Abstract
It is commonly assumed that the propensity to disperse and the dispersal distance of mammals should increase with increasing density and be greater among males than among females. However, most empirical evidence, especially on large mammals, has focused on highly polygynous and dimorphic species displaying female-defence mating tactics. We tested these predictions on roe deer, a weakly polygynous species of large herbivore exhibiting a resource-defence mating tactic at a fine spatial scale. Using three long-term studies of populations that were subject to the experimental manipulation of size, we did not find any support for either prediction, whether in terms of dispersal probability or dispersal distance. Our findings of similar dispersal patterns in both sexes of roe deer suggest that the underlying cause of natal dispersal is not related to inbreeding avoidance in this species. The absence of positive density dependence in fine-scale dispersal behaviour suggests that roe deer natal dispersal is a pre-saturation process that is shaped by heterogeneities in habitat quality rather than by density per se.
Keywords: density dependence, life-history tactic, philopatry, sexual selection
1. Introduction
Natal dispersal describes the definitive movement of an individual from its birth location to the place where it will settle and reproduce (Howard 1960). This process plays a fundamental role in determining the spatial structure (Chesser et al. 1993) and persistence (Gilpin & Hanski 1991) of populations. Indeed, natal dispersal has important consequences for population genetics (Chesser 1991) and demography (Chepko-Sade & Halpin 1987), and has received considerable attention from both theoreticians and empiricists (Clobert et al. 2001). However, the underlying causes of natal dispersal in populations of a given species are still unclear (Johnson & Gaines 1990; Stenseth & Lidicker 1992; Clobert et al. 2001; Lawson Handley & Perrin 2007). While most people would now agree that natal dispersal is a multifactorial process, five major ultimate causes have been proposed to account for observed variation in patterns of natal dispersal. Natal dispersal may have evolved to reduce the cost of inbreeding (Wolf 1993, 1994), to decrease the competition for resources (Greenwood 1980), mates (Dobson 1982) or among kin (Hamilton & May 1977), or in response to habitat instability (McPeek & Holt 1992; Johst & Brandl 1997). These selection pressures are likely to play a role in determining patterns of dispersal in mammalian populations because mammals generally have overlapping generations and complex social structures, are often faced with limiting resources and habitually live in fluctuating environments. In particular, owing to the between-sex differences in allocation to reproductive activities in mammals, with males maximizing mating opportunities and females favouring parental care, we should expect to observe sex-biased natal dispersal in favour of males (Pusey 1987; Lawson Handley & Perrin 2007). In addition, we should also expect the positive density dependence of natal dispersal in most mammalian populations (Matthysen 2005). From long-term monitoring of three populations for which size was experimentally manipulated, we tested whether these general expectations are met in roe deer (Capreolus capreolus), a medium sized slightly dimorphic and weakly polygynous mammalian herbivore in which males are territorial for half of the year and females are highly sedentary (Strandgaard 1972).
2. Material and methods
(a) Populations studied
We analysed patterns of natal dispersal in three populations which were intensively monitored by capture–mark–recapture (CMR) methods for more than 10 years. The Dourdan forest (48°19′ N, 2°01′ E) is located in the Parisian district (France) and comprises an 860 ha open mixed forest of chestnut (Castanea sativa), oak (Quercus sessiflora), beech (Fagus sylvatica) and Scots pine (Pinus sylvestris). Prior to the start of the study in 1979, the population was regularly hunted. During the study period (1979–1988), all hunting ceased and, as a consequence, density (estimated by CMR, Vincent et al. (1991)) increased approximately threefold, from 5.1 to 16.9 deer/100 ha. As a consequence, several life-history traits, including body mass and recruitment (Vincent et al. 1995), early growth (Hewison et al. 2002) and home range size (Kjellander et al. 2004), showed density-dependent responses. Chizé is a deer-fenced forest of 2614 ha in western France (46°05′ N, 0°25′ E), principally composed of oak and beech. Population density (estimated by CMR, Gaillard et al. 2003) fluctuated by approximately threefold, from 5.2 to 16.9 deer/100 ha, during the study period (1979–1998) in response to experimental manipulation (i.e. changes in the number of animals removed for translocation). At Chizé, density-dependent responses have been reported for early growth (Hewison et al. 2002), fawn body mass (Gaillard et al. 1996), fawn survival (Gaillard et al. 1997), the proportion of young females successfully breeding (Gaillard et al. 1992) and adult body mass (Pettorelli et al. 2002). Lastly, the Bogesund area (59°23′ N, 18°15′ E) is situated in east central Sweden and comprises a 2600 ha fragmented habitat of 65% forest and 25% agricultural land, the remainder being rocky areas and bogs. Population density (estimated by CMR, P. Kjellander (2000, unpublished PhD thesis)) varied by approximately fourfold, from 7.5 to 28.1 deer/100 ha, during the study period (1990–1999) in response to experimental manipulation (i.e. changes in hunting pressure). At Bogesund, density-dependent responses have been reported for fawn body mass (Kjellander et al. 2006), recruitment (P. Kjellander 2000, unpublished PhD thesis) and home range size (Kjellander et al. 2004). At Dourdan and Chizé, the climate is mild, with an average winter temperature greater than 3.5°C and a yearly average of less than 5 days winter snow lie. At Bogesund, winter conditions are a bit harsher, with a mean temperature of −3.7°C for the coldest month (January) and a yearly average of 80 days winter snow lie.
