Abstract
We investigated encoding properties of identified auditory interneurons in two not closely related grasshopper species (Acrididae). The neurons can be homologized on the basis of their similar morphologies and physiologies. As test stimuli, we used the species-specific stridulation signals of Chorthippus biguttulus, which evidently are not relevant for the other species, Locusta migratoria. We recorded spike trains produced in response to these signals from several neuron types at the first levels of the auditory pathway in both species. Using a spike train metric to quantify differences between neuronal responses, we found a high similarity in the responses of homologous neurons: interspecific differences between the responses of homologous neurons in the two species were not significantly larger than intraspecific differences (between several specimens of a neuron in one species). These results suggest that the elements of the thoracic auditory pathway have been strongly conserved during the evolutionary divergence of these species. According to the ‘efficient coding’ hypothesis, an adaptation of the thoracic auditory pathway to the specific needs of acoustic communication could be expected. We conclude that there must have been stabilizing selective forces at work that conserved coding characteristics and prevented such an adaptation.
Keywords: spike train metric, neuronal encoding, evolution of nervous system, grasshoppers, acoustic communication
1. Introduction
The evolution of neural networks and in particular the question of how sensory systems could process information in an optimal way has attracted interest for decades (e.g. Barlow 1961; Lewicki 2002). Here, we address these questions by comparing information processing capacities within the auditory pathway of two not closely related grasshopper species. Hearing in orthopteran taxa evolved long ago, and several times independently, in crickets, bush crickets and grasshoppers (Meier & Reichert 1990; Boyan 1993; Hoy et al. 1998; van Staaden & Römer 1998; Stumpner & von Helversen 2001). The origin of grasshopper ears probably dates back more than 200 Myr (Carpenter 1992; Yager 1999), and the associated sensory pathway is used for communication purposes in many species (Stumpner & von Helversen 2001). Acoustic communication often plays a key role in mate finding, and acoustic signals in combination with their correct interpretation by the receiver's auditory pathway often do constitute the major hybridization barrier, as shown in cross-breeding experiments with mute males (Bentley & Hoy 1972; von Helversen & von Helversen 1975; Vedenina & von Helversen 2003; Gottsberger & Mayer 2007). Particularly for females, the priority task must consist of correctly identifying the species-specific signals: to accept a heterospecific partner due to faulty interpretation of his signals would drastically reduce her fitness. There is additional evidence that female grasshoppers use the song signals to infer potential handicaps of males (Kriegbaum 1989), thereby exerting a sexual selection upon male signalling, which may drive speciation (von Helversen & von Helversen 1994; for other Orthoptera, see Hoy 1990; Henry 1994; Gwynne 1995; Gerhardt & Huber 2002; Bailey et al. 2007). Thus, in grasshopper species that rely on acoustic communication, strong selection pressures act on both signal recognition as well as on signal production.
In this paper, we address the question of whether the auditory pathway of acridid grasshoppers may have responded to these selective forces by developing specific adaptations to the processing of communication signals. Evidently, at some stage of auditory processing, a neural network must exist, which enables selective identification of the species-specific signals. But according to the ‘efficient coding’ hypothesis (Barlow 1961), special adaptations to the processing of these behaviourally most relevant signals could be expected on the first stages of the auditory pathway. To investigate this question, we compared the encoding properties of identified auditory neurons in two grasshopper species that are not closely related: Chorthippus biguttulus (Gomphocerinae) and Locusta migratoria (Oedipodinae). A phylogenetic analysis based on mtrRNA gene sequences (Flook & Rowell 1997) clearly supports the monophyly of Oedipodinae and Gomphocerinae (see also Bugrov et al. 2006). The separation of the two taxa probably dates back to the Early Tertiary or even Cretaceous (Flook & Rowell 1997). Hence, the two representative species investigated here were separated in evolution for some 50 million years, and fundamental differences in the stridulatory apparatus indicate that they have evolved acoustic signalling independently (Elsner 1983; Flook & Rowell 1997).
In addition to living in very different habitats, both species rely on acoustic signals to a very different degree. Chorthippus biguttulus uses acoustic signals as a prime cue for mate finding, and therefore its hearing system is under severe selection pressure to correctly identify conspecific signals (Kriegbaum 1989; Machens et al. 2003; Ronacher et al. 2004). By contrast, acoustic communication appears to be of no particular relevance for L. migratoria. Whenever this species shows leg stridulation, short scratching sounds are produced which appear to have mainly a repellent function (Jacobs 1953; Pflüger & Field 1999). However, in Schistocerca gregaria, wing beat noise has been discussed as a possible mechanism for swarm coherence and collision avoidance (Haskell 1957).
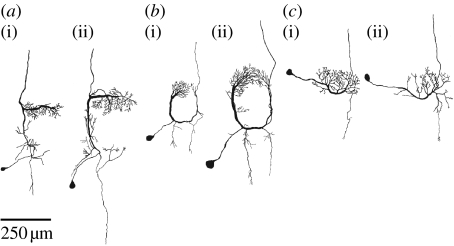
Already in earlier investigations, a large degree of morphological similarity between identified neurons of C. biguttulus and L. migratoria has been observed (Römer et al. 1988; Ronacher & Stumpner 1988; Stumpner 1988), which led to the conclusion that these neurons can putatively be homologized between species (figure 1). This similarity also extends to physiological properties, i.e. temporal filter characteristics and putative transmitter (Ronacher & Stumpner 1988; Sokoliuk et al. 1989).
Figure 1.
Pairs of homologous neurons (a) TN1, (b) AN3 and (c) AN12 in (a(i)–c(i)) C. biguttulus and (a(ii)–c(ii)) L. migratoria.
We now apply a rigorous test procedure—a quantitative measure for the similarity of spiking responses—to investigate whether or not specific adaptations to behaviourally relevant signals exist within the peripheral auditory pathway of these insects, using a spike train metric introduced by van Rossum (2001). As stimuli, we broadcast the species-specific communication signals of C. biguttulus, and recorded spike trains from neurons in both species produced in response to these signals. With this spike train metric method, we found no significant differences between the responses of putatively homologous neurons in both species, i.e. a high correspondence indicating a strong evolutionary conservation.
2. Material and methods
(a) Animals
Electrophysiological experiments were performed on adult male and female L. migratoria and on C. biguttulus. The locusts were obtained from a commercial supplier, and C. biguttulus were caught in the field or F1 reared from our own breeding stock.
