Abstract
Retinal vasoconstriction and reduced retinal blood flow precede the onset of diabetic retinopathy. The pathophysiological mechanisms that underlie increased retinal arteriolar tone during diabetes remain unclear. Normally, local Ca2+ release events (Ca2+-sparks), trigger the activation of large-conductance Ca2+-activated K+(BK)-channels which hyperpolarize and relax vascular smooth muscle cells (VSMCs), thereby causing vasodilatation. In the present study, we examined BK channel function in retinal VSMCs from streptozotocin(STZ)-induced diabetic rats. The BK channel inhibitor, Penitrem A, constricted non-diabetic retinal arterioles (pressurized to 70mmHg) by 28%. The BK current evoked by caffeine was dramatically reduced in retinal arterioles from diabetic animals even though caffeine-evoked [Ca2+]i release was unaffected. Spontaneous BK currents were smaller in diabetic cells, but the amplitude of Ca2+-sparks was larger. The amplitudes of BK currents elicited by depolarizing voltage steps were similar in control and diabetic arterioles and mRNA expression of the pore-forming BKα subunit was unchanged. The Ca2+-sensitivity of single BK channels from diabetic retinal VSMCs was markedly reduced. The BKβ1 subunit confers Ca2+-sensitivity to BK channel complexes and both transcript and protein levels for BKβ1 were appreciably lower in diabetic retinal arterioles. The mean open times and the sensitivity of BK channels to tamoxifen were decreased in diabetic cells, consistent with a downregulation of BKβ1 subunits. The potency of blockade by Pen A was lower for BK channels from diabetic animals. Thus, changes in the molecular composition of BK channels could account for retinal hypoperfusion in early diabetes, an idea having wider implications for the pathogenesis of diabetic hypertension.
Introduction
Diabetes causes changes to the structure and function of blood vessels in the retina leading to visual impairment and blindness1. The cellular and molecular basis of diabetic retinopathy is not wholly understood although large prospective clinical trials have established the importance of hyperglycaemia in precipitating this disease in both type 1 and type 2 diabetic patients2,3. A major pathway through which hyperglycaemia is believed to contribute to retinal microangiopathy is the disruption of retinal blood flow. Patient-based studies have shown that retinal haemodynamic abnormalities occur prior to the onset of clinical diabetic retinopathy4 and that the development and progression of diabetic retinopathy correlates with the extent of the blood flow changes observed5-8. In diabetic patients without retinopathy, retinal arteriolar vasoconstriction9, 10 and decreased total retinal blood flow has been reported4, 11. However, as the disease progresses, the arterioles begin to dilate12 and bulk retinal blood flow increases in proportion to the severity of retinopathy, and thus the degree of retinal ischemia11. Presently, the molecular mechanisms underlying retinal vasoconstriction during early diabetes are unknown, yet an improved understanding of the pathophysiology involved could be crucial to the development of better therapies for the treatment of diabetic retinopathy.
A major factor controlling the contractile state of arterioles is the activity of ion channels on the plasma membranes of the VSMCs13. BK channels are known to play a crucial role in the regulation of arterial smooth muscle tone since blockade of these channels using the specific inhibitor iberiotoxin, causes membrane depolarisation and vasoconstriction in pressurized isolated vessels14. BK channels are activated by local Ca2+ release events, termed Ca2+ sparks, resulting from opening of ryanodine receptor (RyR) channels in the sarcoplasmic reticulum (SR;15). The activation of BK channels results in outward K+ current that opposes VSMC contraction by causing membrane hyperpolarisation. This reduces Ca2+ influx by reducing activation of voltage-dependent Ca2+ channels15. BK channels are composed of α-subunits and accessory β-subunits16. The α-subunit forms the K+ selective pore, while the β subunits influence the kinetics, pharmacology and Ca2+ sensitivity of BK currents17, 18. Four members of the BK β-subunit family have been identified to date (β1-β4) and the β1-subunit is expressed predominantly in VSMCs19. Targeted deletion of the β1-subunit gene reduces the Ca2+ sensitivity of BK channels and the coupling of Ca2+ sparks to BK channel activity in VSMCs from cerebral arteries19, 20. The functional significance of the β1-subunit of VSMC BK channels is underlined by the observation that knockout mice are hypertensive and display enhanced vascular reactivity to application of vasoconstrictors19, 20.
In the present study we have examined the effects of STZ-induced diabetes on the properties of BK channels in VSMCs of the rat retinal microcirculation. A clear advantage of using STZ-diabetic rats is that it is well documented that retinal arteriolar vasoconstriction and decreased retinal blood flow occur in this animal model following several weeks of diabetes21. These animals subsequently exhibit many of the vasodegenerative changes associated with human diabetic retinopathy22. We hypothesised that diabetes causes a downregulation of the α- and/or β1-subunit, thereby reducing the capacity of the BK channels to hyperpolarise retinal VSMCs and resist vasoconstriction. We show that diabetes reduces coupling between Ca2+ release from RyR sensitive Ca2+ stores and BK channel activation. No alteration in the expression of the pore-forming α-subunit was evident, but β1-subunit expression was reduced at both the mRNA and protein level. Consistent with this, we found that BK channels in retinal VSMCs from STZ-diabetic animals exhibit a diminished sensitivity to Ca2+ and we provide pharmacological evidence supporting the idea that the expression of functional α+β1 subunit complexes is reduced in diabetes. These results suggest that changes in the molecular composition of BK channels in retinal VSMCs during diabetes might contribute to the onset and early progression of diabetic retinopathy.
Methods
All procedures with animals were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and the United Kingdom Animals (Scientific Procedures) Act, 1986. Full details of the methods and materials used are in the online data supplement available at http://circres.ahajournals.org.
Results
Characteristics of experimental animals
Diabetic animals had higher mean plasma glucose levels (27.8 ± 1.3mmol/L; n=41) than non-diabetic animals (7.6 ± 0.3mmol/L; n=37 p<0.001). Mean glycosylated haemoglobin values were 5.9 ± 0.2% and 17.6 ± 0.8% in the non-diabetic and diabetic groups, respectively (p<0.001). Both sets of animals gained weight during the 3-month experimental period, but the increase was greater in non-diabetic than diabetic rats (304 ± 17g vs 141 ± 9.7g, respectively; p<0.001).
