Abstract
Transforming growth factor-β (TGF-β) signals through three highly conserved cell surface receptors, the type III TGF-β receptor (TβRIII), the type II TGF-β receptor (TβRII), and the type I TGF-β receptor (TβRI) to regulate diverse cellular processes including cell proliferation, differentiation, migration, and apoptosis. Although TβRI and TβRII undergo ligand-independent endocytosis by both clathrin-mediated endocytosis, resulting in enhanced signaling, and clathrin-independent endocytosis, resulting in receptor degradation, the mechanism and function of TβRIII endocytosis is poorly understood. TβRIII is a heparan sulfate proteoglycan with a short cytoplasmic tail that functions as a TGF-β superfamily co-receptor, contributing to TGF-β signaling through mechanisms yet to be fully defined. We have reported previously that TβRIII endocytosis, mediated by a novel interaction with βarrestin-2, results in decreased TGF-β signaling. Here we demonstrate that TβRIII undergoes endocytosis in a ligand and glycosaminoglycan modification-independent and cytoplasmic domain-dependent manner, with the interaction of Thr-841 in the cytoplasmic domain of TβRIII with β-arrestin2 enhancing TβRIII endocytosis. TβRIII undergoes both clathrin-mediated and clathrin-independent endocytosis. Importantly, inhibition of the clathrin-independent, lipid raft pathway, but not of the clathrin-dependent pathway, results in decreased TGF-β1 induced Smad2 and p38 phosphorylation, supporting a specific role for clathrin-independent endocytosis of TβRIII in regulating both Smad-dependent and Smad-independent TGF-β signaling.
The TGF-β2 superfamily is composed of more than 30 polypeptide growth factors, including the bone morphogenetic proteins and activins, which regulate cell proliferation, differentiation, adhesion, angiogenesis, and embryonic development (1–4). Three highly conserved and tissue-specific TGF-β isoforms signal through heteromeric complexes of three cell surface receptors, the type III TGF-β receptor (TβRIII, or betaglycan), the type II TGF-β receptor (TβRII), and the type I TGF-β receptor (TβRI). Although TβRII and TβRI are both transmembrane serine/threonine kinase receptors, TβRIII is a heparan sulfate proteoglycan with a short cytoplasmic tail that fundamentally contributes to TGF-β signaling through mechanisms yet to be defined. TβRIII binds all three forms of TGF-β ligands specifically; TβRII binds TGF-β1 and TGF-β3 independently, whereas TβRI cannot bind ligand on its own. Upon ligand binding, the constitutively active TβRII recruits TβRI into an active heterotetrameric signaling complex composed of two TβRIs and two TβRIIs and transphosphorylates the glycine and serine rich region of TβRI to activate its kinase function (5). TβRI can then phosphorylate C-terminal serine residues on transcription factors known as Smads, specifically the receptor-Smads, Smad2 and Smad3, which then associate with the common-Smad, Smad4 (6). This association allows accumulation of the complex into the nucleus and transcription of target genes. This classical Smad-dependent TGF-β signaling leads to negative regulation of cell proliferation (1, 7, 8).
Because TβRIII lacked a known signaling motif in its cytoplasmic domain and cells could respond to TGF-β in the absence of TβRIII, TβRIII was initially thought to solely function in presenting ligand to TβRII (9, 10). However, recent studies have challenged this model, demonstrating an essential role of TβRIII in TGF-β2 signaling (11), an essential role in mesenchymal transformation in chick embryonic heart development (12) and an essential role in mediating TGF-β resistance in intestinal goblet cells (13). In addition, TβRIII has been highly conserved evolutionarily with 98% identity between rat and human species, suggesting essential roles for this receptor (14). Recent studies by our laboratory and others have demonstrated frequent loss of TβRIII expression at the mRNA and protein level in human cancer tissues, defining a tumor suppressor role for TβRIII in many cancer types, including cancers of the breast (15), kidney (16), lung (17), ovary (18), pancreas (19), and prostate (20). TβRIII functions as a tumor suppressor primarily through its ability to inhibit cancer cell motility and invasion, as demonstrated in both in vitro and in vivo models. In addition, TβRIII, like other co-receptors, is promiscuous in terms of ligand binding (21), binding inhibin (22), basic fibroblast growth factor (23), and bone morphogenetic protein family members (24) in addition to the TGF-β isoforms, suggesting broader roles for TβRIII in mediating and orchestrating signaling.
We have demonstrated that TβRII can phosphorylate TβRIII on its cytoplasmic domain in vivo (25). This phosphorylation, specifically on residue Thr841, promotes association of the scaffolding protein β-arrestin2 and endocytosis of TβRIII and TβRII with subsequent down-regulation of TGF-β signaling (25). β-Arrestin2 is a member of a family of versatile adapter proteins known to regulate the signaling and internalization of certain activated G-protein-coupled receptors (25). β-Arrestin2 can form specific interactions with members of the endocytic machinery, including clathrin and AP2, resulting in internalization of cell surface receptors, and triggering additional signaling events (26). β-Arrestin2 contains a consensus mitogen-activated protein (MAP) kinase recognition sequence within its C-terminal domain, which enables it to function as a scaffold for two MAP kinase cascades, ERK1/2 and c-Jun NH2-terminal kinase, potentially increasing signaling efficiency of activated receptors (26, 27). For example, the association of an activated β2-adrenergic receptor with β-arrestin allows the recruitment of c-Src and endocytic machinery, triggering internalization and downstream activation of the ERK1/2 pathway (26). Phosphorylation and activation of the ERK1/2 pathway by β-arrestins leads to their translocation into the nucleus where they regulate transcription of genes involved in cell survival, growth, differentiation, cell motility, and invasion (26). TGF-β is known to activate mitogen-activated protein kinase pathways through mechanisms that are yet to be discerned (5, 28).
The two most common methods of endocytosis of cell surface receptors are clathrin-mediated and clathrin-independent or lipid raft/caveolae-mediated endocytosis. Clathrin-mediated endocytosis, the most well studied method, is characterized by formation of clathrin-coated pits at the plasma membrane. Specifically, the formation of clathrin-coated pits is initiated by adaptor protein 2 (AP2) which recruits clathrin and other accessory proteins to the plasma membrane, causing formation of a lattice network. The GTPase dynamin proceeds to “pinch off” the clathrin-coated pit, and the resulting vesicle can recycle its cargo to the plasma membrane, be stripped of clathrin, and enter the early endosomal compartment, resulting in either degradation via the lysosomal pathway or transportation to specific subcellular locations for signaling (29, 30). Lipid raft endocytosis is characterized by plasma membrane domains containing cholesterol, glycosphingolipids, and sometimes caveolin-1. Caveolae, a specialized form of the structures that form on the plasma membrane in lipid raft endocytosis, are abundant in many mammalian cells and closely associate with caveolin-1 (31). This method of endocytosis, whereas not as well studied, is known to be sensitive to cholesterol depletion and has been shown to both cross-talk with and be segregated from common endosomal compartments (32). Previous studies have demonstrated the importance of investigating this method of endocytosis, with a growing number of cell surface receptors undergoing lipid raft-mediated endocytosis: interleukin-2 receptor β (33), autocrine motility factor receptor (34), SV40 (35), TβRI, and TβRII (8, 32).
Although TβRI and TβRII undergo ligand-independent internalization by clathrin-mediated endocytosis, resulting in enhanced signaling, as well as clathrin-independent endocytosis, resulting in receptor degradation, internalization of TβRIII has been largely unexplored (8, 36, 37). Here we examined the mechanism and function of TβRIII internalization.
EXPERIMENTAL PROCEDURES
Cell Culture and Reagents—COS7 cells were maintained in DMEM media supplemented with 10% fetal bovine serum (FBS) (Invitrogen). HepG2 cells were maintained in minimum Eagle's medium supplemented with 10% FBS. L6 stable cells were maintained in DMEM supplemented with 10% FBS and 250 μg/ml G418. Cells were passaged at 37 °C in 5% CO2.
