83:2693–2701. doi: 10.1016/S0006-3495(02)75278-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munro S. Lipid rafts: elusive or illusive? Cell. 2003;115:377–388. doi: 10.1016/s0092-8674(03)00882-1. [DOI] [PubMed] [Google Scholar]
- 9.Janes P.W., Ley S.C., Magee A.I., Kabouridis P.S. The role of lipid rafts in T cell antigen receptor (TCR) signalling. Semin. Immunol. 2000;12:23–34. doi: 10.1006/smim.2000.0204. [DOI] [PubMed] [Google Scholar]
- 10.Harder T., Rentero C., Zech T., Gaus K. Plasma membrane segregation during T cell activation: probing the order of domains. Curr. Opin. Immunol. 2007;19:470–475. doi: 10.1016/j.coi.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Pralle A., Keller P., Florin E.L., Simons K., Horber J.K. Sphingolipid-cholesterol rafts diffuse as small entities in the plasma membrane of mammalian cells. J. Cell Biol. 2000;148:997–1008. doi: 10.1083/jcb.148.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zacharias D.A., Violin J.D., Newton A.C., Tsien R.Y. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science. 2002;296:913–916. doi: 10.1126/science.1068539. [DOI] [PubMed] [Google Scholar]
- 13.Sharma P., Varma R., Sarasij R.C., Ira, Gousset K., Krishnamoorthy G., Rao M., Mayor S. Nanoscale organization of multiple GPI-anchored proteins in living cell membranes. Cell. 2004;116:577–589. doi: 10.1016/s0092-8674(04)00167-9. [DOI] [PubMed] [Google Scholar]
- 14.Murase K. Ultrafine membrane compartments for molecular diffusion as revealed by single molecule techniques. Biophys. J. 2004;86:4075–4093. doi: 10.1529/biophysj.103.035717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lenne P.F. Dynamic molecular confinement in the plasma membrane by microdomains and the cytoskeleton meshwork. EMBO J. 2006;25:3245–3256. doi: 10.1038/sj.emboj.7601214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicolau D.V., Jr., Burrage K., Parton R.G., Hancock J.F. Identifying optimal lipid raft characteristics required to promote nanoscale protein–protein interactions on the plasma membrane. Mol. Cell. Biol. 2006;26:313–323. doi: 10.1128/MCB.26.1.313-323.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hancock J.F. Lipid rafts: contentious only from simplistic standpoints. Nat. Rev. Mol. Cell. Biol. 2006;7:456–462. doi: 10.1038/nrm1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Douglass A.D., Vale R.D. Single-molecule microscopy reveals plasma membrane microdomains created by protein–protein networks that exclude or trap signaling molecules in T cells. Cell. 2005;121:937–950. doi: 10.1016/j.cell.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaus K., Chklovskaia E., Fazekas de St. Groth B., Jessup W., Harder T. Condensation of the plasma membrane at the site of T lymphocyte activation. J. Cell Biol. 2005;171:121–131. doi: 10.1083/jcb.200505047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tavano R., Contento R.L., Baranda S.J., Soligo M., Tuosto L., Manes S., Viola A. CD28 interaction with filamin-A controls lipid raft accumulation at the T-cell immunological synapse. Nat. Cell. Biol. 2006;8:1270–1276. doi: 10.1038/ncb1492. [DOI] [PubMed] [Google Scholar]