(b) Assessment of natal dispersal
In all three populations, we obtained data on natal site location during annual net captures (Dourdan and Chizé) or box trapping (Bogesund) of roe deer that took place from October to March, when fawns (five to ten months old) are still closely associated with their mother (Hewison et al. 1998). Fawns were identified at capture by the presence of milk teeth, notably a tricuspid third premolar, which provides a simple and reliable way to distinguish this age class (Ratcliffe & Mayle 1992). Animals were sexed, marked with collars (radio collars at Dourdan and Bogesund, numbered collars at Chizé) and/or ear tags and released. As natal dispersal of roe deer mainly occurs at approximately 1 year of age (Strandgaard 1972; Wahlström & Liberg 1995), we measured dispersal of individuals that lived for at least 2 years. Dispersal can be measured either as a categorical variable (philopatry versus dispersal) based on a threshold value of the distance between the maternal and offspring home ranges (e.g. Massot & Clobert 2000), or as the absolute distance between these home ranges (e.g. Sutherland et al. 2000). As conclusions might depend on the measure selected, we tested for density and sex effects on dispersal behaviour using both measurements. The assessment of dispersal status for individuals differed among sites owing to differences in the type of monitoring. At Dourdan and Bogesund, all roe deer fawns (N=29 (14 females and 15 males) and N=66 (43 females and 23 males), respectively) were radio collared and subsequently monitored by radio telemetry, so that accurate measures of ranging behaviour were available (Vincent et al. 1995; Kjellander et al. 2004). At Chizé, we used the location of subsequent recaptures to assess dispersal. We defined the area settled by a roe deer from all the capture locations obtained for that animal from 2 years of age onwards. Most roe deer older than 1 year are highly sedentary (more than 95%, Pettorelli et al. 2003), so that they are regularly recaptured in the same area. For the present analysis, we considered only fawns that were subsequently recaptured at least three times as adults in the same sector of the Chizé reserve (N=146 (77 females and 69 males)). To assess dispersal status in the three populations, we considered an animal to have dispersed when successive seasonal ranges were non-overlapping and when their geometric centres of activity were separated by a distance more than 500 m (the approximate radius of an annual home range on these study sites, Kjellander et al. (2004)). Only the categorical measure of dispersal was available at Dourdan because we did not have access to the exact radio-tracking locations of roe deer for that population and used the dispersal status defined from a previous analysis (see Bideau et al. 1987).
(c) Statistical procedures
We tested for the effects of sex and density (the factors of variation) on natal dispersal (the dependent variable) using logistic models (GLM) when natal dispersal was measured as a categorical variable and using linear models when natal dispersal was measured as a distance. Sex was entered as a two-level factor in all the models, whereas density was entered as a continuous covariate. Entering density as a two-level factor (i.e. high versus low density) led to the same conclusions (results not shown). We fitted several models including additive and/or interactive effects of sex and density and selected the best model using the Akaike Information Criterion (AIC) following Burnham & Anderson's (2002) recommendations. The model with the lowest AIC was retained. However, when the AIC of two competing models differed by less than 2, we retained the simplest model in line with the rules of parsimony. We also calculated AIC weights (wi) as a measure of the likelihood that a given model is the best model among a set of fitted models. The ratio of AIC weights between two competing models provides a relative measure of the statistical support for these models. To assess the magnitude of the effect size, we used Wald tests. We also calculated the proportion of variance (linear models) or deviance (GLM) accounted for by the selected model. All calculations were performed with R software (R Development Core Team 2004), using the functions ‘lm’ and ‘glm’.
