Abstract
Life-history theory assumes that animal life histories are a consequence of trade-offs between current activities and future reproductive performance or survival, because resource supply is limited. Empirical evidence for such trade-offs in the wild are common, yet investigations of the underlying mechanisms are rare. Life-history trade-offs may have both physiological and ecological mediated costs. One hypothesized physiological mechanism is that elevated energy metabolism may increase reactive oxygen species production, leading to somatic damage and thus compromising future survival. We investigated the impact of experimentally elevated energy expenditure on oxidative damage, protection and lifespan in short-tailed field voles (Microtus agrestis) maintained in captivity to remove any confounding ecological factor effects. Energy expenditure was elevated via lifelong cold exposure (7±2°C), relative to siblings in the warm (22±2°C). No treatment effect on cumulative mortality risk was observed, with negligible effects on oxidative stress and antioxidant protection. These data suggest that in captive animals physiologically mediated costs on life history do not result from increased energy expenditure and consequent elevations in oxidative stress and reduced survival.
Keywords: lifespan, metabolic rate, oxidative stress, cold exposure, life-history trade-offs, antioxidant
1. Introduction
Life-history theory is based on the assumption that resources are limited and consequently animals are forced to trade off competing activities against one another (Fisher 1930; Stearns 1992; Speakman 2008). Animals face evolutionary decisions, e.g. whether to allocate limited resources (e.g. energy) into current reproductive output or to functions that protect the soma and thereby ensure their continued survival (Stearns 1992; Nussey et al. 2006; Speakman 2008). There is abundant ecological evidence supporting the existence of life-history trade-offs. For example, in red-billed choughs (Pyrrhocorax pyrrhocorax) early life egg laying is negatively correlated with longevity (Reid et al. 2003), and early reproductive effort impacts negatively on lifespan in both North American red squirrel (Tamiasciurus hudsonicus; Descamps et al. 2006) and red deer (Cervus elaphus; Nussey et al. 2006). The idea that animals cannot simultaneously maximize everything (but see Johnston et al. 2006) not only underpins life-history theory but also has ramifications in other areas such as our understanding of the evolutionary basis of ageing, such as the disposable soma theory (Kirkwood & Rose 1991). The disposable soma theory suggests that a high level of reproduction can only be sustained by diversion of resources away from somatic protection. The consequence is a loss of somatic integrity with age, which we see as senescence, results in reduced reproductive performance and increased risk of death (Kirkwood & Rose 1991; Moe et al. 2007).
It is widely assumed that the mechanisms underpinning the trade-offs in life history may include both ecological factors such as changes in predation, disease or starvation risk and physiological effects, such as changes in immune function (Speakman 2008). However, the precise mechanisms that underpin life-history trade-offs are only poorly understood (Partridge 2001; Alonso-Alvarez et al. 2006; Speakman 2008). The involvement of energy as a putative currency that might mediate the link between current activities and future survival was initially suggested by Rubner (1908), who observed that animals with high metabolic rates had short lives and animals with low metabolic rates had long lives. This notion was subsequently incorporated into the ‘rate of living’ theory (Pearl 1928), in which it was proposed that ‘the duration of life varies inversely as the rate of energy expenditure during its continuance’. This theory suggests that the amount of life that any organism has available to it is fixed, and an individual can use that life extravagantly resulting in a short life (living fast and dying young), or more frugally resulting in a long life (Pearl 1928; Speakman et al. 2002). Continuous expenditure of excess energy, whether due to increased reproduction, predator avoidance or thermoregulation appears incompatible with prolonged survival (Hoffman & Parsons 1991).
The free radical/oxidative stress theory of ageing (Harman 1956; Beckman & Ames 1998) provides an attractive candidate mechanism to explain both the increased somatic damage with age, and the proposed negative relationship between increased metabolism and lifespan (Pearl 1928). Free radicals or more specifically reactive oxygen species (ROS) are produced primarily within mitochondria and are unavoidable by-products of aerobic metabolism (Beckman & Ames 1998). This theory predicts that damage to proteins, lipids and nucleic acids by ROS such as superoxide , hydrogen peroxide (H2O2) and the hydroxyl radical (HO·) is the primary mechanism underlying the ageing process. This potential for ROS-induced damage can be reduced by a suite of antioxidant (e.g. catalase, glutathione peroxidase (Gpx), superoxide dismutase (SOD), α-tocopherol, ascorbate) and various repair mechanisms (Beckman & Ames 1998). However, if ROS production exceeds the protection and repair mechanisms then the net result will be oxidative damage, which is correlated with the loss of cellular homeostasis and the functional decline indicative of ageing (Beckman & Ames 1998). Comparative data support the premise that species with higher mass-specific metabolic rates have elevated ROS production (mitochondrial hydrogen peroxide) and tend to have a truncated lifespan (Lopez-Torres et al. 1993). However, fundamental questions relating to how such comparative data are analysed have recently been raised (Speakman et al. 2002), and exceptions to this rule exist. Birds and bats, for example, combine high mass-specific metabolic rates with long lives (e.g. Austad & Fischer 1991; Barja et al. 1994). In birds this appears to be, in part, due to lower mitochondrial ROS production (Barja et al. 1994) and greater resistance of cellular membranes to lipid peroxidation (Pamplona et al. 1999).
Within species the association between metabolic rate and lifespan is similarly confused (Speakman et al. 2002, 2004; Speakman 2005), although forced elevations in expenditure in free-living animals tend to have a negative impact on lifespan (e.g. Schmidt-Hempel & Wolf 1988; Daan et al. 1996). In European kestrels (Falco tinnunculus), an approximate 40% increase in foraging effort during chick provisioning resulted in a lower likelihood of parents returning the following spring (Daan et al. 1996). However, it is not apparent if these costs to lifespan are via increased ROS production, decreased somatic protection and repair and/or ROS-induced oxidative damage. Additionally, it is not established whether ecologically significant factors such as an increased risk of predation due to depleted body condition following such periods of increased metabolism are important (Speakman 2008).
Because ecological and physiological mediated costs are confounded in studies of free-living animals, it is difficult to identify the exact proximate factors that mediate the observed trade-offs. In this study, we explored the role of elevated energy demands and oxidative stress on lifespan in short-tailed field voles (Microtus agrestis). To minimize the potentially confounding effects of ecological factors, we maintained wild-derived animals in captivity (Ricklefs & Cadena 2007) and experimentally elevated their energy requirements by exposing them to cold. An additional advantage in this approach is that it is highly unlikely that these wild-derived animals would have had sufficient time to experience inadvertent selection for short generation times and/or high fecundity observed in long-term laboratory maintained animals (Reznick 2005). We elevated metabolic rate in singly housed voles via lifelong cold exposure (7±2°C) compared with same-sex siblings maintained in the warm (22±2°C). Acute cold exposure (h d−1) increases oxidative stress (Barja de Quiroga et al. 1991; Selman et al. 2002a) and radical production in mammals (Barja de Quiroga et al. 1991) but chronic lifelong effects are unclear. Only one previous study has examined the effect of cold exposure on lifespan, reporting no lifespan effect in laboratory rats forced to stand in cold water (23°C) for 4 h d−1, 5 days per week (Holloszy & Smith 1986), although energy metabolism and oxidative stress were not directly assayed.