- 21.de Wet B., Harder T. Are rafts involved in T-cell receptor signalling? Introduction to the Talking Point on the involvement of lipid rafts in T-cell activation. EMBO Rep. 2008;9:523–524. doi: 10.1038/embor.2008.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kenworthy A.K. Have we become overly reliant on lipid rafts? Talking Point on the involvement of lipid rafts in T-cell activation. EMBO Rep. 2008;9:531–535. doi: 10.1038/embor.2008.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He H.T., Marguet D. T-cell antigen receptor triggering and lipid rafts: a matter of space and time scales. Talking Point on the involvement of lipid rafts in T-cell activation. EMBO Rep. 2008;9:525–530. doi: 10.1038/embor.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown D.A., Rose J.K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- 25.Foster L.J., De Hoog C.L., Mann M. Unbiased quantitative proteomics of lipid rafts reveals high specificity for signaling factors. Proc. Natl. Acad. Sci. USA. 2003;100:5813–5818. doi: 10.1073/pnas.0631608100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Haller P.D., Donohoe S., Goodlett D.R., Aebersold R., Watts J.D. Mass spectrometric characterization of proteins extracted from Jurkat T cell detergent-resistant membrane domains. Proteomics. 2001;1:1010–1021. doi: 10.1002/1615-9861(200108)1:8<1010::AID-PROT1010>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 27.Razzaq T.M., Ozegbe P., Jury E.C., Sembi P., Blackwell N.M., Kabouridis P.S. Regulation of T-cell receptor signalling by membrane microdomains. Immunology. 2004;113:413–426. doi: 10.1111/j.1365-2567.2004.01998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis M.M., Boniface J.J., Reich Z., Lyons D., Hampl J., Arden B., Chien Y. Ligand recognition by alpha beta T cell receptors. Annu. Rev. Immunol. 1998;16:523–544. doi: 10.1146/annurev.immunol.16.1.523. [DOI] [PubMed] [Google Scholar]
- 29.Reth M. Antigen receptor tail clue. Nature. 1989;338:383–384. doi: 10.1038/338383b0. [DOI] [PubMed] [Google Scholar]
- 30.Irving B.A., Chan A.C., Weiss A. Functional characterization of a signal transducing motif present in the T cell antigen receptor zeta chain. J. Exp. Med. 1993;177:1093–1103. doi: 10.1084/jem.177.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lanzavecchia A., Iezzi G., Viola A. From TCR engagement to T cell activation: a kinetic view of T cell behavior. Cell. 1999;96:1–4. doi: 10.1016/s0092-8674(00)80952-6. [DOI] [PubMed] [Google Scholar]
- 32.Holst J. Scalable signaling mediated by T cell antigen receptor-CD3 ITAMs ensures effective negative selection and prevents autoimmunity. Nat. Immunol. 2008;9:658–666. doi: 10.1038/ni.1611. [DOI] [PubMed] [Google Scholar]
- 33.Latour S., Veillette A. Proximal protein tyrosine kinases in immunoreceptor signaling. Curr. Opin. Immunol. 2001;13:299–306. doi: 10.1016/s0952-7915(00)00219-3. [DOI] [PubMed] [Google Scholar]
- 34.Molina T.J. Profound block in thymocyte development in mice lacking p56lck. Nature. 1992;357:161–164. doi: 10.1038/357161a0. [DOI] [PubMed] [Google Scholar]
- 35.Straus D.B., Weiss A. Genetic evidence for the involvement of the Lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell. 1992;70:585–593. doi: 10.1016/0092-8674(92)90428-f. [DOI] [PubMed] [Google Scholar]
- 36.Chan A.C., Iwashima M., Turck C.W., Weiss A. ZAP-70: a 70 kDa protein-tyrosine kinase that associates with the TCR zeta chain. Cell. 1992;71:649–662. doi: 10.1016/0092-8674(92)90598-7. [DOI] [PubMed] [Google Scholar]
- 37.Zhang W., Sloan-Lancaster J., Kitchen J., Trible R.P., Samelson L.E. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92:83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 38.Zhang W. Essential role of LAT in T cell development. Immunity. 1999;10:323–332. doi: 10.1016/s1074-7613(00)80032-1. [DOI] [PubMed] [Google Scholar]
- 39.Mustelin T., Altman A. Dephosphorylation and activation of the T cell tyrosine kinase pp56lck by the leukocyte common antigen (CD45) Oncogene. 1990;5:809–813. [PubMed] [Google Scholar]
