Abstract
The etiology of Parkinson disease (PD) is unclear but may involve environmental toxins such as pesticides leading to dysfunction of the ubiquitin proteasome system (UPS). Here, we measured the relative toxicity of ziram (a UPS inhibitor) and analogs to dopaminergic neurons and examined the mechanism of cell death. UPS (26 S) activity was measured in cell lines after exposure to ziram and related compounds. Dimethyl- and diethyldithiocarbamates including ziram were potent UPS inhibitors. Primary ventral mesencephalic cultures were exposed to ziram, and cell toxicity was assessed by staining for tyrosine hydroxylase (TH) and NeuN antigen. Ziram caused a preferential damage to TH+ neurons and elevated α-synuclein levels but did not increase aggregate formation. Mechanistically, ziram altered UPS function through interfering with the targeting of substrates by inhibiting ubiquitin E1 ligase. Sodium dimethyldithiocarbamate administered to mice for 2 weeks resulted in persistent motor deficits and a mild reduction in striatal TH staining but no nigral cell loss. These results demonstrate that ziram causes selective dopaminergic cell damage in vitro by inhibiting an important degradative pathway implicated in the etiology of PD. Chronic exposure to widely used dithiocarbamate fungicides may contribute to the development of PD, and elucidation of its mechanism would identify a new potential therapeutic target.
Parkinson disease (PD)2 is a common neurodegenerative disease characterized by relatively selective degeneration of dopaminergic (DA) neurons in the substantia nigra (nigrostriatal neurons). The etiology probably involves both environmental and genetic factors including pesticide exposure (1–3). Hundreds of pesticides are used alone or in combinations making it difficult to separate individual effects. Because no individual pesticide has been established by epidemiologic studies, we chose to perform an unbiased screen of potential toxicants for their ability to interfere with the ubiquitin-proteasome system (UPS), a biological pathway implicated in the etiology of PD. Impaired UPS activity has been reported in the brains of patients with PD, and mutations in two UPS genes, Parkin and UCHL-1, cause rare genetic forms of PD (4). Although these results are not universally reproduced (5–7), in some studies administration of UPS inhibitors to rodents recapitulates many of the clinical and pathological aspects of PD (8–10). We hypothesized that chronic pesticide exposure may increase the risk of developing PD by inhibiting the UPS. We screened several pesticides for their ability to inhibit the UPS and found a number of toxicants that can lower activity at relevant concentrations (11). We then focused on dithiocarbamate fungicides because they were found to be one of the most potent UPS inhibitors and are widely used in crop protection.
In the present study, zinc dimethyldithiocarbamate (ziram) was one of several dimethyl- and diethyldithiocarbamates found to inhibit the UPS at 0.15–1 μm. Furthermore, ziram increased α-synuclein expression in DA cells, induced relatively selective DA cell damage in vitro, and inhibited the UPS by interfering with ubiquitin E1 ligase activity. In vivo, systemic administration of the more soluble sodium dimethyldithiocarbamate (NaDMDC) in mice resulted in motor deficits and damage to the nigrostriatal pathway. These findings help explain how chronic pesticide exposure could increase the risk of developing PD.
EXPERIMENTAL PROCEDURES
Chemicals—The test compounds (see Table 1) were from Chem Service (West Chester, PA), Sigma-Aldrich, or other commercial sources as the highest available purity, except for 7 and 8, which were synthesized by Karl Fisher in the Casida laboratory.
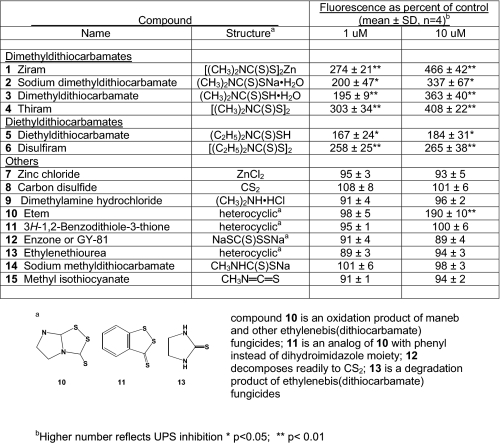
TABLE 1.