Functional Significance of BK channels in retinal VSMCs
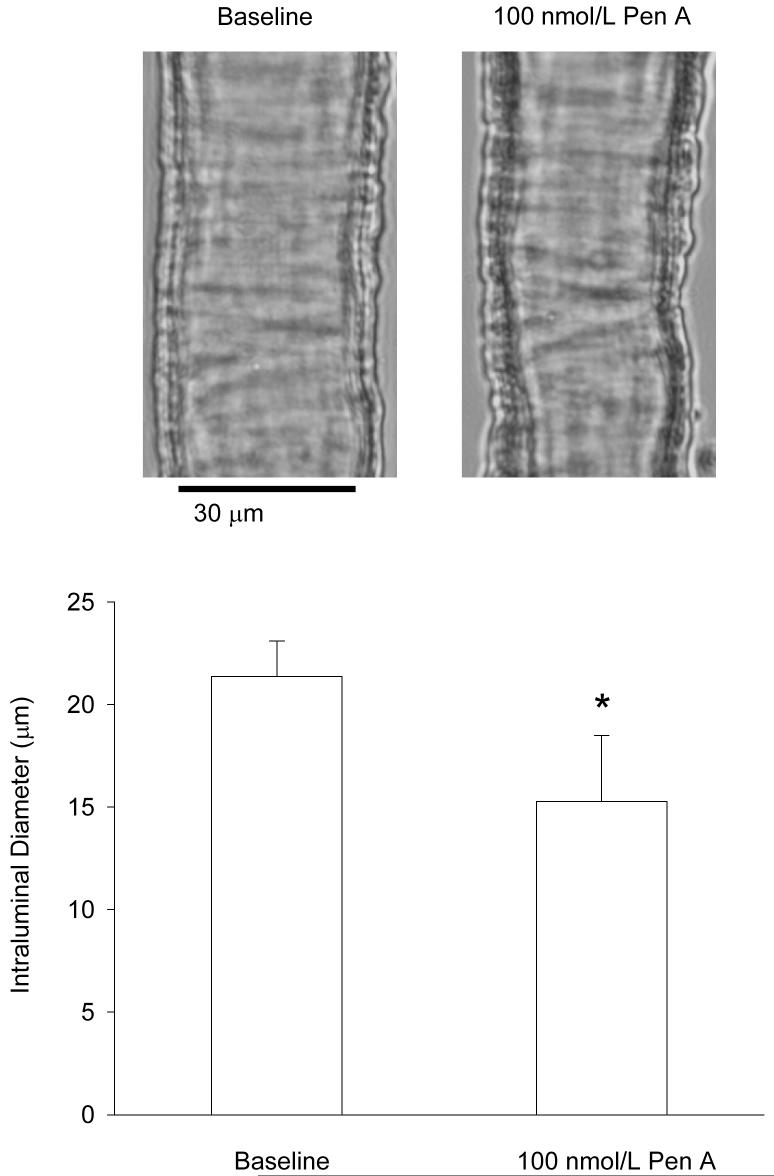
We examined the contribution of BK channels to the regulation of retinal arteriolar tone by pressurizing freshly isolated retinal arterioles from non-diabetic animals to 70mmHg and measuring the change in internal diameter induced by 100nmol/L Penitrem A (Pen A), a potent inhibitor of BK channels23. Pen A caused a 28% decrease in the diameter of pressurized retinal vessels (Fig 1). These results suggest that BK activity plays an important vasodilatory role in retinal blood vessels.
Figure 1. Physiological significance of BK channels in retinal arterioles.
Top panel, representative photomicrographs of a pressurized retinal arteriole (70mmHg) before and 10-min after the application of 100nmol/L Pen A. Bottom panel, mean ± SEM of the Pen A-induced constriction in non-diabetic retinal arterioles (n=8 vessels, p<0.05; paired t-test).
Caffeine-induced BK currents, but not Ca2+ activated Cl- currents, are smaller in retinal VSMCs from diabetic animals
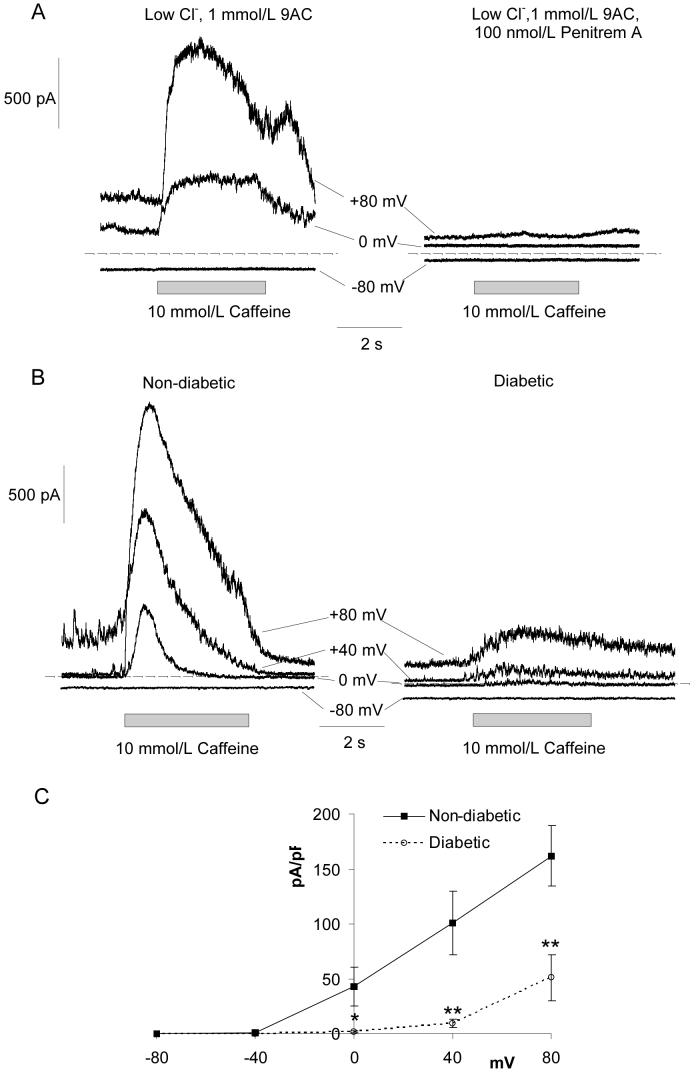
To investigate BK channel activity during diabetes we tested activation following Ca2+ release from caffeine-sensitive Ca2+ stores. Retinal arterioles were bathed in low Cl- Hanks’ solution containing 1mmol/L 9-anthracene carboxylic acid (9AC) to block Ca2+ activated Cl- channels (ClCa channels). Application of 10mmol/L caffeine for 5 seconds evoked large, noisy transient outward currents at positive membrane potentials (Fig 2A,C). These currents were fully abolished by addition of 100nmol/L Pen A (Fig 2A; n=6). Fig 2B shows typical caffeine-induced BK currents in retinal VSMCs from non-diabetic and diabetic animals. Fig 2C shows the average peak current density plotted as a function of voltage for the caffeine-induced BK currents. BK currents evoked by Ca2+ release from caffeine-sensitive Ca2+ stores were dramatically reduced in VSMCs from diabetic animals.
Figure 2. Caffeine-induced BK currents recorded from retinal VSMCs of non-diabetic and diabetic animals.
(A) Left, whole-cell records showing caffeine-induced outward currents in a non-diabetic vessel held at a range of potentials. The vessel was bathed in low Cl- Hanks’ solution with 1mmol/L 9AC to block ClCa currents. Right, the BK channel inhibitor, Pen A (100nmol/L), completely abolished the caffeine-induced currents. (B) Typical traces showing caffeine-induced BK currents at -80, 0, +40 and +80 mV in non-diabetic and diabetic retinal arterioles. (C) Summary current-voltage relationships for the caffeine-induced BK currents in non-diabetic (for each point, n=5-13) and diabetic (n=7-19) vessels.