Cell Surface ELISA—55,000–60,000 COS7 cells × n (number of samples) were plated in a 10-cm dish. At 80% confluence 300 ng/sample of each DNA construct was transfected using FuGENE 6 (Roche Diagnostics) according to the manufacturer's protocol (2 μl of FuGENE:1 μg of DNA). 24 h post-transfection cells were trypsinized, centrifuged, and re-plated into 4-well dishes. 24 h later cells were put on ice and rinsed once with PBS. Samples were then incubated with a TβRIII extracellular specific antibody (αRIII-EC) (R&D Systems, Minneapolis, MN) at 1:250 in DMEM serum-free media for 30 min, rocking at 4 °C. Cells were rinsed once with PBS and replaced with 1 ml of DMEM serum-free media, DMEM + 10% FBS, or DMEM serum-free media + 150 pm TGF-β1. Cells were either kept on ice (0 min samples) or put at 37 °C over the time course of interest. Cells were then returned to ice, rinsed once with PBS, fixed with 2% formaldehyde for 10 min, and rinsed once more with PBS. Cells were rocked at room temperature while incubated with secondary antibody, α-goat-horseradish peroxidase at 1:1000 (in 5% bovine serum albumin in PBS-Tween) for 15 min, rocking. Cells were then rinsed 2–3 times with PBS and rocked at room temperature for 30 min with 100 μl of a 1-Step Turbo TMB-ELISA substrate (Pierce) immediately followed by the addition of 100 μl of 1 m sulfuric acid. Quantification of cell surface TβRIII expression was accomplished by measuring the Absorbance @450 nm using a Wallac Victor Counter (PerkinElmer Life Sciences) normalized to background.
Trypsin Internalization Assay—COS7 cells were plated in 6-cm dishes to reach 60% confluence followed by transfection of 2 μg of DNA per plate of indicated constructs using FuGENE 6 according to the manufacturer's protocol. 48 h later cells were put on ice and rinsed three times with ice-cold PBS. Then cells were incubated with 0.5 mg/ml Sulfo-NHS-LC-Biotin (Pierce) in Hepes buffer (150 mm NaCl, 5 mm KCl, 1.3 mm CaCl2, 1.2 mm MgCl2, and 10 mm Hepes), rocking at 4 °C for 2 h. Cells on ice were rinsed twice with ice-cold PBS. 1 ml of room temperature media (DMEM + 10% FBS or DMEM serum-free media) was added to select samples followed by a time course at 37 °C. Cells were then rinsed once with PBS on ice and incubated at 37 °C with trypsin for ≈1 min followed by immediate addition of DMEM + 10% FBS on ice. Cells were lysed on ice with 1 ml of RIPA plus protease inhibitors (leupeptin, pepstatin, phenylmethylsulfonyl fluoride, and dithiothreitol), kept on ice for 30 min, and centrifuged at 14,000 rpm for 30 min at 4 °C. Pellets were removed, and 60 μl of each sample was added to 30-μl of 2× sample buffer for β-actin. Samples were then added to either 10 μl of α820 or αRIII-EC antibodies with 25 μl of protein A sepharose or protein G sepharose beads, respectively, and immunoprecipitated at 4 °C overnight. Immunoprecipitations were rinsed 3 times with radioimmune precipitation assay buffer plus protease inhibitors (at 14,000 rpm for 2 min at 4 °C) followed by the addition of 2× sample buffer. All samples were boiled for 10 min and run on a 7.5% SDS-PAGE gel. Western blots were performed using strep-horseradish peroxidase at 1:10,000 and αβ-actin at 1:500–1:2000 followed by secondary α-mouse at 1:1000 and detected using a Pico West chemiluminescent substrate (Pierce).
Time Constant Calculations—Data were plotted as the percentage of initially labeled receptor remaining on the cell surface over a time course at 37 °C using Microsoft Excel. A single exponential decay function was then fit to the data (time points of 10 min and longer for ELISA and 5 min or longer for trypsin assays) of the form y = 100e-t/τ. The equation was then solved for the time constant τ.
Sucrose Density Fractionation—COS7 and HepG2 cells were plated in 10-cm dishes to obtain ∼1 × 108 cells in total when transfection was performed. COS7 cells were infected at 30 plaque-forming units/cell with Adeno-HA-hTβRIII or Adeno-HA-hTβRIIIΔgag. Cells were put on ice, rinsed once with PBS, collected, and centrifuged for 3 min; supernatant was aspirated and replaced with 1 ml of homogenization buffer (25 mm HEPES, pH 6.5, 1 mm EDTA, and 150 mm NaCl) plus 0.5% Triton X-100 and protease inhibitors. Cells were then passed 10 times through a 25-gauge needle and incubated on ice for 30 min. 1 ml of this cell solution was added to the bottom of centrifuge tubes followed by the addition of 1 ml of an 80% sucrose solution, 6.5 ml of a 30% sucrose solution, and 2.5 ml of a 5% sucrose solution. Samples were centrifuged at 38,000 rpm using a SW-40 swing-bucket rotor for ∼20 h at 4 °C. On ice, 900-μl top-to-bottom fractions were removed. The cell pellet (final fraction) was suspended in 900 μl of homogenization buffer and sonicated. A mix of 300 μl of each fraction was added to 60 μl of 6× sample buffer and run on 8 and 10% SDS-PAGE gels to Western blot for TβRIII, clathrin, caveolin-1, flotillin, and the cholera-toxin B subunit.
Down-regulation—Cells were plated in 6-well dishes to obtain 70% confluence on the start of the experiment. Cells were rinsed with 1 ml of warmed binding buffer (0.25 g of bovine serum albumin into 50 ml of KRH) followed by the addition of 2 ml of binding buffer for 30 min at 37 °C. Cells were moved to 4 °C, and 150 pm 125I-labeled TGF-β1 was added for 3 h, rocking. Cells were then rinsed 4 times with KRH followed by the addition of 0.05 mg/μl 2,2-dimethyl-2-silapentanesulfonic acid in DMSO solution for 15 min at 4 °C, rocking. 1 m glycine was added for 10 min to quench the 2,2-dimethyl-2-silapentanesulfonic acid/DMSO solution followed by 3 rinses with KRH. Warmed DMEM + 10% FBS was added to cells followed by a time course of interest at 37 °C. Cells were returned to 4 °C, rinsed once with KRH, lysed with hot 2× sample buffer, and boiled for 5 min. Samples were then run on a 7.5% SDS-PAGE gel, dried, and exposed to a phosphorimaging screen.
To examine the lipid raft endocytic pathway, COS7 cells were transfected with TβRIIIΔgag using FuGENE 6 according to the manufacturer's protocol. After 48 h cells were exposed to 250 pm 125I-TGF-β1 binding and cross-linking as described above. After 2.5 h, select cells were pretreated for 30 min with 25, 50, or 100 μg/ml nystatin ± 0.25 mm cholesterol and exposed to the same concentrations of nystatin ± 0.25 mm cholesterol for the first hour of the 5-h time course. Buffers contained 0.025 mg/ml cycloheximide throughout the experiment. Cells were lysed and analyzed as described above. Densitometry was performed using ImageJ.
Examination of the clathrin-mediated endocytic pathway was done by FuGENE 6 transfection of COS7 cells with 2 μg of TβRIIIΔgag or TβRIII-FL or HepG2 cells with transfection of a control dynamin wild-type construct or mutant dynamin K44A. Binding and cross-linking was performed as described above, with ranges of 25–150 pm 125I-TGF-β1 used followed by a 5- or 22-h time course at 37 °C. Buffers contained 0.025 mg/ml cycloheximide throughout the experiment. Cells were lysed with 2× sample buffer, boiled, separated on a 7.5% SDS-page gel, and exposed to a phosphorimaging screen. β-Actin served as a loading control, and densitometry was performed using ImageJ.