(b) Neurophysiology
The experimental procedures were conventional and are described in detail elsewhere (Wohlgemuth & Ronacher 2007). Head, legs, wings and gut were removed and the animals fixed ventrally onto a free standing holder. The thorax was opened dorsally and the whole torso was filled with locust Ringer's solution. During the experiments, the preparation was kept at a constant temperature (30±2°C). Intracellular recordings were obtained from auditory interneurons within the metathoracic ganglion using glass capillaries, the tips of which were filled with a 3–5% Lucifer yellow solution (Sigma-Aldrich, Taufkirchen, Germany) in 0.5 M LiCl. Neural responses were amplified (SEC-05LX; NPI Electronic, Tamm, Germany) and recorded by a data acquisition board (PCI-MIO-16E-1; National Instruments, Munich, Germany) with a sampling rate of 20 kHz. After completion of the stimulation protocol, dye was iontophoretically injected into the recorded neuron. The ganglia were removed, fixed in 4% paraformaldehyde, dehydrated and cleared in methyl salicylate. The stained cells were identified according to their characteristic morphology (Römer & Marquart 1984; Stumpner & Ronacher 1991). All experiments were performed in a Faraday cage lined with foam prisms to minimize echoes. We analysed recordings of seven neuron types: three local interneurons, TN1, BSN1 and SN3, and four ascending interneurons, AN1, AN2, AN3 and AN12. These neurons cover a broad range of physiological response types and also reflect consecutive processing levels.
(c) Stimulation
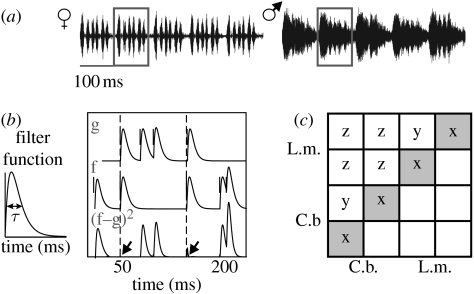
We used songs of a C. biguttulus male and female as acoustic stimuli (figure 2a). The signals of the two sexes share the basic pattern of syllables separated by pauses, with a major difference: the syllables of the female song consist of distinct pulses separated by small gaps, which are masked in the male song (figure 2a). These different temporal patterns can influence the responses of some neurons (see §3). Each stimulus was repeated 8–10 times. Acoustic stimuli were stored digitally and delivered by custom-made software (LabView; National Instruments). Following a 100 kHz D/A conversion (PCI-MIO-16E-1; National Instruments), the stimulus was routed through a computer-controlled attenuator (PA5; Tucker-Davis Technologies, Gainesville, FL, USA) and an audio amplifier (Mercury 2000; Jensen, Pulheim, Germany). Acoustic stimuli were broadcast unilaterally by speakers (D-28/2; Dynaudio, Skanderborg, Denmark) situated at a distance of 30 cm from the preparation. Sound intensity was calibrated with a half inch microphone (type 4133; Brüel & Kjær, Nærum, Denmark) and a measuring amplifier (type 2209, Brüel & Kjær), positioned at the site of the preparation. Depending on the neuron type, we used sound intensities between 55 and 80 dB SPL.
Figure 2.
Acoustic stimuli and principle of spike train metric. (a) Song of a C. biguttulus female and male; one syllable is marked in each song. (b) Principle of the spike train metric analysis according to van Rossum (2001); see text. (c) Scheme for the intra- and interspecific comparison of spike trains of two neurons in each of both species. The shaded squares along the diagonal, denoted with ‘x’, contain the distance values between the 8 and 10 spike trains obtained from a single cell, and represent a measure for the trial-to-trial variability of single neurons. The ‘y’ squares represent the dissimilarities obtained when comparing different specimens of a particular neuron type recorded in a given species, while the ‘z’ squares denote the spike train dissimilarities between the two species.
(d) Analysis of spike train distances according to van Rossum (2001)
Spike times were extracted from the digitized recordings by means of a voltage threshold criterion and provided the basis for further analysis. A spike train segment of 500 ms was used to determine metric distances between spike trains. This window size has been found to be sufficient for a correct signal classification (Wohlgemuth & Ronacher 2007). To compute the distance between two spike trains, the spikes were first convolved with a filter function (figure 2b). Then the two convolved spike train traces (‘g’ and ‘f’, figure 2b) were subtracted, the result was squared and the integral was computed (van Rossum 2001). The rationale of this is as follows: obviously two spike trains are similar in which many spikes occur at the same time. Now, by the subtraction process the contribution to the integral of coincident spikes cancels out (see arrows in the lowest trace of figure 2b), resulting in a low metric distance—equivalent to a high similarity. Thus, this metric yields a measure for the (dis)similarity between spike trains (for details, see van Rossum 2001; Machens et al. 2003; Wohlgemuth & Ronacher 2007).
By varying the width τ of the filter function, the temporal resolution of the evaluation procedure can be adjusted (inset, figure 2b). Since earlier investigations have shown that the timing of spikes plays an important role for the encoding of acoustic signals at the level of thoracic neurons (e.g. Stumpner et al. 1991; Stumpner & Ronacher 1994; Franz & Ronacher 2002), we adjusted the resolution parameter of the spike train metric so as to focus on the temporal patterns of spiking responses. For the present analysis, we used a temporal resolution of τ=5 ms, which has been found to sufficiently encompass the coding properties of auditory neurons (Machens et al. 2003; Wohlgemuth & Ronacher 2007). Hence, the spike trains were compared with a resolution that emphasizes differences in the spike-timing pattern. The metric distances between all spike trains of a given cell type and stimulus were computed, which enabled us to quantify intraspecific (i.e. spike train dissimilarities between specimens of a neuron type in either C. biguttulus or L. migratoria) and interspecific distances (between species; ‘z’, figure 2c).
(e) Clustering algorithms and statistical analysis
The statistical evaluation of the intra- and interspecific distance distributions is not straightforward, since these distributions were necessarily based on multiple comparisons within the same dataset. Each spike train served as a template for all other spike trains, resulting in excess sampling of similarity values [n(n−1)/2]. We therefore decided to use two different cluster algorithms to test the differences in neuronal responses for their statistical significance.
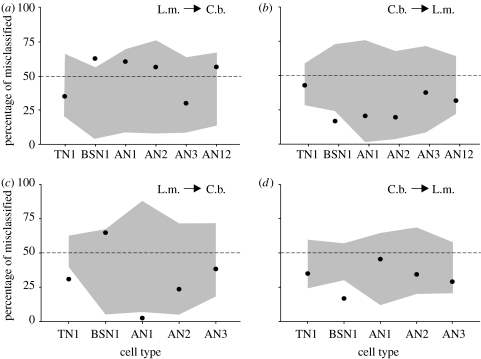
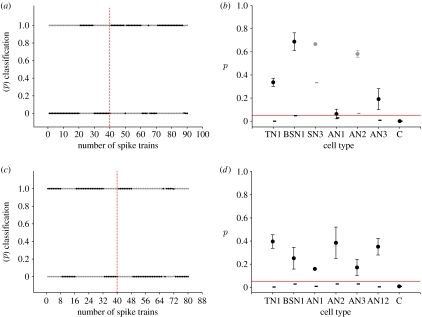
The clustering algorithm developed by Machens et al. (2003) is based on the distance matrix achieved with the spike train metric described above. Once the distances between all spike trains of a certain cell type were computed, clustering was examined by randomly picking for each species a spike train as a ‘template’. All remaining spike trains were classified by assigning them to that template to which they exhibited the smallest distance. This procedure was repeated for all possible template permutations, resulting in an average probability of correct classification (for details see Machens et al. (2003) and Wohlgemuth & Ronacher (2007)). The null hypothesis was that correct classifications should be around chance level if the spike trains are as similar between the species as within the species. To obtain confidence limits, we then shuffled the responses of C. biguttulus with those of L. migratoria in a way that the spike trains of one cell specimen were kept together (Grewe et al. 2003). This shuffling procedure was repeated 10 times independently, which allowed us to determine a ‘domain of uncertainty’ (enclosed by the grey-shaded area in figure 5). Classification values enclosed within this domain are not significantly different from chance classification.