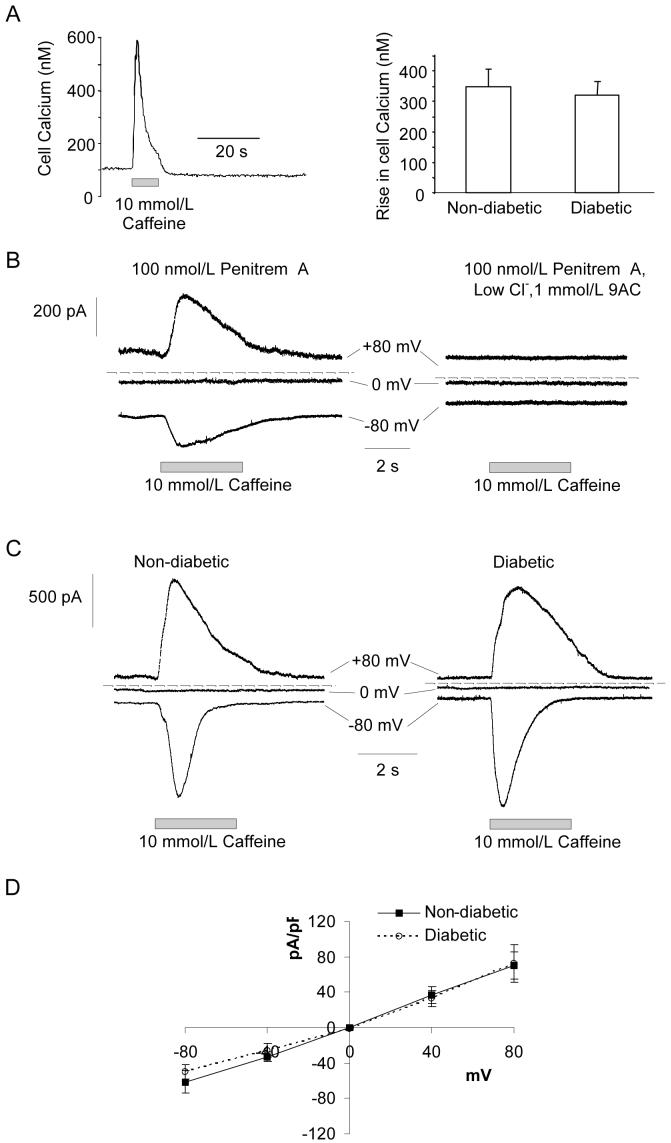
The attenuated BK currents in arterioles from diabetic rats could be explained by decreased Ca2+ release from the caffeine-sensitive stores. To test this, global [Ca2+]i responses were measured in retinal VSMCs from non-diabetic and diabetic animals using fura 2 based Ca2+ microfluorimetry (Fig 3A). No differences in the peak amplitude of [Ca2+]i transients evoked by 10mmol/L caffeine were observed. We also compared the size of caffeine-evoked ClCa currents at a range of voltages in myocytes from non-diabetic and diabetic arterioles bathed in normal Hanks’ solution containing 100nmol/L Pen A to block BK channels. Application of 10mmol/L caffeine evoked currents that reversed close to ECl (0mV; Fig 3B,D). These were completely abolished in low Cl- Hanks’ solution containing the Cl- channel inhibitor, 9AC (Fig 3B; n=6). Isolation of the caffeine-evoked Cl- currents in diabetic arterioles required longer pre-incubation times with Pen A (∼20 min as compared to ∼5-min for non-diabetic vessels). Fig 3C,D show representative traces and summary data for the caffeine-evoked Cl- currents in non-diabetic and diabetic retinal VSMCs. The mean peak current densities were similar for normal and diabetic cells at all voltages tested. Thus, it appears that the smaller caffeine-induced BK currents in diabetic retinal VSMCs reflect reduced coupling efficiency between Ca2+ release and BK channel activation rather than defective Ca2+ release.
Figure 3. Caffeine-evoked global [Ca2+]i transients and ClCa currents in non-diabetic and diabetic retinal VSMCs.
(A) Left, time-course record showing the effects of 10mmol/L caffeine on global [Ca2+]i in a non-diabetic retinal arteriole segment. Right, mean data showing that caffeine-induced [Ca2+]i transients were unaffected by diabetes. (B) Left, whole-cell currents elicited by 10 mmol/L caffeine in a non-diabetic vessel held at a range of test potentials and bathed in normal Hanks’ solution containing the BK channel antagonist, Pen A (100nmol/L). Right, the ClCa channel inhibitor, 9AC (1mmol/L), completely blocked the caffeine-evoked transient inward and outward currents. (C) Original records showing caffeine-induced ClCa currents at -80, 0 and +80mV in non-diabetic and diabetic vessels. (D) Plot showing the mean peak current density against voltage for the caffeine-evoked ClCa currents in non-diabetic (n=12) and diabetic (n=10) retinal VSMCs.
Ca2+ sparks are larger, but STOCs are smaller in diabetic retinal VSMCs
From a physiological perspective it is well established that Ca2+ sparks activate BK channels generating spontaneous transient outward K+ currents (STOCs), modulating vascular tone15, 24. STOCs were recorded from retinal VSMCs of non-diabetic and diabetic animals at test potentials between -80mV to +80mV (increased in 40mV increments) using the perforated patch clamp technique. STOCs were evident at membrane potentials positive to -40mV and were completely abolished in the presence of 100nmol/L Pen A (n=6, non-diabetic VSMCs). Individual STOC events were superimposed (Fig 4A) precluding an accurate assessment of STOC amplitudes and frequencies, so we calculated the integral of the STOC densities for recordings lasting 5-10 minutes. Visual inspection of the original traces suggested that STOC amplitudes and frequencies were greatly reduced in diabetic retinal VSMCs, and integrated current densities were considerably smaller (Fig 4A). These data confirm that retinal VSMCs from diabetic animals demonstrate less spontaneous BK current activity than cells from non-diabetic animals.
Figure 4. STOC activity is reduced but Ca2+ sparks are greater in retinal VSMCs from diabetic animals.
(A) Top panel, whole-cell recordings of STOC activity in a non-diabetic and diabetic vessel at a holding potential of +40mV. Bottom panel, graph showing the mean integrated current density versus voltage for STOCs from non-diabetic (n=8) and diabetic (n=8) retinal arterioles. (B) Top panel, line-scan image recorded from a non-diabetic retinal VSMC showing two consecutive Ca2+ sparks originating from the same Ca2+ spark site. The graph below plots the fractional fluorescence change (F/F0) for this panel. Bottom panel, line scan image and graph on slower time scales from another non-diabetic cell in which Ca2+ sparks amalgamate to produce a cell-wide global Ca2+ oscillation. (C) Representative line-scan images of basal Ca2+ sparks in non-diabetic and diabetic retinal VSMCs. Bottom, average temporal profile for each spark has been plotted and the traces superimposed.