2. Material and methods
(a) Study species and experimental protocol
Short-tailed field voles (M. agrestis) were captured from a wild population located 15 miles northwest of Aberdeen, UK (57° N), and subsequently maintained at The University of Aberdeen, Aberdeen, UK. Experimental animals were F2 offspring derived from the original wild population. All individuals were born at 22±2°C, which is slightly below the thermoneutral zone (25–30°C; McDevitt & Speakman 1994) of this arvicoline rodent. Individuals were weaned at 16 days of age and kept in same-sex sibling groups until approximately 21 days of age. They were subsequently individually housed in cages (48×15×13 cm3) at 22±2°C and under a 16 L : 8 D light/dark cycle (lights on at 0600 hours), with ad libitum access to standard mouse diet (rat and mouse breeder and grower diet (CRM (P), Special Diets Services, BP Nutrition, UK) and water). As M. agrestis are not inbred, we used same-sex sibling pairs to reduce genetic variability between our experimental groups. At 30 days of age, one individual per sibling pair was randomly assigned to remain at 22±2°C, while the other individual was transferred to a controlled temperature room maintained at 7±2°C (Atlantic Cooling Services, UK). Cold exposed animals were given access to bedding material to facilitate acclimation to 7±2°C, but this was gradually removed by 60 days of age (30 days of cold exposure) until no bedding material remained.
(b) Lifespan analysis
A total of 35 male and 21 female sibling pairs of voles were used in the lifespan analysis. Individuals were monitored daily, with food intake and body mass (BM) measured (ages indicated below) but otherwise left undisturbed until they died naturally. If death appeared, imminent individuals were weighed and examined for macroscopic pathologies and euthanized, with this considered to be the date of death. Kaplan–Meier survival curves were constructed using the known of birth and death dates of each individual, with p values calculated using the log-rank test. Maximum lifespan was calculated as the mean age of the oldest 10% of mice from each group.
(c) Metabolic rate analyses
Resting metabolic rate (RMR) and daily energy expenditure (DEE) were determined in a subsample of animals used in the longevity study using open-flow respirometry and the doubly labelled water (DLW) technique, respectively. Individuals had ad libitum access to food and water prior to, but not during RMR measurements. In brief, voles were weighed (0.01 g) and subsequently placed within an airtight Plexiglas chamber in a temperature-controlled incubator (INL-401N-010, Weiss-Gallenkamp, Loughborough, UK) at their relevant housing temperature (7±0.1°C or 22±0.1°C) for 3 hours. Silica gel-dried air was pumped (Charles Austin Ltd, Weybridge UK) through the chamber at 600–800 ml min−1 (DM3A, Alexander Wright Flow Meter, London, UK) and subsequently re-dried. A subsample (approx. 150 ml min−1) was then passed through a paramagnetic oxygen analyser (Series 1100, Servomex Group Ltd, Crowburgh, UK) and an infrared carbon dioxide analyser (Series 1400, Servomex Group Ltd, UK). RMR (ml O2 min−1), corrected for standard temperature and pressure, was calculated using the lowest 10 consecutive readings in oxygen concentration (equivalent to 5 min within the chamber; Hayes et al. 1992).
DEE was estimated at 11 and 20 months of age by the DLW technique (Lifson et al. 1955; Speakman 1998), which has been validated by comparison with indirect calorimetry in a range of small mammals (e.g. Speakman & Krol 2005a) and provides an accurate measure of DEE over periods of several days (Speakman et al. 1994; Berteaux et al. 1996). On day 1, animals were weighed (±0.1 g, sartorius, Epsom, UK) and approximately 0.15 ml of DLW (30APE 18O, 15APE 2H) was administered intraperitoneally. Syringes were weighed before and after administration (±0.0001 g, sartorius) to calculate the exact mass of injectate. Initial blood samples (approx. 80 μl) were collected after 1 hours of isotope equilibration to estimate initial isotope enrichments of 2H and 18O. Final blood samples (approx. 80 μl) were collected either 24 or 48 hours after the initial blood sample to estimate isotope elimination rates. Background enrichments were evaluated from blood samples taken from unlabelled animals under the same protocol (Speakman & Racey 1987: method C). Capillaries containing the blood samples were vacuum distilled and water from the resulting distillate were analysed using gas source isotope ratio mass spectrometry (Optima, Micromass IRMS, UK). Three high enrichment standards were run daily alongside the samples, with raw data corrected to these standards to minimize inlet cross contamination. Isotope enrichments were converted to values of DEE using a single pool model, as recommended for this size of animal (Speakman 1993). There are several alternative approaches for the treatment of evaporative water loss (Visser & Schekkerman 1999). We chose the assumption of a fixed evaporation of 25% of the water flux (eqn. (7.17), Speakman 1997), as this has been established to minimize error in a range of conditions (Visser & Schekkerman 1999).
(d) Oxidative stress markers
A subsample of male and female voles not used in the longevity or RMR/DEE measurements was used to examine the effects of lifelong cold exposure on various oxidative stress and antioxidant parameters. A total of 12 pairs (six male and six female) were killed at 4 and 11 months of age and 7 pairs (two male and five female) were killed at 20 months of age. These animals were recruited at the same time and maintained under exactly the same conditions as those in the longevity study described above.
The ‘modified’ Comet assay was employed to determine hepatic (20 months of age only) and lymphocyte (11 and 20 months of age) DNA oxidative damage as previously described (Selman et al. 2006). The modification step used lesion-specific bacterial repair enzymes endonuclease III (ENDO III) and formamidopyrimidine-DNA glycosylase (FPG) to increase the sensitivity and specificity of the Comet assay (Collins 2004). ENDO III induces breaks at the sites of oxidized pyrimidines and FPG induces breaks at the sites of purines, including 8-oxo-guanine (Collins 2004). Damage was scored visually, with 100 comets on each slide that were assigned a score from 0 to 4 depending on the fraction of DNA pulled out into the tail. Therefore, the overall score for each slide was between 0 (undamaged) and 400 (completely damaged). Hepatic lipid peroxidation was estimated by measuring thiobarbituric acid reactive substances (TBARS) using high performance liquid chromatography (HPLC) in 4-, 11- and 20-month-old animals as previously described (Selman et al. 2002b) and expressed as ηmol malonaldehyde per mg protein.
(e) Antioxidant analyses
The levels of α-tocopherol, ascorbic acid and retinol were measured in liver homogenates in 4-, 11- and 20-month-old voles using reverse-phase HPLC and a fluorescent/visible detection method (Selman et al. 2006). Catalase, selenium-dependent Gpx and total SOD were measured in heart, liver, kidney, duodenum, skeletal muscle (gastrocnemius) and brown adipose tissue (BAT) at 4, 11 and 20 months of age. The protocols are described fully elsewhere (Selman et al. 2000). Absorbance changes were measured using a SPECTRAmax Plus microplate spectrophotometer (Molecular Devices Corp., Sunnyvale, USA) and SoftMax Pro software (Molecular Devices Corp., USA).
(f) Statistical analysis
All statistics were performed using Minitab (Version 13, Minitab Ltd, Coventry, UK) and GraphPad (Prism 4, GraphPad Software Inc., San Diego, USA) software. Data were analysed using general linear model, Cox regression, one-way ANOVA and paired t-test where appropriate. Kaplan–Meier mortality curves were constructed and differences in mortality rate were determined using the log-rank test. All values reported are mean±standard error of the mean (s.e.m.), except where indicated and p<0.05 were regarded as statistically significant. In all analyses described below, non-significant interaction effects (p>0.05) were subsequently removed from each analysis in order to obtain the best-fitted model in each case. For the analyses of oxidative stress and antioxidant protection we did not have a priori information on individual variation sufficient to design the experiments with power in mind. Consequently, we performed post hoc power analysis to establish the power of our experiments to detect treatment effects (for the appropriate sample size) for all variables.