3. Results
In all three populations and for both measures of natal dispersal, the selected model was the constant model, including neither the effects of sex nor density. Thus, neither the proportion of roe deer that dispersed nor the average distance between the maternal and offspring home range centres differed between the sexes or in relation to population density (table 1).
Table 1.
Model selection for testing the influence of sex (SEX) and population density (DEN), as well as a possible interaction between these factors (INT) on (a) the proportion of roe deer that dispersed from their natal home range and (b) the dispersal distance, in three populations (Dourdan, Chizé and Bogesund). (The table gives the AIC scores and the AIC weights in brackets. The selected model appears in italics. X indicates the terms that are included in the model.)
| (a) | |||||
|---|---|---|---|---|---|
| DEN | SEX | INT | Dourdan | Chizé | Bogesund |
| 36.16 (0.29) | 180.71 (0.37) | 93.25 (0.40) | |||
| X | 38.10 (0.11) | 181.59 (0.24) | 94.99 (0.17) | ||
| X | 35.67 (0.37) | 182.08 (0.19) | 95.24 (0.15) | ||
| X | X | 37.65 (0.14) | 182.86 (0.13) | 96.99 (0.06) | |
| X | X | X | 38.35 (0.10) | 183.84 (0.08) | 94.41 (0.22) |
| (b) | |||||
|---|---|---|---|---|---|
| DEN | SEX | INT | Chizé | Bogesund | |
| 594.20 (0.29) | 1159.49 (0.49) | ||||
| X | 595.56 (0.15) | 1161.44 (0.18) | |||
| X | 594.40 (0.27) | 1161.23 (0.21) | |||
| X | X | 595.64 (0.14) | 1163.15 (0.08) | ||
| X | X | X | 595.62 (0.15) | 1164.40 (0.04) | |
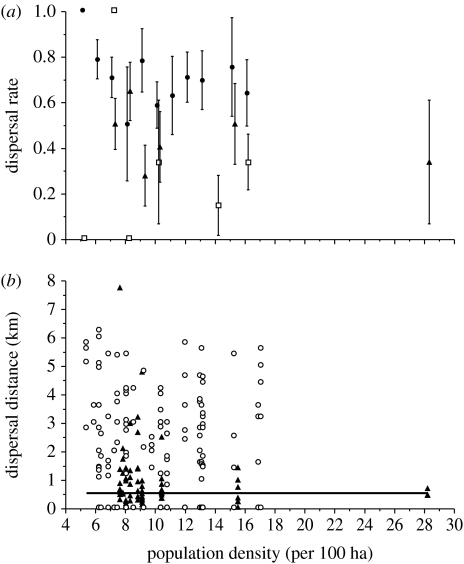
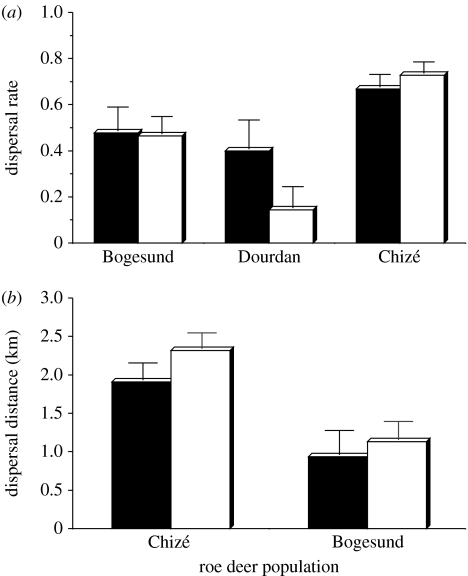
When measuring natal dispersal as the proportion of roe deer that dispersed, we did not find any evidence for interactive effects of sex and density (support for the models including an interaction term was two to three times lower than that for the constant model). Population density did not influence natal dispersal of roe deer (support for the models including a density term was 1.5 to greater than 2 times lower than that for the constant model and the effect size for density (measured as the slope on a logit scale) was not different from 0: 0.004±0.014, p=0.77 at Dourdan; −0.028±0.056, p=0.61 at Bogesund; and −0.059±0.055, p=0.29 at Chizé; figure 1a). Lastly, we did not detect any evidence for between-sex differences in natal dispersal of roe deer (figure 2a). Males tended to disperse more than females at Dourdan (support for the model including a sex term was 1.3 times greater than that for the constant model, effect size of −1.386±0.928, p=0.135, males as a reference), while the reverse trend was observed at Chizé (support for the model including a sex term was half that for the constant model, effect size of 0.288±0.362, p=0.43, males as a reference), and both sexes dispersed in almost exactly the same proportion at Bogesund (support for the model including a sex term was less than half that for the constant model, effect size of 0.053±0.517, p=0.92, males as a reference). The selected constant model, without any effects of sex and density, did not show any evidence of over-dispersion (ĉ of 1.22 at Dourdan, 1.40 at Bogesund and 1.23 at Chizé). From these models, the mean proportion of roe deer fawns that dispersed was highly variable among populations (Chizé: 69.87%, 95% CI [61.96; 76.75]; Bogesund: 46.98%, 95% CI [35.32; 58.98]; and Dourdan: 27.60%, 95% CI [20.10; 36.60]).