Using confocal imaging techniques, we have recently described the presence of two distinct populations of spontaneous Ca2+ sparks in retinal VSMCs: “basal” sparks that arise from resting fluorescence levels (i.e. from F/Fo = 0.95-1.05) and “sparks on oscillations” that overlay global Ca2+ transients25. Fig 4B shows representative images of basal Ca2+ sparks, Ca2+ sparks on oscillations and global Ca2+ oscillations in non-diabetic arteriolar myocytes. We considered the possibility that the lower STOC activity in diabetic retinal VSMCs may reflect reduced spontaneous sub-cellular [Ca2+]i signaling in these cells. Fig 4C shows typical images of basal Ca2+ sparks in non-diabetic and diabetic arteriolar myocytes. Below the image panels, the time course of the normalized fluorescence for each event has been plotted and the traces superimposed. It is evident that the peak amplitude of the Ca2+ spark in the diabetic cell is around twice that of the non-diabetic. Quantitative data for basal Ca2+ sparks, Ca2+ sparks on oscillations and global oscillations in non-diabetic and diabetic VSMCs are summarized in Table 1 of the online data supplement. The peak amplitude of both populations of Ca2+ sparks was substantially larger in diabetic than in non-diabetic VSMCs (ΔF/Fo basal sparks, 0.92 ± 0.06 and 0.42 ± 0.03, respectively; ΔF/Fo sparks on oscillations, 1.56 ± 0.21 and 0.36 ± 0.04, respectively; p<0.001 in both cases), while the frequency and duration (FDHM) of these events remained unchanged. No differences were observed in amplitude, frequency or duration of global Ca2+ oscillations between non-diabetic and diabetic VSMCs. These results show that decreased sub-cellular Ca2+ signaling activity cannot explain the decreased STOC activity observed in diabetic VSMCs.
Diabetes reduces the Ca2+ sensitivity of BK channels in diabetic retinal VSMCs
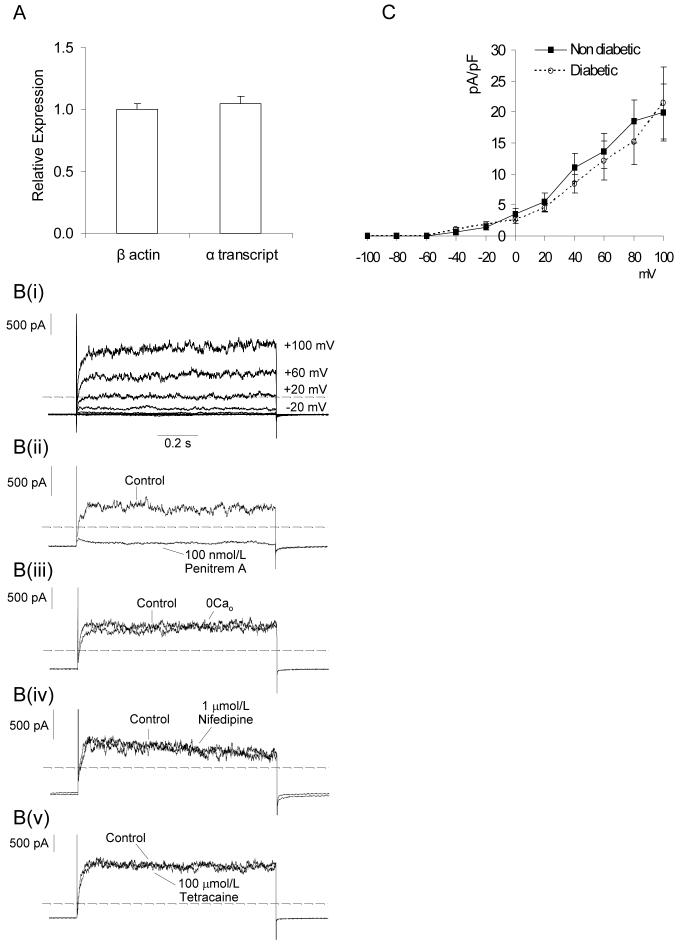
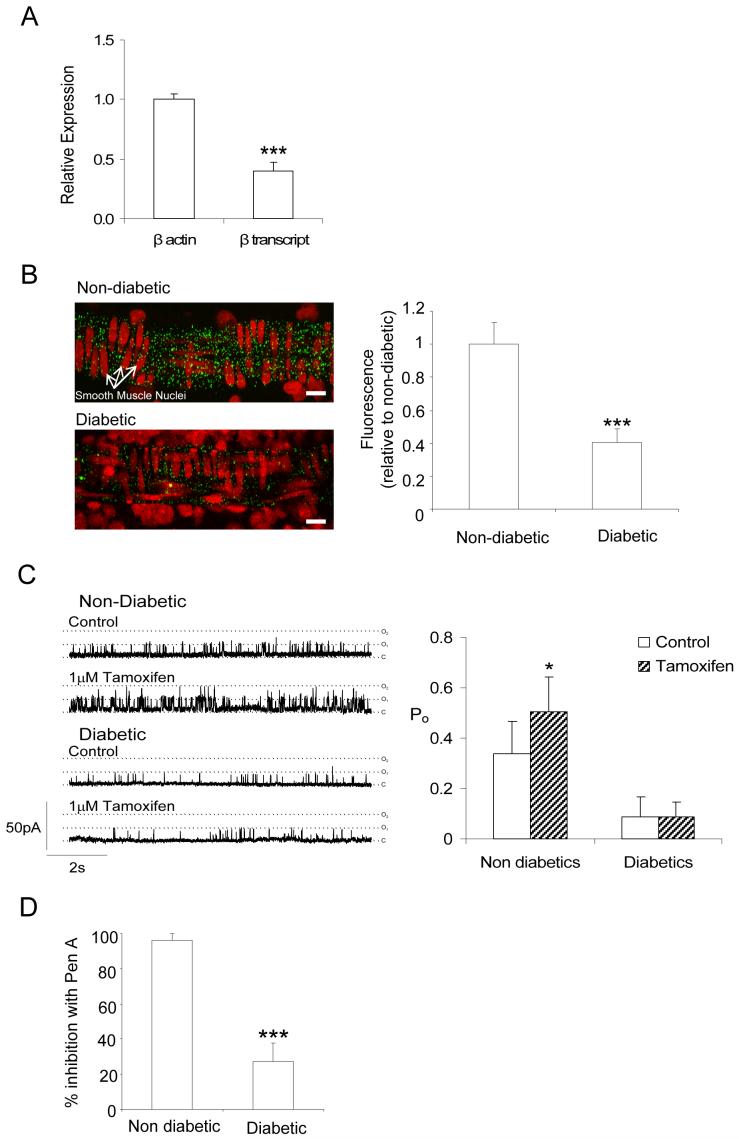
Both the comparisons of caffeine-evoked currents and Ca2+ transients, and of STOC and spark activity, suggest decreased coupling between Ca2+ release and BK channel activation in retinal VSMCs after short-term diabetes. This might be explained by a reduced number of functional BK channels in diabetic myocytes. Quantitative RT-PCR, however, failed to reveal any differences in BKα transcript expression in non-diabetic and diabetic retinal arterioles (Fig 5A). To assess BK channel density, we also compared depolarization dependent whole-cell BK currents (Fig 5Bi,ii). Vessels were bathed in low Cl- Hanks’ solution containing 1mmol/L 9AC and 10mmol/L 4-aminopyridine (4AP) to block ClCa and Kv channels26, respectively. 10mmol/L 4AP has no effect on BK channels in retinal arterioles; caffeine-evoked BK currents at +40mV in the absence and presence of 10mmol/L 4AP were 115 ± 29.8 and 114 ± 26pA/pF, respectively (n=4; p=0.84; paired t-test). Voltage-activated BK currents in control VSMCs were unaffected by the removal of extracellular Ca2+, inhibition of voltage-dependent Ca2+ channels with 10μmol/L nifedipine or blockade of RyR receptors with 100μmol/L tetracaine (Fig 5Biii-v). This demonstrates that BK currents evoked by depolarisation are independent of Ca2+ influx and Ca2+ store release in these cells i.e. they appear to be entirely dependent on voltage gating of the BK channels. As such, the peak current density should be proportional to the number of functional BK channels, assuming that the single-channel conductance and the voltage dependence of activation remain constant. Single channel conductance’s of BK channels were similar for non-diabetic and diabetic VSMCs (160 ± 2pS and 160 ± 4pS, respectively; n=21 and 15 patches, p>0.05). Fig 5C shows the average peak current density as a function of voltage for the voltage-activated BK currents in retinal VSMCs from non-diabetic and diabetic animals. No differences were observed, suggesting that BK channel density is unaltered in retinal VSMCs during diabetes. Consistent with this, in single channel recordings we found no difference in the number of BK channels per membrane patch in non-diabetic (2.4 ± 0.2 channels) and diabetic (2.1 ± 0.2 channels) retinal VSMCs (p>0.05, n=40 patches).