Figure 5.
Misclassifications demonstrate similarities between species. (a,c) Evaluation of how many of the locusts' spike trains were incorrectly classified as stemming from C. biguttulus due to their higher similarities to the C. biguttulus templates (cf. §2) if we allowed for only two classes. Note the high proportions of wrong classifications (points), indicating that the responses of these neurons within the two species cannot be separated. Grey-shaded areas indicate the domain of uncertainty that has been determined by shuffling spike trains (see text). As a control, we also performed the shuffling procedure with 100 repetitions, but the uncertainty domains did not change. (b,d) Proportion of misclassifications of C. biguttulus spike trains as stemming from the locust. (a,b) Male song as stimulus and (c,d) female song as stimulus. For SN3, there were too few recordings (2 and 1) to include this neuron in this kind of evaluation. Only few data points outside the uncertainty range show a significant deviation from the 50 : 50 chance expectation (see text for details).
As a second approach, we applied information-based clustering to the distance matrix (Slonim et al. 2005). This clustering algorithm maximizes the average similarity within all classes. The elements of our data sample were clustered into two classes in a way in which the elements within one class are as similar to each other as possible. If the intraspecific distances between spike trains were smaller than the interspecific distances, the C. biguttulus spike trains should be assigned to one class and the L. migratoria ones should fall into the other class. To verify this statistically, we performed a Monte Carlo analysis on the clustered spike trains. If we had, for example, recordings from five C. biguttulus neurons and six locust neurons, we choose randomly one spike train for each cell and applied Fisher–Yates exact probability test in order to test whether the distribution of this sample of 11 spike trains deviated from a chance distribution. We repeated this procedure 100 000 times to obtain a mean p value. Higher (1 000 000) and lower (50 000) numbers of repetitions yielded similar results.
3. Results
(a) Principle of analysis
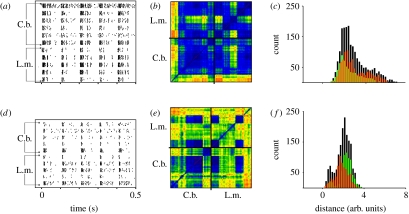
The evaluation scheme will be exemplified with the data of two interneurons, TN1 (figure 3a–c) and AN12 (figure 3d–f). TN1 is a tonically responding neuron whose arborizations are restricted to the thoracic and abdominal ganglia, while AN12 is a phasically responding neuron with an axon that ascends to the brain (Stumpner & Ronacher 1991; Stumpner et al. 1991). Figure 3a,d shows the raster plots of the spike responses to repeated presentations of the song of a C. biguttulus male (each dot represents a spike). Data stem from five to six specimens of TN1 and AN12 in C. biguttulus and L. migratoria, respectively. Figure 3b,e shows the respective spike train distances in a colour-coded two-dimensional matrix that contains the distances of each spike train to all other spike trains (note that in figure 3e, data of AN12, the colour code for distances is different from figure 3b). Comparing a spike train with itself yields a zero distance (figure 3b, dark purple points on the diagonal). The small squares along the diagonal (cf. ‘x’, figure 2c) represent the distances between the spike trains of one neuron. By their mostly dark colour, they indicate similar responses of a single cell to repeated presentations of the same stimulus. The large squares on the lower left and upper right (marked by black lines) represent the intraspecific comparisons, i.e. between the spike trains of two specimens of TN1 in C. biguttulus (or in L. migratoria, upper right), and are characterized by the occurrence of larger distances. The 5×6 block on the upper left (and symmetrical also on the lower right) visualizes the interspecific comparison, between the TN1 specimens of C. biguttulus compared with those of L. migratoria (cf. ‘z’ values in figure 2c). The spike train distances in this block are not distinctly larger than those between the neurons of C. biguttulus (lower left), or of L. migratoria (upper right).
Figure 3.
Comparison between spike trains of two neuron types, in two species: (a–c) TN1 and (d–f) AN12. (a,d) Raster plots of spike trains for the male song pattern as stimulus. (b,e) Corresponding spike train distances. (c,f) Distributions of spike train distances. (a) Data of six specimens of TN1 in C. biguttulus (C.b.) and five specimens in L. migratoria (L.m.; eight stimulus presentations per neuron). (b) Metric distances between all spike trains in a colour-coded diagram. Dark purple and blue indicate low distances (=high similarities), while yellow to orange indicate large distances (dissimilarities) between spike trains. The small squares represent the comparison between two cells (or, on the diagonal between the eight spike trains produced by one cell; cf. figure 2c); data points within the lower left large square marked by black lines represent comparisons between all TN1 specimens recorded in C. biguttulus, and those in the upper right square represent the intraspecific comparison for the locust. The 5×6 block on the upper left shows the comparison between the two species. Dark purple, 0; blue, 2; green, 4; orange, 6. (d,e) Same evaluation for the AN12 neuron as in (a,b). Dark purple, 0; blue, 1; green, 2; orange, 3. (c,f) Comparison of the distance distributions for C. biguttulus (orange) and L. migratoria (green), and for the interspecific comparison (black). See text for further explanations.
To allow for a quantitative comparison, we plotted all distance values (figure 3c,f), separately for C. biguttulus (orange), L. migratoria (green) and the interspecific comparison (black). Two important results can be read from these figures. (i) The two distributions of intraspecific distances do overlap to a large degree, indicating a similar variation in the responses of different specimens of a neuron type in the two species investigated. (ii) Most importantly, however, the distribution of interspecific distances (black) encompasses the two intraspecific distributions, and this was true for other neurons as well (data not shown). At first glance, this may appear as a trivial result, but this is not the case, as can be immediately seen when referring to a geographical example. The beeline distance between London and Oxford is approximately the same as that between Zurich and Basel; however, the distances between London and Zurich, or Oxford and Basel, are roughly 10-fold larger. Similarly, the distribution of interspecific spike train distances could be located at much higher values, even if the two intraspecific distributions overlap completely. The overlapping of the three distributions in figure 3c,f demonstrates a high similarity between the spike trains of the two species, for the neurons investigated. Notably, this result refers to the temporal patterns of spiking of the neurons, as the spike train dissimilarities were evaluated with a high temporal resolution (τ=5 ms), and thus the evaluation is sensitive to potential differences in the timing of spikes, not only to spike-count differences.