Figure 1.
Relationship between natal dispersal ((a) dispersal rate and (b) dispersal distance) and population density in three populations of roe deer ((a) filled circles and (b) open circles, Chizé; open squares, Dourdan; filled triangles, Bogesund). The full line in panel (b) corresponds to the threshold distance separating philopatry from dispersal (500 m).
Figure 2.
Sex-specific natal dispersal ((a) dispersal rate and (b) dispersal distance) in three populations of roe deer (filled bars, males; open bars, females).
When measuring natal dispersal as the distance between the geometric centres of maternal and offspring home ranges at Chizé and Bogesund, we did not find any evidence for interactive effects of sex and density (support for the models with an interaction term was 2 (Chizé) and 10 (Bogesund) times lower than that for the constant model). Likewise, population density did not influence natal dispersal distance of roe deer (support for the models including a density term was half that for the constant model and the effect size for density (measured as the slope) was not different from 0: −0.037±0.047, p=0.43 at Chizé and −0.0089±0.042, p=0.83 at Bogesund; figure 1b). Lastly, we did not detect any evidence for between-sex differences in natal dispersal distances of roe deer (support for the models including a sex term was equal to (Chizé) or half that (Bogesund) for the constant model, effect size of 0.405 km±0.303, p=0.18 and −0.201 km±0.401, p=0.62 at Chizé and Bogesund, respectively, males as a reference; figure 2b). From the selected constant model, without any effects of sex and density, the mean dispersal distance of roe deer fawns was 2.124±0.152 km at Chizé and 1.062±0.190 km at Bogesund.
4. Discussion
Contrary to our expectations based on current evolutionary theories of dispersal, the rate and distance of natal dispersal in roe deer did not differ markedly between the sexes and did not vary in relation to population density, despite the wide range of densities encountered within each of our three contrasting populations. However, marked variation in the magnitude of dispersal did occur among these populations.
The absence of between-sex differences in dispersal behaviour of roe deer contrasts with most previous studies of mammals. As recently pointed out by Lawson Handley & Perrin (2007) from a literature survey, sex-biased dispersal is almost a ubiquitous feature of mammalian life history. Populations of large herbivores fit this general pattern, with a high rate of natal dispersal among males and a high level of philopatry among females (mule deer Odocoileus hemionus: Bunnell & Harestad 1983; moose Alces alces: Labonté et al. 1998; chamois Rupicapra rupicapra: Loison et al. 1999; white-tailed deer Odocoileus virginianus: Etter et al. 2002; red deer Cervus elaphus: Catchpole et al. 2004). However, in all these species, males exhibit a mating tactic of female-defence polygyny. By contrast, substantial natal dispersal in females has been reported in both horse Equus caballus (Monard & Duncan 1996) and guanacos Lama guanicoe (Sarno et al. 2003). In the weakly dimorphic horse exhibiting resource-defence polygyny, no between-sex difference occurs in natal dispersal, while in monomorphic guanacos approximately 50% of offspring of both sexes are forced to disperse by territorial males. The absence of a between-sex difference in dispersal behaviour that we report here is thus consistent with the low sexual size dimorphism, the mating tactic of resource defence and the low level of polygyny exhibited by roe deer (Vanpé et al. 2008). Note, however, that our results do not support Greenwood's (1980) model which hypothesized that when adult males defend resource territories (rather than sexual partners) they should disperse less than females. Indeed, we did not find any support for sex-biased dispersal in favour of females in any of the three populations we studied (see also Coulon et al. (2006) for similar conclusions based on an analysis of sex-specific inter-individual genetic distances). Instead, as suggested first by Dobson (1982), and advocated on more theoretical grounds by Perrin & Goudet (2001), we suggest that the degree of skew towards male-biased dispersal is linked to the intensity of female defence in a given species. Thus, in resource-defence polygyny where males are often territorial and invest little time in female defence, male-biased dispersal is not expected to occur. In this case, following Greenwood's model, female dispersal is expected in order to avoid inbreeding (Pusey 1987). Our results clearly indicate that roe deer females do not disperse more or farther than males, leading to a potential risk of inbreeding. However, recent evidence has suggested that a substantial proportion of roe deer females undergo excursions during the rut, potentially to mate with unrelated males (J. D. C. Linnell 1994, unpublished PhD thesis; San José & Lovari 1998; Richard et al. in press). We speculatively suggest that a consequence of this kind of breeding dispersal during adulthood could be that inbreeding risk is not a significant selection pressure driving the evolution of natal dispersal in roe deer.
