Abstract
Leks are classic models for studies of sexual selection due to extreme variance in male reproductive success, but the relative influence of intrasexual competition and female mate choice in creating this skew is debatable. In the lekking lance-tailed manakin (Chiroxiphia lanceolata), these selective episodes are temporally separated into intrasexual competition for alpha status and female mate choice among alpha males that rarely interact. Variance in reproductive success between status classes of adult males (alpha versus non-alpha) can therefore be attributed to male–male competition whereas that within status largely reflects female mate choice. This provides an excellent opportunity for quantifying the relative contribution of each of these mechanisms of sexual selection to the overall opportunity for sexual selection on males (Imales). To calculate variance in actual reproductive success, we assigned genetic paternity to 92.3% of 447 chicks sampled in seven years. Reproduction by non-alphas was rare and apparently reflected status misclassifications or opportunistic copulations en route to attaining alpha status rather than alternative mating strategies. On average 31% (range 7–44%, n=6 years) of the total Imales was due to variance in reproductive success between alphas and non-alphas. Similarly, in a cohort of same-aged males followed for six years, 44–58% of the total Imales was attributed to variance between males of different status. Thus, both intrasexual competition for status and female mate choice among lekking alpha males contribute substantially to the potential for sexual selection in this species.
Keywords: sexual selection, reproductive skew, lek, cooperation, mate choice, male–male competition
1. Introduction
Lek mating systems provide some of the best empirical models for studies of sexual selection (Höglund & Alatalo 1995). On a lek, females visit male display territories solely for the purpose of mating, and males provide nothing but genes to the resulting offspring (Bradbury 1981). In lek mating systems, the opportunity for sexual selection is thought to be particularly high, because reproductive success among displaying males on leks is generally highly skewed: a small number of males are apparently responsible for the majority of matings.
Sexual selection can arise from male–male competition, female choice or a combination of the two (Darwin 1871). The relative importance of these processes in determining mating success affects male reproductive strategies and may vary among species or in relation to ecological circumstances within species or populations (Bradbury & Davies 1987). Both male competition and female choice have been identified as creating skew in male mating success on leks (Borgia 1985; Pruett-Jones & Pruett-Jones 1990; Gibson 1996). However, the importance of male competition may often have been underestimated in lek mating systems because comparisons usually consider only males observed displaying on lekking grounds (Höglund & Alatalo 1995). When males compete to obtain display sites or status, the distinction between displaying and non-displaying males can be viewed as the outcome of male–male competition for time at the lek. If the analysis of sexual selection is restricted to males that have already obtained a position in the lek, a potentially important component of sexual selection, namely that due to male competition to obtain a display site, is a priori neglected (Koenig & Albano 1986).
The relative strength of intra- and intersexual selection classically has been tested by identifying variables correlated with mating success, and then experimentally determining their influence on breeding females or male competitors (Arak 1983; Johnson 1988; Benson & Basolo 2006). However, the potential for evolutionary change can more generally be quantified via the opportunity for selection, I, which is the variance in relative fitness (Arnold & Wade 1984b). In polygynous mating systems, the opportunity for sexual selection is usually calculated as Imales; the variance in male reproductive success divided by the squared mean of male reproductive success (Arnold & Wade 1984b; Shuster & Wade 2003). This measure quantifies the potential for selection to operate, separate from the evolutionary response to selective pressure and without requiring precise knowledge of the mechanism by which selection occurs (Arnold & Wade 1984a; Shuster & Wade 2003).
We examined the relative strength of inter- and intrasexual selection on an exploded lek of lance-tailed manakins (Chiroxiphia lanceolata). Leks in this species comprise assemblages of alpha males, each of which may be assisted in paired courtship displays by beta helpers that do not mate with females that the pair attracts (DuVal 2007a,b). Male lance-tailed manakins exhibit delayed plumage maturation, first displaying adult plumage in the breeding season of their fourth year after hatching (DuVal 2005). Most males with adult plumage do not hold any defined social status, but may nevertheless participate in duet song and dance displays when no females are present. Some of these adult males subsequently enter into long-term social alliances with dominant individuals, and these alpha and beta partners perform duet songs and cooperative courtship displays for females in traditional display areas. Alpha males are distinguished from other males in the population by consistent presence at display areas, performance of solo courtship displays for females and distinctive vocalizations during paired displays (DuVal 2007c).
In many lekking species, competition among territory holders continues on the lek and may directly affect female mate choice (e.g. Trail 1985). By contrast, alpha male lance-tailed manakins rarely interact with each other, and social status is established long before female visitation. Alphas' display areas are dispersed through available habitat to form an exploded lek of alpha males; alphas are in auditory but not visual contact with other displaying alphas. Females observe courtship displays by flying among several dispersed alpha males (E. H. DuVal 2002, 2007, unpublished data). This separation of inter- and intrasexual selection is underscored by a study of congeneric long-tailed manakins (Chiroxiphia linearis) showing that early interactions among males predict social status nearly five years later (McDonald 2007). In lance-tailed manakins, sexual selection can therefore be thought of as occurring in two distinct episodes: male–male competition to obtain alpha status and female mate choice among displaying alpha males.