(b) The dependence of metric distances on differences in spike count
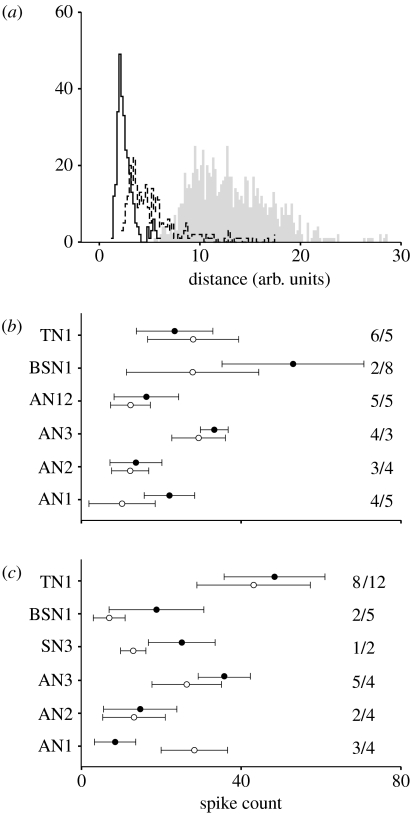
As can be inferred from figure 2b, the overall metric distance between two spike trains will depend not only on their temporal structure but also on the number of spikes present within the evaluation window. Hence, in general, the van Rossum distance depends on spike count, and large differences in overall spike count will cause high dissimilarity values. Since the spike rates of a neuron type were not always the same, even within one species (cf. figure 3a,d), spike-count differences might be a confounding factor that could compromise the comparison between species. The influence of large spike-count differences on the distance measure is quantified and visualized in figure 4a. We compared the spike trains produced by the locust's TN1, in response to a female C. biguttulus song pattern, for which we had recordings from 18 specimens that covered a range of spike rates, due to different stimulus intensities and differences in individual thresholds. We applied the evaluation procedure to a subsample of three specimens each, with the lowest (35±8 Hz, mean±s.d.) and highest spike rates (100±20 Hz). The distributions of distances between low spike-count trains, and those high spike-count trains show a partial overlap (figure 4a). By contrast, the distances between the spike trains with low and high spike count (grey) are shifted to much higher values, well beyond the centres of the other two distributions (note that these data were different from those shown in figure 3a–c, since the latter were obtained with the male song stimulus). This example, in which spike trains from a single neuron type within a single species were compared, shows first that the dissimilarity between the spike trains can be strongly influenced by large differences in spike count, whereas the comparison between species should focus on samples with roughly similar spike numbers. In addition, figure 4a shows the size of the expected shift of the ‘interspecific’ differences, if two samples differ considerably, and thus further illustrates the close correspondence in the distributions of distances in figure 3c,f.
Figure 4.
Influence of spike rate on the metric data. (a) Comparison of distance distributions for six specimens of the TN1 neuron in the locust that exhibited very different spike rates (low count sample, 35±8 Hz; high count sample, 100±20 Hz; mean±s.d.). Stimulus was the female song pattern, while in figure 3a the stimulus was the male song. Solid line, low spike-count data; dashed line, high spike-count data; grey area, comparison of the low- and high-rate ‘inter’ data. Note the shift in the grey distribution to higher distances. Spike rates of our samples for (b) male and (c) female songs as stimuli. Filled circles, data of C. biguttulus; open circles, data of L. migratoria (mean±s.d., based on single spike trains). The number of recorded cells in both species indicated in figures (N/M, C.b. first). On the x-axis, the spike counts in the 500 ms evaluation window are shown. Note that the differences in spike count between (b,c) are mainly due to differences in stimulus structure (figure 2a).
Based on these observations (figure 4a) and additional model calculations with Poisson spike trains (data not shown), we excluded from the analysis a few cells with strongly deviating spike rates, in an attempt to ensure that the mean spike count of one species lay within the standard deviation of the spike count of the other species (figure 4b,c). However, for some neuron types for which we had only a few recordings (SN3, BSN1, AN1), we decided to tolerate somewhat higher differences in spike count, in order to not restrict our sample of neurons too much. The ranges of spike count of our final samples of neurons are shown in figure 4b,c, for the tests with male and female songs, respectively. With the few exceptions mentioned above (BSN1, SN3, AN1), our dataset thus contained spike trains with roughly similar spike counts between species. In total, we excluded from the analysis, for the male song stimulus (C.b./L.m.): 0/3, TN1; 0/1, BSN1; 3/3, AN3; and 1/1, AN2; for the female song stimulus: 1/6, TN1 (figure 4a); 0/2, BSN1; 1/3, AN3; and 2/0, AN1. It should be emphasized, however, that the inclusion of these cells mainly added noise to the evaluation, but did not change the main conclusion.
(c) Interspecific comparison reveals unchanged coding properties
To quantitatively compare the spike trains of a neuron type between species, we performed two types of cluster analyses, both based on the spike train distances. First, we asked how many of the spike trains of L. migratoria neurons would be misclassified as stemming from C. biguttulus, and vice versa, if we assign the spike trains of one neuron type to two classes only (figure 5). The points in figure 5a show the percentage of L. migratoria (‘L.m.’) spike trains that were by their similarity values ‘erroneously’ classified as stemming from C. biguttulus. Figure 5b shows the percentage of C. biguttulus (‘C.b.’) spike trains that were classified to the group of L. migratoria. Figure 5c,d shows the respective data for tests with the female song model as stimulus. In the case of identical response patterns between the two species, one would expect values of approximately 50% misclassification. In particular, the L. migratoria spike trains approached or even exceeded this chance level (figure 5a), while for C. biguttulus misclassifications were in the range between 20 and 47%. For most neuron types, a chance performance becomes evident (points lying within the uncertainty range). There were only three exceptions, in which the percentage of misclassifications was outside the uncertainty range: BSN1 in figure 5a,b,d; and TN1 and AN1 in figure 5c. In the case of BSN1, it should be mentioned, however, that our sample for C. biguttulus consisted of only two neurons of this type. Furthermore, this neuron exists as twin neurons with somewhat different response types—one strongly phasic and the other phasic–tonic (Stumpner 1989)—which was probably the reason for the deviation visible in figure 5. Since for male songs AN1 showed a misclassification rate over 50%, we do not consider this single result as statistically relevant (similar for TN1 in figure 5c).