Confocal Microscopy—Approximately 2 × 105 COS7 cells were plated on poly-d-lysine-coated coverslips in a 6-well dish. 2 μg of each construct, TβRIII-FL and Rab9 or SARA (smad-anchor for receptor activation), was transfected using FuGENE 6 according to manufacturer's protocol. After 48 h cells were rinsed 3 times with PBS and fixed with 4% paraformaldehyde in PBS at room temperature for 20 min. Cells were rinsed 3 times with PBS, permeabilized for 5 min at room temperature with 0.1% Triton X-100 in PBS, rinsed twice, blocked for 1 h at room temperature with 1% bovine serum albumin in PBS, and again rinsed twice. Samples were then incubated for 1 h in primary antibody diluted in 1% bovine serum albumin in PBS. TβRIII-FL was detected using an antibody specific to the extracellular domain, αRIII-EC, at 1:250. SARA was detected using a monoclonal α-FLAG M2 antibody (Sigma) at 1:500. Endogenous clathrin was detected using a monoclonal α-clathrin heavy chain antibody (Sigma) at 1:100. Endogenous caveolin was detected using a monoclonal α-caveolin 1 antibody (BD Biosciences) at 1:100. Endogenous flotillin was detected using α-flotillin-1 antibody (BD Biosciences) at 1:100. Cells were rinsed 3 × 5 min with PBS followed by a 20-min incubation with rabbit α-goat Alexa 594 or rabbit α-mouse Alexa 488 (Molecular Probes, Eugene, OR) secondary antibody at 1:500 or 1:1000 dilutions in 1% bovine serum albumin in PBS covered in foil. Cells were rinsed 3 × 5 min with PBS followed by a 10-min incubation with 4′,6-diamidino-2-phenylindole (0.03% in PBS). Cells were rinsed three times in PBS followed by a rinse with distilled H2O and mounted onto slides using ProLong Gold Antifade reagent (Invitrogen). Analysis was done using a Zeiss LSM 510 inverted confocal microscope, and colocalization was determined by % pixel overlap using the LSM Image Browser.
Co-immunoprecipitation—COS-7 cells were transfected with Lipofectamine 2000 according to the manufacturer's protocol. 48 h post-transfection cells expressing vector control (pcDNA3.1) or RIII constructs (TβRIII-FL, TβRIIIΔGAG, and TβRIIIΔcyto) were washed with PBS then lysed on ice with lysis buffer (20 mm HEPES, pH 7.4, 150 mm NaCl, 0.5% Nonidet P-40, 2 mm EDTA, 10 mm NaF, 10% w/v glycerol) supplemented with protease inhibitors (Sigma protease inhibitor mixture) and phosphatase inhibitors (Sigma phosphatase inhibitor mixture). The lysates were precleared by centrifugation and incubated with an RIII-EC antibody for 4 h at 4 °C then with protein G-agarose beads for an additional 2 h. The immunoprecipitates were collected by centrifugation, and pellets were washed with lysis buffer and stored in 2× sample buffer before Western blot analyses.
Signaling—Untransfected HepG2 or L6 stables were serum-starved overnight or for 1–2 h, respectively. Select samples were either untreated or pretreated for 3 h with 50 μg/ml nystatin ± 0.25 mm cholesterol. All samples underwent a 10-min dose course of TGF-β1 treatment and lysis with 2× sample buffer. Western blot analysis was performed using p-Smad2, total Smad2, p-p38, and total p38 antibodies (Cell Signaling, Danvers, MA).
RESULTS
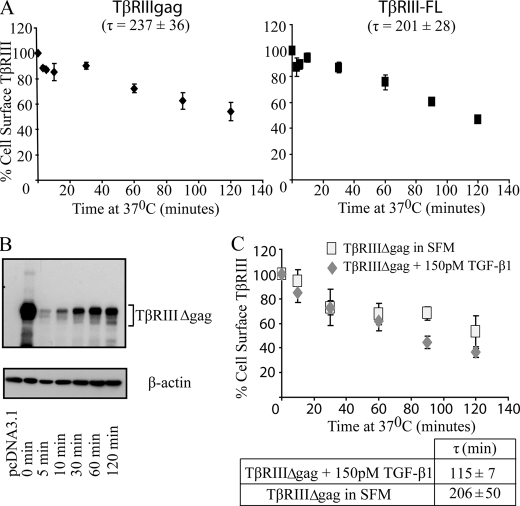
Time Course of TβRIII Internalization—Because TβRIII internalization has not been characterized previously, we first investigated the time course of TβRIII internalization utilizing two complementary methods; that is, a cell surface ELISA, which assesses loss of cell surface receptors from an initially labeled pool of cell surface receptors over a time course, and a trypsin-biotinylation internalization assay, which assesses internalized receptors from an initially labeled pool of biotinylated cell surface receptors. Because endogenous TGF-β receptors, including TβRIII, are very difficult to detect and to characterize TβRIII in isolation from other TGF-β superfamily receptors, we initially evaluated TβRIII internalization in overexpression models. Full-length TβRIII (TβRIII-FL) or TβRIIIΔgag (in which both glycosaminoglycan attachment sites are mutated preventing glycosaminoglyan modification of the extracellular domain) were expressed in COS7 cells, which express very low levels of TGF-β superfamily receptors. Receptor internalization was then followed using a cell surface ELISA over a 120-min time course at 37 °C, as preliminary time courses revealed maximum internalization at 120 min, after which cell surface levels stabilized (data not shown). Both TβRIII-FL and TβRIIIΔgag receptors were lost from the cell surface, reaching a maximum internalization of 46 ± 7% for TβRIIIΔgag and 53 ± 1% for TβRIII-FL (Fig. 1A). The data were fit to a single exponential decay function from which an average endocytic time constant, τ, could be determined. TβRIII-FL and TβRIIIΔgag internalized with similar kinetics, with endocytic time constants of τ = 201 ± 28 for TβRIII-FL and τ = 237 ± 36 min for TβRIIIΔgag (Fig. 1A), suggesting that the glycosaminoglycan modifications do not significantly alter TβRIII internalization. In a complementary manner, the trypsin-biotinylation internalization assay demonstrated time-dependent internalization of TβRIII and TβRIIIΔgag, with τ = 200 min (Fig. 1B, data not shown).
FIGURE 1.
TβRIII internalization is ligand-independent. A, a cell-surface ELISA was performed with COS7 cells transiently expressing TβRIIIΔgag or TβRIII-FL in full media over a 2-h time course at 37 °C. Averages from three or more independent experiments of TβRIII remaining on the cell surface at each time point normalized to empty vector control are plotted. The time constant values, τ, were obtained by fitting data to a single exponential decay function; bars = S.E. B, a trypsin internalization assay was performed in COS7 cells transiently expressing TβRIIIΔgag over the indicated time course at 37 °C. C, the effect of ligand on internalization was determined using a cell-surface ELISA in the presence of 150 pm TGF-β1 or serum-free media. Time constant values were obtained as described in A. Data represent the average of three or more independent experiments.
TβRIII Internalization Is Ligand-independent—Although some signaling pathways, including the epidermal growth factor receptor pathway, require ligand for initiation of receptor internalization and down-regulation, other signaling pathways, including the type I (TβRI) and type II (TβRII) TGF-β receptors undergo ligand-independent internalization (38, 39), with more complex processes regulating receptor turnover. To assess the effect of TGF-β on TβRIII internalization, we performed a cell surface ELISA assay and quantitated TβRIIIΔgag internalization in the presence and absence of 150 pm TGF-β. Here TβRIIIΔgag internalized with a time constant of τ = 206 ± 50 min and maximum internalization of 47 ± 13%, whereas in the presence of TGF-β, TβRIIIΔgag internalized with a time constant of τ = 115 ± 7 and maximum internalization of 64 ± 4% (Fig. 1C). Although there was a trend for TGF-β1 to enhance the rate of TβRIIIΔgag internalization, this was not statistically significant (p = 0.15, one-tailed t test). In addition, when comparing the time constant values of TβRIII in serum-free DMEM (τ = 206 ± 50 min) to DMEM + 10% serum (τ = 237 ± 36 min), containing TGF-β as well as other growth factors, there was no statistically significant difference, further supporting a model for ligand-independent internalization of TβRIII.