A previous study of cooperative behaviour by beta male lance-tailed manakins showed that betas rarely sire young during their beta tenure (DuVal 2007a). In that study, however, fathers were identified for only 29% of sampled offspring, and only alpha or beta males were considered as possible sires. If males that were neither alpha nor beta sired the many unassigned offspring, our understanding of the mating system would be drastically changed. Therefore, a key goal of the present study was to expand genetic paternity analyses to comprehensively address paternity of chicks in the study population and examine the prevalence of cryptic reproductive strategies.
The temporal separation of status acquisition and female choice in lance-tailed manakins creates an ideal situation in which to quantify separately the opportunity for sexual selection from male competition and female mate choice. This separation allows total variance in fitness among males due to sexual selection (Imales) to be partitioned into two components that represent distinct selective episodes. The variance in mating success within status (Iwithin-status; mostly within the class of alpha males) will be largely reflective of female mate choice, whereas the variance in mating success between the classes of alpha and non-alpha males (Ibetween-status) will reflect the combination of male competition and survival-based (natural) selection.
We combined genetic measures of paternity with behavioural observations of male status in a seven-year study of wild lance-tailed manakins to quantify (i) the prevalence of cryptic reproductive strategies, (ii) the distribution of reproductive success among males in the population within and across years and (iii) the relative opportunity for sexual selection from male–male competition and female mate choice.
2. Material and methods
(a) Study species and field protocols
We studied a wild population of lance-tailed manakins on 46 ha of Isla Boca Brava, an approximately 3000 ha island in Chiriquí, Panamá (8°12′ N, 82°12′ W). Using mistnets, we captured a total of 618 individuals from 1999 to 2006 during 3256 mistnet hours (1.12 m net open for 1 hour). All birds were colour banded with unique band combinations, and approximately 20 μl of blood were taken from the brachial wing vein and stored in lysis buffer. Male age was determined by prior capture in predefinitive plumage, or a minimum age was assigned for individuals first captured in adult plumage (DuVal 2005). Male status was determined by behavioural observations of presence, duet singing and display interactions of banded males at display sites. Detailed descriptions of observation protocols and the quantitative method of defining male status are provided elsewhere (DuVal 2007c). Behavioural observations and nest searching began in March of each year and lasted until July in 2000–2002, May in 2003, April in 2004 and late June in 2005–2006, capturing the peak of lance-tailed manakin breeding activity on this site (DuVal 2007b).
Nests were located by daily searches of understorey vegetation and monitored every other day until they fledged or failed. Clutches consisted of no more than two eggs (DuVal 2007a). To maximize genetic sampling of chicks despite high nest predation, small blood samples (approx. 5 μl) were taken on day 2 after hatching; larger samples (approx. 20 μl) were drawn when chicks were banded on day 12–14. Nevertheless, 33.3–62.7% of active nests found each year failed before sampling. We assume that this nest failure occurred randomly and does not bias our results. The adult female attending a nest was assigned as the female parent of the chicks in that nest. In total, 447 chicks were genetically sampled and mothers were identified for 435 of these chicks. In every case, mothers shared at least one allele at each typed locus with the chicks in their nests, indicating that conspecific brood parasitism did not occur. To determine the distances females travelled between nests and mates, we recorded locations of nests and of the main display perch on which each alpha male performed courtship displays, using a handheld GPS unit.
(b) DNA isolation and microsatellite genotyping
Genomic DNA was extracted from samples using the Dneasy tissue kit (QUIAGEN, Inc.) or GFX Genomic Blood DNA purification kit (GE Healthcare). DNA was amplified from 20 variable microsatellite loci in multiplexed PCRs that included 5–20 ng DNA and four fluorescently labelled primer pairs (DuVal 2007a; DuVal et al. 2007). PCR products were visualized on an ABI 3110 genotyper (Applied Biosystems), with one negative and two positive controls included in each genotyper run. Alleles scored were using Genemapper v. 3.7 (Applied Biosystems), and ambiguous samples rerun until a consensus allele call was reached.
The 20 microsatellite loci used in this study amplified 2–14 alleles per locus (mean±s.d.=7.0±3.3 alleles, 1026 individuals typed). These loci had a combined second-parent exclusion probability of Pre=0.999986, and Pre=0.997942 when no maternal information was available.
(c) Paternity analyses
Paternity was analysed using the maximum-likelihood program Cervus v. 2.0 (Marshall et al. 1998). Fathers were assigned to chicks only if, given the mother's genotype, fathers mismatched the target chick at less than one locus, and the candidate father was assigned as the most likely father with higher than 95% confidence. This confidence level represents the difference in log-likelihood scores of the most likely and next most likely candidate father, based on simulation (10 000 cycles), given the number of candidate fathers in each year and locus-specific population allele frequencies (Marshall et al. 1998).
We first analysed paternity considering all live males as candidate fathers in each year, regardless of age (n=144–177 candidates per year). Live males were defined as males captured or observed in a given year, or in years before and after the target analysis year (i.e. males that were alive though not directly observed in the target year were included). Paternity was assigned only when chicks genotypically matched no more than one male, resulting in paternity assignment for 83.7% of 435 chicks. Only 1 (0.3%) of 364 assigned chicks was assigned to a male in subadult plumage, supporting behavioural observations that suggest young males generally do not mate. Furthermore, the tenure of successful alphas probably includes several years in which their sons are also present in the population; this exceptional case of a subadult siring offspring occurred in the first year of the study and it thus seems most likely that the young male's father was the actual sire but was unsampled. The final paternity analysis therefore considered as candidate sires all adult-plumage males alive in the population in each year.