As a second approach, we used information-based clustering (Slonim et al. 2005) to test whether all spike trains of one cell type were assigned to two classes, according to the two species. Figure 6a,c illustrates the clustering results for AN3 (female song) and AN12 (male song), respectively. In this figure, the L. migratoria and the C. biguttulus spike trains are shown on the left hand side and right hand side, respectively, of the vertical dashed line. If significant interspecific differences exist, then the spike trains of the two species should be assigned separately to the two classes. However, the spike trains of one species are not assigned to only one class but are rather scattered between the two classes (figure 6a,c). To verify whether cells stemming from one species were significantly more often assigned to the same class, we applied a Monte Carlo analysis based on the Fisher–Yates exact test (see §2). The results are illustrated in figure 6b,d. For all tested cells, the mean p values (black circles) were above the p=0.05 level (for the female song, p=0.062–0.69; for the male song, p=0.16–0.40), indicating that the neuronal responses did not differ significantly between species. For some cell types (controls and male AN1 and SN3), no standard deviations are indicated since there was no intracellular variation in cluster assignment. The small horizontal bars indicate the achievable significance levels, which is an important control for the statistical power of our approach. Owing to the differences in sample size between the cells, these values may differ. Only for the SN3 and the AN2 (female; indicated as grey circles), had we not enough data points to achieve significant results. As a control, we applied this evaluation to two different cell types within one species (TN1 and BSN1 of L. migratoria for female song, and AN3 and AN12 of C. biguttulus for male song). These controls yielded highly significant differences (p<0.01 and 0.001, respectively), and thus demonstrate the principal power of this approach by showing that the spike trains of the different cell types were separately assigned to different classes based on the differences in their neuronal response.
Figure 6.
Information-based clustering. The assignment probabilities of AN3 spike trains stimulated with a (a) female song and of AN12 spike trains tested with a (c) male song. The two classes are indicated with grey (cluster 1) and black (cluster 2) dots, one for each spike train. On the x-axis, the number of spike trains is shown. Tick intervals indicate single specimens. The difference in tick intervals between (a,c) is due to different numbers of stimulus repetitions (10 and 8 repetitions, respectively). Vertical dashed red lines indicate the border between the two species, L. migratoria spike trains and the C. biguttulus, respectively, on the left hand side and right hand side (b,d) Mean p values (black circles) and standard deviation for all tested cells types as stemming from the female or male song. The achievable significance levels are indicated with short horizontal bars for each cell type. Control calculations for TN1 and BSN1 cells or AN3 and AN12 cells, respectively. Horizontal red lines, 0.05 significance level.
In summary, our analyses showed that the response properties of putatively homologous neurons do not differ more between the two species investigated than among different specimens within one species.
4. Discussion
A major goal of this study was to explore to what degree a sensory system might have adapted during evolution to the processing of a class of particularly relevant stimuli, the conspecific communication signals. To this aim we compared the thoracic auditory pathways between a species that relies heavily on acoustic communication, C. biguttulus, and a species that does not, L. migratoria.
We compared the coding properties of putatively homologous auditory neurons in these two not closely related species in a most rigorous way. The spike train metric of van Rossum (2001) has been used in different fields of neuroscience (e.g. Grewe et al. 2003; Machens et al. 2003; Narayan et al. 2007; Wohlgemuth & Ronacher 2007). This method to measure the similarity of spike trains yields a most critical test to uncover any differences in the information-bearing capacities of neurons. The main result of this study is that the homologous neurons are not only morphologically similar between the two species but also in their coding properties for acoustic signals. Our analysis reveals a high degree of conservation in this nervous system in spite of the fact that the two taxa, to which these species belong, split up more than 50 Myr ago (Flook & Rowell 1997), and that stridulation must have evolved independently in both groups, as the stridulatory apparatus are very different in the two taxa (Elsner 1983; Stumpner & von Helversen 2001).
However, before we explore the possible reasons for this evolutionary conservation of neuronal coding properties, first the problem of homology of neurons should be discussed.
(a) Putative homology of neurons
There has been quite some debate on the question of what should be considered as sufficient evidence for the homology of neurons across species. In a purist's view, it would be necessary to perform a lineage analysis and to trace back neurons to their, common, progenitor in the embryo, the ganglion mother cell (e.g. Boyan 1993). However, as in most cases, this technique is nearly impossible to apply with the methods available to date; in this paper, we take a pragmatic point of view and consider the auditory neurons as putatively homologous between species based on their highly similar morphology (figure 1), as has been done before by several authors. For example, Römer et al. (1988) considered the AN3 neuron in three different grasshopper species as homologous, due to their striking similarity with respect to soma position, branching patterns and course of the axon. Also, the omega neuron (ON1) has been homologized across cricket and bush cricket species, on the basis of its characteristic morphology and its exclusive use of the so-called omega tract located anterior to the acoustic neuropile (Zhantiev & Korsunovskaya 1983; Stumpner & von Helversen 2001; Molina & Stumpner 2005). The putative homology of neurons of C. biguttulus and L. migratoria is further substantiated by the finding of GABA-like immunoreactivity in the TN1 neuron of both species (Sokoliuk et al. 1989). Hence, at least for TN1, the physiological similarity is not restricted to the spike trains but extends also to the putative neurotransmitter, further justifying the treatment as homologous across species.
(b) Evolution of the auditory system: between adaptation and conservation of neuronal processing?
Remember that our stimuli were chosen in order to represent highly relevant signals, containing features that are crucial for recognition of species and sex, for C. biguttulus only. Thus, for C. biguttulus, the demands of acoustic communication constitute an additional, sexual, selection pressure upon the processing of these signals, which in this form does not exist for L. migratoria. However, these ‘meaningless’ stimuli were processed by the locust's auditory system in exactly the same, and quantitatively undistinguishable, manner as by C. biguttulus. For another auditory interneuron, the AN4, similar gap-detection properties in C. biguttulus and L. migratoria have been described earlier (Ronacher & Stumpner 1988).
What could be the reason for this strong conservation of encoding properties, and the corresponding lack of any obvious adaptations of the thoracic auditory pathway to specific requirements for the processing of communication signals? We must conclude that other stabilizing selective forces exist, which prevented an adaptation to the specific demands of acoustic communication, the most likely candidate being predator avoidance. Indeed, it is thought that grasshoppers ears evolved to assist predator avoidance (Stumpner & von Helversen 2001), and that intraspecific communication developed much later, making use of an existing auditory system (cf. also Riede 1987). Indeed, evasive steering during flight in response to high-frequency sounds is documented and has been interpreted as bat avoidance for L. migratoria (Robert 1989; Dawson et al. 2004), as well as for other insects (see Hoy 1992; Fullard 1998; Faure & Hoy 2000; Marsat & Pollack 2006). However, even though C. biguttulus also possess high-frequency receptors (tuned to more than 20 kHz), nowadays bat avoidance is unlikely to be important since this species is diurnal and does not fly over large distances.