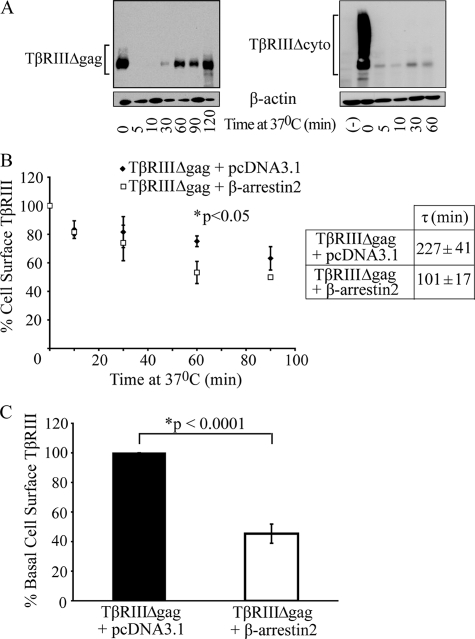
The Cytoplasmic Domain of TβRIII Is Necessary for Internalization—In addition to ligand, association with interacting proteins is another mechanism by which internalization can be regulated. In this regard the short 43-amino acid cytoplasmic domain of TβRIII contains binding sites for β-arrestin2 and GIPC (GAIP-interacting protein, C terminus), which we have demonstrated have opposing effects on TβRIII cell surface levels (25, 40). Thus, we aimed to investigate the significance of the cytoplasmic domain on internalization by utilizing COS7 cells overexpressing a mutant form of the receptor missing the cytoplasmic domain, TβRIIIΔcyto, in the trypsin-biotinylation internalization assay. Results consistently demonstrated a dramatic and statistically significant slower rate of internalization of TβRIIIΔcyto, as compared with TβRIII, with time constant values of τ ≥ 2000 and quantitatively <11% of TβRIIIΔcyto internalized (Fig. 2A). These data support an essential role for the TβRIII cytoplasmic domain in its internalization.
FIGURE 2.
The cytoplasmic domain of TβRIII is necessary for TβRIII internalization. A, TβRIIIΔgag or TβRIIIΔcyto was transfected into COS7 cells, and internalization was examined using a trypsin-internalization assay. Data are representative of three independent experiments. B, a cell-surface ELISA was performed with COS7 cells transiently expressing TβRIIIΔgag and either empty vector (pcDNA3.1) or β-arrestin2. Time constant values, τ, were obtained by fitting data to a single exponential decay function (p < 0.05, independent two-tailed t test). C, basal cell surface levels of either TβRIIIΔgag+pcDNA3.1 or TβRIIIΔgag+β-arrestin2, normalized to TβRIIIΔgag+pcDNA3.1, are shown (p < 0.001, independent two-tailed t test). Average of five independent experiments; bars = S.E.
β-Arrestin2 Enhances TβRIII Internalization—Given that the cytoplasmic domain of TβRIII is critical for receptor internalization, we assessed whether TβRIII-interacting protein(s) might be necessary for internalization. Because we have previously demonstrated that TβRII can phosphorylate the TβRIII cytoplasmic domain in vivo, allowing association with β-arrestin2, co-internalization, and co-localization (25), we focused on this interaction by performing a cell-surface ELISA in COS7 cells overexpressing TβRIIIΔgag ± β-arrestin2. Increasing β-arrestin2 expression enhanced TβRIII internalization, with the time constant decreasing from 227 ± 41 to 101 ± 17 min (p < 0.05, independent two-tailed t test, Fig. 2B). In addition, expression of β-arrestin2 reduced the basal cell surface TβRIII levels to 45 ± 6.5% that of the vector-transfected controls (p < 0.001, independent two-tailed t test, Fig. 2C). Taken together, these data demonstrate that β-arrestin2 enhances TβRIII internalization, resulting in decreased basal cell surface TβRIII levels.
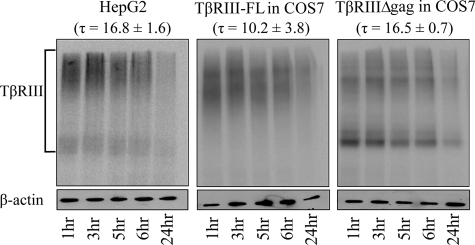
Steady-state Levels of Endogenous TβRIII Are Down-regulated in a Time-dependent Manner—Although these studies characterized TβRIII internalization in isolation, to examine endogenous TβRIII internalization in context of the other TGF-β receptors, we focused on HepG2 cells, as they contain endogenous TβRI, TβRII, and TβRIII at levels that can be easily detected using 125I-labeled TGF-β1 binding and cross-linking. Because we established a time course for TβRIII ligand-independent internalization and the importance of the cytoplasmic domain, we examine endogenous TβRIII down-regulation. Receptor down-regulation is a complex process, involving receptor internalization, endocytosis, and receptor degradation. This process normally occurs over a longer time course than receptor internalization alone and results in a steady-state decrease in receptor protein expression. Using densitometry, normalized to β-actin levels, we determined the time constant, τ, for endogenous TβRIII down-regulation is 16.8 ± 1.6 h. Importantly, utilizing this same assay, we were able to compare down-regulation of endogenous TβRIII to TβRIII-FL and TβRIIIΔgag overexpressed in COS7 cells, which had time constants of 10.2 ± 3.8 and 16.5 ± 0.7 h, respectively, which were not significantly different (p ≥ 0.15, independent two-tailed t test, Fig. 3). These results suggest that our overexpression studies closely mirror the internalization of endogenous TβRIII and that the glycosaminoglycan side chains do not significantly regulate TβRIII endocytosis or down-regulation.
FIGURE 3.
TβRIII undergoes down-regulation. HepG2 cells endogenously expressing TβRIII or COS7 cells transiently expressing TβRIII-FL or TβRIIIΔgag were bound and cross-linked with 125I-labeled TGF-β1. Cells were placed at 37 °C for 1–24 h. Densitometry was performed using ImageJ, and values were normalized to β-actin. Data were fit to a single exponential decay function from which the time constant, τ, was determined. Data are representative of two or more independent experiments.
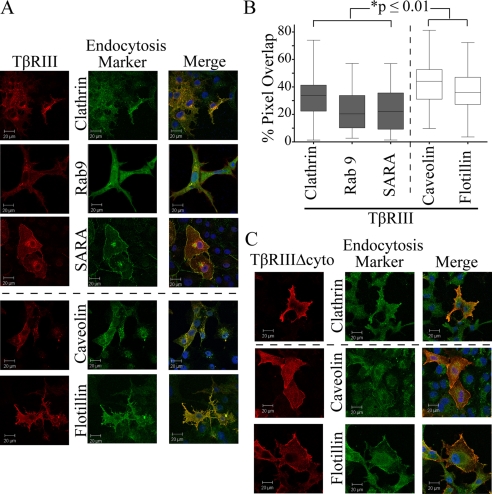
TβRIII Colocalizes with Markers of Both Clathrin-dependent and Clathrin-independent Endocytic Pathways—Endocytosis, the internalization of cell surface receptors and nutrient/growth factors into internal membrane compartments which lead to recycling, signaling, or degradation, is a key mechanism through which receptors can be regulated at the protein level and occurs through two main pathways, clathrin-dependent and clathrin-independent (or lipid raft/caveolae) endocytosis. Previous studies established that TβRI and TβRII undergo clathrin-mediated endocytosis, resulting in downstream signaling and lipid-raft endocytosis, resulting in receptor degradation (8, 38). To determine the method(s) of TβRIII endocytosis and resulting consequence(s) on downstream signaling or receptor degradation, we first utilized confocal microscopy to examine localization of TβRIII with markers of the clathrin-dependent and clathrin-independent (lipid raft) pathways. Clathrin-dependent markers included the heavy chain (clathrin-HC), a key component of clathrin-coated pits, Rab9, a marker of the late endosome, and SARA, a marker of the early endosome. Although both SARA and Rab9 were overexpressed, an antibody to clathrin was utilized to examine endogenous protein. Clathrin-independent, lipid raft-dependent markers included antibodies to endogenous proteins; caveolin-1, a common component of caveolae, and flotillin, a component of lipid-rafts. Cells were analyzed based on the extent of co-localization (as determined by % pixel overlap) of TβRIII with the aforementioned markers, and representative images are presented (Fig. 4A). Although TβRIII-FL co-localized with both markers of the clathrin-dependent and clathrin-independent pathways, TβRIII-FL displayed a significantly greater degree of co-localization with markers of the clathrin-independent pathway. Specifically, TβRIII-FL and caveolin-1 demonstrated a mean of 43% pixel overlap, and TβRIII-FL and flotillin demonstrated 37% pixel overlap, whereas TβRIII-FL demonstrated 23% pixel overlap with both SARA and Rab9 and 32% pixel overlap with the clathrin-HC (Fig. 4B, p ≤ 0.01, unpaired two-tailed t test). A similar trend was observed with TβRIIIΔgag (data not shown). These studies were repeated examining the localization of the TβRIII mutant lacking its cytoplasmic domain, TβRIIIΔcyto, with endogenous markers of both clathrin-mediated and lipid-raft-mediated endocytosis. TβRIIIΔcyto demonstrated a mean pixel overlap of 31% with clathrin-HC, 35% with flotillin, and 37% with caveolin-1. Although all these values are below those obtained when examining TβRIII-FL, the only statistically significant decrease in % pixel overlap between TβRIII-FL and TβRIIIΔcyto was seen with caveolin-1 (p < 0.01, unpaired two-tailed t test). In addition, there was a dramatic shift in where TβRIII-FL and TβRIIIΔcyto localized with TβRIIIΔcyto localizing to a much greater extent on the cell surface than TβRIII-FL and TβRIIIΔgag, providing support that internalization of TβRIIIΔcyto is impaired (Fig. 4C). Taken together, these data indicate that TβRIII can associate with both clathrin-dependent and -independent pathways but co-localizes more frequently with markers of the clathrin-independent (lipid raft) pathway.