Paternity was determined as described above, but was also assigned when (i) a chick perfectly matched its nest-mate's sire, but was previously unassigned owing to low statistical confidence in this match and (ii) a chick matched more than one male perfectly, but could be assigned to one candidate father with higher than 95% confidence. The second scenario allowed the assessment of paternity for chicks with unknown maternal ID (mother not caught), which always matched several males completely (n=12 chicks). We tested the accuracy of assignment in the absence of maternal information by reassessing the paternity of assigned chicks from 2006 (n=89 chicks) when known maternal information was withheld. Assignments made without maternal information matched previous paternity assignments in 62 of 66 cases (94% of chicks assigned with higher than 95% confidence), suggesting that paternity could be accurately assigned when female identity was not available.
Mistnetting effort in each year targeted unbanded adult males, and so the proportion of candidate males sampled was estimated as the number of adult males identified in the target year divided by that number plus the number of unbanded adult males captured in the following year (0.83 in 2000; 0.95–0.99 in other years of the study). As this estimate is affected by variability in netting effort and because some males may have evaded capture for multiple years, we defined the proportion of sampled candidates in Cervus as 0.90 in all years except 2000, for which we used 0.83. The proportion of loci typed was 0.997, and the genotyping error rate was 0.01, based on empirical error estimates calculated for nine of the loci involved (DuVal 2007a). To determine whether unassigned chicks could substantially affect our estimates of the distribution of reproductive success (i.e. whether one male sired all unassigned chicks), we used the program DadShare (www.zoo.cam.ac.uk/zoostaff/amos; Hoffman et al. 2003) to infer paternal sibships from genotypes of mother–offspring pairs of chicks that could not be assigned to known fathers. This program graphically estimates sibship groups by creating a clustering dendrogram based on pairwise relatedness coefficients (Queller & Goodnight 1989).
(d) Opportunity for sexual selection
Female lance-tailed manakins attend courtship displays of multiple alpha males and paternity analyses showed that by far, the majority of reproducing males were alphas, but alpha status itself is determined through male–male interactions that occur long before females observe male displays. We therefore considered Imales in lance-tailed manakins to consist of the opportunity for selection within alpha males (Iwithin-status, reflecting female mate choice) and that between alpha and non-alpha males (Ibetween-status, reflecting male competition for status). To estimate these components of Imales, we calculated yearly variance in reproductive success (number of sired offspring) within and between male status classes, following Shuster & Wade (2003). The variance in reproductive success within status classes equals the average of the variances for each status class, so that Vwithin-status=ΣpiVi, where pi is the proportion of males belonging to the ith status class and Vi is the variance in number of offspring sired for the ith status class. The variance in reproductive success between status classes equals the variance in the average fitness for each status class, so that Vbetween-status=Σpi(Y−Yi)2, where pi is the proportion of males belonging to the ith status class, Y is the average number of offspring sired across all males and Yi is the average number of offspring sired for males of the ith status class. These variance components were then used to calculate Ibetween-status and Iwithin-status, respectively. This approach recognizes that, at least in some years, the observed variance in reproductive success was non-zero within non-alpha males; within-status variance estimates therefore include variance in reproductive success of alphas and non-alphas rather than only that within alphas. Data were from all adult males alive in each year of the study, including males with no detected reproductive success (no chicks sired). Because our goal was to quantify the opportunity for sexual selection on males and because both genetic analyses (see above) and behavioural observations suggest that males with subadult plumage do not reproduce, we excluded subadults from these analyses.
Note that the tendency for males to have reproductive tenures lasting multiple years is likely to make any single-year estimate of sexual selection conservative. To determine whether cross-sectional analyses of opportunity for sexual selection reflect selective pressure experienced in the lifetimes of individual males, we therefore examined the cumulative reproductive success of a cohort of same-aged males. This cohort consisted of all males in their third year after hatching (when males last display easily scored age-specific plumage) in 2000, to maximize the number of adult years subsequently observed. We partitioned variance into within- and between-status components, as described above.
Variance in reproductive success due to status may be overestimated if adult reproduction is age linked and a large number of adult males are relatively young. Status is in part influenced by individual age (DuVal 2007c). Our analyses limited such inflation of variance between status classes in two ways. First, we calculated variances using only data from definitive-plumage (adult) males, as both behavioural and genetic data suggested that males in predefinitive plumage do not mate. Second, we examined the cumulative reproductive success of a cohort of same-aged males (explained above).
Reproductive success is quantified here as the number of chicks sired. Results were qualitatively similar when using the number of nests in which a male sired young or the number of mates per year (details not shown).
3. Results
(a) Paternity assignment
When all adult-plumage males were considered as candidate sires, 92.3% of 447 chicks were assigned to a known male, representing 253 nests and 144 unique females (table 1). Of the assigned chicks, 85.7% matched only one male at all 20 loci (n=354); 9.4% mismatched the most likely candidate at only one locus and were assigned with higher than 95% statistical confidence to that male (n=39); and 4.8% perfectly matched two or more males but were still assigned due to high statistical confidence (n=12, including 9 with no associated maternal genotype) or because one of the matching males also sired their nest-mate (n=8). The remaining chicks were unassigned because chicks mismatched the most likely sire at more than one locus (n=28; see below), or matched more than one male completely and none with high statistical confidence (n=6).