Figure 5. Diabetes does not affect BK channel density.
(A) Histogram showing relative BKα transcript expression in non-diabetic and diabetic retinal arterioles as determined by quantitative PCR. Amplifications were performed in triplicate. 9 non-diabetic and 9 diabetic rats were used in total and RNA was isolated from 15-25 retinal arterioles collected from 3 non-diabetic and 3 diabetic animals per replicate. BKα transcript expression was normalized to β-actin. (Bi) Family of whole-cell voltage clamp currents evoked in a non-diabetic retinal VSMC by voltage steps ranging between -100 mV to +100mV from an initial holding potential of -80mV in the presence of 9AC (1 mmol/L) and 4AP (10mmol/L). (Bii) The voltage-dependent current was abolished by the BK channel inhibitor, Pen A (100nmol/L; % change, -99.3 ± 1.1%; p<0.05; n=4), but was unaffected by (Biii) the removal of extracellular Ca2+ (2.5± 8.5%; p>0.05; n=4), (Biv) the L-type Ca2+ channel inhibitor, nifedipine (10μmol/L; -1.6 ± 17.2%; p>0.05 n=5) and (Bv) the RyR antagonist tetracaine (100μmol/L; 3.4 ± 15.2%; p>0.05; n=5). Responses were constant across the full voltage range. For clarity current records are presented for single steps between -80 to +80mV. Dashed lines: zero current (C) Average peak current density as a function of voltage for the voltage-activated BK current in non-diabetic (n=13) and diabetic (n=13) vessels.
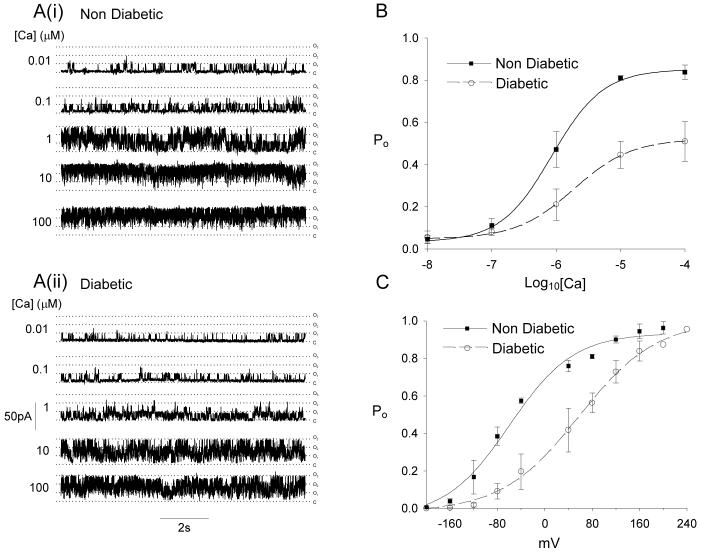
Another possible explanation for the reduced coupling between Ca2+ release and BK channel activation is a decrease in the sensitivity of the BK channels to activation by Ca2+. We examined the Ca2+-sensitivity of BK channels using inside-out membrane patches from retinal VSMCs (Fig 6). The open probability (Po) at +80mV was determined for a range of Ca2+ concentrations between 0.01-100μmol/L (Fig 6A,B). The activity of BK channels from both non-diabetic and diabetic animals increased with increasing Ca2+ concentrations, but the Po vs Ca2+ curve was shifted to the right and the Hill slope was reduced for diabetics (Fig 6B). No differences in Po’s were observed at Ca2+ concentrations of 0.01 and 0.1μmol/L, and this may explain the similarity in voltage-activated, whole cell BK-currents described above. Po vs voltage relations were also determined at a single Ca2+ concentration, 10μmol/L (Fig 6C). There was a strong rightward shift along the voltage axis (>100mV) for BK channels from diabetic animals.
Figure 6. Ca2+ sensitivity of single BK channels is reduced in diabetes.
(A) Representative single BK channel records in inside-out patches (holding potential +80mV) from non-diabetic and diabetic VSMCs exposed to increasing [Ca2+]. (B) Summary data of the mean ± SEM Po at the five Ca2+ concentrations tested. Non-diabetic, n=8-11; diabetics, n=8-11. Curves are fitted with the Hill equation as described in the supplementary methods. Fit parameters are as follows: non-diabetic (Kd = 0.86 μmol/L, Hill slope 1.1), diabetic (Kd = 1.9 μmol/L, Hill slope 0.97). (C) Po-V relations determined at 10μmol/L Ca2+. Non-diabetic, n=2-11; diabetics, n=1-11. Curves are Boltzmann fits with the following parameters: non-diabetic (V1/2 = -59.7 mV); diabetic (V1/2 = 57.9 mV).
β1 expression and function is lower in diabetic retinal VSMCs
The results above suggest that BK channels in diabetic VSMCs have a reduced Ca2+ sensitivity. Since the Ca2+ sensitivity of BK channels is known to be dependent on the presence of β1 accessory subunits17, 18, a downregulation of the β1 subunit could explain the changes observed in diabetes. Expression of the BKβ1 subunit in retinal arterioles from non-diabetic and diabetic animals was investigated at the mRNA level. β1 transcripts were approximately 60% less abundant in diabetic than in non-diabetic arterioles (Fig 7A). We also estimated changes in expression of the β1 subunit by immunohistochemistry. A punctuate distribution of BKβ1-associated fluorescence was apparent throughout the VSMC layer of retinal arterioles from non-diabetic animals (Fig 7B). Consistent with the RT-PCR analysis, BKβ1 immunostaining decreased dramatically in retinal arterioles of diabetic rats (Fig 7B) i.e. BKβ1 expression is reduced in retinal VSMC in diabetes.