FIGURE 4.
TβRIII associates with both the clathrin-dependent and clathrin-independent endocytic pathways. A, cells transiently transfected with TβRIII and markers of the endocytic pathways, Rab9 and SARA, or endogenous clathrin, caveolin1, or flotillin were imaged using a Zeiss LSM 510 inverted confocal microscope. Confocal images are representative of three or more experiments. B, “% pixel overlap” was determined using the LSM Image Examiner. *, p ≤ 0.01, unpaired two-tailed t test between each pair of samples. C, immunofluorescence was performed on cells transiently expressing TβRIIIΔcyto and endogenous clathrin-HC, flotillin, and caveolin-1. Confocal images analyzed at 63× using a Zeiss LSM 510 inverted confocal microscope. Data are representative of three independent experiments.
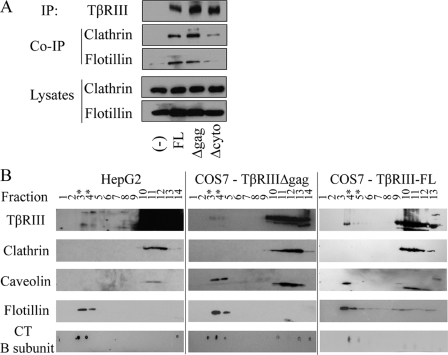
The Cytoplasmic Domain of TβRIII Is Necessary for TβRIII Interaction with Flotillin and Clathrin—To further characterize the method(s) of TβRIII endocytosis and provide additional support for the confocal microscopy studies, we performed co-immunoprecipitation studies in COS7 cells overexpressing TβRIII-FL, TβRIIIΔgag, or TβRIIIΔcyto with endogenous components of the clathrin-dependent and clathrin-independent (lipid-raft) endocytic pathways, clathrin heavy chain, and flotillin, respectively. Both TβRIII-FL and TβRIIIΔgag co-immunoprecipitated endogenous clathrin and flotillin, further supporting an interaction of TβRIII with both the clathrin-dependent and clathrin-independent endocytic pathways (Fig. 5A). In contrast, there was a marked decrease in the ability of TβRIIIΔcyto to co-immunoprecipitate clathrin and flotillin when compared with TβRIII-FL and TβRIIIΔgag (Fig. 5A). These data suggest an additional possible mechanism for the essential role for the cytoplasmic domain of TβRIII in mediating TβRIII receptor endocytosis (Fig. 2).
FIGURE 5.
TβRIII associates with lipid raft domains. A, TβRIII constructs were overexpressed in COS7 cells, and TβRIII was immunoprecipitated (IP) and then blotted for endogenous clathrin and flotillin in the immunoprecipitates. B, HepG2 cells endogenously expressing TβRIII or COS7 cells adenovirally infected with either TβRIII-FL or TβRIIIΔgag were lysed and subjected to sucrose density fractionation. Fractions were analyzed by Western blot with antibodies to markers of the lipid-raft pathway, caveolin-1, flotillin-1, and the cholera toxin (CT) B subunit, to confirm lipid raft fractions and an antibody for clathrin-mediated endocytosis, clathrin-heavy chain. Results are representative of two independent experiments; asterisks (*) represent visible lipid raft fractions.
TβRIII Associates with Lipid Raft Domains—Another commonly employed biochemical approach, sucrose density fractionation, was utilized to determine whether either endogenous TβRIII or overexpressed TβRIII associates with lipid raft domains. Fractionations were performed with HepG2 cells expressing endogenous TβRIII in the context of endogenous TβRII and TβRI as well as adenovirus-infected COS7 cells overexpressing either TβRIII-FL or TβRIIIΔgag. Lipid raft fractions were confirmed by Western blots establishing that they expressed endogenous components of lipid rafts, caveolin-1, flotillin, and the cholera toxin B-subunit, and lacked expression of an endogenous component of clathin-dependent endocytosis, clathrin-HC. We established that in all cases, both endogenous and overexpressed TβRIII associates with lipid rafts (Fig. 5B), supporting a role for clathrin-independent TβRIII endocytosis as well as supporting the relevance of TβRIII overexpression studies to endogenous TβRIII.
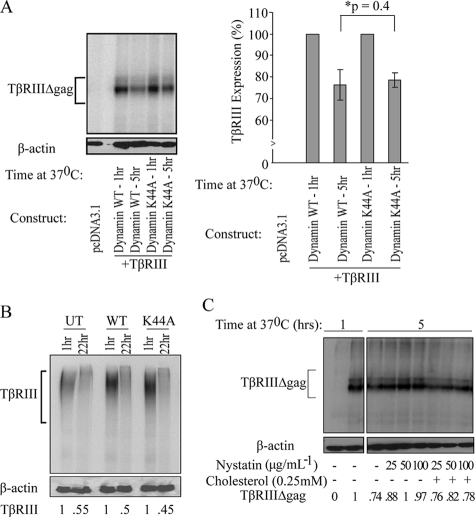
TβRIII Down-regulation Is Not Blocked by Inhibitors of Clathrin-mediated Endocytosis—Sucrose density fractionation only verifies association of proteins with lipid rafts, but provides no information regarding association with the clathrin-dependent endocytic pathway. To examine the function of the clathrin-dependent pathway on TβRIII trafficking, we performed a 125I-labeled TGF-β1 binding and cross-linking down-regulation assay in the presence of a dominant-negative dynamin I mutant, dynamin K44A, which is deficient in GTP binding and GTPase activity, both of which are required functions for clathrin-mediated endocytosis (41). The dynamin K44A mutant has been extensively used to specifically block the clathrin-mediated endocytic pathway (42–44). We independently confirmed by fluorescence-activated cell sorter analysis that transferrin internalization was significantly blocked using dynamin K44A compared with the wild-type control (data not shown). In COS7 cells transfected with TβRIIIΔgag (or TβRIII-FL) and either dynamin wild-type (dynamin WT) or dynamin K44A, blocking clathrin-mediated endocytosis with the GTPase-deficient dynamin mutant, dynamin K44A, was unable to block TβRIII down-regulation, with 24 ± 7% TβRIIIΔgag down-regulation in the presence of dynamin WT and 21 ± 4% down-regulation in the presence of dynamin K44A (p = 0.4, independent one-tailed t test, Fig. 6A). We further explored the role of clathrin-mediated endocytosis utilizing HepG2 cells expressing endogenous TβRIII, TβRII, and TβRI. In this model, as in our overexpression system, inhibiting clathrin-mediated endocytosis by dynamin K44A had no effect on the ability of TβRIII to be down-regulated. Wild-type dynamin showed 50% down-regulation compared with 55% down-regulation in cells expressing the dynamin K44A mutant after a 22-h time course (Fig. 6B). Furthermore, similar results were obtained utilizing an EPS15 mutant (DIII) to block clathrin-mediated endocytosis, which resulted in no significant difference in TβRIII down-regulation as compared with an EPS15 control vector (D3Δ2, data not shown).
FIGURE 6.