Table 1.
Paternity assignment success and paternal sibships of unassigned chicks. (Males that sired chicks and were suspected of alpha status in 2000 and 2001 were considered as non-alpha adult males (see text)).
| year | chicks genotyped | paternities assigned | critical delta LOD statistic | no. of candidates (% siring chicks) | no. of males siring unassigned chicksa (no. of chicks) | |||
|---|---|---|---|---|---|---|---|---|
| chicks | nests | females | all adult males | lekking males (alphas) | ||||
| 2000 | 39 | 33 (84.6%) | 20 | 19 | 1.83 | 77 (13%) | 18 (22%) | 4 (6) |
| 2001 | 54 | 52 (96.3%) | 31 | 27 | 1.04 | 101 (18%) | 17 (76%) | 1 (2) |
| 2002 | 66 | 60 (90.9%) | 38 | 34 | 1.02 | 130 (13%) | 27 (59%) | 3 (4) |
| 2003 | 60 | 53 (88.3%) | 30 | 30 | 1.02 | 124 (15%) | 27 (67%) | 4 (6) |
| 2004 | 17 | 16 (94.1%) | 13 | 13 | 0.96 | 104 (11%) | 20 (55%) | 1 (1) |
| 2005 | 105 | 103 (98.1%) | 64 | 57 | 0.95 | 121 (21%) | 30 (77%) | 0 (0) |
| 2006 | 106 | 96 (90.6%) | 57 | 52 | 1.09 | 123 (16%) | 31 (65%) | 3 (4) |
| total | 447 | 413 (92.3%) | 253 | 144b | 216 (29%)b | 63 (86%)b | 11 (23)b | |
Chicks of known maternity only; paternal sibships estimated in the program DadShare.
Totals represent the number of unique individuals.
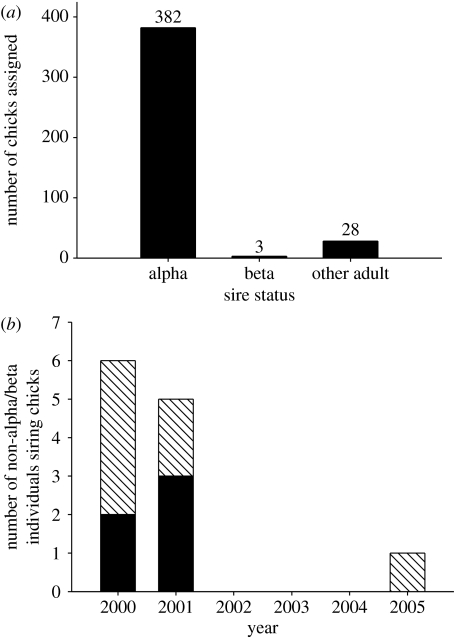
Overwhelmingly, chicks were sired by alpha males (92.5% of 413 chicks; figure 1a). Less than 1% of chicks were sired by beta males (0.7% of 413 chicks; two beta sires). Twelve males that were neither alphas nor betas sired 28 chicks; seven of these sires were identified as alpha in the following year, and therefore may have gained paternity as they transitioned between status classes or they may have been misclassified in their first year of alpha status (figure 1b). Five males were assigned as sires though they were not observed as alphas during the year they sired chicks, or in the following year. Detailed behaviour histories for two of these sires show that they became betas in later years, and one ascended to alpha status five years after he apparently sired a chick as a non-alpha.
Figure 1.
The reproductive success of males of different behavioural status. (a) Sampled chicks were overwhelmingly sired by alphas. (b) Seven of twelve males that sired chicks while neither alpha nor beta were identified behaviourally as alpha in the following breeding season, and this tended to occur in the early years of the study, suggesting that incomplete behavioural sampling of male display areas may have occurred in these years or that males gained paternity as they transitioned between status classes. Solid bars, sire not alpha in next year; hatched bars, sire alpha in next year.
Six per cent of chicks (n=28 chicks from 19 nests) could not be assigned paternity despite known maternity because they mismatched all candidate males at more than one locus (4.1±1.3 loci mismatching, mean±s.d.). These 28 chicks comprised 12 paternal sibships, with males siring one to five chicks each and no more than three chicks in any one year. Unassigned chicks are therefore not expected to substantially affect variance in male reproductive success, and further analyses consider only assigned chicks.
(b) Opportunity for sexual selection
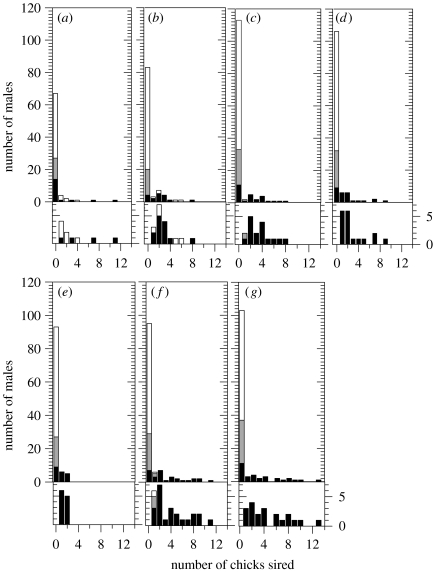
We calculated the variance in male reproductive success based on all adult-plumage males present in the population, including those that did not sire any chicks. On average 60.1±18.7% of alphas sired chicks each year, whereas only 3.1±3.8% of non-alphas did so (mean±s.d., n=7 years; table 1, figure 2). Partitioning the variance in Imales within and between alpha and non-alpha status classes revealed that the opportunity for sexual selection due to status was on average 31±13% (range 7–44%) of the total opportunity for sexual selection (table 2). The yearly opportunity for sexual selection (Imales) was on average 9.32±2.82. Because non-alpha reproduction was so rare, we combined beta and non-alpha/beta males into a ‘non-alpha’ status category. If beta males were instead considered as a distinct status category, the total variance in fitness differed by less than 0.02 and our conclusions were unchanged.