Figure 7. BKβ1 subunit expression and function.
(A) Downregulation of BKβ1 mRNA in retinal VSMC cells from diabetic arterioles. BKβ1 expression in diabetic arterioles is presented relative to non-diabetic vessels. Amplifications were performed in triplicate (same samples as for Fig 5A) and normalized as described for BKα transcripts (B) Left, confocal images of non-diabetic and diabetic retinal arterioles embedded within retinal flatmount preparations and labeled with anti-BKβ1 Ab (green) and propidium iodide (red: nuclear label). Labeling of the circular smooth muscle is reduced in the tissue from the diabetic animal. Right, summary data showing statistically significant reduction in anti-BKβ1 fluorescence for diabetic samples (n=6 retinas, 30 vessels) relative to non-diabetics (n=6 retinas, 25 vessels). (C) Sensitivity of single BK channels in inside out patches to 1μmol/L tamoxifen (holding potential +80mV; 1μmol/L free [Ca2+]) from non-diabetic and diabetic retinal VSMCs. Right, summary data showing the differential effects of tamoxifen on the Po of single BK channels from non-diabetic (n=7) and diabetic (n=8) vessels. (D) Pharmacology of single BK channels from non-diabetic (n=5) and diabetic (n=9) retinal VSMCs exposed to Pen A. Mean data is expressed as the % inhibition of Po after 5-min of exposure to 100nmol/L Pen A.
Besides increasing the Ca2+ sensitivity of the BKα-subunit, the BKβ1-subunit also modifies the kinetics and pharmacological properties of BK channels18. The β1 subunit increases the stability of BK channel open states27. If there is a decrease in the coupling ratio of α/β1 subunits in diabetes then BK channel open times should be reduced. We compared the open times of BK channels from control and diabetic retinal VSMCs by constructing open time histograms at +80 mV with 10 μmol//L Ca2+. Histograms were fitted with a single exponential function. BK channels from diabetic myocytes (τopen = 2.6 ± 1.5ms) had shorter open times than those from control VSMCs (τopen = 6.36 ± 0.54ms; p<0.05), supporting the view that β1 subunit function is decreased during diabetes. Recently it has been shown that the xenoestrogen, tamoxifen, markedly increases the Po of BK channels but only when they are associated with a β1 subunit28. Exposure of BK channels from non-diabetic cells to 1μmol/L tamoxifen increased the Po by approximately 1.5-fold but had no significant effect on BK channels from diabetic retinal VSMCs (Fig 7C). From these data, we conclude that the reduced coupling efficiency between Ca2+ release and BK channel activation in diabetic retinal VSMCs results from reduced Ca2+ sensitivity of the BK channels, reflecting a decrease in the functional expression of the BKβ1 accessory subunit.
It has also previously been reported that BKα+β channels are markedly less sensitive to blockade by iberiotoxin when compared to BKα channels alone17. To determine whether the BKβ1 subunit might affect the sensitivity of BK channels to Pen A, we compared the effects of this inhibitor on the Po of BK channels from non-diabetic and diabetic retinal VSMCs (Fig 7D). Application of 100nmol/L Pen A to BK channels from non-diabetic cells caused close to 100% block of channel activity. By contrast, the Po of BK channels from diabetic cells was reduced by only 27%. These results are consistent with the possibility that BKβ1 increases the sensitivity of BK channels to Pen A, although further studies utilizing heterologous expression systems are required to confirm this.
Discussion
It has been recognised for the past 25 years that abnormal blood flow to the retina occurs during early diabetes and that this may contribute to the pathogenesis of diabetic retinopathy12. Despite this, surprisingly little is known about the precise mechanisms linking chronic hyperglycaemia to retinal arteriolar vasoconstriction and reduced retinal blood flow prior to the onset of overt retinopathy. In the present study, we have identified a major pathophysiological mechanism that may play a specific role in the development of retinal perfusion abnormalities in diabetes. We have obtained molecular and functional data to suggest that during diabetes, the BKβ1 subunit is downregulated in retinal VSMCs, while the expression of the pore-forming α-subunit remains unaltered. Consistent with this, we observed a marked reduction in the Ca2+ sensitivity of BK channels and an uncoupling of BK channel activation from Ca2+ release in diabetic retinal VSMCs. Of particular note, the decreased expression of the β1 subunit drastically reduced the ability of spontaneous Ca2+ sparks to activate BK channel-mediated STOCs. Because STOCs act to hyperpolarise and relax VSMCs15, 29, loss of STOC activity could well underlie the observed arteriolar vasoconstriction seen in the development of diabetic retinopathy.
Information is generally lacking regarding the effects of diabetes on vascular BK channel function. Coronary microvessels from diabetic dyslipidemic swine exhibit an uncoupling in the relationship between Ca2+ sparks and STOC activation30 and whole-cell BK current density is reduced in microvascular smooth muscle cells of mesenteric arteries from Zucker diabetic fatty (ZDF) rats31 and fructose-fed, insulin resistant rats32. The precise mechanisms underlying the changes in vascular BK channel activity were not resolved in these studies, although it is interesting that an alteration in BKβ1 expression was not apparent in ZDF rats31. This discrepancy with the present study could possibly be attributed to the different animal models used and the origins of the microvessels studied. In general terms, however, the findings from the current work do strengthen the view that BK channel function is impaired in VSMCs of the microcirculation during diabetes. Intriguingly, impaired BK channel function may be limited to the microvasculature since the Po of BK channels is increased in thoracic aortic VSMCs of STZ-induced diabetic mice33.
Hypertension is approximately twice as frequent in patients with diabetes compared to patients without the disease34. Furthermore work linked to the United Kingdom Prospective Diabetes Study (UKPDS) showed that the incidence of micro- and macrovascular diabetic complications was strongly associated with elevated blood pressure (BP)35. Presently the etiology of hypertension in diabetic patients is not fully understood, although loss of the normal vascular relaxation to insulin in both type 1 (insulin-deficiency) and type 2 (insulin-resistance) diabetes may contribute36. The present study is the first to raise the possibility that there is a selective downregulation of BKβ1 resulting in an impairment of RyR-BK channel signaling which could be an important component in the pathogenesis of hypertension in diabetic patients. However, detailed profiling of BKβ1 expression in a range of vascular tissues during diabetes, particularly in small arteries and arterioles that are central to BP regulation37, is required before any firm conclusions can be made. It is likely that a widespread downregulation of BKβ1 in diabetes could be sufficient to induce hypertension since BKβ1 knockout mice are indeed hypertensive19, 20. Furthermore, downregulation of BKβ1 has been reported in animal models of both acquired and genetic hypertension38, 39. Also, a recent population-based epidemiological study has reported that a gain-of-function polymorphism (E65K) in the BKβ1 subunit, which increased the apparent Ca2+- and voltage-sensitivity of the pore-forming BKα subunit, is associated with a low prevalence of diastolic hypertension in this population40. Future work should be directed towards a clearer understanding of the role BKβ1 downregulation in the development of hypertension in diabetic patients as well as elucidating the nature of the signaling mechanisms that regulate BKβ1 expression during diabetes.