Inhibitors of lipid-raft mediated endocytosis block TβRIII down-regulation. A, COS7 cells transiently expressing TβRIIIΔgag and wild-type dynamin or mutant dynamin-K44A were bound and cross-linked with 125I-labeled TGF-β1 followed by a 5-h time course at 37 °C. TβRIII down-regulation was quantified by densitometry (ImageJ). The average of three independent experiments was graphed (right); bars = S.E. B, HepG2 cells either untransfected (UT) or transfected with wild-type dynamin (WT) or dynamin K44A (K44A) were bound and cross-linked with 125I-labeled TGF-β1 followed by a 22-h time course at 37 °C, and TβRIII was immunoprecipitated with a TβRIII antibody. TβRIII down-regulation was quantified by densitometry and normalized to β-actin (ImageJ). C, COS7 cells transiently expressing TβRIIIΔgag were bound and cross-linked with 125I-labeled TGF-β1 and either untreated or treated with increasing amounts of nystatin ± cholesterol over a time course at 37 °C.
Cholesterol Depletion Blocks TβRIII Internalization and Down-regulation—Given that there was no affect of blocking the clathrin-dependent pathway on receptor down-regulation (Fig. 6, A and B) and we did see association of TβRIII with markers of the clathrin-independent pathway (Fig. 4), we examined whether the clathrin-independent/lipid raft endocytic pathway was involved in receptor down-regulation, as has been previously established for TβRI and TβRII (8). Accordingly, 125I-labeled TGF-β1 binding and cross-linking was performed to examine TβRIII down-regulation in the presence of nystatin, a cholesterol-sequestering drug. Nystatin is a commonly employed tool used to block lipid raft-mediated endocytosis, and although it can have off-target effects at high concentrations, the concentrations we utilized have been shown previously to not affect clathrin-coated pits or clathrin-mediated endocytosis (45–47). In the absence of nystatin, 26% of 125I-labeled TGF-β1 cross-linked TβRIII was down-regulated after 5 h at 37 °C. However, in the presence of 25, 50, or 100 μg/ml nystatin, there was an inhibition of TβRIII down-regulation, with only 12, 0.3, and 3% down-regulation, respectively. Importantly, the addition of cholesterol to samples containing nystatin was able to rescue down-regulation, restoring levels to base line, reaching 24, 18, and 22% down-regulation with 25, 50, or 100 μg/ml nystatin + 0.25 mm cholesterol, respectively (Fig. 6C). Taken together, this data supports a role for lipid-raft mediated endocytosis in TβRIII trafficking and down-regulation.
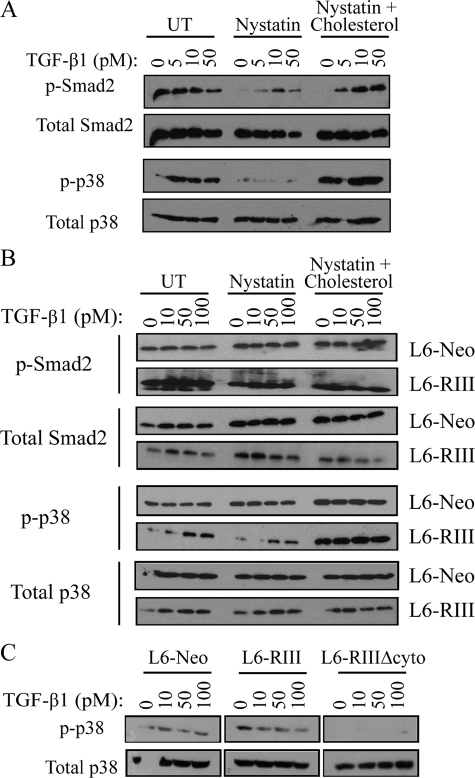
Clathrin-independent TβRIII Endocytosis Is Important for Smad-dependent and -independent Signaling—A major function of endocytosis is regulation of downstream signaling, resulting in either receptor degradation and down-regulation of signaling or increasing signaling through unclear mechanisms. To explore what effect TβRIII endocytosis through both clathrin-dependent and -independent pathways had on downstream TGF-β signaling, we utilized the endogenous HepG2 system in which cells were either untreated (control) or treated with 50 μg/ml nystatin ± 0.25 mm cholesterol followed by TGF-β1 stimulation and assessment of Smad2 and p38 phosphorylation. Although high levels of basal Smad2 phosphorylation was observed in the untreated condition, likely due to autocrine TGF-β signaling, blocking clathrin-independent endocytosis with nystatin resulted in a decrease in both p-Smad2 and p-p38 (Fig. 7A). Importantly, these effects could be rescued by the addition of cholesterol (Fig. 7A, right panel).
FIGURE 7.
Clathrin-independent TβRIII endocytosis regulates TβRIII-mediated Smad-dependent and Smad-independent signaling. A, HepG2 cells were either untreated (UT) or treated with 50 μg/ml nystatin ± 0.25 mm cholesterol followed by a 10-min stimulation with the indicated concentrations of TGF-β1 and analyzed by Western blot for p-Smad2, p-p38, total Smad2, and total p-38. Blots are representative of three independent experiments. B, L6-Neo and L6-RIII cells were treated as described in A and blotted for p-Smad2, p-p38, total Smad2, and total p-38. Blots are representative of three independent experiments. C, L6-Neo, L6-RIII, and L6-RIIIΔcyto stable expressing cell lines were treated for 10 min with 0, 10, 50, or 100 pm TGF-β1 and analyzed by Western blot for p-p38 and total p38. Blots are representative of three independent experiments.
To determine whether this effect was TβRIII-specific, we utilized L6 cells stably expressing either empty vector (L6-Neo) or TβRIII (L6-RIII). Although L6-Neo cells again exhibited high basal Smad2 and p38 activation, likely due to autocrine TGF-β signaling, there were no dramatic differences in Smad2 or p38 phosphorylation with or without the addition of nystatin to block clathrin-independent endocytosis (Fig. 7B). In contrast, L6-RIII cells, which also exhibited high basal Smad2 phosphorylation, displayed slightly decreased Smad2 and dramatically decreased p38 phosphorylation in response to nystatin (Fig. 7B), corroborating what was seen in HepG2 cells (Fig. 7A). Again, these effects could be rescued by the addition of cholesterol (Fig. 7B, right panel). The results in the L6 cells support that the effects of nystatin were via regulation of TβRIII endocytosis. Identical studies were also carried out in both cell systems overexpressing dynamin wild type, dynamin K44A, the EPS15 mutant (DIII), or EPS15 control vector (D3Δ2). In no case was there any appreciable difference in Smad2 or p38 phosphorylation seen with inhibition of the clathrin-mediated endocytic pathway either by K44A or DIII (data not shown), suggesting a specific role for the clathrin-independent/lipid raft pathway in mediating both Smad-dependent and Smad-independent signaling downstream of TβRIII.
To further characterize the role of TβRIII on downstream signaling, we utilized L6 cells stably expressing the TβRIIIΔcyto mutant, which is primarily localized at the cell surface (Fig. 4C) and is not internalized (Fig. 2A). Compared with L6-Neo and L6-RIII cells, L6-TβRIIIΔcyto cells demonstrated markedly reduced p38 activation in response to TGF-β1 (Fig. 7C), consistent with previous results (48). This further supports a role of TβRIII cytoplasmic domain in regulating receptor internalization and downstream Smad-dependent and Smad-independent signaling.
DISCUSSION
This study for the first time characterizes the mechanism of TβRIII endocytosis. We demonstrate that TβRIII internalizes in a ligand-independent and glycosaminoglycan modification-independent manner, which is consistent with previous data that the GAG chains do not affect the TβRIII ligand binding abilities or cell surface expression (49). Based on the biochemical and immunofluorescent microscopy studies, TβRIII undergoes both clathrin-dependent and clathrin-independent/lipid raft-dependent endocytosis. Confocal microscopy studies and the down-regulation assays support the clathrin-independent/lipid raft pathway as the preferential mechanism for TβRIII endocytosis, with this endocytosis proving to be important for both TβRIII-mediated signaling and for subsequent receptor down-regulation. In contrast, we have yet to define a function for TβRIII endocytosis through the clathrin-dependent pathway, as inhibiting this pathway had no effects either on TβRIII-mediated signaling or receptor down-regulation. In terms of how endocytosis through the clathrin-independent/lipid raft-dependent pathway could both be necessary for signaling as well as for down-regulation, we hypothesize that endocytosis is initially important for localization of TβRIII and/or its respective signaling receptors in an intracellular location required for efficient signaling but that after mediating their signal the receptors are then trafficked via this pathway to the late endosome, where they fuse with the lysosome and are degraded. This latter aspect is consistent with data obtained for TβRII and TβRI (8). This coordinated endocytic role would allow for an additional mode of regulation of TGF-β signaling. The mechanism by which endocytosis through the clathrin-independent/lipid raft pathway regulates both TβRIII-mediated signaling and receptor down-regulation, including the effects of TβRIII on the endocytosis and signaling other TGF-β superfamily receptors and their respective ligands, remains an active area of investigation.