In acridid grasshoppers, a large set of different auditory interneurons has been described, approximately 10–15 types of local neurons, and at least 15–20 ascending interneurons (C. biguttulus: Stumpner 1988, Stumpner & Ronacher 1991; locusts: Römer & Marquart 1984, Marquart 1985, Stumpner 1988, Baader 1991). One might argue that for some primary-like local interneurons, it is not surprising to find no differences in coding properties between the two species. Perhaps the basic function of a cell such as TN1 is merely to translate tonic excitation into tonic inhibition, while maintaining the precise temporal structure of all kind of signals in its spike pattern. Nevertheless, one could imagine that among the set of ascending neurons at least some neuron types could have been freed from the constraints once imposed by predator detection and could have shifted their encoding properties to optimize the processing of species-specific communication signals. Since we have investigated at least two ascending neurons that are supposed to be involved in processing specific song features of C. biguttulus (AN12: Stumpner et al. 1991; for the interspecific comparison of AN4, see Ronacher & Stumpner 1988), and two involved in directional hearing (AN1 and AN2: Stumpner & Ronacher 1994), it seems, however, unlikely that at this processing stage, species-specific adaptations have occurred. However, we want to emphasize that within the supraoesophageal ganglion, the probable site of species-specific recognition filters, there must exist auditory neurons that respond selectively to species-specific (and sex-specific) song features.
This appears to be different for Ensifera, in which hearing and acoustic signalling probably evolved from vibratory communication and was tightly coupled to the context of communication and mate finding (e.g. Otte 1992; von Helversen & von Helversen 1995; Stumpner & von Helversen 2001). In two cricket species, there is evidence for an adaptation of neuronal coding properties to signal parameters. The omega neuron (ON1) of Gryllus texensis appears to be adapted to the relatively high pulse rates this species produce in their songs, while the properties of ON1 of Teleogryllus oceanicus are in accord with the lower pulse rates of its songs (Tunstall & Pollack 2005). This observation underlines our conclusion that in grasshoppers there must have been stabilizing selective forces that kept the peripheral auditory system of the Gomphocerinae and the Oedipodinae in a quasi-‘encapsulated’ state during millions of years, in spite of additional selective forces imposed by communication (cf. also Fullard et al. 2007; Lehmann et al. 2007). Probably, the main function of the thoracic auditory neurons is to extract and provide salient cues for more central processing levels. A common feature of all gomphocerine songs is their pulsed structure. This might be a consequence of the general preference of some interneurons such as AN3 and AN12 (Stumpner & Ronacher 1991) for pulsed rather than homogeneous stimuli (von Helversen & von Helversen 1994; Krahe et al. 2002). We conclude that the communication signals of gomphocerine grasshoppers have evolved in a way to optimally exploit the pre-existing processing capacities of the (thoracic) auditory pathway rather than vice versa (sensory bias hypothesis; see Ryan et al. 1990).
With a different approach on efficient coding of sound in vertebrates, Lewicki came to a similar conclusion: ‘Efficient representation of speech is nearly identical to that of natural sounds combined with vocalizations, suggesting an evolutionary adaptation of speech to make maximally efficient use of the coding properties of a prelinguistic auditory system’ (Lewicki 2002, p. 361). The present study, therefore, also has implications for claims along the ‘efficient coding hypothesis’ presented recently for grasshoppers: ‘that the coding strategies of sensory neurons are heavily influenced by differences in behavioural relevance among natural stimuli’ (Machens et al. 2005). Our comparison between two not closely related species, in stark contrast, indicates that essential differences in behavioural relevance did not lead to changes in the coding strategies of thoracic auditory neurons. The conservation of processing features at this peripheral stage is probable due to enduring, so far unknown, selective forces that act in similar ways in both species.
Acknowledgments
The experimental protocol complied with German laws governing animal care.
We thank Christian Machens for providing the spike train metric programs, and Matthias Hennig for discussions and helpful comments on the manuscript. This work was supported by grants to D.N. (Doc-FFORTE from the Austrian Academy of Science) and B.R. (DFG: SFB 618).
References
- Baader A. Auditory interneurons in locusts produce directional head and abdomen movements. J. Comp. Physiol. A. 1991;169:87–100. doi:10.1007/BF00198175 [Google Scholar]
- Bailey N.W, Gwynne D.T, Bailey W.V, Ritchie M.G. Multiple differences in calling songs and other traits between solitary and gregarious Mormon crickets from allopatric mtDNA clades. BMC Evol. Biol. 2007;7:5. doi: 10.1186/1471-2148-7-5. doi:10.1186/1471-2148-7-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H.B. Possible principles underlying the transformation of sensory messages. In: Rosenblith W.A, editor. Sensory communication. MIT Press; Cambridge, MA: 1961. pp. 217–234. [Google Scholar]
- Bentley D.R, Hoy R.R. Genetic control of the neuronal network generating cricket (Teleogryllus gryllus) song patterns. Anim. Behav. 1972;20:478–492. doi: 10.1016/s0003-3472(72)80012-5. doi:10.1016/S0003-3472(72)80012-5 [DOI] [PubMed] [Google Scholar]
- Boyan G.S. Another look at insect audition: the tympanic receptors as an evolutionary specialization of the chordotonal system. J. Insect Physiol. 1993;39:187–200. doi:10.1016/0022-1910(93)90088-9 [Google Scholar]
- Bugrov A, Novikova O, Mayorov V, Adkinson L, Blinov A. Molecular phylogeny of Palaearctic genera of Gomphocerinae grasshoppers (Orthoptera, Acrididae) Syst. Entomol. 2006;31:362–368. doi:10.1111/j.1365-3113.2005.00317.x [Google Scholar]
- Carpenter, F. M. 1992 Superclass Hexapoda. In Treatise on invertebrate paleontology. Part R. Arthropoda 4, vols 3 and 4 (ed. R. L. Kaesler), pp. 1–655. Boulder, CO: Geological Society of America.