3. Results
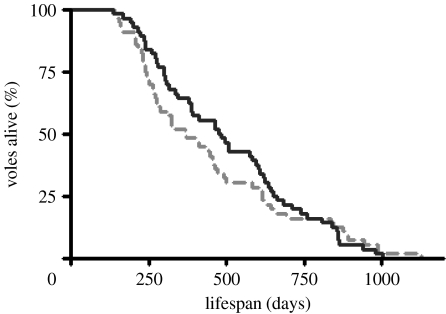
There was no significant sex effect on lifespan between the warm- and cold-exposed voles (F1,112=0.146, p=0.702) and no sex×treatment interaction (F1,112=0.030, p=0.862), so data from male and female voles were pooled. Using the log-rank test, we observed no effect of lifelong cold exposure on cumulative mortality risk (figure 1; X2=0.331, p=0.565, N=112), with median lifespan being 368 days in the warm-exposed and 477 days in the cold-exposed voles. Mean±s.e.m. lifespan was 448±35 and 503±32 days for warm- and cold-exposed voles, respectively. This lack of a significant effect on lifespan was observed despite median lifespan being 30% and mean lifespan being 12% greater in the cold. A large range in lifespan was seen (139–1128 and 137–1004 days for warm- and cold-exposed voles, respectively), with no difference (X2=1.077, p=0.299, N=12) in maximum lifespan between the warm-exposed (969±37 days) and the cold-exposed (918±27 days) voles. In addition, we also analysed the lifespan data using a paired t-test as we used sibling pairs of voles in the lifespan study, but again no treatment effect was detected (T56=1.078, p=0.286). However, as indicated by the Kaplan–Meier lifespan curves (figure 1), there was a significant (X2=3.892, p=0.049, N=94) difference in cumulative mortality risk in voles until 750 days of age, this being greater in the warm. However, this mortality effect was subsequently lost when we included those individuals that survived beyond 750 days in the analysis (figure 1).
Figure 1.
Lifespan of voles (N=56 sibling pairs, 35 male and 21 female pairs) following lifelong exposure to warm (22±2°C) or cold (7±2°C) ambient temperature. No significant sex or sex×treatment interaction effects were observed on lifespan (p>0.05). Using the log-rank test on male and female pooled lifespan data indicated no significant effect of cold exposure on mortality (log-rank test, X2=0.331, p=0.565). Median lifespan was 368 and 477 days in the warm-exposed (dashed line) and cold-exposed (solid line) voles, respectively, and mean±s.e.m. lifespan was 448±35 and 503±32 days for warm- and cold-exposed voles, respectively.
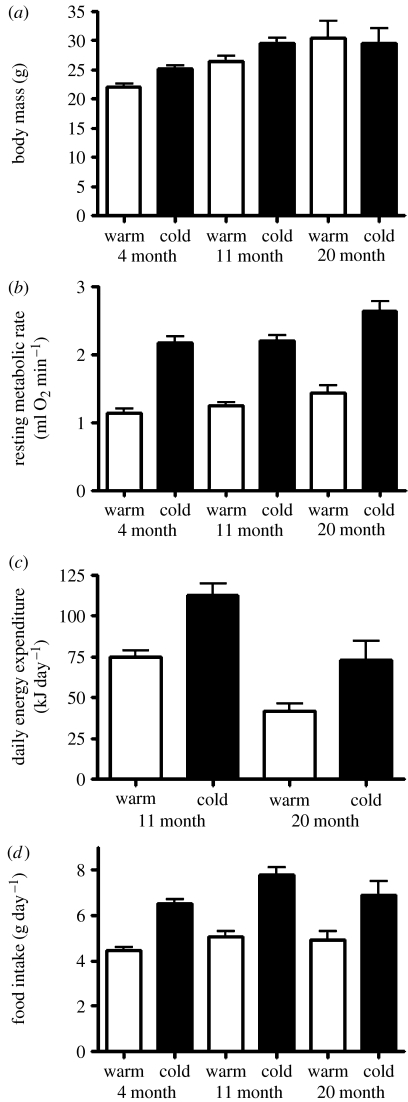
In the following analyses, we employed a general linear model with age, sex and treatment (warm and cold) as factors and BM included as a covariate. In all cases, non-significant interactions (p>0.05) were removed in order to obtain the best-fitted model in each case and only significant interactions (p<0.05) are reported below. Mean BM increased significantly with age (figure 2a; F2,230=21.612, p<0.001) and males were significantly heavier than females (F1,230=6.949, p=0.009). Animals in the cold were significantly heavier (F1,230=21.454, p<0.001), with mean BM being 22, 26 and 30 g in voles in the warm compared with 25, 30 and 30 g in the cold at 4, 11 and 20 months of age, respectively. A significant age×sex interaction effect on BM was also observed (F2,230=6.723, p=0.001). With regard to metabolism, significant age (F2,116=7.184, p=0.001), treatment (F1,116=209.784, p<0.001) and BM (F1,116=16.706, p<0.001; R2=0.039) effects on RMR were identified (figure 2b), although sex was non-significant (F1,116=1.976, p=0.163). RMR was approximately 80% higher in cold-exposed than warm-exposed voles across all ages, and increased in an age-dependent manner. DEE (figure 2c) was also significantly elevated in the cold (F1,43=27.244, p<0.001), but actually declined with age (F1,43=28.765, p<0.001; 75±4 kJ day and 113±7 kJ day at 11 months; 42±4 kJ day and 73±12 kJ day at 20 months, for warm- and cold-exposed voles, respectively). There was no significant BM (F1,43=3.371, p=0.074) or sex effect (F1,43=0.108, p=0.744) on DEE. Daily food intake (figure 2d) increased with increasing BM (F1,230=7.742, p=0.006; R2=0.021), increasing age (F2,230=4.739, p=0.01) and was significantly higher in cold-exposed animals (F1,230=90.845, p<0.001), but did not differ between sexes (F1,230=3.425, p=0.066).
Figure 2.
(a) Mean BM increased significantly in the cold, increased with age and was higher in male voles (N=51, 50 and 8 for warm and 59, 56 and 8 for cold maintained voles at 4, 11 and 20 months of age, respectively). (b) RMR was significantly higher in the cold, increased significantly with increasing age and increasing BM, although no sex effects were seen (N=12, 31 and 10 for warm- and cold-exposed voles at 4, 11 and 20 months of age, respectively). (c) DEE was significantly higher in the cold-exposed voles, but declined significantly with age (N=15 and 7 for both warm- and cold-exposed voles at 11 and 20 months of age, respectively). No significant BM or sex effects on DEE were observed. (d) Daily food intake was significantly higher in the cold, and increased with both increasing BM and increasing age (N=51, 50 and 8 for warm and 59, 56 and 8 for cold maintained voles at 4, 11 and 20 months of age, respectively).