How might TβRIII associate with lipid rafts? Our studies support an important role for the cytoplasmic domain of TβRIII in receptor endocytosis. In addition to binding β-arrestin2, the cytoplasmic domain also appears to mediate interactions of TβRIII with clathrin and flotillin either directly or indirectly (Fig. 5A). The interaction with flotillin could mediate the interaction with lipid rafts. In addition, sequence analysis of TβRIII reveals predicted myristoylation sites on TβRIII, which have been highly conserved over evolution. Myristoylation, a mechanism through which a myristate is added to a glycine commonly on the N terminus or a protein, allows receptors to become more hydrophobic and associate with lipid bilayer domains. Whether TβRIII undergoes myristoylation in vitro and in vivo remains to be investigated, but this provides another potential explanation for the TβRIII association with lipid rafts.
The cytoplasmic domain of TβRIII was found to have a critical role in endocytosis, with its removal virtually abolishing receptor internalization. In addition, the association of β-arrestin2 with TβRIII, within its cytoplasmic domain, leads to enhanced internalization. This interaction with β-arrestin2 could be a potential mechanism through which TβRIII acts to regulate the dual function TGF-β exerts during tumorigenesis to suppress invasion and migration (17, 20). More specifically, β-arrestin2 has been linked to cancer metastasis through its regulation of p38 activity. Decreased cancer cell metastasis has been demonstrated previously in HeLa and human embryonic kidney 293 cells that have reduced β-arrestin2 levels (50). Association of β-arrestin2 with TβRIII could lead to down-regulation of β-arrestin2 and, therefore, a decrease in cell motility, which we have observed in TβRIII expressing prostate, lung, and breast cancer cell lines in vitro (15, 17, 20). Another protein known to interact with TβRIII on its cytoplasmic domain, GIPC, has opposing effects on cell surface levels of TβRIII when compared with β-arrestin2. Although the link between GIPC and cancer is not clear, the effect of GIPC on TβRIII internalization and the consequences of this interaction on the role of TβRIII in tumorigenesis are currently being explored.
The finding that TβRIII internalization is ligand-independent begs the questions as to the role of ligand stimulation. Although there is evidence that some receptors, including the insulin-like growth factor II receptor (IGF-II receptor), are able to internalize and recycle in the absence of ligand, this is still an emerging area of research (52). Within the TGF-β field numerous studies have demonstrated that internalization rates of TβRI and TβRII are not altered by the addition of TGF-β (38, 53, 54). However, clearly both ligand binding and receptor internalization are important for downstream signaling. How then could this regulation occur in a ligand-independent internalization model? A couple possible explanations have been suggested. On the one hand, constitutive recycling of the “unstimulated/unbound” receptors could regulate steady-state cell surface receptor levels and, therefore, regulate cell sensitivity to extracellular ligands (38). This is particularly relevant to TβRIII, which binds all isoforms of TGF-β but also binds inhibin, BMPs, and basic fibroblast growth factor. Alternatively, perhaps only the receptors bound to ligand traffic into endosomal compartments that result in enhanced signaling. Along these lines, only TGF-β bound heteromeric (and not homomeric) complexes of TβRI and TβRII were effectively able to undergo both endocytosis and signaling (55). Together, this implies that although ligand is not necessary for receptor internalization, ligand might be required for the association of TβRIII with cytoplasmic proteins to enhance specificity of downstream signaling, recycling, or degradation.
Regulation of receptor internalization could directly impact the amount of soluble TβRIII produced by altering the time at which the receptor is on the cell surface and allowing extended contact with metalloproteases and enzymes likely responsible for cleavage of TβRIII. Indeed, studies performed in human embryonic kidney cells examining the amyloid precursor protein (APP) found that blocking clathrin-dependent endocytosis with a dynamin K44A mutant enhanced the amount of extracellular soluble APP produced and the amount of APP present on the cell surface (51). Additionally, because soluble TβRIII is able to bind ligand, the regulation of its production by receptor endocytosis could be a mechanism through which the signaling alterations outlined in this study were seen. Specifically, we found that by blocking clathrin-independent endocytosis with nystatin, there was a decrease in p-Smad2 and p-p38. Whether enhanced soluble TβRIII production and ligand sequestration is due to increased cell surface expression of TβRIII, observed when blocking the clathrin-independent endocytic pathway, is currently being explored. Taken together, these results provide a foundation for further studies examining TβRIII internalization, specifically investigating the biological consequences of internalization, including potential dysregulation during tumorigenesis.
Acknowledgments
We thank Dr. Robert Lefkowitz for the dynamin constructs, Dr. Alexandre Benmerah for the EPS15 constructs, Dr. Jeff Wrana for the SARA construct, and Dr. Suzanne Pfeffer for the Rab constructs.
This work was supported, in whole or in part, by National Institutes of Health Grant R01-CA106307 (NCI; to G. C. B.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: TGF-β, transforming growth factor-β;TβR, TGF-β receptor; DMEM, Dulbecco's modified Eagle's medium; FBS, fetal bovine serum; ELISA, enzyme-linked immunosorbent assay; PBS, phosphate-buffered saline; FL, full-length; ERK, extracellular signal-regulated kinase; KRH, Krebs Ringer HEPES buffer; HC, heavy chain.