- 40.Reynolds P.J., Hurley T.R., Sefton B.M. Functional analysis of the SH2 and SH3 domains of the Lck tyrosine protein kinase. Oncogene. 1992;7:1949–1955. [PubMed] [Google Scholar]
- 41.Kishihara K. Normal B lymphocyte development but impaired T cell maturation in CD45-exon6 protein tyrosine phosphatase-deficient mice. Cell. 1993;74:143–156. doi: 10.1016/0092-8674(93)90302-7. [DOI] [PubMed] [Google Scholar]
- 42.Koretzky G.A., Picus J., Thomas M.L., Weiss A. Tyrosine phosphatase CD45 is essential for coupling T-cell antigen receptor to the phosphatidyinositol pathway. Nature. 1990;346:66–68. doi: 10.1038/346066a0. [DOI] [PubMed] [Google Scholar]
- 43.Thomas M.L., Brown E.J. Positive and negative regulation of Src-family membrane kinases by CD45. Immunol. Today. 1999;20:406–411. doi: 10.1016/s0167-5699(99)01506-6. [DOI] [PubMed] [Google Scholar]
- 44.D’Oro U., Ashwell J.D. Cutting edge: the CD45 tyrosine phosphatase is an inhibitor of Lck activity in thymocytes. J. Immunol. 1999;162:1879–1883. [PubMed] [Google Scholar]
- 45.McNeill L. The differential regulation of Lck kinase phosphorylation sites by CD45 is critical for T cell receptor signaling responses. Immunity. 2007;27:425–437. doi: 10.1016/j.immuni.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 46.Campi G., Varma R., Dustin M.L. Actin and agonist MHC-peptide complex-dependent T cell receptor microclusters as scaffolds for signaling. J. Exp. Med. 2005;202:1031–1036. doi: 10.1084/jem.20051182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yokosuka T., Sakata-Sogawa K., Kobayashi W., Hiroshima M., Hashimoto-Tane A., Tokunaga M., Dustin M.L., Saito T. Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP-76. Nat. Immunol. 2005;6:1253–1262. doi: 10.1038/ni1272. [DOI] [PubMed] [Google Scholar]
- 48.Varma R., Campi G., Yokosuka T., Saito T., Dustin M.L. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity. 2006;25:117–127. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bromley S.K. The immunological synapse. Annu. Rev. Immunol. 2001;19:375–396. doi: 10.1146/annurev.immunol.19.1.375. [DOI] [PubMed] [Google Scholar]
- 50.van der Merwe P.A. The TCR triggering puzzle. Immunity. 2001;14:665–668. doi: 10.1016/s1074-7613(01)00155-8. [DOI] [PubMed] [Google Scholar]
- 51.Freiberg B.A., Kupfer H., Maslanik W., Delli J., Kappler J., Zaller D.M., Kupfer A. Staging and resetting T cell activation in SMACs. Nat. Immunol. 2002;3:911–917. doi: 10.1038/ni836. [DOI] [PubMed] [Google Scholar]
- 52.Depoil D. Immunological synapses are versatile structures enabling selective T cell polarization. Immunity. 2005;22:185–194. doi: 10.1016/j.immuni.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 53.Lee K.H. The immunological synapse balances T cell receptor signaling and degradation. Science. 2003;302:1218–1222. doi: 10.1126/science.1086507. [DOI] [PubMed] [Google Scholar]
- 54.Burack W.R., Lee K.H., Holdorf A.D., Dustin M.L., Shaw A.S. Cutting edge: quantitative imaging of raft accumulation in the immunological synapse. J. Immunol. 2002;169:2837–2841. doi: 10.4049/jimmunol.169.6.2837. [DOI] [PubMed] [Google Scholar]
- 55.Tavano R., Gri G., Molon B., Marinari B., Rudd C.E., Tuosto L., Viola A. CD28 and lipid rafts coordinate recruitment of Lck to the immunological synapse of human T lymphocytes. J. Immunol. 2004;173:5392–5397. doi: 10.4049/jimmunol.173.9.5392. [DOI] [PubMed] [Google Scholar]