Figure 2.
(a–g) Yearly distribution of reproductive success among adult males of different social status. Alpha males are represented by black bars, betas by light grey and adult males that were neither alpha nor beta by white bars. Lower histogram for each year shows an expanded view of males with non-zero reproductive success. (a) 2000, (b) 2001, (c) 2002, (d) 2003, (e) 2004, (f) 2005 and (g) 2006.
Table 2.
Yearly estimates of opportunity for sexual selection, partitioned into within- and between-status components. (Ibetween-status/Imales represents the proportion of the total opportunity for selection due to male–male competition. Numbers of adult males and alphas in each year as well as number of chicks assigned paternity are given in table 1. The year 2004 is excluded from variance component and I analyses because only few nests were sampled during a short field season. Results are unchanged and variance values differ by less than 0.02 if non-alpha males are further divided into ‘beta’ and ‘non-pair’ (neither alpha nor beta) status categories.)
| year | average number of chicks sired | variance in reproductive success for each status class | variance components | opportunity for sexual selection | Ibetween-status/Imales | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| overall | alpha | non-alpha | alpha | non-alpha | Vwithin-status | Vbetween-status | Vtotal | Iwithin-status | Ibetween-status | Imales | ||
| 2000 | 0.43 | 1.22 | 0.19 | 9.01 | 0.43 | 2.44 | 0.19 | 2.63 | 13.17 | 1.03 | 14.20 | 0.07 |
| 2001 | 0.51 | 2.12 | 0.19 | 3.86 | 0.81 | 1.32 | 0.52 | 1.84 | 5.09 | 2.00 | 7.09 | 0.28 |
| 2002 | 0.46 | 2.19 | 0.01 | 5.70 | 0.01 | 1.19 | 0.78 | 1.97 | 5.63 | 3.70 | 9.33 | 0.40 |
| 2003 | 0.43 | 1.96 | 0.00 | 5.96 | 0.00 | 1.30 | 0.65 | 1.95 | 7.02 | 3.54 | 10.56 | 0.34 |
| 2004 | 0.15 | 0.80 | 0.00 | 0.69 | 0.00 | — | — | — | — | — | — | — |
| 2005 | 0.85 | 3.33 | 0.03 | 10.37 | 0.03 | 2.59 | 2.03 | 4.62 | 3.59 | 2.81 | 6.40 | 0.44 |
| 2006 | 0.78 | 3.10 | 0.00 | 12.89 | 0.00 | 3.25 | 1.81 | 5.06 | 5.34 | 2.98 | 8.32 | 0.36 |
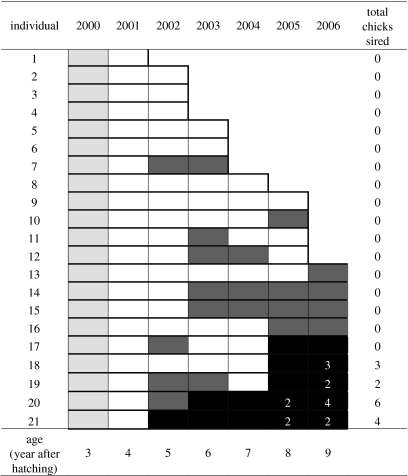
We identified a cohort of 21 third-year males captured in 2000 and resighted after they attained definitive adult plumage in the following year (figure 3). Males in this cohort were observed for 1–6 additional years (mode=6; mean±s.d.=4.4±1.7 years alive after 2000). Twelve males disappeared before becoming alphas and were assumed to be dead. Nine males were known to be alive in 2006, and five of these became alphas and held this status for 2–5 years. Males that became alphas sired an average of 3.0±2.2 chicks (±s.d.) from 2000 to 2006; only one male that was an alpha sired no chicks. By contrast, males in this cohort that did not become alphas were never identified as sires. Of 21, 20 males survived past the age at which the first male in the cohort attained alpha status. The opportunity for sexual selection for this cohort of males was somewhat lower than that found in the yearly analyses (Imales-cohort=5.5), and the opportunity for selection between status classes was high relative to that within status classes: Ibetween-status=3.20, Iwithin-status=2.33; Ibetween-status/Itotal=0.58. When Ibetween-status is calculated using only those males that survived for the entire observation period, it is greatly reduced but remains a large component of the overall opportunity for sexual selection (table 3).
Figure 3.
Survival and reproductive success of a cohort of 21 same-aged males followed from 2000 to 2006. White rectangles indicate adult-plumage birds that were known to be alive but were not identified as alphas or betas in the target year. Dark grey rectangles indicate that the male held beta status and black rectangles indicate alpha status in a particular year. The yearly number of chicks genetically assigned to each male is designated by numbers in the appropriate rectangle. Light grey rectangles in 2000 indicate males observed in third-year subadult plumage during the breeding season (DuVal 2005).