Post hoc power analysis established that the mean±s.e.m. power of our experiments to detect treatment effects in our oxidative stress and antioxidant parameters was 21±3%. Using the lesion-specific enzyme ENDO III (identifies oxidized pyrimidines) we observed that lymphocyte DNA oxidative damage (table 1) was unaffected by cold exposure (F1,28=0.106, p=0.748), age (F1,28=0.080, p=0.780), sex (F1,28=0.816, p=0.375) or BM (F1,28=0.620, p=0.439). Lymphocyte DNA oxidative damage estimated using the lesion-specific enzyme FPG (table 1; FPG; identifies oxidized purines) was similarly unaffected by treatment (F1,26=0.465, p=0.502), age (F1,26=2.621, 0.120), sex (F1,26=0.021, p=0.886) or BM (F1,26=0.049, p=0.827). Hepatic DNA oxidative damage was determined in voles only at 20 months of age. Using the lesion-specific enzyme ENDO III (table 1), hepatocyte DNA oxidative damage was increased both in the cold (F1,12=8.280, p=0.018) and in female voles (F1,12=5.981, p=0.037), although no significant BM effect was seen (F1,12=4.169, p=0.072). No treatment (F1,12=0.810, p=0.391), sex (F1,12=0.001, p=0.978) or BM effect (F1,12=0.001, p=0.990) was observed on hepatocyte DNA oxidative damage when using FPG (table 1). Hepatic lipid peroxidation, determined using the TBARS assay, decreased significantly with advancing age (table 1; F2,54=3.666, p=0.033), but was unaffected by treatment (F1,54=0.388, p=0.536), sex (F1,54=2.472, p=0.122) or BM (F1,54=0.093, p=0.762).
Table 1.
Mean (±s.e.m.) oxidative stress and antioxidant parameters. (Lymphocyte and hepatocyte DNA oxidative damage (Arbitrary units), hepatic lipid peroxidation (ηmol mg protein−1), hepatic ascorbic acid levels (μmol mg protein−1), hepatic α-tocopherol levels (ηg mg protein−1) and hepatic retinol levels (ηg mg protein−1).)
| group | 4 month | 11 month | 20 month | n | |
|---|---|---|---|---|---|
| ENDO III | warm | — | 35±7.4 | 34±9.8 | 11,5 |
| lymphocytes | cold | — | 40±7.0 | 31±9.1 | 7,6 |
| FPG | warm | — | 80±7.0 | 57±12.8 | 11,5 |
| lymphocytes | cold | — | 95±26.0 | 63±21.7 | 6,5 |
| ENDO III | warm | — | — | 7.9±3.5 | 7 |
| hepatocytes | cold | 31±9.5 | 6 | ||
| FPG | warm | — | — | 22±5.0 | 7 |
| hepatocytes | cold | — | — | 35±13 | 6 |
| TBARS | warm | 2.2±0.5 | 1.8±0.4 | 0.5±0.1 | 12,8,7 |
| cold | 1.9±0.4 | 1.3±0.1 | 0.9±0.2 | 12,9,7 | |
| ascorbic acid | warm | 3.6±0.9 | 2.9±1.1 | 5.4±1.1 | 12,8,7 |
| cold | 4.4±1.1 | 4.1±0.8 | 4.0±0.4 | 12,9,7 | |
| α-tocopherol | warm | 51±3.9 | 38±5.1 | 150±30.0 | 12,8,7 |
| cold | 72±24.9 | 52±5.1 | 92±10.6 | 12,9,7 | |
| retinola | warm | 11±0.8 | 10±0.7 | 18±2.7 | 12,8,7 |
| cold | 15±2.0 | 14±1.7 | 16±1.5 | 12,9,7 |
Absolute retinol levels rescaled (100× higher than values presented).
Cold exposure had little effect on various antioxidant parameters measured. Hepatic ascorbic acid levels (table 1) were not altered by age (F2,54=1.088, p=0.345), treatment (F1,54=0.040, p=0.845) or by sex (F1,54=0.114, p=0.738), although a significant BM effect was seen with levels being higher in heavier animals (F=4.810, p=0.033; R2=0.083). The levels of hepatic α-tocopherol (table 1) were significantly altered by age (F2,54=6.450, p=0.003) being higher in 20-month-old animals, but not by treatment (F1,54=0.009, p=0.925), sex (F1,54=0.173, p=0.679) or BM (F1,54=0.025, p=0.874). Retinol levels in the liver (table 1) were significantly altered with age (F2,54=5.260, p=0.009) but not treatment (F1,54=4.017, p=0.051), BM (F1,54=3.852, p=0.055) or sex (F1,54=0.757, p=0.389), although there appeared to be a trend towards significance for treatment and BM. In addition, we determined the activities of the antioxidant enzymes catalase, Gpx and total SOD in heart, liver, kidney, skeletal muscle, duodenum and BAT of warm- and cold-exposed voles at 4, 11 and 20 months of age (table 2). Catalase (liver, kidney and muscle), Gpx (liver, kidney and BAT) and SOD (heart, liver, kidney, muscle, duodenum and BAT) were all significantly altered by age (table 2). However, in the majority of cases, these age-related changes did not show a consistent pattern of increase or decrease in enzyme activity with age, but rather the highest enzyme activities were observed in 11-month-old animals. Only one significant treatment effect was observed, with SOD activity in BAT being significantly higher in cold-exposed animals (F1,44=8.399, p=0.006; table 2). No significant sex effects were detected (table 2) and BM had a significant effect only on liver Gpx activity (F1,44=5.586, p=0.023; R2=0.035; table 2). Significant interaction effects were observed in heart Gpx (table 2) for treatment×age (F2,44=4.979; p=0.015), age×sex (F2,44=5.091; p=0.014) and treatment×age×sex×BM interactions (F11,44=2.212; p=0.049).
Table 2.
Mean (±s.e.m.) tissue antioxidant enzyme activities (catalase), glutathione peroxidase (Gpx), superoxide dismutase (total SOD) in heart, liver, kidney, skeletal muscle, duodenum and brown adipose tissue (BAT) of warm and cold maintained voles at 4, 11 and 20 months of age. (Activities are expressed in International Bergmeyer units (U) per mg protein (U (min−1 mgprotein−1)). Int.=Interaction. Significant p values are indicated in italics. *0.014=p value of the significant age×sex interaction effect, **0.015=p value of the significant treatment×age interaction effect, ***0.049=p value of the significant treatment×age×sex×BM interaction effect.)