- Dawson J.W, Kutsch W, Robertson R.M. Auditory-evoked evasive manoeuvres in free-flying locusts and moths. J. Comp. Physiol. A. 2004;190:69–84. doi: 10.1007/s00359-003-0474-3. doi:10.1007/s00359-003-0474-3 [DOI] [PubMed] [Google Scholar]
- Elsner N. A neuroethological approach to the phylogeny of leg stridulation in gomphocerine grasshoppers. In: Huber F, Markl H, editors. Neuroethology and behavioural physiology. Springer; Berlin, Germany; New York, NY: 1983. pp. 54–68. [Google Scholar]
- Faure P.A, Hoy R.R. The sounds of silence: cessation of singing and song pausing are ultrasound-induced acoustic startle behaviours in the katydid Neoconocephalus ensiger (Orthoptera, Tettigoniidae) J. Comp. Physiol. A. 2000;186:129–142. doi: 10.1007/s003590050013. doi:10.1007/s003590050013 [DOI] [PubMed] [Google Scholar]
- Flook P.K, Rowell C.H.F. The phylogeny of the Caelifera (Insecta, Orthoptera) as deduced from mtrRNA gene sequences. Mol. Genet. Evol. 1997;8:89–103. doi: 10.1006/mpev.1997.0412. doi:10.1006/mpev.1997.0412 [DOI] [PubMed] [Google Scholar]
- Franz A, Ronacher B. Temperature dependence of time resolution in an insect nervous system. J. Comp. Physiol. A. 2002;188:261–271. doi: 10.1007/s00359-002-0298-6. doi:10.1007/s00359-002-0298-6 [DOI] [PubMed] [Google Scholar]
- Fullard J.H. The sensory coevolution of moths and bats. In: Hoy R.R, Popper A.N, Fay R.R, editors. Comparative hearing: insects. Springer; New York, NY: 1998. pp. 279–326. [Google Scholar]
- Fullard J.H, Ratcliffe J.M, ter Hofstede H. Neural evolution in the bat-free habitat of Tahiti: partial regression in an anti-predator auditory system. Biol. Lett. 2007;3:26–28. doi: 10.1098/rsbl.2006.0550. doi:10.1098/rsbl.2006.0550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt H.C, Huber F. University of Chicago Press; Chicago, IL; London, UK: 2002. Acoustic communication in insects and anurans. [Google Scholar]
- Gottsberger B, Mayer F. Behavioral sterility of hybrid males in acoustically communicating grasshoppers (Acrididae, Gomphocerinae) J. Comp. Physiol. A. 2007;193:703–714. doi: 10.1007/s00359-007-0225-y. doi:10.1007/s00359-007-0225-y [DOI] [PubMed] [Google Scholar]
- Grewe J, Kretzberg J, Warzecha A.-K, Egelhaaf M. Impact of photon noise on the reliability of a motion-sensitive neuron in the fly's visual system. J. Neurosci. 2003;23:10 776–10 783. doi: 10.1523/JNEUROSCI.23-34-10776.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwynne D.T. A phylogeny of Ensifera (Orthoptera): a hypothesis supporting multiple origins of acoustical signalling, complex spermatophores and maternal care in crickets, katydids and weta. J. Orthoptera Res. 1995;4:203–218. doi:10.2307/3503478 [Google Scholar]
- Haskell P.T. The influence of flight noise on behaviour in the desert locust Schistocerca gregaria (Forsk.) J. Insect Physiol. 1957;1:52–75. doi:10.1016/0022-1910(57)90023-9 [Google Scholar]
- Henry C.S. Singing and cryptic speciation in insects. Trends Ecol. Evol. 1994;9:388–392. doi: 10.1016/0169-5347(94)90061-2. doi:10.1016/0169-5347(94)90061-2 [DOI] [PubMed] [Google Scholar]
- Hoy R.R. Evolutionary innovation in behaviour and speciation: opportunities for behavioural neuroethology. Brain Behav. Evol. 1990;36:141–153. doi: 10.1159/000115303. doi:10.1159/000115303 [DOI] [PubMed] [Google Scholar]
- Hoy R.R. The evolution of hearing in insects as an adaptation to predation from bats. In: Webster D.B, Fay R.R, Popper A.N, editors. The evolutionary biology of hearing. Springer; New York, NY: 1992. pp. 115–129. [Google Scholar]
- Hoy R.R, Popper A.N, Fay R.R. Springer; New York, NY: 1998. Comparative hearing: insects. [Google Scholar]
- Jacobs W. Verhaltensbiologische Studien an Feldheuschrecke. Z. Tierpsychol. Beiheft. 1953;1:1–228. [Google Scholar]
- Krahe R, Budinger E, Ronacher B. Coding of a sexually dimorphic song feature by auditory interneurons of grasshoppers: the role of leading inhibition. J. Comp. Physiol. A. 2002;187:977–985. doi: 10.1007/s00359-001-0268-4. doi:10.1007/s00359-001-0268-4 [DOI] [PubMed] [Google Scholar]
- Kriegbaum H. Female choice in the grasshopper Chorthippus biguttulus: mating success is related to song characteristics of the male. Naturwissenschaften. 1989;76:81–82. doi:10.1007/BF00396715 [Google Scholar]
- Lehmann G.U.C, Strauß J, Lakes-Harlan R. Listening when there is no sexual signalling? Maintenance of hearing in the asexual bushcricket Poecilimon intermedius. J. Comp. Physiol. A. 2007;193:537–545. doi: 10.1007/s00359-007-0209-y. doi:10.1007/s00359-007-0209-y [DOI] [PubMed] [Google Scholar]
- Lewicki M.S. Efficient coding of natural sounds. Nat. Neurosci. 2002;5:356–363. doi: 10.1038/nn831. doi:10.1038/nn831 [DOI] [PubMed] [Google Scholar]
- Machens C.K, Schütze H, Franz A, Stemmler M.B, Ronacher B, Herz A.V.M. Auditory receptor neurons preserve characteristic differences between conspecific communication signals. Nat. Neurosci. 2003;6:341–342. doi: 10.1038/nn1036. doi:10.1038/nn1036 [DOI] [PubMed] [Google Scholar]
- Machens C.K, Gollisch T, Kolesnikova O, Herz A.V.M. Testing the efficiency of sensory coding with optimal stimulus ensembles. Neuron. 2005;47:447–456. doi: 10.1016/j.neuron.2005.06.015. doi:10.1016/j.neuron.2005.06.015 [DOI] [PubMed] [Google Scholar]
- Marquart, V. 1985 Auditorische Interneurone im thorakalen Nervensystem von Heuschrecken: Morphologie, Physiologie und synaptische Verbindungen. PhD thesis, Universität Bochum.