The absence of response of roe deer natal dispersal behaviour to changes in density, despite the large variations experienced by the three populations we studied, contrasts markedly with previous studies on deer species (Strandgaard 1972; Wahlström & Liberg 1995 on roe deer; Clutton-Brock et al. 2002 on red deer). Indeed, the propensity to disperse was not related to population density in any of the three populations we studied. A supposed positive relationship between natal dispersal and population density has previously been suggested to be the basis of a regulatory process in roe deer populations (Strandgaard 1972). However, while marked intra-male competition for territories at high density could potentially lead to higher dispersal rates among young males (Wahlström 1994), if roe deer populations were to be regulated by density-dependent dispersal of non-territorial females, this would require a voluntary decision to track temporal changes in resource availability. Whatever the case, the strong discrepancy between Strandgaard's results in Denmark, who reported a clear positive density dependence in natal dispersal of roe deer, and our results on three different populations appears to be puzzling at first sight. However, differences in the spatial scale studied could account for this contrast. Roe deer in the Danish study were likely emigrating from the 900 ha reserve in order to colonize vacant areas of high habitat quality in the surrounding, intensively hunted, landscape, so that natal dispersal was analysed at the population scale. By contrast, in our study populations, emigration is not possible (Chizé) or is difficult (Dourdan and Bogesund) because these forests are surrounded by barriers (a motorway and a railway in Dourdan, sea in Bogesund and deer fencing in Chizé). Natal dispersal was thus analysed at a fine scale (home range), within the population, so that dispersal rates cannot play a role in population regulation. Such contrasting patterns of natal dispersal in relation to the spatial scale studied provide strong support for Ronce et al.'s (2001) statement that selective pressures on dispersal, and thereby dispersal patterns, are likely to depend on the spatial scale considered. The occurrence of substantial dispersal, even at low population density, that we reported here indicates that roe deer exhibit a pattern of pre-saturation dispersal (sensu Swenson et al. 1998), which varied highly in magnitude among the three populations we studied. Although differences in the methods of assessing dispersal patterns might partly account for this variability, the magnitude of the differences in dispersal rate (from 28 to 70%) and distance (from 1 to 2 km, on average) are extremely pronounced. We therefore suggest that the pattern of highly variable dispersal behaviour that we observed among the three studied populations corresponds to marked between-population heterogeneity in range quality. Although we did not have detailed measurements to compare range quality among the three study sites, between-population differences of fawn body mass in winter can be used as a proxy for range quality in large herbivores (Saether & Heim 1993; Gaillard et al. 1996). For a given density, fawn body mass was lower at Chizé than at Bogesund (Kjellander et al. 2006), suggesting that dispersal rate at the population level could be inversely related to range quality. Further work will be required to investigate the determinants of natal dispersal patterns of roe deer at a fine scale.
Acknowledgments
We thank Jean-Paul Vincent and Bernard Boisaubert for having initiated the long-term monitoring of roe deer at Dourdan and Chizé, respectively. This work was supported by the GICC2 program.