Structure-activity relationships for ziram-related compounds on 26 S UPS with 24-h treatment of SK-N-MC cells
Measurement of 26 S Proteasome Activity and Cell Death in Cell Lines—26 and 20 S UPS activity and cell death were measured in human embryonic kidney (HEK) and neuroblastoma SK-N-MC cells by flow cytometry as previously described (12). Fluorescence of the green fluorescent protein degron fusion protein (GFP-U) was measured and expressed as a percentage of control.
Rat Primary Ventral Mesencephalic Cultures (VMC)—VMC were prepared by using a protocol adapted from Rayport et al. (13). Briefly, the cultures were prepared in two stages. In the first stage, cortical glial feeder cells were established on polyornithine/laminin-coated coverslips, which formed the base of a 10-mm-diameter well cut into 35-mm culture dishes, until they reached confluency in ∼6 days. Fluorodeoxyuridine was then added to prevent additional glial proliferation. In the second stage, postnatal day 2 or 3 pups were anesthetized, and 1 mm3 mesencephalic blocks containing the ventral tegmental area were dissected from sagittal sections taken along the midline of the brain. The cells were dissociated and plated onto pre-established glial feeder cells at densities of 1 × 105/coverslip. The mixed cultures were grown in chemically defined media containing fluorodeoxyuridine for 10 days before treatment and analysis.
Immunocytochemistry in VMCs and Cell Counts—At the conclusion of the experiment, the cultures were immediately washed and fixed in 4% paraformaldehyde for 30 min. The cultures were then incubated with anti-tyrosine hydroxylase (TH) antibodies (1:500; Calbiochem) and anti-NeuN antibodies (1:100; Chemicon) overnight at 4 °C. The cells were stained for 2 h with fluorescein isothiocyanate- and Cy3-conjugated secondary antibodies (Jackson ImmunoResearch). In some experiments, the cells were stained with anti-TH and anti-α-synuclein (1:500; Zymed Laboratories Inc.) antibodies. After staining and prior to counting, the coverslips were randomly assigned an identification number. TH- and NeuN-immunoreactive neuron counts were then determined manually by raters blinded to the experimental conditions. For TH, the entire coverslip was counted but for NeuN+ neurons, representative fields from each coverslip were counted and averaged because of their large number.
Determination of Dopamine Content in VMCs—VMCs were homogenized in 0.1 m perchloric acid containing 0.1% EDTA. Insoluble debris was removed by centrifugation, and the supernatant was filtered through a Millipore MC cartridge. The filtrate was analyzed for dopamine by high pressure liquid chromatography with electrochemical detection (Antec Leyden, Leiden, The Netherlands) using a mobile phase consisting of sodium acetate (75 mm), sodium dodecane sulfonate (0.75 mm), EDTA (10 mm), triethylamine (0.01%), acetonitrile (12%), methanol (12%), and tetrahydrofuran (1%), pH 5.5, pumped at a rate of 200 μl/min (model LC-10AD; Shimadzu, Columbia, MD) through a 100 × 2 mm column (3 μm, Hypersil C18; Keystone Scientific, Bellefonte, PA). The data were collected and analyzed using ChromPerfect software (Justice Innovations, Mountain View, CA).
Quantitation of α-Synuclein Expression—TH-positive cells in each coverslip were identified under 10×, and digital images were obtained using a 40× objective for TH and α-synuclein immunoreactivity. Exposure times were held constant between coverslips. After acquisition, the images were randomly assigned an identification number, and the experimental conditions were blinded. All of the images were stacked into a single sequence, a polygonal region of interest was manually drawn around the neuron, and the mean image intensity, total image intensity, and the area inside the region of interest were evaluated for each cell. Data analysis was performed with IPLab ver 3.x for Windows (Scanalytics Inc.) compensating for bleed-through between fluorescein isothiocyanate and Cy3 channels.
To determine whether ziram treatment leads to high molecular weight α-synuclein species, we performed Western blots on the detergent-soluble fractions of culture lysates. VMCs treated with either 1 μm ziram or 5 μm lactocystin were lysed in buffer containing 1% Triton X-100 and 0.1% SDS. The lysates were sonicated, and insoluble debris was removed by centrifugation. The protein concentrations were determined, and 10 μg/lane were subjected to SDS-PAGE. The separated proteins were transferred to nitrocellulose membranes and incubated with an anti-α-synuclein antibody (BD Transduction Laboratories; 1:1000) followed by an anti-tubulin antibody for normalization.