- 56.Owen D.M., Lanigan P.M., Dunsby C., Munro I., Grant D., Neil M.A., French P.M., Magee A.I. Fluorescence lifetime imaging provides enhanced contrast when imaging the phase-sensitive dye di-4-ANEPPDHQ in model membranes and live cells. Biophys. J. 2006;90:L80–82. doi: 10.1529/biophysj.106.084673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Glebov O.O., Nichols B.J. Lipid raft proteins have a random distribution during localized activation of the T-cell receptor. Nat. Cell Biol. 2004;6:238–243. doi: 10.1038/ncb1103. [DOI] [PubMed] [Google Scholar]
- 58.Harder T., Kuhn M. Selective accumulation of raft-associated membrane protein LAT in T cell receptor signaling assemblies. J. Cell Biol. 2000;151:199–208. doi: 10.1083/jcb.151.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kabouridis P.S., Magee A.I., Ley S.C. S-Acylation of LCK protein tyrosine kinase is essential for its signalling function in T lymphocytes. EMBO J. 1997;16:4983–4998. doi: 10.1093/emboj/16.16.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang W., Trible R.P., Samelson L.E. LAT palmitoylation: its essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity. 1998;9:239–246. doi: 10.1016/s1074-7613(00)80606-8. [DOI] [PubMed] [Google Scholar]
- 61.Xavier R., Brennan T., Li Q., McCormack C., Seed B. Membrane compartmentation is required for efficient T cell activation. Immunity. 1998;8:356–360. doi: 10.1016/s1074-7613(00)80577-4. [DOI] [PubMed] [Google Scholar]
- 62.Kabouridis P.S., Janzen J., Magee A.L., Ley S.C. Cholesterol depletion disrupts lipid rafts and modulates the activity of multiple signaling pathways in T lymphocytes. Eur. J. Immunol. 2000;30:954–963. doi: 10.1002/1521-4141(200003)30:3<954::AID-IMMU954>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 63.Jury E.C., Isenberg D.A., Mauri C., Ehrenstein M.R. Atorvastatin restores Lck expression and lipid raft-associated signaling in T cells from patients with systemic lupus erythematosus. J. Immunol. 2006;177:7416–7422. doi: 10.4049/jimmunol.177.10.7416. [DOI] [PubMed] [Google Scholar]
- 64.Hillyard D.Z., Cameron A.J., McDonald K.J., Thomson J., MacIntyre A., Shiels P.G., Panarelli M., Jardine A.G. Simvastatin inhibits lymphocyte function in normal subjects and patients with cardiovascular disease. Atherosclerosis. 2004;175:305–313. doi: 10.1016/j.atherosclerosis.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 65.Chichili G.R., Rodgers W. Clustering of membrane raft proteins by the actin cytoskeleton. J. Biol. Chem. 2007;282:36682–36691. doi: 10.1074/jbc.M702959200. [DOI] [PubMed] [Google Scholar]
- 66.Viola A., Gupta N. Tether and trap: regulation of membrane-raft dynamics by actin-binding proteins. Nat. Rev. Immunol. 2007;7:889–896. doi: 10.1038/nri2193. [DOI] [PubMed] [Google Scholar]
- 67.Ike H., Kosugi A., Kato A., Iino R., Hirano H., Fujiwara T., Ritchie K., Kusumi A. Mechanism of Lck recruitment to the T-cell receptor cluster as studied by single-molecule-fluorescence video imaging. ChemPhysChem. 2003;4:620–626. doi: 10.1002/cphc.200300670. [DOI] [PubMed] [Google Scholar]
- 68.Kosugi A., Sakakura J., Yasuda K., Ogata M., Hamaoka T. Involvement of SHP-1 tyrosine phosphatase in TCR-mediated signaling pathways in lipid rafts. Immunity. 2001;14:669–680. doi: 10.1016/s1074-7613(01)00146-7. [DOI] [PubMed] [Google Scholar]
- 69.Kabouridis P.S. Selective interaction of LAT (linker of activated T cells) with the open-active form of Lck in lipid rafts reveals a new mechanism for the regulation of Lck in T cells. Biochem. J. 2003;371:907–915. doi: 10.1042/BJ20021578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Torgersen K.M. Release from tonic inhibition of T cell activation through transient displacement of C-terminal Src kinase (Csk) from lipid rafts. J. Biol. Chem. 2001;276:29313–29318. doi: 10.1074/jbc.C100014200. [DOI] [PubMed] [Google Scholar]