References
- Bideau E, Vincent J.P, Maublanc M.L, Gonzalez R. Dispersion chez le jeune chevreuil (Capreolus capreolus L.): étude sur une population en milieu forestier. Acta Oecologica-Oecologica Appl. 1987;8:135–148. [Google Scholar]
- Bunnell F.L, Harestad A.S. Dispersal and dispersion of black-tailed deer. Models and observations. J. Mammal. 1983;64:201–209. doi:10.2307/1380549 [Google Scholar]
- Burnham K.P, Anderson D.R. Springer; New York, NY: 2002. Model selection and multi-model inference: a practical information-theoretic approach. [Google Scholar]
- Catchpole E.A, Fan Y, Morgan B.J.T, Clutton-Brock T.H, Coulson T. Sexual dimorphism, survival and dispersal in red deer. J. Agric. Biol. Environ. Stat. 2004;9:1–26. doi:10.1198/1085711043172 [Google Scholar]
- Chepko-Sade B.D, Halpin Z.T. University of Chicago Press; Chicago, IL: 1987. Mammalian dispersal patterns. The effects of social structure on population genetics. [Google Scholar]
- Chesser R.K. Gene diversity and female philopatry. Genetics. 1991;127:437–447. doi: 10.1093/genetics/127.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesser R.K, Sugg D.W, Rhodes O.E, Nowak J.M, Smith M.H. Evolution of mammalian social structure. Acta Theriol. 1993;38:163–174. [Google Scholar]
- Clobert J, Danchin E, Dhondt A.A, Nichols J.D. Oxford University Press; New York, NY: 2001. Dispersal. [Google Scholar]
- Clutton-Brock T.H, Coulson T.N, Milner-Gulland E.J, Thomson D, Armstrong H.M. Sex differences in emigration and mortality affect optimal management of deer populations. Nature. 2002;415:633–637. doi: 10.1038/415633a. doi:10.1038/415633a [DOI] [PubMed] [Google Scholar]
- Coulon A, Cosson J.-F, Morellet N, Angibault J.-M, Cargnelutti B, Galan M, Aulagnier S, Hewison A.J.M. Dispersal is not female biased in a resource-defence mating ungulate, the European roe deer. Proc. R. Soc. B. 2006;273:341–348. doi: 10.1098/rspb.2005.3329. doi:10.1098/rspb.2005.3329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson F.S. Competition for mates and predominant juvenile male dispersal in mammals. Anim. Behav. 1982;30:1183–1192. doi:10.1016/S0003-3472(82)80209-1 [Google Scholar]
- Etter D.R, Hollis K.M, Van Deelen T.R, Ludwig D.R, Chelsvig J.E, Anchor C.L, Warner R.E. Survival and movements of white-tailed deer in suburban Chicago, Illinois. J. Wildl. Manage. 2002;66:500–510. doi:10.2307/3803183 [Google Scholar]
- Gaillard J.M, Sempéré A.J, Boutin J.M, Van Laere G, Boisaubert B. Effects of age and body weight on the proportion of females breeding in a population of roe deer (Capreolus capreolus) Can. J. Zool. 1992;70:1541–1545. [Google Scholar]
- Gaillard J.M, Delorme D, Boutin J.M, Van Laere G, Boisaubert B. Body mass of roe deer fawns during winter in two contrasting populations. J. Wildl. Manage. 1996;60:29–36. doi:10.2307/3802036 [Google Scholar]
- Gaillard J.M, Boutin J.M, Delorme D, Van Laere G, Duncan P, Lebreton J.D. Early survival of roe deer: causes and consequences of cohort variation in two contrasted populations. Oecologia. 1997;112:502–513. doi: 10.1007/s004420050338. doi:10.1007/s004420050338 [DOI] [PubMed] [Google Scholar]
- Gaillard J.M, Duncan P, Delorme D, Van Laere G, Pettorelli N, Maillard D, Renaud G. Effects of hurricane Lothar on the population dynamics of European roe deer. J. Wildl. Manage. 2003;67:767–773. doi:10.2307/3802684 [Google Scholar]
- Gilpin M.E, Hanski I. Academic Press; London, UK: 1991. Metapopulation dynamics: empirical and theoretical investigations. [Google Scholar]
- Greenwood P.J. Mating systems, philopatry and dispersal in birds and mammals. Anim. Behav. 1980;28:1140–1162. doi:10.1016/S0003-3472(80)80103-5 [Google Scholar]
- Hamilton W.D, May R.M. Dispersal in stable habitats. Nature. 1977;269:578–581. doi:10.1038/269578a0 [Google Scholar]