Evaluation of E1 Ligase Activity—The effects of ziram on E1 ligase activity were investigated using Western blot analysis of treated cellular extracts to determine E1/E1-ubiquitin ratios and using purified enzyme preparations. Ziram-treated SK-N-MC cells were washed with phosphate-buffered saline and then lysed in a thiol stabilizing buffer using the method of Jha et al. (14). The samples were sonicated for 10 s, centrifuged for 15 min at 13,000 × g, mixed with 2 parts thiol gel buffer (33 mm Tris-HCl, pH 6.8, 2.7 m urea, 2.5% sodium dodecyl sulfate, and 13% glycerol), and boiled for 2 min, and 10 μg of protein/lane was loaded on a 12% SDS-PAGE gel (15). The proteins were electrophoretically transblotted onto nitrocellulose paper, and immunoblots were performed as previously described (16) using anti-E1 ligase antibody (BIOMOL, Plymouth Meeting, PA). Antigen-antibody interactions for immunoblots were visualized using horseradish peroxidase-conjugated secondary antibody and chemoluminesence substrate (Pierce).
For purified enzyme assays, human recombinant ubiquitin-activating E1 enzyme and biotinylated ubiquitin (both from BIOMOL) were incubated for 5 or 10 min in thiolester buffer as per the manufacturer's protocol. The reactions were stopped using thiol-stabilizing buffer, and the proteins were subjected to SDS-PAGE and transblotted onto nitrocellulose paper. E1-ubiquitin conjugates were determined using streptavidin-horseradish peroxidase and an enhanced chemiluminescence kit. Band densities were measured using a scanning densitometer.
Animal Studies—Male C57BL/6 mice were treated for 2 weeks by subcutaneous osmotic minipumps with NaDMDC 50 mg/kg/day) in phosphate-buffered saline or with phosphate-buffered saline only. The behavior of the mice was evaluated using the pole test as described by Fleming et al. (17) except that a cut-off time of 60 s was used. One week after the last behavioral testing, the mice were perfused with fixative, and their brains were sectioned for TH staining. Fiber density was measured with a computer-assisted image analysis system as previously described (18).
For stereological analysis in the substantia nigra pars compacta (SNc), the neurons were counted using the optical fractionator method with the Stereo Investigator software (Microbrightfield, Colchester, VT) coupled to a Leica DM-LB microscope with a Ludl XYZ motorized stage and z axis microcator (MT12; Heidenheim, Traunreut, Germany). The SNc was delineated at 5× objective using previously reported criteria (19, 20). After delineation at low magnification, every fourth section throughout the SNc was counted at 100× magnification.
Statistical Analysis—Statistical analysis was performed with GraphPad Prism software. Statistical significance was determined using one-way ANOVA with Dunnett's multiple comparison post-test and Bonferroni's multiple comparison post-test as appropriate. For histochemistry and behavior, statistical analysis was performed with GB-Stat software (Dynamic Microsystems, Inc., Silver Spring, MD, 2000) for Macintosh. A 2 × 3 mixed design ANOVA was followed by post hoc analysis with Fisher's least significant difference. To maintain homogeneity of variance, an inverse transform was calculated (21) for each score in the pole test. The level of significance was set at p < 0.05.
RESULTS
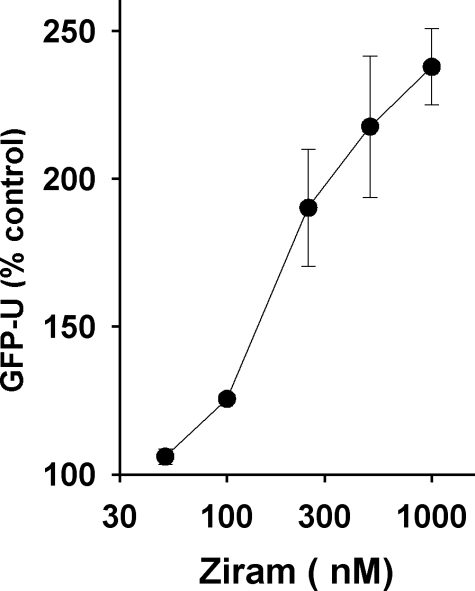
Ziram Inhibits 26 S Proteasome Activity in HEK Cells—We reported earlier that ziram inhibits 26 S UPS in SK-N-MC cells expressing a GFP-degron reporter system (11). To determine whether ziram causes a similar inhibition in a non-neuronal cell line, HEK cells were exposed, and GFP accumulation was measured using flow cytometry. We found that HEK cells were similarly sensitive to the 26 S inhibitory effects of ziram with an IC50 of 161 nm (Fig. 1).