| group | 4 month | 11 month | 20 month | age effect, p | treatment effect, p | BM effect, p | sex effect, p | interaction effect, p | |
|---|---|---|---|---|---|---|---|---|---|
| catalasea | |||||||||
| heart | warm | 3.8±0.42 | 2.7±0.24 | 3.5±0.74 | 0.068 | 0.769 | 0.334 | 0.751 | — |
| cold | 3.4±0.15 | 2.5±0.12 | 4.5±1.11 | ||||||
| liver | warm | 6.1±0.64 | 4.5±0.40 | 5.3±0.37 | 0.008 | 0.684 | 0.107 | 0.800 | — |
| cold | 6.2±0.63 | 4.9±0.74 | 4.7±0.35 | ||||||
| kidney | warm | 7.4±0.55 | 5.7±0.37 | 7.2±0.53 | 0.006 | 0.261 | 0.903 | 0.472 | — |
| cold | 6.9±0.55 | 5.2±0.46 | 6.7±0.42 | ||||||
| muscle | warm | 3.1±0.46 | 3.6±0.40 | 3.5±0.57 | 0.010 | 0.979 | 0.209 | 0.444 | — |
| cold | 2.2±0.33 | 4.8±0.26 | 3.1±0.86 | ||||||
| duodenum | warm | 4.9±1.04 | 5.1±0.64 | 5.5±0.88 | 0.153 | 0.221 | 0.570 | 0.386 | — |
| cold | 3.4±0.57 | 4.2±0.47 | 5.6±0.54 | ||||||
| BAT | warm | 16±1.97 | 15±1.97 | 16±3.39 | 0.109 | 0.988 | 0.651 | 0.059 | — |
| cold | 13±0.96 | 23±2.36 | 13±1.08 | ||||||
| Gpxa | |||||||||
| heart | warm | 19±1.44 | 14±0.85 | 21±2.35 | 0.083 | 0.096 | 0.176 | 0.530 | 0.015** |
| cold | 19±1.10 | 12±1.00 | 20±1.82 | 0.014* | |||||
| 0.049*** | |||||||||
| liver | warm | 33±4.10 | 70±6.71 | 28±1.07 | <0.001 | 0.840 | 0.023 | 0.438 | — |
| cold | 27±4.55 | 73±6.38 | 25±1.68 | ||||||
| kidney | warm | 31±3.47 | 19±0.65 | 33±1.74 | <0.001 | 0.773 | 0.856 | 0.272 | — |
| cold | 33±1.80 | 18±0.75 | 33±2.40 | ||||||
| muscle | warm | 13±2.24 | 13±1.16 | 14±1.68 | 0.192 | 0.317 | 0.757 | 0.467 | — |
| cold | 18±1.56 | 15±0.95 | 10±2.00 | ||||||
| duodenum | warm | 11±1.88 | 7.1±0.65 | 6.0±0.64 | 0.063 | 0.923 | 0.958 | 0.251 | — |
| cold | 9.6±1.27 | 6.8±0.74 | 7.9±1.81 | ||||||
| BAT | warm | 44±7.20 | 57±6.57 | 46±4.98 | 0.020 | 0.696 | 0.255 | 0.109 | — |
| cold | 40±6.0 | 58±2.17 | 41±7.21 | ||||||
| total SODa | |||||||||
| heart | warm | 3.7±0.29 | 9.1±0.43 | 7.4±0.55 | <0.001 | 0.110 | 0.798 | 0.306 | — |
| cold | 3.3±0.21 | 6.2±1.00 | 7.9±0.82 | ||||||
| liver | warm | 5.9±0.49 | 7.6±0.51 | 5.6±0.33 | <0.001 | 0.610 | 0.665 | 0.716 | — |
| cold | 6.0±0.54 | 8.2±0.55 | 4.0±0.61 | ||||||
| kidney | warm | 5.4±0.43 | 8.0±0.33 | 7.8±0.21 | <0.001 | 0.367 | 0.070 | 0.671 | — |
| cold | 5.4±0.22 | 8.2±0.32 | 8.2±0.39 | ||||||
| muscle | warm | 3.0±0.44 | 14±0.62 | 4.9±0.78 | <0.001 | 0.980 | 0.782 | 0.442 | — |
| cold | 3.3±0.28 | 14±0.83 | 4.6±0.65 | ||||||
| duodenum | warm | 7.6±1.48 | 17±2.54 | 12±1.25 | <0.001 | 0.758 | 0.387 | 0.204 | — |
| cold | 6.2±0.57 | 16±1.87 | 16±3.74 | ||||||
| BAT | warm | 8.0±1.06 | 13±2.32 | 11±2.01 | <0.001 | 0.006 | 0.305 | 0.251 | — |
| cold | 8.7±1.79 | 23±1.81 | |||||||
Absolute Catalase, Gpx and SOD activities were rescaled (1000×, 1000× and 100× higher, respectively than values presented).
4. Discussion
Life-history trade-offs may be mediated by both physiological and ecological mechanisms (Speakman 2008). However, while physiological factors are often presumed to underlie many life-history trade-offs (Stearns 1992), the precise nature of these physiological mechanisms remain largely unknown (Partridge 2001; Alonso-Alvarez et al. 2006; Speakman 2008). One commonly assumed physiological mechanism is that increased energy demands associated with current activities may compromise future survival owing to their effects on ROS production and levels of somatic damage. To try and disassociate physiologically mediated life-history costs from ecologically mediated costs such as predation and starvation, we maintained short-tailed field voles (M. agrestis) in captivity (Ricklefs & Cadena 2007) and exposed them to cold conditions to elevate their energetic demands. Our direct measures of energy demands demonstrated that the experimental treatment successfully increased metabolic rate in the voles with DEEs being over 50% (11 months of age) and over 70% (20 months of age) higher in the cold. However, despite this significant elevation in demands, we failed to detect a negative relationship between metabolic rate and lifespan as predicted. Indeed, up until 750 days of age the mortality rate was significantly higher in the animals maintained in the warm conditions than in the cold. These data also provide further direct evidence refuting the rate of living theory (Pearl 1928), which suggests that an inverse relationship exists between metabolic rate and lifespan, and supports earlier work on cold-exposed rats (Holloszy & Smith 1986). Our experimental design extends these previous data by removing one major criticism of previous tests of this theory, specifically the failure to simultaneously determine metabolic rate and assay lifespan within the same individuals and under the same environmental conditions (Van Voorhies et al. 2004).
The disposable soma theory provides an evolutionary explanation for ageing through the partitioning of limited resources to either reproduction or somatic maintenance (Kirkwood & Rose 1991). The main physiological mediated cost of reproduction is likely to be energetic in nature (Speakman 2008), with even the indirect energetic consequences of mounting an immune response apparently diverting limited resources away from both reproduction and survival (Hanssen et al. (2004)—but see Speakman (2008) for an alternative explanation of these observations). We predicted that any increase in resource allocation towards metabolism, irrespective of the cause of this increase, would result in somatic (oxidative) damage. It has been suggested that metabolically mediated somatic damage may result from an increase in oxidative damage to lipids, proteins and nucleic acids induced by ROS production, as ROS are inevitable consequences of aerobic metabolism (Harman 1956; Beckman & Ames 1998). However, in agreement with the lack of an effect on lifespan, we observed no consistent effect in various oxidative stress parameters following lifelong cold exposure despite the significant elevation of metabolism in the cold. Hepatic DNA oxidative damage did increase in aged voles (20 months old) in the cold but only when using one of our site-specific enzymes (ENDO III) and not the other (FPG). No significant effect of cold exposure was observed on either lymphocyte DNA damage or hepatic lipid peroxidation. In addition, the age effects were complex in that hepatic lipid peroxidation decreased with age and antioxidant enzyme levels tended to be higher at 11 months rather than at 4 or 20 months of age. It is certainly possible that perhaps due to differential mortality and our cross-sectional experimental design that the older age classes included better quality individuals, i.e. individual with relatively less oxidative damage and/or reduced requirement for antioxidant protection. It is also possible that by studying animals older than 20 months of age we may have identified a greater age effect in the parameters measured.