- Marsat G, Pollack G.S. A behavioral role for feature detection by sensory bursts. J. Neurosci. 2006;26:10 542–10 547. doi: 10.1523/JNEUROSCI.2221-06.2006. doi:10.1523/JNEUROSCI.2221-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier T, Reichert H. Embryonic development and evolutionary origin of the Orthopteran auditory organs. J. Neurobiol. 1990;21:592–610. doi: 10.1002/neu.480210407. doi:10.1002/neu.480210407 [DOI] [PubMed] [Google Scholar]
- Molina J, Stumpner A. Effects of pharmacological treatment and photoinactivation on the directional responses of an insect neuron. J. Exp. Zool. A. 2005;303:1085–1103. doi: 10.1002/jez.a.228. doi:10.1002/jez.a.228 [DOI] [PubMed] [Google Scholar]
- Narayan R, Best V, Ozmeral E, McClaine E, Dent M, Shinn-Cunningham B, Sen K. Cortical interference effects in the cocktail party problem. Nat. Neurosci. 2007;10:1601–1607. doi: 10.1038/nn2009. doi:10.1038/nn2009 [DOI] [PubMed] [Google Scholar]
- Otte D. Evolution of cricket songs. J. Orthoptera Res. 1992;1:25–46. doi:10.2307/3503559 [Google Scholar]
- Pflüger H.J, Field L.H. A locust chordotonal organ coding for proprioceptive and acoustic stimuli. J. Comp. Physiol. A. 1999;184:169–183. doi:10.1007/s003590050316 [Google Scholar]
- Riede K. A comparative study of mating behaviour in some neotropical grasshoppers (Acridoidea) Ethology. 1987;76:265–296. [Google Scholar]
- Robert D. The auditory behaviour of flying locusts. J. Exp. Biol. 1989;147:279–301. [Google Scholar]
- Römer H, Marquart V. Morphology and physiology of auditory interneurons in the metathoracic ganglion of the locust. J. Comp. Physiol. A. 1984;155:249–262. doi:10.1007/BF00612642 [Google Scholar]
- Römer H, Marquart V, Hardt M. Organization of a sensory neuropile in the auditory pathway of two groups of Orthoptera. J. Comp. Neurol. 1988;275:201–215. doi: 10.1002/cne.902750204. doi:10.1002/cne.902750204 [DOI] [PubMed] [Google Scholar]
- Ronacher B, Stumpner A. Filtering of behaviourally relevant temporal parameters of a grasshopper song by an auditory interneuron. J. Comp. Physiol. A. 1988;163:517–523. doi:10.1007/BF00604905 [Google Scholar]
- Ronacher B, Franz A, Wohlgemuth S, Hennig H. Variability of spike trains and the processing of temporal patterns of acoustic signals: problems, constraints, and solutions. J. Comp. Physiol. A. 2004;190:257–277. doi: 10.1007/s00359-004-0494-7. doi:10.1007/s00359-004-0494-7 [DOI] [PubMed] [Google Scholar]
- Ryan M.J, Fox J.H, Wilczynski W, Rand A.S. Sexual selection for sensory exploitation in the frog Physalaemus pustulosus. Nature. 1990;343:66–67. doi: 10.1038/343066a0. doi:10.1038/343066a0 [DOI] [PubMed] [Google Scholar]
- Slonim N, Atwal G.S, Tkacik G, Bialek W. Information-based clustering. Proc. Natl Acad. Sci. USA. 2005;102:18 297–18 302. doi: 10.1073/pnas.0507432102. doi:10.1073/pnas.0507432102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoliuk T, Stumpner A, Ronacher B. GABA-like immunoreactivity suggests an inhibitory function of the thoracic-low-frequency-neuron (TN1) in grasshoppers. Naturwissenschaften. 1989;76:223–225. doi:10.1007/BF00627695 [Google Scholar]
- Stumpner, A. 1988 Auditorische thorakale Interneurone von Chorthippus biguttulus L: Morphologische und physiologische Charakterisierung und Darstellung ihrer Filtereigenschaften für verhaltensrelevante Lautattrappen. PhD thesis, Universiät Erlangen-Nürnberg.
- Stumpner A. Physiological variability of auditory neurons in a grasshopper. Naturwissenschaften. 1989;76:427–429. doi:10.1007/BF00366170 [Google Scholar]
- Stumpner A, Ronacher B. Auditory interneurons in the metathoracic ganglion of the grasshopper Chorthippus biguttulus 1. Morphological and physiological characterization. J. Exp. Biol. 1991;158:391–410. [Google Scholar]
- Stumpner A, Ronacher B. Neurophysiological aspects of song pattern recognition and sound localization in grasshoppers. Am. Zool. 1994;34:696–705. doi:10.1093/icb/34.6.696 [Google Scholar]
- Stumpner A, von Helversen D. Evolution and function of auditory systems in insects. Naturwissenschaften. 2001;88:159–170. doi: 10.1007/s001140100223. doi:10.1007/s001140100223 [DOI] [PubMed] [Google Scholar]
- Stumpner A, Ronacher B, von Helversen O. Auditory interneurons in the metathoracic ganglion of the grasshopper Chorthippus biguttulus 2. Processing of temporal patterns of the song of the male. J. Exp. Biol. 1991;158:411–430. [Google Scholar]
- Tunstall D.N, Pollack G.S. Temporal and directional processing by an identified interneuron, ON1, compared in cricket species that sing with different tempos. J. Comp. Physiol. A. 2005;191:363–372. doi: 10.1007/s00359-004-0591-7. doi:10.1007/s00359-004-0591-7 [DOI] [PubMed] [Google Scholar]
- van Rossum M. A novel spike distance. Neural Comput. 2001;13:751–763. doi: 10.1162/089976601300014321. doi:10.1162/089976601300014321 [DOI] [PubMed] [Google Scholar]
- van Staaden M, Römer H. Evolutionary transition from stretch to hearing organs in ancient grasshoppers. Nature. 1998;394:773–776. doi:10.1038/29517 [Google Scholar]
- Vedenina V.Y, von Helversen O. Complex courtship in a bimodal grasshopper hybrid zone. Behav. Ecol. Sociobiol. 2003;54:44–54. doi:10.1007/s00265-003-0595-2 [Google Scholar]
- von Helversen D, von Helversen O. Verhaltensgenetische Untersuchungen am akustischen Kommunikationssystem der Feldheuschrecken (Orthoptera, Acrididae). I Der Gesang von Artbastarden zwischen Chorthippus biguttulus und C. mollis. J. Comp. Physiol. A. 1975;104:273–299. doi:10.1007/BF01379053 [Google Scholar]
- von Helversen O, von Helversen D. Forces driving coevolution of song and song recognition in grasshoppers. In: Schildberger K, Elsner N, editors. Neural basis of behavioural adaptations. G. Fischer Verlag; Stuttgart, Germany: 1994. pp. 253–284. [Google Scholar]
- von Helversen D, von Helversen O. Acoustic pattern recognition and orientation in orthopteran insects: parallel or serial processing. J. Comp. Physiol. A. 1995;177:767–774. doi:10.1007/BF00187635 [Google Scholar]
- Wohlgemuth S, Ronacher B. Auditory discrimination of amplitude modulations based on metric distances of spike trains. J. Neurophysiol. 2007;97:3082–3092. doi: 10.1152/jn.01235.2006. doi:10.1152/jn.01235.2006 [DOI] [PubMed] [Google Scholar]
- Yager D.D. Structure, development, and evolution of insect auditory systems. Microsc. Res. Tech. 1999;47:380–400. doi: 10.1002/(SICI)1097-0029(19991215)47:6<380::AID-JEMT3>3.0.CO;2-P. doi:10.1002/(SICI)1097-0029(19991215)47:6<380::AID-JEMT3>3.0.CO;2-P [DOI] [PubMed] [Google Scholar]
- Zhantiev R.D, Korsunovskaya O.S. Structure and functions of two auditory neurons in the bush cricket Tettigonia cantans Fuess. (Orthoptera, Tettigoniidae) Revue d' Entomologie de L'URSS. 1983;62:462–469. [Google Scholar]