Table 3.
Reproductive variance and opportunity for selection in a cohort of same-aged males followed for six years of adulthood. (Detailed histories of these 21 individuals are presented in figure 3.)
| all males in same-aged cohort identified in 2000 | cohort males surviving until 2006 | |
|---|---|---|
| number of males | 21 | 9 |
| proportion ever alpha | 0.24 | 0.56 |
| total chicks sired | 15 | 15 |
| average number of chicks per male | 0.71 | 1.67 |
| average chicks per alpha | 3 | 3 |
| average chicks per non-alpha | 0 | 0 |
| Vtotal | 2.82 | 5.00 |
| Vwithin-status | 1.19 | 2.78 |
| Vbetween-status | 1.63 | 2.22 |
| Itotal | 5.53 | 1.80 |
| Ibetween-status | 3.20 | 0.80 |
| Iwithin-status | 2.33 | 1.00 |
| Ibetween-status/Iwithin-status | 0.58 | 0.44 |
4. Discussion
In this seven year study of genetic reproductive success in a population of lance-tailed manakins, we demonstrated that lekking alpha males are responsible for siring almost all chicks and males have few if any options for reproduction before attaining alpha status. The incidence of beta paternity was even lower than that estimated in a previous paternity analysis using 9 instead of 20 microsatellite markers (DuVal 2007a). Males that sired chicks while neither alpha nor beta nevertheless attained these statuses later; non-alpha reproduction apparently represents rare opportunistic behaviour while moving towards alpha status, rather than a distinct and widely successful mating strategy. Partitioning reproductive variance into within- and between-status components revealed that both female mate choice (within-status variance) and competition for status (between-status variance) contributed substantially to the total opportunity for sexual selection in this lekking species.
A combined influence of male–male competition and female choice is probably the norm on avian leks (Bradbury & Davies 1987). In lance-tailed manakins, male status is determined by male–male interactions (in combination with intrinsic individual characteristics such as longevity or experience), while females essentially choose mates from among alpha males only. This separation of sexual selection mechanisms may also occur in other lekking species. For example, variation in mating success among lekking great snipe (Gallinago media) males is attributed primarily to female choice; females select particular males rather than particular display sites within leks (Sæther et al. 2005). However, if some males are competitively excluded from holding any display site or if competition among males occurs off the lek (Wegge et al. 2005), a large component of selection is neglected by focusing only on males that hold lek positions.
Both limited reproduction by non-alpha males and the possible misclassification of some males' status in early years of the study introduce non-zero variance in reproductive success of non-alpha individuals. Misclassifications of male status were logically more likely to have occurred early than late in the study, as field monitoring techniques were well established in later years. Correspondingly, the first two of seven years reported the lowest reproductive variance between status classes of males (table 2). As suggested earlier, the opportunity for selection within status categories was mostly due to variation within the alpha male status class.
To what extent does opportunity for sexual selection attributed here to male–male competition reflect natural selection for longevity? Natural and sexual selection can be considered a continuum of selection on individuals (Kokko et al. 2002). Survival of males to adulthood will be an important component of total selection on males, but we do not have any reason to expect that survival to adulthood is affected by the mechanisms of sexual selection. However, a large part of lifetime reproductive success of male lance-tailed manakins once they become adults will be determined by the combination of age at initial alpha status (which is highly variable; DuVal 2007c) and tenure in that alpha position, which will be affected by a combination of sexual selection through male–male interactions and natural selection on survival. Our cohort analysis indicates that many males never become alphas. Only non-reproducing non-alpha males disappeared from the cohort in the observed time period, and so the opportunity for selection due to status was obviously lower when only males surviving for six years were considered. Though this suggests that a portion of between-status variance can be attributed to differential survival, 20 of 21 males survived past the age at which the first male in the cohort attained alpha status. Whether males differ in life-history strategies with respect to age at which they attain alpha status remains to be determined. For example, males may experience a trade-off between the age at which they can attain alpha status and how long they can keep this status. The characteristics that determine individuals' success in attaining and maintaining alpha status are an interesting area for further research. It further remains to be determined whether the same traits influence the outcome of male–male interactions and female choice.
Two levels of female choice can be distinguished. First, our data suggest that females almost exclusively mate with alpha males. This can be viewed as an extremely strong choice, either directly for alpha males or indirectly for traits associated with alpha status (Sæther et al. 2005). Limiting copulations to alphas may efficiently allow females to mate with the highest quality males (‘good genes’ process): alphas are generally competent at performing courtship displays and at surviving to a certain age (sires in this study were in at least their sixth year post hatching). Second, females apparently choose among the pool of alpha males and may gain additional benefits from this choice. Why do females mate with particular alpha males? One possibility is that females employ a compatibility criterion to choose the most appropriate mate from among the pool of alpha males (Neff & Pitcher 2005; Kempenaers 2007). In effect, such a choice system would create nested levels of sexual selection for both good genes and genetic compatibility; mating only with alpha males would limit mate choice to high-quality males, and increase the efficiency of fine-scale compatibility assessments by decreasing the number of males to be assessed. Alternatively, females may choose at random from among high-quality males (alphas), and observed variance in reproductive success could result from variation in placement (i.e. accessibility) of males' display areas. Finally, some variation in male reproductive success could result from post-copulatory processes (i.e. sperm competition or cryptic female choice). This could occur if females mate with multiple males and males differ in fertilization capability, or females select sperm from specific males. Fine-scale monitoring of female choice behaviour is necessary to address how mate choice actually occurs, and hence to determine its evolutionary significance.