- Hewison A.J.M, Vincent J.P, Reby D. Social organisation of European roe deer. In: Andersen R, Duncan P, Linnell J.D.C, editors. The European roe deer: the biology of success. Scandinavian University Press; Oslo, Norway: 1998. pp. 189–219. [Google Scholar]
- Hewison A.J.M, Gaillard J.M, Angibault J.M, Van Laere G, Vincent J.P. The influence of density on post-weaning winter growth in roe deer Capreolus capreolus fawns. J. Zool. 2002;257:303–309. doi:10.1017/S0952836902000900 [Google Scholar]
- Howard W.E. Innate and environmental dispersal of individual vertebrates. Am. Midl. Nat. 1960;63:152–161. doi:10.2307/2422936 [Google Scholar]
- Johnson M.L, Gaines M.S. Evolution of dispersal: theoretical models and empirical tests using birds and mammals. Annu. Rev. Ecol. Syst. 1990;21:449–480. doi:10.1146/annurev.es.21.110190.002313 [Google Scholar]
- Johst K, Brandl R. Evolution of dispersal: the importance of the temporal order of reproduction and dispersal. Proc. R. Soc. B. 1997;264:23–30. doi:10.1098/rspb.1997.0004 [Google Scholar]
- Kjellander P, Hewison A.J.M, Liberg O, Angibault J.M, Bideau E, Cargnelutti B. Experimental evidence for density-dependence of home-range size in roe deer (Capreolus capreolus L.): a comparison of two long-term studies. Oecologia. 2004;139:478–485. doi: 10.1007/s00442-004-1529-z. doi:10.1007/s00442-004-1529-z [DOI] [PubMed] [Google Scholar]
- Kjellander P, Gaillard J.M, Hewison A.J.M. Density-dependent responses of fawn cohort body mass in two contrasting roe deer populations. Oecologia. 2006;146:521–530. doi: 10.1007/s00442-005-0188-z. doi:10.1007/s00442-005-0188-z [DOI] [PubMed] [Google Scholar]
- Labonté J, Ouellet J.P, Courtois R, Belisle F. Moose dispersal and its role in the maintenance of harvested populations. J. Wildl. Manage. 1998;62:225–235. doi:10.2307/3802282 [Google Scholar]
- Lawson Handley L.J, Perrin N. Advances in our understanding of mammalian sex-biased dispersal. Mol. Ecol. 2007;16:1559–1578. doi: 10.1111/j.1365-294X.2006.03152.x. doi:10.1111/j.1365-294X.2006.03152.x [DOI] [PubMed] [Google Scholar]
- Loison A, Jullien J.M, Menaut P. Subpopulation structure and dispersal in two populations of chamois. J. Mammal. 1999;80:620–632. doi:10.2307/1383306 [Google Scholar]
- Massot M, Clobert J. Processes at the origin of similarities in dispersal behaviour among siblings. J. Evol. Biol. 2000;13:707–719. doi:10.1046/j.1420-9101.2000.00202.x [Google Scholar]
- Matthysen E. Density-dependent dispersal in birds and mammals. Ecography. 2005;28:403–416. doi:10.1111/j.0906-7590.2005.04073.x [Google Scholar]
- McPeek M.A, Holt R.D. The evolution of dispersal in spatially and temporally varying environments. Am. Nat. 1992;140:1010–1027. doi:10.1086/285453 [Google Scholar]
- Monard A.M, Duncan P. Consequences of natal dispersal in female horses. Anim. Behav. 1996;52:565–579. doi:10.1006/anbe.1996.0198 [Google Scholar]
- Perrin N, Goudet J. Inbreeding, kinship, and the evolution of natal dispersal. In: Clobert J, Danchin E, Dhondt A.A, Nichols J.D, editors. Dispersal. Oxford University Press; New York, NY: 2001. pp. 127–146. [Google Scholar]
- Pettorelli N, Gaillard J.M, Van Laere G, Duncan P, Maillard D. Cohort effects and spatial variation in adult mass of roe deer. Proc. R. Soc. B. 2002;269:747–753. doi: 10.1098/rspb.2001.1791. doi:10.1098/rspb.2001.1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettorelli N, Gaillard J.M, Duncan P, Maillard D, Van Laere G, Delorme D. Age and density modify the effects of habitat quality on survival and movements of roe deer. Ecology. 2003;84:3307–3316. doi:10.1890/02-0602 [Google Scholar]
- Pusey A.E. Sex-biased dispersal and inbreeding avoidance in birds and mammals. Trends Ecol. Evol. 1987;2:295–299. doi: 10.1016/0169-5347(87)90081-4. doi:10.1016/0169-5347(87)90081-4 [DOI] [PubMed] [Google Scholar]
- Ratcliffe, P. R. & Mayle, B. 1992 Roe deer biology and management Bulletin no. 105, London, UK: Forestry Commission, HMSO Publications.