It is suggested by the free radical/oxidative theory of ageing (Harman 1956; Beckman & Ames 1998) that a dynamic equilibrium exists between antioxidant and repair defences and ROS production, with the net effect being oxidative stress and ageing if these protective mechanisms are insufficient to prevent ROS-induced damage to macromolecules (Beckman & Ames 1998). Therefore, limited investment in the antioxidant protection and endogenous repair mechanisms may result in somatic damage following elevations in metabolic rate (Alonso-Alvarez et al. 2006). Wiersma et al. (2004) manipulated reproductive effort in zebra finches (Taeniopygia guttata) and showed that antioxidant enzyme activities (SOD and Gpx) decreased in the pectoral muscle of parents with experimentally increased brood sizes. These findings suggest that limited resources may have been channelled to fuel the increased metabolism to the detriment of somatic protection by the antioxidant enzymes, resulting in increased oxidative damage and fitness costs. However, we do not know if oxidative stress or lifespan were affected in those individuals with the larger broods, but resistance of red blood cells to oxidative stress decreases with increasing breeding effort in zebra finches (Alonso-Alvarez et al. 2006), with this trade-off perhaps being modulated via antioxidant (carotenoid) availability (Bertrand et al. 2006). In agreement with our oxidative stress assays, no effect of cold exposure on catalase, Gpx and SOD activity was observed in the various tissues measured, except for SOD activity in BAT that was higher in the cold. Non-shivering thermogenesis is the main mechanism for heat generation in small mammals in the cold and ROS production increases significantly in BAT of cold-exposed rats (Barja de Quiroga et al. 1991). However, we have previously reported that protein carbonyl levels in BAT do not increase following acute cold exposure in voles, unlike levels in skeletal muscle and liver (Selman et al. 2002a). Consequently, it is possible that the cold-exposed voles responded to elevated superoxide in BAT by adaptively increasing SOD activity. The lack of an increase in pectoral muscle antioxidant activities in zebra finches (Wiersma et al. 2004) following brood enlargement may then simply reflect that this tissue was not the one most affected by the subsequent elevations in energy demand. However, the relationship between metabolism, ROS and antioxidant protection is highly complex (Barja 2007). For example, acute bouts of intense metabolism such as forced treadmill running may actually decrease skeletal muscle antioxidant enzyme activity (Ji 1993).
It is also conceivable that the lack of a lifespan effect in the cold, despite a lifelong elevation in metabolic rate, was because the ratio between metabolic rate and ROS production is not fixed. Indeed, there is a general decline in absolute and relative (per unit O2 consumed) ROS production in mitochondria during voluntary exercise, for example (for discussion see Barja 2007). The ‘uncoupling to survive’ theory (Brand 2000) suggests exactly this, that mitochondrial uncoupling results in a lowering of proton motive force and decreased superoxide production. However, uncoupling reduces mitochondrial efficiency and consequently leads to an increased respiration rate (Brand 2000; Speakman et al. 2002). No treatment effect was seen in hepatic levels of the antioxidants retinol, ascorbic acid or α-tocopherol. These findings demonstrating no clear induction of antioxidant protection despite a significant increase in metabolic rate (see Wiersma et al. 2004) perhaps indicates that the endogenous antioxidants were sufficient to cope with the elevated metabolism we imposed. Indeed, the elevation in protection may be specifically targeted to tissues where the greatest energetic demands occur, such as BAT during cold exposure.
Although we observed no effect of our manipulations on either oxidative damage or lifespan in these animals, it remains possible that physiological aspects of energy balance do mediate the negative trade-off between reproductive activity and survival. This might occur, for example, if the critical feature causing this trade-off is the maximal rate of energy demand, rather than the time-averaged rate. During cold exposure, we continuously exposed the voles to an elevated rate of energy expenditure (over 50–70% depending on age). During reproduction, however, the energy costs are more transient but for short periods substantially higher (Johnson et al. 2001; Krol et al. 2003; Speakman & Krol 2005b). During these short periods of intense metabolic activity, the level of ROS production may overwhelm the capacity of the defence system to cope (Ji 1993), and it is during these periods that oxidative damage may occur. This may not have been picked up by our experimental approach where energy demands, although lifelong, were not elevated to such transiently high levels.
Another possibly important factor is that during cold exposure adverse effects of ROS production may be mitigated by the elevation in levels of mitochondrial uncoupling, which facilitates heat production in the cold. It is interesting that until 750 days of age the cold-exposed animals in spite of their much greater energy demands actually had lower mortality consistent with the uncoupling to survive idea (Brand 2000). By contrast, during the peak period of reproduction, levels of uncoupling proteins are highly suppressed (Trayhurn 1989), which may imply greater levels of ROS are generated for any given level of energy expenditure. It would be interesting therefore to repeat this work by elevating energetic demands transiently via repeated reproductive events, to measure directly the effects of peak lactation energy demands on mitochondrial uncoupling, ROS production, oxidative damage and protection. The lack of an effect of reproductive activity on survival and lifespan in captive animals (Ricklefs & Cadena 2007) suggests that the difference in patterning of energy demands and different levels of uncoupling may not be that important.
In conclusion, while the idea that elevated energy demands linked to ROS production may be an attractive hypothetical mechanism underpinning the empirically observed life-history trade-offs in animals, we were unable to find evidence for any such mechanism when we experimentally elevated energy expenditure by cold exposure in captive animals. These findings may be because the relationship between metabolic rate and ROS production is far more complex than the simple one-to-one relationship often inferred (see Barja 2007), perhaps due to alterations in mitochondrial uncoupling in the cold (Brand 2000). Our data suggest that physiological costs of increased metabolic rate are not incurred via oxidative damage at least when metabolism is consistently elevated by over 50% in non-resource limited captive voles. However, we should also highlight that that post hoc power analysis calculated for the mean power to detect significant treatment effects was approximately 21% for our oxidative stress and antioxidant assays. Therefore, care should be taken in such cases not to definitively accept any null hypothesis outright. More transient but greater elevations in metabolism that characterize periods such as reproduction (Krol et al. 2003; Speakman 2008) may have different effects that require further investigation. We suggest that studies that will measure potential costs of elevated metabolism and lifespan in resource limited wild-living populations are now essential as the competing demands for these limited resources, driven by both physiological and ecological mediated costs, are likely to be significant (Speakman 2008). The ability to trade resources between life-history choices that may potentially induce damage or protect the soma are likely to be far more restrictive on individual animals simultaneously facing inclement environmental conditions, experiencing intense metabolic challenges, such as reproduction and with limited access to resources. There is now a requirement to test these ideas formally under field conditions.
Acknowledgments
All experiments were carried out under local ethical review (University of Aberdeen) and under licence from the UK Home Office.
We thank Duncan Wood, Shona Fleming, Jim Levenie, Jodie Fleming, Susanne Lumsden, Paula Redman and Peter Thompson (all University of Aberdeen), and Nik Vaughan, Sharon Wood and Philip Morrice (all Rowett Research Institute, Aberdeen) for their technical help. We thank Prof. Gustavo Barja (Complutense University, Spain) for the antioxidant enzyme protocols. Research was funded by a BBSRC (SAGE-1 initiative) grant to J.R.S. The authors declare no competing financial interests.