- 71.Kawabuchi M., Satomi Y., Takao T., Shimonishi Y., Nada S., Nagai K., Tarakhovsky A., Okada M. Transmembrane phosphoprotein Cbp regulates the activities of Src-family tyrosine kinases. Nature. 2000;404:999–1003. doi: 10.1038/35010121. [DOI] [PubMed] [Google Scholar]
- 72.Brdicka T. Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adaptor protein, binds the protein tyrosine kinase Csk and is involved in regulation of T cell activation. J. Exp. Med. 2000;191:1591–1604. doi: 10.1084/jem.191.9.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brdickova N. LIME: a new membrane raft-associated adaptor protein involved in CD4 and CD8 coreceptor signaling. J. Exp. Med. 2003;198:1453–1462. doi: 10.1084/jem.20031484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hur E.M., Son M., Lee O.H., Choi Y.B., Park C., Lee H., Yun Y. LIME, a novel transmembrane adaptor protein, associates with p56lck and mediates T cell activation. J. Exp. Med. 2003;198:1463–1473. doi: 10.1084/jem.20030232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Davidson D., Bakinowski M., Thomas M.L., Horejsi V., Veillette A. Phosphorylation-dependent regulation of T-cell activation by PAG/Cbp, a lipid raft-associated transmembrane adaptor. Mol. Cell. Biol. 2003;23:2017–2028. doi: 10.1128/MCB.23.6.2017-2028.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yasuda K. Fyn is essential for tyrosine phosphorylation of Csk-binding protein/phosphoprotein associated with glycolipid-enriched microdomains in lipid rafts in resting T cells. J. Immunol. 2002;169:2813–2817. doi: 10.4049/jimmunol.169.6.2813. [DOI] [PubMed] [Google Scholar]
- 77.Dobenecker M.W., Schmedt C., Okada M., Tarakhovsky A. The ubiquitously expressed Csk adaptor protein Cbp is dispensable for embryogenesis and T-cell development and function. Mol. Cell. Biol. 2005;25:10533–10542. doi: 10.1128/MCB.25.23.10533-10542.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oneyama C., Hikita T., Enya K., Dobenecker M.W., Saito K., Nada S., Tarakhovsky A., Okada M. The lipid raft-anchored adaptor protein Cbp controls the oncogenic potential of c-Src. Mol. Cell. 2008;30:426–436. doi: 10.1016/j.molcel.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 79.Gregoire C. Deletion of the LIME adaptor protein minimally affects T and B cell development and function. Eur. J. Immunol. 2007;37:3259–3269. doi: 10.1002/eji.200737563. [DOI] [PubMed] [Google Scholar]
- 80.Edmonds S.D., Ostergaard H.L. Dynamic association of CD45 with detergent-insoluble microdomains in T lymphocytes. J. Immunol. 2002;169:5036–5042. doi: 10.4049/jimmunol.169.9.5036. [DOI] [PubMed] [Google Scholar]
- 81.Irles C., Symons A., Michel F., Bakker T.R., van der Merwe P.A., Acuto O. CD45 ectodomain controls interaction with GEMs and Lck activity for optimal TCR signaling. Nat. Immunol. 2003;4:189–197. doi: 10.1038/ni877. [DOI] [PubMed] [Google Scholar]
- 82.Ishii T., Ohnuma K., Murakami A., Takasawa N., Kobayashi S., Dang N.H., Schlossman S.F., Morimoto C. CD26-mediated signaling for T cell activation occurs in lipid rafts through its association with CD45RO. Proc. Natl. Acad. Sci. USA. 2001;98:12138–12143. doi: 10.1073/pnas.211439098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Viola A., Schroeder S., Sakakibara Y., Lanzavecchia A. T Lymphocyte costimulation mediated by reorganization of membrane microdomains. Science. 1999;283:680–682. doi: 10.1126/science.283.5402.680. [DOI] [PubMed] [Google Scholar]