References
- 1.Shi, Y., and Massague, J. (2003) Cell 113 685-700 [DOI] [PubMed] [Google Scholar]
- 2.Massague, J. (1998) Annu. Rev. Biochem. 67 753-791 [DOI] [PubMed] [Google Scholar]
- 3.Leof, E. B. (2000) Trends Cell Biol. 10 343-348 [DOI] [PubMed] [Google Scholar]
- 4.Gordon, K. J., and Blobe, G. C. (2008) Biochim. Biophys. Acta 1782 197-228 [DOI] [PubMed] [Google Scholar]
- 5.Siegel, P. M., and Massague, J. (2003) Nat. Rev. Cancer 3 807-821 [DOI] [PubMed] [Google Scholar]
- 6.Engel, M. E., McDonnell, M. A., Law, B. K., and Moses, H. L. (1999) J. Biol. Chem. 274 37413-37420 [DOI] [PubMed] [Google Scholar]
- 7.Derynck, R., and Zhang, Y. E. (2003) Nature 425 577-584 [DOI] [PubMed] [Google Scholar]
- 8.Di Guglielmo, G. M., Le Roy, C., Goodfellow, A. F., and Wrana, J. L. (2003) Nat. Cell Biol. 5 410-421 [DOI] [PubMed] [Google Scholar]
- 9.Lopez-Casillas, F., Wrana, J. L., and Massague, J. (1993) Cell 73 1435-1444 [DOI] [PubMed] [Google Scholar]
- 10.Wang, X. F., Lin, H. Y., Ng-Eaton, E., Downward, J., Lodish, H. F., and Weinberg, R. A. (1991) Cell 67 797-805 [DOI] [PubMed] [Google Scholar]
- 11.Blobe, G. C., Schiemann, W. P., Pepin, M. C., Beauchemin, M., Moustakas, A., Lodish, H. F., and O'Connor-McCourt, M. D. (2001) J. Biol. Chem. 276 24627-24637 [DOI] [PubMed] [Google Scholar]
- 12.Brown, C. B., Boyer, A. S., Runyan, R. B., and Barnett, J. V. (1999) Science 283 2080-2082 [DOI] [PubMed] [Google Scholar]
- 13.Deng, X., Bellis, S., Yan, Z., and Friedman, E. (1999) Cell Growth Differ. 10 11-18 [PubMed] [Google Scholar]
- 14.Kirkbride, K. C., and Blobe, G. C. (2003) Expert Opin. Biol. Ther. 3 251-261 [DOI] [PubMed] [Google Scholar]
- 15.Dong, M., How, T., Kirkbride, K. C., Gordon, K. J., Lee, J. D., Hempel, N., Kelly, P., Moeller, B. J., Marks, J. R., and Blobe, G. C. (2007) J. Clin. Investig. 117 206-217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Copland, J. A., Luxon, B. A., Ajani, L., Maity, T., Campagnaro, E., Guo, H., LeGrand, S. N., Tamboli, P., and Wood, C. G. (2003) Oncogene 22 6109-6118 [DOI] [PubMed] [Google Scholar]
- 17.Finger, E. C., Turley, R. S., Dong, M., How, T., Fields, T. A., and Blobe, G. C. (2008) Carcinogenesis 29 528-535 [DOI] [PubMed] [Google Scholar]
- 18.Hempel, N., How, T., Dong, M., Murphy, S. K., Fields, T. A., and Blobe, G. C. (2007) Cancer Res. 67 5231-5238 [DOI] [PubMed] [Google Scholar]
- 19.Gordon, K. J., Dong, M., Chislock, E. M., Fields, T. A., and Blobe, G. C. (2007) Carcinogenesis 29 252-262 [DOI] [PubMed] [Google Scholar]
- 20.Turley, R. S., Finger, E. C., Hempel, N., How, T., Fields, T. A., and Blobe, G. C. (2007) Cancer Res. 67 1090-1098 [DOI] [PubMed] [Google Scholar]
- 21.Kirkbride, K. C., Ray, B. N., and Blobe, G. C. (2005) Trends Biochem. Sci. 30 611-621 [DOI] [PubMed] [Google Scholar]
- 22.Esparza-Lopez, J., Montiel, J. L., Vilchis-Landeros, M. M., Okadome, T., Miyazono, K., and Lopez-Casillas, F. (2001) J. Biol. Chem. 276 14588-14596 [DOI] [PubMed] [Google Scholar]
- 23.Andres, J. L., De Falcis, D., Noda, M., and Massague, J. (1992) J. Biol. Chem. 267 5927-5930 [PubMed] [Google Scholar]
- 24.Kirkbride, K. C., Townsend, T. A., Bruinsma, M. W., Barnett, J. V., and Blobe, G. C. (2008) J. Biol. Chem. 283 7628-7637 [DOI] [PubMed] [Google Scholar]
- 25.Chen, W., Kirkbride, K. C., How, T., Nelson, C. D., Mo, J., Frederick, J. P., Wang, X. F., Lefkowitz, R. J., and Blobe, G. C. (2003) Science 301 1394-1397 [DOI] [PubMed] [Google Scholar]
- 26.McDonald, P. H., and Lefkowitz, R. J. (2001) Cell. Signal. 13 683-689 [DOI] [PubMed] [Google Scholar]
- 27.Luttrell, L. M., and Lefkowitz, R. J. (2002) J. Cell Sci. 115 455-465 [DOI] [PubMed] [Google Scholar]
- 28.Elliott, R. L., and Blobe, G. C. (2005) J. Clin. Oncol. 23 2078-2093 [DOI] [PubMed] [Google Scholar]
- 29.McPherson, P. S., Kay, B. K., and Hussain, N. K. (2001) Traffic 2 375-384 [DOI] [PubMed] [Google Scholar]
- 30.Neel, N. F., Schutyser, E., Sai, J., Fan, G. H., and Richmond, A. (2005) Cytokine Growth Factor Rev. 16 637-658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirkham, M., and Parton, R. G. (2005) Biochim. Biophys. Acta 1745 273-286 [DOI] [PubMed] [Google Scholar]
- 32.Le Roy, C., and Wrana, J. L. (2005) Nat. Rev. Mol. Cell Biol. 6 112-126 [DOI] [PubMed] [Google Scholar]
- 33.Lamaze, C., Dujeancourt, A., Baba, T., Lo, C. G., Benmerah, A., and Dautry-Varsat, A. (2001) Mol. Cell 7 661-671 [DOI] [PubMed] [Google Scholar]
- 34.Benlimame, N., Le, P. U., and Nabi, I. R. (1998) Mol. Biol. Cell 9 1773-1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pelkmans, L., Kartenbeck, J., and Helenius, A. (2001) Nat. Cell Biol. 3 473-483 [DOI] [PubMed] [Google Scholar]
- 36.Razani, B., Zhang, X. L., Bitzer, M., von Gersdorff, G., Bottinger, E. P., and Lisanti, M. P. (2001) J. Biol. Chem. 276 6727-6738 [DOI] [PubMed] [Google Scholar]
- 37.Penheiter, S. G., Mitchell, H., Garamszegi, N., Edens, M., Dore, J. J., Jr., and Leof, E. B. (2002) Mol. Cell. Biol. 22 4750-4759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitchell, H., Choudhury, A., Pagano, R. E., and Leof, E. B. (2004) Mol. Biol. Cell 15 4166-4178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dore, J. J., Jr., Yao, D., Edens, M., Garamszegi, N., Sholl, E. L., and Leof, E. B. (2001) Mol. Biol. Cell 12 675-684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blobe, G. C., Liu, X., Fang, S. J., How, T., and Lodish, H. F. (2001) J. Biol. Chem. 276 39608-39617 [DOI] [PubMed] [Google Scholar]
- 41.Damke, H., Baba, T., Warnock, D. E., and Schmid, S. L. (1994) J. Cell Biol. 127 915-934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun, T. X., Van Hoek, A., Huang, Y., Bouley, R., McLaughlin, M., and Brown, D. (2002) Am. J. Physiol. Renal Physiol. 282 998-1011 [DOI] [PubMed] [Google Scholar]
- 43.Gaborik, Z., Szaszak, M., Szidonya, L., Balla, B., Paku, S., Catt, K. J., Clark, A. J., and Hunyady, L. (2001) Mol. Pharmacol. 59 239-247 [DOI] [PubMed] [Google Scholar]
- 44.Yang, W., Wang, D., and Richmond, A. (1999) J. Biol. Chem. 274 11328-11333 [DOI] [PubMed] [Google Scholar]
- 45.Simons, K., and Toomre, D. (2000) Nat. Rev. Mol. Cell Biol. 1 31-39 [DOI] [PubMed] [Google Scholar]
- 46.Rothberg, K. G., Heuser, J. E., Donzell, W. C., Ying, Y. S., Glenney, J. R., and Anderson, R. G. (1992) Cell 68 673-682 [DOI] [PubMed] [Google Scholar]
- 47.Schnitzer, J. E., Oh, P., Pinney, E., and Allard, J. (1994) J. Cell Biol. 127 1217-1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.You, H. J., Bruinsma, M. W., How, T., Ostrander, J. H., and Blobe, G. C. (2007) Carcinogenesis 28 2491-2500 [DOI] [PubMed] [Google Scholar]
- 49.Cheifetz, S., and Massague, J. (1989) J. Biol. Chem. 264 12025-12028 [PubMed] [Google Scholar]
- 50.Buchanan, F. G., and DuBois, R. N. (2006) Cell Cycle 5 2060-2063 [DOI] [PubMed] [Google Scholar]
- 51.Carey, R. M., Balcz, B. A., Lopez-Coviella, I., and Slack, B. E. (2005) BMC Cell Biol. 6 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oka, Y., and Czech, M. P. (1986) J. Biol. Chem. 261 9090-9093 [PubMed] [Google Scholar]
- 53.Ehrlich, M., Shmuely, A., and Henis, Y. I. (2001) J. Cell Sci. 114 1777-1786 [DOI] [PubMed] [Google Scholar]
- 54.Yao, D., Ehrlich, M., Henis, Y. I., and Leof, E. B. (2002) Mol. Biol. Cell 13 4001-4012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anders, R. A., Arline, S. L., Dore, J. J., and Leof, E. B. (1997) Mol. Biol. Cell 8 2133-2143 [DOI] [PMC free article] [PubMed] [Google Scholar]