References
- Alonso-Alvarez C, Bertrand S, Devevey G, Prost J, Faivre B, Chastel O, Sorci G. An experimental manipulation of life-history trajectories and resistance to oxidative stress. Evolution. 2006;60:1913–1924. doi:10.1111/j.0014-3820.2006.tb00534.x [PubMed] [Google Scholar]
- Austad S.N, Fischer K.E. Mammalian aging, metabolism, and ecology: evidence from the bats and marsupials. J. Gerontol. 1991;46:B47–B53. doi: 10.1093/geronj/46.2.b47. [DOI] [PubMed] [Google Scholar]
- Barja G. Mitochondrial oxygen consumption and reactive oxygen species production are independently modulated: implications for aging studies. Rejuvenation Res. 2007;10:215–224. doi: 10.1089/rej.2006.0516. doi:10.1089/rej.2006.0516 [DOI] [PubMed] [Google Scholar]
- Barja G, Cadenas S, Rojas C, Perez-Campo R, Lopez-Torres M. Low mitochondrial free radical production per unit O2 consumption can explain the simultaneous presence of high longevity and high aerobic metabolic rate in birds. Free Radic. Res. 1994;21:317–327. doi: 10.3109/10715769409056584. doi:10.3109/10715769409056584 [DOI] [PubMed] [Google Scholar]
- Barja de Quiroga G, Lopez-Torres M, Perez-Campo R, Abelenda M, Paz Nava M, Puerta M.L. Effect of cold acclimation on GSH, antioxidant enzymes and lipid peroxidation in brown adipose tissue. Biochem. J. 1991;277(Pt 1):289–292. doi: 10.1042/bj2770289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman K.B, Ames B.N. The free radical theory of aging matures. Physiol. Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- Berteaux D, Thomas D.W, Bergeron J.-M, Lapierre H. Repeatability of daily field metabolic rate in female meadow voles (Microtus pennsylvanicus) Funct. Ecol. 1996;10:751–759. doi:10.2307/2390510 [Google Scholar]
- Bertrand S, Alonso-Alvarez C, Devevey G, Faivre B, Prost J, Sorci G. Carotenoids modulate the trade-off between egg production and resistance to oxidative stress in zebra finches. Oecologia. 2006;147:576–584. doi: 10.1007/s00442-005-0317-8. doi:10.1007/s00442-005-0317-8 [DOI] [PubMed] [Google Scholar]
- Brand M.D. Uncoupling to survive? The role of mitochondrial inefficiency in ageing. Exp. Gerontol. 2000;35:811–820. doi: 10.1016/s0531-5565(00)00135-2. doi:10.1016/S0531-5565(00)00135-2 [DOI] [PubMed] [Google Scholar]
- Collins A.R. The comet assay for DNA damage and repair: principles, applications, and limitations. Mol. Biotechnol. 2004;26:249–261. doi: 10.1385/MB:26:3:249. doi:10.1385/MB:26:3:249 [DOI] [PubMed] [Google Scholar]
- Daan S, Deerenberg C, Dijkstra C. Increased daily work precipitates natural death in the kestrel. J. Anim. Ecol. 1996;65:539–544. doi:10.2307/5734 [Google Scholar]
- Descamps S, Boutin S, Berteaux D, Gaillard J.M. Best squirrels trade a long life for an early reproduction. Proc. Biol. Sci. 2006;273:2369–2374. doi: 10.1098/rspb.2006.3588. doi:10.1098/rspb.2006.3588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R.A. Claredon Press; Oxford, UK: 1930. The genetical theory of natural selection. [Google Scholar]
- Hanssen S.A, Hasselquist D, Folstad I, Erikstad K.E. Costs of immunity: immune responsiveness reduces survival in a vertebrate. Proc. Biol. Sci. 2004;271:925–930. doi: 10.1098/rspb.2004.2678. doi:10.1098/rspb.2004.2678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Hayes J.P, Speakman J.R, Racey P.A. Sampling bias in respirometry. Physiol. Zool. 1992;65:604–619. [Google Scholar]
- Hoffman A.R, Parsons P.A. Oxford University Press; Oxford, UK: 1991. Evolutionary genetics and environmental stress. [Google Scholar]
- Holloszy J.O, Smith E.K. Longevity of cold-exposed rats: a reevaluation of the “rate-of-living theory”. J. Appl. Physiol. 1986;61:1656–1660. doi: 10.1152/jappl.1986.61.5.1656. [DOI] [PubMed] [Google Scholar]
- Ji L.L. Antioxidant enzyme response to exercise and aging. Med. Sci. Sports Exerc. 1993;25:225–231. [PubMed] [Google Scholar]
- Johnson M.S, Thomson S.C, Speakman J.R. Limits to sustained energy intake. III. Effects of concurrent pregnancy and lactation in Mus musculus. J. Exp. Biol. 2001;204:1947–1956. doi: 10.1242/jeb.204.11.1947. [DOI] [PubMed] [Google Scholar]
- Johnston S.L, et al. Having it all: historical energy intakes do not generate the anticipated trade-offs in fecundity. Proc. Biol. Sci. 2006;273:1369–1374. doi: 10.1098/rspb.2005.3456. doi:10.1098/rspb.2005.3456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood T.B.L, Rose M.R. Evolution of senescence: late survival sacrificed for reproduction. Phil. Trans. R. Soc. B. 1991;332:15–24. doi: 10.1098/rstb.1991.0028. doi:10.1098/rstb.1991.0028 [DOI] [PubMed] [Google Scholar]
- Krol E, Johnson M.S, Speakman J.R. Limits to sustained energy intake. VIII. Resting metabolic rate and organ morphology of laboratory mice lactating at thermoneutrality. J. Exp. Biol. 2003;206:4283–4291. doi: 10.1242/jeb.00676. doi:10.1242/jeb.00676 [DOI] [PubMed] [Google Scholar]
- Lifson N, Gordon G.B, McClintock R. Measurement of total carbon dioxide production by means of D2O18. J. Appl. Physiol. 1955;7:704–710. doi: 10.1152/jappl.1955.7.6.704. [DOI] [PubMed] [Google Scholar]
- Lopez-Torres M, Perez-Campo R, Rojas C, Cadenas S, Barja G. Maximum life span in vertebrates: relationship with liver antioxidant enzymes, glutathione system, ascorbate, urate, sensitivity to peroxidation, true malondialdehyde, in vivo H2O2, and basal and maximum aerobic capacity. Mech. Ageing Dev. 1993;70:177–199. doi: 10.1016/0047-6374(93)90047-u. doi:10.1016/0047-6374(93)90047-U [DOI] [PubMed] [Google Scholar]
- McDevitt R.M, Speakman J.R. Long photophase is not a sufficient stimulus to reduce thermogenic capacity in winter-acclimatized short-tailed field voles (Microtus agrestis) during long-term cold acclimation. J. Comp. Physiol. [B] 1994;164:159–164. doi: 10.1007/BF00301659. [DOI] [PubMed] [Google Scholar]
- Moe B, Angelier F, Bech C, Chastel O. Is basal metabolic rate influenced by age in a long-lived seabird, the snow petrel? J. Exp. Biol. 2007;210:3407–3414. doi: 10.1242/jeb.005090. doi:10.1242/jeb.005090 [DOI] [PubMed] [Google Scholar]
- Nussey D.H, Kruuk L.E, Donald A, Fowlie M, Clutton-Brock T.H. The rate of senescence in maternal performance increases with early-life fecundity in red deer. Ecol. Lett. 2006;9:1342–1350. doi: 10.1111/j.1461-0248.2006.00989.x. doi:10.1111/j.1461-0248.2006.00989.x [DOI] [PubMed] [Google Scholar]