- 84.Janes P.W., Ley S.C., Magee A.I. Aggregation of lipid rafts accompanies signaling via the T cell antigen receptor. J. Cell Biol. 1999;147:447–461. doi: 10.1083/jcb.147.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Boerth N.J., Sadler J.J., Bauer D.E., Clements J.L., Gheith S.M., Koretzky G.A. Recruitment of SLP-76 to the membrane and glycolipid-enriched membrane microdomains replaces the requirement for linker for activation of T cells in T cell receptor signaling. J. Exp. Med. 2000;192:1047–1058. doi: 10.1084/jem.192.7.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Veri M.C. Membrane raft-dependent regulation of phospholipase C-gamma-1 activation in T lymphocytes. Mol. Cell. Biol. 2001;21:6939–6950. doi: 10.1128/MCB.21.20.6939-6950.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bi K., Tanaka Y., Coudronniere N., Sugie K., Hong S., van Stipdonk M.J., Altman A. Antigen-induced translocation of PKC-theta to membrane rafts is required for T cell activation. Nat. Immunol. 2001;2:556–563. doi: 10.1038/88765. [DOI] [PubMed] [Google Scholar]
- 88.Rentero C. Functional implications of plasma membrane condensation for T cell activation. PLoS One. 2008;3:e2262. doi: 10.1371/journal.pone.0002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tuosto L., Parolini I., Schroder S., Sargiacomo M., Lanzavecchia A., Viola A. Organization of plasma membrane functional rafts upon T cell activation. Eur. J. Immunol. 2001;31:345–349. doi: 10.1002/1521-4141(200102)31:2<345::aid-immu345>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 90.Tani-Ichi S. Structure and function of lipid rafts in human activated T cells. Int. Immunol. 2005;17:749–758. doi: 10.1093/intimm/dxh257. [DOI] [PubMed] [Google Scholar]
- 91.Larbi A., Dupuis G., Khalil A., Douziech N., Fortin C., Fulop T., Jr. Differential role of lipid rafts in the functions of CD4+ and CD8+ human T lymphocytes with aging. Cell Signal. 2006;18:1017–1030. doi: 10.1016/j.cellsig.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 92.Larbi A., Douziech N., Dupuis G., Khalil A., Pelletier H., Guerard K.P., Fulop T., Jr. Age-associated alterations in the recruitment of signal-transduction proteins to lipid rafts in human T lymphocytes. J. Leukoc. Biol. 2004;75:373–381. doi: 10.1189/jlb.0703319. [DOI] [PubMed] [Google Scholar]
- 93.Jury E.C., Kabouridis P.S., Flores-Borja F., Mageed R.A., Isenberg D.A. Altered lipid raft-associated signaling and ganglioside expression in T lymphocytes from patients with systemic lupus erythematosus. J. Clin. Invest. 2004;113:1176–1187. doi: 10.1172/JCI20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jury E.C., Kabouridis P.S., Abba A., Mageed R.A., Isenberg D.A. Increased ubiquitination and reduced expression of LCK in T lymphocytes from patients with systemic lupus erythematosus. Arthr. Rheum. 2003;48:1343–1354. doi: 10.1002/art.10978. [DOI] [PubMed] [Google Scholar]
- 95.Baechler E.C. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc. Natl. Acad. Sci. USA. 2003;100:2610–2615. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cedeno S., Cifarelli D.F., Blasini A.M., Paris M., Placeres F., Alonso G., Rodriguez M.A. Defective activity of ERK-1 and ERK-2 mitogen-activated protein kinases in peripheral blood T lymphocytes from patients with systemic lupus erythematosus: potential role of altered coupling of Ras guanine nucleotide exchange factor hSos to adapter protein Grb2 in lupus T cells. Clin. Immunol. 2003;106:41–49. doi: 10.1016/s1521-6616(02)00052-9. [DOI] [PubMed] [Google Scholar]