Another novel observation in the current study was that Ca2+ spark amplitudes were greater in retinal VSMCs from diabetic animals, even though the frequency and duration of these events were unaltered. It seems unlikely that this represents a compensatory response to the downregulation of BKβ1 and loss of Ca2+ spark/BK channel coupling since the spatiotemporal properties of Ca2+ sparks were unaltered in VSMCs of BKβ1 knockout animals19, 20. Ca2+ spark amplitude should depend on the number and activity (open times) of RyR channels in each spark unit and the driving force for Ca2+ efflux from the SR. It seems unlikely that the larger Ca2+ spark amplitudes can be attributed to changes in the SR Ca2+ load, since caffeine-evoked [Ca2+]i transients and caffeine-activated ClCa currents were similar in retinal VSMCs from non-diabetic and diabetic animals. Diabetic retinal vessels are known to accumulate increased levels of advanced glycation end-products41 and these adducts are known to accumulate heavily on RyR channels during diabetes42. The possible contribution of AGE crosslinking of RyRs to alterations in Ca2+ spark activity in diabetic retinal VSMCs deserves further investigation.
In summary, we have presented data that strongly supports the hypothesis that diabetes downregulates the expression of the BKβ1 subunit and consequently decreases Ca2+ dependent activity of BK channels in retinal VSMCs. Our findings may have important implications with respect to the early pathogenesis of diabetic retinopathy. It is also fascinating to speculate that this mechanism might contribute to the development of hypertension in diabetic patients, although more extensive studies are needed to fully evaluate this possibility.
Supplementary Material
Acknowledgements
This work was supported by grants from The Juvenile Diabetes Research Foundation, USA; Fight for Sight, UK; and The Wellcome Trust.
Reference List
- (1).Curtis TM, Scholfield CN. The role of lipids and protein kinase Cs in the pathogenesis of diabetic retinopathy. Diabetes Metab Res Rev. 2004;20(1):28–43. doi: 10.1002/dmrr.431. [DOI] [PubMed] [Google Scholar]
- (2).The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- (3).UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352(9131):837–53. [PubMed] [Google Scholar]
- (4).Bursell SE, Clermont AC, Kinsley BT, Simonson DC, Aiello LM, Wolpert HA. Retinal blood flow changes in patients with insulin-dependent diabetes mellitus and no diabetic retinopathy. Invest Ophthalmol Vis Sci. 1996;37(5):886–97. [PubMed] [Google Scholar]
- (5).Clermont AC, Aiello LP, Mori F, Aiello LM, Bursell SE. Vascular endothelial growth factor and severity of nonproliferative diabetic retinopathy mediate retinal hemodynamics in vivo: a potential role for vascular endothelial growth factor in the progression of nonproliferative diabetic retinopathy. Am J Ophthalmol. 1997;124(4):433–46. doi: 10.1016/s0002-9394(14)70860-8. [DOI] [PubMed] [Google Scholar]
- (6).Cunha-Vaz JG, Fonseca JR, de A, Jr., Lima JJ. Studies on retinal blood flow. II. Diabetic retinopathy. Arch Ophthalmol. 1978;96(5):809–11. doi: 10.1001/archopht.1978.03910050415001. [DOI] [PubMed] [Google Scholar]
- (7).Kohner EM, Hamilton AM, Saunders SJ, Sutcliffe BA, Bulpitt CJ. The retinal blood flow in diabetes. Diabetologia. 1975;11(1):27–33. doi: 10.1007/BF00422814. [DOI] [PubMed] [Google Scholar]
- (8).Yoshida A, Feke GT, Morales-Stoppello J, Collas GD, Goger DG, McMeel JW. Retinal blood flow alterations during progression of diabetic retinopathy. Arch Ophthalmol. 1983;101(2):225–7. doi: 10.1001/archopht.1983.01040010227008. [DOI] [PubMed] [Google Scholar]
- (9).Klein R, Klein BE, Moss SE, Wong TY, Hubbard L, Cruickshanks KJ, Palta M. Retinal vascular abnormalities in persons with type 1 diabetes: the Wisconsin Epidemiologic Study of Diabetic Retinopathy: XVIII. Ophthalmology. 2003;110(11):2118–25. doi: 10.1016/S0161-6420(03)00863-7. [DOI] [PubMed] [Google Scholar]
- (10).Wong TY, Klein R, Sharrett AR, Schmidt MI, Pankow JS, Couper DJ, Klein BE, Hubbard LD, Duncan BB. Retinal arteriolar narrowing and risk of diabetes mellitus in middle-aged persons. JAMA. 2002;287(19):2528–33. doi: 10.1001/jama.287.19.2528. [DOI] [PubMed] [Google Scholar]
- (11).Ciulla TA, Harris A, Latkany P, Piper HC, Arend O, Garzozi H, Martin B. Ocular perfusion abnormalities in diabetes. Acta Ophthalmol Scand. 2002;80(5):468–77. doi: 10.1034/j.1600-0420.2002.800503.x. [DOI] [PubMed] [Google Scholar]
- (12).Schmetterer L, Wolzt M. Ocular blood flow and associated functional deviations in diabetic retinopathy. Diabetologia. 1999;42(4):387–405. doi: 10.1007/s001250051171. [DOI] [PubMed] [Google Scholar]
- (13).Jackson WF. Ion channels and vascular tone. Hypertension. 2000;35(1 Pt 2):173–8. doi: 10.1161/01.hyp.35.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Brayden JE, Nelson MT. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992;256(5056):532–5. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- (15).Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270(5236):633–7. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- (16).Knaus HG, Folander K, Garcia-Calvo M, Garcia ML, Kaczorowski GJ, Smith M, Swanson R. Primary sequence and immunological characterization of beta-subunit of high conductance Ca(2+)-activated K+ channel from smooth muscle. J Biol Chem. 1994;269(25):17274–8. [PubMed] [Google Scholar]
- (17).Dworetzky SI, Boissard CG, Lum-Ragan JT, McKay MC, Post-Munson DJ, Trojnacki JT, Chang CP, Gribkoff VK. Phenotypic alteration of a human BK (hSlo) channel by hSlobeta subunit coexpression: changes in blocker sensitivity, activation/relaxation and inactivation kinetics, and protein kinase A modulation. J Neurosci. 1996;16(15):4543–50. doi: 10.1523/JNEUROSCI.16-15-04543.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).McManus OB, Helms LM, Pallanck L, Ganetzky B, Swanson R, Leonard RJ. Functional role of the beta subunit of high conductance calcium-activated potassium channels. Neuron. 1995;14(3):645–50. doi: 10.1016/0896-6273(95)90321-6. [DOI] [PubMed] [Google Scholar]