Could variance in reproductive success among alphas simply be due to local differences in female nesting density? To estimate whether females could sample most alphas on the study site, we examined the distance between nest sites and sire display areas and compared it with the longest axis of our triangularly shaped study site (approx. 1025 m). The average distance (±s.d.) from nest to sire was 313±159 m (range 31–960 m; n=174 nests), whereas the average distance to the nearest alpha male's display site was 47.7±33.8 m (range 10–211 m; n=166 nests). For females that had chicks sired by two males (15% of 147 nests with both chicks sampled), the distance between the two sires was on average 355±234 m (range 92–818 m, n=22 sire pairs). These data suggest that females moved outside their immediate nesting area to find a mate, and males' reproductive success was not greatly affected by female limitations in sampling males across the study area, though the influence of display site location on male reproductive success remains an interesting question for future research.
How do our results compare with those from other lekking species? Our estimate of Imales was somewhat lower than that reported in the congeneric long-tailed manakins (37.2 in long-tailed manakins (McDonald 1989); and 9.32 in lance-tailed manakins, this study). However, direct measures of Imales are sensitive to differences in mean fitness, so it has been suggested that interspecific comparisons should be approached with caution (Downhower et al. 1987). Similarly, the differences in estimates of Imales between years of this study, albeit small, may be attributable to differences in sampling conditions (e.g. length of field season and the corresponding number of nests sampled) rather than differences in the reproductive behaviour of the birds.
In conclusion, reproductive success of male lance-tailed manakins is highly dependent on social status, but most alpha males apparently sire some chicks during their alpha tenure. When the opportunity for sexual selection is partitioned into components representing reproductive variance within and between status classes of males, Iwithin-status consistently contributes more than half of the total opportunity for selection. This suggests a strong role for female mate choice among alpha males in generating variance in reproductive success. Though relatively lower, Ibetween-status contributed on average 31% of the total opportunity for selection in yearly measures and somewhat more in our cohort analysis. Thus, intrasexual competition for both status and female mate choice among lekking males substantially contribute to the potential for sexual selection in this species.
Acknowledgments
This research was conducted in accordance with the ethical guidelines of the Max Planck Institute for Ornithology and all procedures were approved by the Autoridad Nacional del Ambiente (ANAM) of Panama´.
We greatly appreciate the assistance of seven volunteer field crews whose members contributed to data used here. We thank K. Foerster and M. Valcu for their many useful discussions, and A. Jacot and one anonymous reviewer for their constructive comments on an earlier draft of this manuscript. We are particularly grateful to S. Shuster for his comments and help with the calculations. Funding was provided by the Max Planck Institute for Ornithology, and from granting agencies that contributed to previous stages of this long-term research. J. Garcia and the staff of ANAM, Panamá, assisted with research permits, and F. Köhler and E. Y. Köhler allowed field site access.
References
- Arak A. Sexual selection by male–male competition in natterjack toad choruses. Nature. 1983;306:261–262. doi:10.1038/306261a0 [Google Scholar]
- Arnold S.J, Wade M.J. On the measurement of natural and sexual selection—applications. Evolution. 1984a;38:720–734. doi: 10.1111/j.1558-5646.1984.tb00345.x. doi:10.2307/2408384 [DOI] [PubMed] [Google Scholar]
- Arnold S.J, Wade M.J. On the measurement of natural and sexual selection—theory. Evolution. 1984b;38:709–719. doi: 10.1111/j.1558-5646.1984.tb00344.x. doi:10.2307/2408383 [DOI] [PubMed] [Google Scholar]
- Benson K.E, Basolo A.L. Male–male competition and the sword in male swordtails, Xiphophorus helleri. Anim. Behav. 2006;71:129–134. doi:10.1016/j.anbehav.2005.05.004 [Google Scholar]
- Borgia G. Bower destruction and sexual competition in the satin bowerbird (Ptilonorhynchus violaceus) Behav. Ecol. Sociobiol. 1985;18:91–100. doi:10.1007/BF00299037 [Google Scholar]
- Bradbury J.W. The evolution of leks. In: Alexander R.D, Tinkle D.W, editors. Natural selection and social behavior. Chiron; New York, NY: 1981. pp. 138–169. [Google Scholar]