- Pamplona R, Portero-Otin M, Requena J.R, Thorpe S.R, Herrero A, Barja G. A low degree of fatty acid unsaturation leads to lower lipid peroxidation and lipoxidation-derived protein modification in heart mitochondria of the longevous pigeon than in the short-lived rat. Mech. Ageing Dev. 1999;106:283–296. doi: 10.1016/s0047-6374(98)00121-3. doi:10.1016/S0047-6374(98)00121-3 [DOI] [PubMed] [Google Scholar]
- Partridge L. Evolutionary theories of ageing applied to long-lived organisms. Exp. Gerontol. 2001;36:641–650. doi: 10.1016/s0531-5565(00)00232-1. doi:10.1016/S0531-5565(00)00232-1 [DOI] [PubMed] [Google Scholar]
- Pearl R.L. Alfred Knopf; New York, NY: 1928. The rate of living. [Google Scholar]
- Reid J.M, Bignal E.M, Bignal S, McCracken D.I, Monaghan P. Age-specific reproductive performance in red-billed choughs Pyrrhocorax pyrrhocorax: patterns and processes in a natural population. J. Anim. Ecol. 2003;72:765–776. doi:10.1046/j.1365-2656.2003.00750.x [Google Scholar]
- Reznick D.N. The genetic basis of aging: an evolutionary biologist's perspective. Sci. Aging Knowl. Environ. 2005;2005:pe7. doi: 10.1126/sageke.2005.11.pe7. doi:10.1126/sageke.2005.11.pe7 [DOI] [PubMed] [Google Scholar]
- Ricklefs R.E, Cadena C.D. Lifespan is unrelated to investment in reproduction in populations of mammals and birds in captivity. Ecol. Lett. 2007;10:867–872. doi: 10.1111/j.1461-0248.2007.01085.x. doi:10.1111/j.1461-0248.2007.01085.x [DOI] [PubMed] [Google Scholar]
- Rubner M. Oldenbourg; Munchen, Germany: 1908. Das problem der lebensdauer und seine beziehungen zum wachstum und ernahrung. [Google Scholar]
- Schmidt-Hempel P, Wolf T. Foraging effort and life span of workers in a social insect. J. Anim. Ecol. 1988;57:500–521. [Google Scholar]
- Selman C, McLaren J.S, Himanka M.J, Speakman J.R. Effect of long-term cold exposure on antioxidant enzyme activities in a small mammal. Free Radic. Biol. Med. 2000;28:1279–1285. doi: 10.1016/s0891-5849(00)00263-x. doi:10.1016/S0891-5849(00)00263-X [DOI] [PubMed] [Google Scholar]
- Selman C, Grune T, Stolzing A, Jakstadt M, McLaren J.S, Speakman J.R. The consequences of acute cold exposure on protein oxidation and proteasome activity in short-tailed field voles, Microtus agrestis. Free Radic. Biol. Med. 2002a;33:259–265. doi: 10.1016/s0891-5849(02)00874-2. doi:10.1016/S0891-5849(02)00874-2 [DOI] [PubMed] [Google Scholar]
- Selman C, McLaren J.S, Collins A.R, Duthie G.G, Speakman J.R. Antioxidant enzyme activities, lipid peroxidation, and DNA oxidative damage: the effects of short-term voluntary wheel running. Arch. Biochem. Biophys. 2002b;401:255–261. doi: 10.1016/S0003-9861(02)00050-4. doi:10.1016/S0003-9861(02)00050-4 [DOI] [PubMed] [Google Scholar]
- Selman C, McLaren J.S, Meyer C, Duncan J.S, Redman P, Collins A.R, Duthie G.G, Speakman J.R. Life-long vitamin C supplementation in combination with cold exposure does not affect oxidative damage or lifespan in mice, but decreases expression of antioxidant protection genes. Mech. Ageing Dev. 2006;127:897–904. doi: 10.1016/j.mad.2006.09.008. doi:10.1016/j.mad.2006.09.008 [DOI] [PubMed] [Google Scholar]
- Speakman J.R. How should we calculate CO2 production in doubly labeled water studies of animals? Funct. Ecol. 1993;7:746–750. [Google Scholar]
- Speakman J.R. Chapman and Hall; London, UK: 1997. Doubly labelled water. Theory and practice. [Google Scholar]
- Speakman J.R. The history and theory of the doubly labeled water technique. Am. J. Clin. Nutr. 1998;68:932S–938S. doi: 10.1093/ajcn/68.4.932S. [DOI] [PubMed] [Google Scholar]
- Speakman J.R. Body size, energy metabolism and lifespan. J. Exp. Biol. 2005;208:1717–1730. doi: 10.1242/jeb.01556. doi:10.1242/jeb.01556 [DOI] [PubMed] [Google Scholar]
- Speakman J.R. The physiological costs of reproduction in small mammals. Phil. Trans. R. Soc. B. 2008;363:375–398. doi: 10.1098/rstb.2007.2145. doi:10.1098/rstb.2007.2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman J.R, Krol E. Comparison of different approaches for the calculation of energy expenditure using doubly labeled water in a small mammal. Physiol. Biochem. Zool. 2005a;78:650–667. doi: 10.1086/430234. doi:10.1086/430234 [DOI] [PubMed] [Google Scholar]
- Speakman J.R, Krol E. Limits to sustained energy intake IX: a review of hypotheses. J. Comp. Physiol. [B] 2005b;175:375–394. doi: 10.1007/s00360-005-0013-3. [DOI] [PubMed] [Google Scholar]
- Speakman J.R, Racey P.A. The equilibrium concentration of O-18 in body-water - implications for the accuracy of the doubly-labeled water technique and a potential new method of measuring RQ in free-living animals. J. Theor. Biol. 1987;127:79–95. doi:10.1016/S0022-5193(87)80162-5 [Google Scholar]
- Speakman J.R, Racey P.A, Haim A, Webb P.I, Ellison G.T.H, Skinner J.D. Interindividual and intraindividual variation in daily energy expenditure of the pouched mouse (Saccostomus campestris) Funct. Ecol. 1994;8:336–342. doi:10.2307/2389826 [Google Scholar]
- Speakman J.R, Selman C, McLaren J.S, Harper E.J. Living fast, dying when? The link between aging and energetics. J. Nutr. 2002;132:1583S–1597S. doi: 10.1093/jn/132.6.1583S. [DOI] [PubMed] [Google Scholar]
- Speakman J.R, et al. Uncoupled and surviving: individual mice with high metabolism have greater mitochondrial uncoupling and live longer. Aging Cell. 2004;3:87–95. doi: 10.1111/j.1474-9728.2004.00097.x. doi:10.1111/j.1474-9728.2004.00097.x [DOI] [PubMed] [Google Scholar]
- Stearns S.C. Oxford University Press; Oxford, UK: 1992. The evolution of life histories. [Google Scholar]
- Trayhurn P. Thermogenesis and the energetics of pregnancy and lactation. Can. J. Physiol. Pharmacol. 1989;67:370–375. doi: 10.1139/y89-060. [DOI] [PubMed] [Google Scholar]
- Van Voorhies W.A, Khazaeli A.A, Curtsinger J.W. Testing the “rate of living” model: further evidence that longevity and metabolic rate are not inversely correlated in Drosophila melanogaster. J. Appl. Physiol. 2004;97:1915–1922. doi: 10.1152/japplphysiol.00505.2004. doi:10.1152/japplphysiol.00505.2004 [DOI] [PubMed] [Google Scholar]
- Visser G.H, Schekkerman H. Validation of the doubly labeled water method in growing precocial birds: the importance of assumptions concerning evaporative water loss. Physiol. Biochem. Zool. 1999;72:740–749. doi: 10.1086/316713. doi:10.1086/316713 [DOI] [PubMed] [Google Scholar]
- Wiersma P, Selman C, Speakman J.R, Verhulst S. Birds sacrifice oxidative protection for reproduction. Proc. Biol. Sci. 2004;271(Suppl. 5):S360–S363. doi: 10.1098/rsbl.2004.0171. doi:10.1098/rsbl.2004.0171 [DOI] [PMC free article] [PubMed] [Google Scholar]