- R Development Core Team. R Foundation for Statistical Computing; Vienna, Austria: 2004. R: a language and environment for statistical computing. [Google Scholar]
- Richard, E., Morellet, N., Cargnelutti, B., Angibault, J. M., Vanpe, C. & Hewison, A. J. M. In press. Ranging behaviour and rut excursions of female roe deer: evidence for female mate choice? Behav. Proc (doi:10.1016/j.beproc.2008.04.008) [DOI] [PubMed]
- Ronce O, Olivieri I, Clobert J, Danchin E. Perspective on the studies of dispersal evolution. In: Clobert J, Danchin E, Dhondt A.A, Nichols J.D, editors. Dispersal. Oxford University Press; New York, NY: 2001. pp. 314–357. [Google Scholar]
- Saether B.E, Heim M. Ecological correlates of individual variation in age at maturity in female moose (Alces alces)—the effects of environmental variability. J. Anim. Ecol. 1993;62:482–489. doi:10.2307/5197 [Google Scholar]
- San José C, Lovari S. Ranging movements of female roe deer: do home-loving does roam to mate? Ethology. 1998;104:721–728. [Google Scholar]
- Sarno R.J, Bank M.S, Stern H.S, Franklin W.L. Forced dispersal of juvenile guanacos (Lama guanicoe): causes, variation, and fates of individuals dispersing at different times. Behav. Ecol. Sociobiol. 2003;54:22–29. doi:10.1007/s00265-003-0604-5 [Google Scholar]
- Stenseth N.C, Lidicker W.Z., Jr. Chapman & Hall; London, UK: 1992. Animal dispersal: small mammals as a model. [Google Scholar]
- Strandgaard H. The roe deer (Capreolus capreolus) population at Kalø and the factors regulating its size. Dan. Rev. Game Biol. 1972;7:1–205. [Google Scholar]
- Sutherland G.D, Harestad A.S, Price K, Lertzman K.P. Scaling of natal dispersal distances in terrestrial birds and mammals. Conserv. Ecol. 2000;4:16. [Google Scholar]
- Swenson J.E, Sandegren F, Soderberg A. Geographic expansion of an increasing brown bear population: evidence for presaturation dispersal. J. Anim. Ecol. 1998;67:819–826. doi:10.1046/j.1365-2656.1998.00248.x [Google Scholar]
- Vanpé C, Kjellander P, Galan M, Cosson J.F, Aulagnier S, Liberg O, Hewison A.J.M. Mating system, sexual dimorphism and the opportunity for sexual selection in a territorial ungulate. Behav. Ecol. 2008;19:309–316. doi:10.1093/beheco/arm132 [Google Scholar]
- Vincent J.P, Gaillard J.M, Bideau E. Kilometric index as biological indicator for monitoring forest roe deer populations. Acta Theriol. 1991;36:315–328. [Google Scholar]
- Vincent J.P, Bideau E, Hewison A.J.M, Angibault J.M. The influence of increasing density on body weight, kid production, home range and winter grouping in roe deer (Capreolus capreolus) J. Zool. 1995;236:371–382. [Google Scholar]
- Wahlström L.K. The significance of male–male aggression for yearling dispersal in roe deer (Capreolus capreolus) Behav. Ecol. Sociobiol. 1994;35:409–412. doi:10.1007/BF00165843 [Google Scholar]
- Wahlström L.K, Liberg O. Patterns of dispersal and seasonal migration in roe deer (Capreolus capreolus) J. Zool. 1995;235:455–467. [Google Scholar]
- Wolff J.O. What is the role of adults in mammalian juvenile dispersal? Oikos. 1993;68:173–175. doi:10.2307/3545324 [Google Scholar]
- Wolff J.O. More on juvenile dispersal in mammals. Oikos. 1994;71:349–352. doi:10.2307/3546284 [Google Scholar]