- Bradbury J.W, Davies N.K. Relative role of intra- and intersexual selection. In: Bradbury J.W, Andersson M.B, editors. Sexual selection: testing the alternatives. Wiley; New York, NY: 1987. pp. 143–163. [Google Scholar]
- Darwin C. John Murray; London, UK: 1871. The descent of man and selection in relation to sex. [Google Scholar]
- Downhower J.F, Blumer L.S, Brown L. Opportunity for selection—an appropriate measure for evaluating variation in the potential for selection. Evolution. 1987;41:1395–1400. doi: 10.1111/j.1558-5646.1987.tb02476.x. doi:10.2307/2409103 [DOI] [PubMed] [Google Scholar]
- DuVal E.H. Age-based plumage changes in the lance-tailed manakin: a two-year delay in plumage maturation. Condor. 2005;107:917–922. doi:10.1650/7793.1 [Google Scholar]
- DuVal E.H. Adaptive advantages of cooperative courtship for subordinate male lance-tailed manakins. Am. Nat. 2007a;169:423–432. doi: 10.1086/512137. doi:10.1086/512137 [DOI] [PubMed] [Google Scholar]
- DuVal E.H. Cooperative display and lekking behavior of the lance-tailed manakin (Chiroxiphia lanceolata) Auk. 2007b;124:1168–1185. doi:10.1642/0004-8038(2007)124[1168:CDALBO]2.0.CO;2 [Google Scholar]
- DuVal E.H. Social organization and variation in cooperative alliances among male lance-tailed manakins. Anim. Behav. 2007c;73:391–401. doi:10.1016/j.anbehav.2006.05.017 [Google Scholar]
- DuVal E.H, Teltscher K, Kempenaers B. Isolation and characterization of novel microsatellite loci for parentage assessment in the lance-tailed manakin (Chiroxiphia lanceolata) Mol. Ecol. Notes. 2007;7:1111–1113. doi:10.1111/j.1471-8286.2007.01795.x [Google Scholar]
- Gibson R.M. Female choice in sage grouse: the roles of attraction and active comparison. Behav. Ecol. Sociobiol. 1996;39:55–59. doi:10.1007/s002650050266 [Google Scholar]
- Hoffman J.I, Boyd I.L, Amos W. Male reproductive strategy and the importance of maternal status in the Antarctic fur seal Arctocephalus gazella. Evolution. 2003;57:1917–1930. doi: 10.1111/j.0014-3820.2003.tb00598.x. doi:10.1554/02-530 [DOI] [PubMed] [Google Scholar]
- Höglund J, Alatalo R.V. Princeton University Press; Princeton, NJ: 1995. Leks. [Google Scholar]
- Johnson K. Sexual selection in pinyon jays I: female choice and male–male competition. Anim. Behav. 1988;36:1038–1047. doi:10.1016/S0003-3472(88)80063-0 [Google Scholar]
- Kempenaers B. Mate choice and genetic quality: a review of the heterozygosity theory. Adv. Study Behav. 2007;37:189–278. [Google Scholar]
- Koenig W.D, Albano S.S. On the measurement of sexual selection. Am. Nat. 1986;127:403–409. doi:10.1086/284491 [Google Scholar]
- Kokko H, Brooks R, McNamara J.M, Houston A.I. The sexual selection continuum. Proc. R. Soc. B. 2002;269:1331–1340. doi: 10.1098/rspb.2002.2020. doi:10.1098/rspb.2002.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall T.C, Slate J, Kruuk L.E.B, Pemberton J.M. Statistical confidence for likelihood-based paternity inference in natural populations. Mol. Ecol. 1998;7:639–655. doi: 10.1046/j.1365-294x.1998.00374.x. doi:10.1046/j.1365-294x.1998.00374.x [DOI] [PubMed] [Google Scholar]
- McDonald D.B. Cooperation under sexual selection: age-graded changes in a lekking bird. Am. Nat. 1989;134:709–730. doi:10.1086/285007 [Google Scholar]
- McDonald D.B. Predicting fate from early connectivity in a social network. Proc. Natl Acad. Sci. USA. 2007;104:10 910–10 914. doi: 10.1073/pnas.0701159104. doi:10.1073/pnas.0701159104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff B.D, Pitcher T.E. Genetic quality and sexual selection: an integrated framework for good genes and compatible genes. Mol. Ecol. 2005;14:19–38. doi: 10.1111/j.1365-294X.2004.02395.x. doi:10.1111/j.1365-294X.2004.02395.x [DOI] [PubMed] [Google Scholar]
- Pruett-Jones S.G, Pruett-Jones M.A. Sexual selection through female choice in Lawes parotia, a lek-mating bird of paradise. Evolution. 1990;44:486–501. doi: 10.1111/j.1558-5646.1990.tb05934.x. doi:10.2307/2409431 [DOI] [PubMed] [Google Scholar]
- Queller D.C, Goodnight K.F. Estimating relatedness using genetic markers. Evolution. 1989;43:258–275. doi: 10.1111/j.1558-5646.1989.tb04226.x. doi:10.2307/2409206 [DOI] [PubMed] [Google Scholar]
- Sæther S.A, Baglo R, Fiske P, Ekblom R, Höglund J, Kålås J.A. Direct and indirect mate choice on leks. Am. Nat. 2005;166:145–157. doi: 10.1086/431248. doi:10.1086/431248 [DOI] [PubMed] [Google Scholar]
- Shuster, S. M. & Wade, M. J. 2003 Mating systems and strategies Monographs in Behavior and Ecology. Princeton, NJ: Princeton University Press.
- Trail P.W. Courtship disruption modifies mate choice in a lek-breeding bird. Science. 1985;227:778–780. doi: 10.1126/science.227.4688.778. doi:10.1126/science.227.4688.778 [DOI] [PubMed] [Google Scholar]
- Wegge P, Eliassen S, Finne M.H, Odden M. Social interactions among Capercaillie Tetrao urogallus males outside the lek during spring. Ornis Fenn. 2005;82:147–154. [Google Scholar]