- 97.Flores-Borja F., Kabouridis P.S., Jury E.C., Isenberg D.A., Mageed R.A. Altered lipid raft-associated proximal signaling and translocation of CD45 tyrosine phosphatase in B lymphocytes from patients with systemic lupus erythematosus. Arthr. Rheum. 2007;56:291–302. doi: 10.1002/art.22309. [DOI] [PubMed] [Google Scholar]
- 98.Wagner D.H., Jr., Vaitaitis G., Sanderson R., Poulin M., Dobbs C., Haskins K. Expression of CD40 identifies a unique pathogenic T cell population in type 1 diabetes. Proc. Natl. Acad. Sci. USA. 2002;99:3782–3787. doi: 10.1073/pnas.052247099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vaitaitis G.M., Wagner D.H., Jr. High distribution of CD40 and TRAF2 in Th40 T cell rafts leads to preferential survival of this auto-aggressive population in autoimmunity. PLoS One. 2008;3:e2076. doi: 10.1371/journal.pone.0002076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Noseworthy J.H., Lucchinetti C., Rodriguez M., Weinshenker B.G. Multiple sclerosis. N. Engl. J. Med. 2000;343:938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 101.Cayrol R. Activated leukocyte cell adhesion molecule promotes leukocyte trafficking into the central nervous system. Nat. Immunol. 2008;9:137–145. doi: 10.1038/ni1551. [DOI] [PubMed] [Google Scholar]
- 102.Monti E. Identification and expression of NEU3, a novel human sialidase associated to the plasma membrane. Biochem. J. 2000;349:343–351. doi: 10.1042/0264-6021:3490343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nan X., Carubelli I., Stamatos N.M. Sialidase expression in activated human T lymphocytes influences production of IFN-gamma. J. Leukoc. Biol. 2007;81:284–296. doi: 10.1189/jlb.1105692. [DOI] [PubMed] [Google Scholar]
- 104.Moore M.L., Chi M.H., Zhou W., Goleniewska K., O’Neal J.F., Higginbotham J.N., Peebles R.S., Jr. Cutting edge: oseltamivir decreases T cell GM1 expression and inhibits clearance of respiratory syncytial virus: potential role of endogenous sialidase in antiviral immunity. J. Immunol. 2007;178:2651–2654. doi: 10.4049/jimmunol.178.5.2651. [DOI] [PubMed] [Google Scholar]
- 105.Unruh T.L., Li H., Mutch C.M., Shariat N., Grigoriou L., Sanyal R., Brown C.B., Deans J.P. Cholesterol depletion inhibits src family kinase-dependent calcium mobilization and apoptosis induced by rituximab crosslinking. Immunology. 2005;116:223–232. doi: 10.1111/j.1365-2567.2005.02213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Janas E., Priest R., Wilde J.I., White J.H., Malhotra R. Rituxan (anti-CD20 antibody)-induced translocation of CD20 into lipid rafts is crucial for calcium influx and apoptosis. Clin. Exp. Immunol. 2005;139:439–446. doi: 10.1111/j.1365-2249.2005.02720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ehrenstein M.R., Jury E.C., Mauri C. Statins for atherosclerosis—as good as it gets? N. Engl. J. Med. 2005;352:73–75. doi: 10.1056/NEJMe048326. [DOI] [PubMed] [Google Scholar]
- 108.Greenwood J., Steinman L., Zamvil S.S. Statin therapy and autoimmune disease: from protein prenylation to immunomodulation. Nat. Rev. Immunol. 2006;6:358–370. doi: 10.1038/nri1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Weber M.S., Steinman L., Zamvil S.S. Statins—treatment option for central nervous system autoimmune disease? Neurotherapeutics. 2007;4:693–700. doi: 10.1016/j.nurt.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ponce J., de la Ossa N.P., Hurtado O., Millan M., Arenillas J.F., Davalos A., Gasull T. Simvastatin reduces the association of NMDA receptors to lipid rafts: a cholesterol-mediated effect in neuroprotection. Stroke. 2008;39:1269–1275. doi: 10.1161/STROKEAHA.107.498923. [DOI] [PubMed] [Google Scholar]