- (19).Brenner R, Perez GJ, Bonev AD, Eckman DM, Kosek JC, Wiler SW, Patterson AJ, Nelson MT, Aldrich RW. Vasoregulation by the beta1 subunit of the calcium-activated potassium channel. Nature. 2000;407(6806):870–6. doi: 10.1038/35038011. [DOI] [PubMed] [Google Scholar]
- (20).Pluger S, Faulhaber J, Furstenau M, Lohn M, Waldschutz R, Gollasch M, Haller H, Luft FC, Ehmke H, Pongs O. Mice with disrupted BK channel beta1 subunit gene feature abnormal Ca(2+) spark/STOC coupling and elevated blood pressure. Circ Res. 2000;87(11):E53–E60. doi: 10.1161/01.res.87.11.e53. [DOI] [PubMed] [Google Scholar]
- (21).Takagi C, Bursell SE, Lin YW, Takagi H, Duh E, Jiang Z, Clermont AC, King GL. Regulation of retinal hemodynamics in diabetic rats by increased expression and action of endothelin-1. Invest Ophthalmol Vis Sci. 1996;37(12):2504–18. [PubMed] [Google Scholar]
- (22).Engerman RL, Kern TS. Retinopathy in animal models of diabetes. Diabetes Metab Rev. 1995;11(2):109–20. doi: 10.1002/dmr.5610110203. [DOI] [PubMed] [Google Scholar]
- (23).Knaus HG, McManus OB, Lee SH, Schmalhofer WA, Garcia-Calvo M, Helms LM, Sanchez M, Giangiacomo K, Reuben JP, Smith AB., III Tremorgenic indole alkaloids potently inhibit smooth muscle high-conductance calcium-activated potassium channels. Biochemistry. 1994;33(19):5819–28. doi: 10.1021/bi00185a021. [DOI] [PubMed] [Google Scholar]
- (24).Jaggar JH, Porter VA, Lederer WJ, Nelson MT. Calcium sparks in smooth muscle. Am J Physiol Cell Physiol. 2000;278(2):C235–C256. doi: 10.1152/ajpcell.2000.278.2.C235. [DOI] [PubMed] [Google Scholar]
- (25).Curtis TM, Tumelty J, Dawicki J, Scholfield CN, McGeown JG. Identification and spatiotemporal characterization of spontaneous Ca2+ sparks and global Ca2+ oscillations in retinal arteriolar smooth muscle cells. Invest Ophthalmol Vis Sci. 2004;45(12):4409–14. doi: 10.1167/iovs.04-0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).McGahon MK, Dawicki JM, Scholfield CN, McGeown JG, Curtis TM. A-type potassium current in retinal arteriolar smooth muscle cells. Invest Ophthalmol Vis Sci. 2005;46(9):3281–7. doi: 10.1167/iovs.04-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Nimigean CM, Magleby KL. The beta subunit increases the Ca2+ sensitivity of large conductance Ca2+-activated potassium channels by retaining the gating in the bursting states. J Gen Physiol. 1999;113(3):425–40. doi: 10.1085/jgp.113.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Dick GM, Rossow CF, Smirnov S, Horowitz B, Sanders KM. Tamoxifen activates smooth muscle BK channels through the regulatory beta 1 subunit. J Biol Chem. 276(37):34594–9. doi: 10.1074/jbc.M104689200. 200. [DOI] [PubMed] [Google Scholar]
- (29).Jaggar JH, Porter VA, Lederer WJ, Nelson MT. Calcium sparks in smooth muscle. Am J Physiol Cell Physiol. 2000;278(2):C235–C256. doi: 10.1152/ajpcell.2000.278.2.C235. [DOI] [PubMed] [Google Scholar]
- (30).Mokelke EA, Dietz NJ, Eckman DM, Nelson MT, Sturek M. Diabetic dyslipidemia and exercise affect coronary tone and differential regulation of conduit and microvessel K+ current. Am J Physiol Heart Circ Physiol. 2005;288(3):H1233–H1241. doi: 10.1152/ajpheart.00732.2004. [DOI] [PubMed] [Google Scholar]
- (31).Burnham MP, Johnson IT, Weston AH. Reduced Ca2+-dependent activation of large-conductance Ca2+-activated K+ channels from arteries of Type 2 diabetic Zucker diabetic fatty rats. Am J Physiol Heart Circ Physiol. 2006;290(4):H1520–H1527. doi: 10.1152/ajpheart.00827.2005. [DOI] [PubMed] [Google Scholar]
- (32).Dimitropoulou C, Han G, Miller AW, Molero M, Fuchs LC, White RE, Carrier GO. Potassium (BK(Ca)) currents are reduced in microvascular smooth muscle cells from insulin-resistant rats. Am J Physiol Heart Circ Physiol. 2002;282(3):H908–H917. doi: 10.1152/ajpheart.00382.2001. [DOI] [PubMed] [Google Scholar]
- (33).Ye CL, Shen B, Ren XD, Luo RJ, Ding SY, Yan FM, Jiang JH. An increase in opening of BK(Ca) channels in smooth muscle cells in streptozotocin-induced diabetic mice. Acta Pharmacol Sin. 2004;25(6):744–50. [PubMed] [Google Scholar]
- (34).Sowers JR, Epstein M, Frohlich ED. Diabetes, hypertension, and cardiovascular disease: an update. Hypertension. 2001;37(4):1053–9. doi: 10.1161/01.hyp.37.4.1053. [DOI] [PubMed] [Google Scholar]
- (35).Adler AI, Stratton IM, Neil HA, Yudkin JS, Matthews DR, Cull CA, Wright AD, Turner RC, Holman RR. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;321(7258):412–9. doi: 10.1136/bmj.321.7258.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Sowers JR, Khoury S, Standley P, Zemel P, Zemel M. Mechanisms of hypertension in diabetes. Am J Hypertens. 1991;4(2 Pt 1):177–82. doi: 10.1093/ajh/4.2.177. [DOI] [PubMed] [Google Scholar]
- (37).Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995;268(4 Pt 1):C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- (38).Amberg GC, Bonev AD, Rossow CF, Nelson MT, Santana LF. Modulation of the molecular composition of large conductance, Ca(2+) activated K(+) channels in vascular smooth muscle during hypertension. J Clin Invest. 2003;112(5):717–24. doi: 10.1172/JCI18684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Amberg GC, Santana LF. Downregulation of the BK channel beta1 subunit in genetic hypertension. Circ Res. 2003;93(10):965–71. doi: 10.1161/01.RES.0000100068.43006.36. [DOI] [PubMed] [Google Scholar]
- (40).Fernandez-Fernandez JM, Tomas M, Vazquez E, Orio P, Latorre R, Senti M, Marrugat J, Valverde MA. Gain-of-function mutation in the KCNMB1 potassium channel subunit is associated with low prevalence of diastolic hypertension. J Clin Invest. 2004;113(7):1032–9. doi: 10.1172/JCI20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Stitt AW, Li YM, Gardiner TA, Bucala R, Archer DB, Vlassara H. Advanced glycation end products (AGEs) co-localize with AGE receptors in the retinal vasculature of diabetic and of AGE-infused rats. Am J Pathol. 1997;150(2):523–31. [PMC free article] [PubMed] [Google Scholar]
- (42).Bidasee KR, Nallani K, Yu Y, Cocklin RR, Zhang Y, Wang M, Dincer UD, Besch HR., Jr. Chronic diabetes increases advanced glycation end products on cardiac ryanodine receptors/calcium-release channels. Diabetes. 2003;52(7):1825–36. doi: 10.2337/diabetes.52.7.1825. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.