- 111.Ifergan I. Statins reduce human blood–brain barrier permeability and restrict leukocyte migration: relevance to multiple sclerosis. Annu. Neurol. 2006;60:45–55. doi: 10.1002/ana.20875. [DOI] [PubMed] [Google Scholar]
- 112.Ghittoni R., Patrussi L., Pirozzi K., Pellegrini M., Lazzerini P.E., Capecchi P.L., Pasini F.L., Baldari C.T. Simvastatin inhibits T-cell activation by selectively impairing the function of Ras superfamily GTPases. FASEB J. 2005;19:605–607. doi: 10.1096/fj.04-2702fje. [DOI] [PubMed] [Google Scholar]
- 113.Lee J.Y., Kim J.S., Kim J.M., Kim N., Jung H.C., Song I.S. Simvastatin inhibits NF-kappaB signaling in intestinal epithelial cells and ameliorates acute murine colitis. Int. Immunopharmacol. 2007;7:241–248. doi: 10.1016/j.intimp.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 114.Flanagan K., Modrusan Z., Cornelius J., Chavali A., Kasman I., Komuves L., Mo L., Diehl L. Intestinal epithelial cell up-regulation of LY6 molecules during colitis results in enhanced chemokine secretion. J. Immunol. 2008;180:3874–3881. doi: 10.4049/jimmunol.180.6.3874. [DOI] [PubMed] [Google Scholar]
- 115.Bensinger S.J. LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell. 2008;134:97–111. doi: 10.1016/j.cell.2008.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fan Y.Y., McMurray D.N., Ly L.H., Chapkin R.S. Dietary (n−3) polyunsaturated fatty acids remodel mouse T-cell lipid rafts. J. Nutr. 2003;133:1913–1920. doi: 10.1093/jn/133.6.1913. [DOI] [PubMed] [Google Scholar]
- 117.Zeyda M., Staffler G., Horejsi V., Waldhausl W., Stulnig T.M. LAT displacement from lipid rafts as a molecular mechanism for the inhibition of T cell signaling by polyunsaturated fatty acids. J. Biol. Chem. 2002;277:28418–28423. doi: 10.1074/jbc.M203343200. [DOI] [PubMed] [Google Scholar]
- 118.Zeyda M., Szekeres A.B., Saemann M.D., Geyeregger R., Stockinger H., Zlabinger G.J., Waldhausl W., Stulnig T.M. Suppression of T cell signaling by polyunsaturated fatty acids: selectivity in inhibition of mitogen-activated protein kinase and nuclear factor activation. J. Immunol. 2003;170:6033–6039. doi: 10.4049/jimmunol.170.12.6033. [DOI] [PubMed] [Google Scholar]
- 119.Geyeregger R., Zeyda M., Zlabinger G.J., Waldhausl W., Stulnig T.M. Polyunsaturated fatty acids interfere with formation of the immunological synapse. J. Leukoc. Biol. 2005;77:680–688. doi: 10.1189/jlb.1104687. [DOI] [PubMed] [Google Scholar]
- 120.Chapkin R.S., Wang N., Fan Y.Y., Lupton J.R., Prior I.A. Docosahexaenoic acid alters the size and distribution of cell surface microdomains. Biochim. Biophys. Acta. 2008;1778:466–471. doi: 10.1016/j.bbamem.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sanderson P., Calder P.C. Dietary fish oil appears to prevent the activation of phospholipase C-gamma in lymphocytes. Biochim. Biophys. Acta. 1998;1392:300–308. doi: 10.1016/s0005-2760(98)00044-7. [DOI] [PubMed] [Google Scholar]
- 122.Fan Y.Y., Ly L.H., Barhoumi R., McMurray D.N., Chapkin R.S. Dietary docosahexaenoic acid suppresses T cell protein kinase C theta lipid raft recruitment and IL-2 production. J. Immunol. 2004;173:6151–6160. doi: 10.4049/jimmunol.173.10.6151. [DOI] [PubMed] [Google Scholar]
- 123.Li Q., Zhang Q., Zhang M., Wang C., Zhu Z., Li N., Li J. Effect of n−3 polyunsaturated fatty acids on membrane microdomain localization of tight junction proteins in experimental colitis. FEBS J. 2008;275:411–420. doi: 10.1111/j.1742-4658.2007.06210.x. [DOI] [PubMed] [Google Scholar]