FIGURE 1.
Ziram inhibits UPS in HEK cells with 24-h incubation prior to analysis by flow cytometry. The IC50 for ziram was 161 nm.
Structure-Activity Relationships of Ziram-related Compounds for UPS Inhibition—Ziram and 14 related compounds were tested at 1 and 10 μm for their ability to inhibit 26 S UPS activity in GFP-U-transfected SK-N-MC cells (Table 1). Ziram (1) and the other DMDC derivatives (sodium DMDC (2), free acid (3), and bis-disulfide (4)) were similarly very active. Two diethyldithiocarbamates (5 and 6) were also very potent. Thiram (4) and disulfiram (6) are cleaved into DMDC (3) and diethyldithiocarbamate (5), respectively. Ziram in aqueous solution dissociates to zinc ion (7) and forms dithio acid (3), which breaks down into carbon disulfide (8) and dimethylamine (9), all of which were inactive. The disulfide functionality of etem (10) (an ethylenebis(dithiocarbamate) fungicide oxidation product) can cleave to a dithiocarbamic acid possibly associated with its activity, whereas that of the phenyl analog (11) cannot give a dithiocarbamate. The carbon disulfide progenitor enzone (12) was also inactive. Environmental degradation converts ethylenebis(dithiocarbamate) fungicides to ethylenethiourea (13) and sodium methyldithiocarbamate (14) to methyl isothiocyanate (15), all of which were inactive. Thus, the dialkyldithiocarbamate moiety is required to alter UPS activity. Furthermore, preincubation of ziram with the reducing agents GSH, dithiothreitol, or N-acetylcysteine completely abolished its inhibitory activity (data not shown).
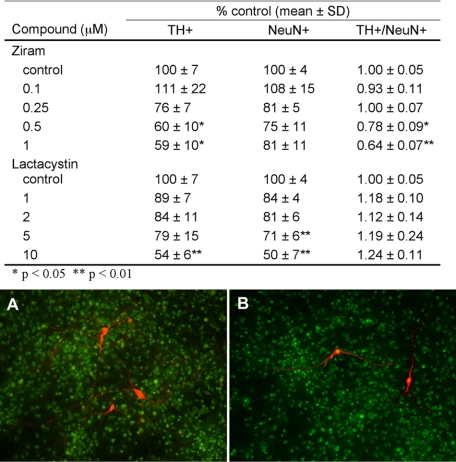
Ziram Causes Dopaminergic Cell Damage in Primary VMC—Because proteasome inhibition has been implicated in the etiology of PD and ziram causes UPS dysfunction, dopaminergic cells might be selectively vulnerable to the toxicity of ziram. Primary VMC were exposed to ziram for 48 h, and then cellular toxicity was measured by counting TH immuno-positive (TH+) cells and NeuN immuno-positive (NeuN+) cells (a nonspecific neuronal marker). Ziram at 5 and 10 μm was highly toxic to all cells, including the glial bed, causing the cell layers to lift off the coverslip after treatment or during immunostaining (data not shown). At lower doses Ziram exposure had a significant overall effect on TH+ cell survival (F4,110 = 4.52, p < 0.005) reducing TH+ cell number at 0.5 and 1 μm (p < 0.05, Dunnet's post-hoc test versus control; Fig. 2). The small, nonsignificant decrease in total NeuN+ cells measured after ziram treatment therefore represents primarily the loss of the TH+ subset of the entire NeuN+ pool. The loss of TH+ cells is likely reflective of cell death and not simply the loss of expression because TH levels were increased in the surviving positive cells (data not shown).
FIGURE 2.
Ziram- and lactacystin-induced tyrosine hydroxylase cell damage in primary mesencephalic cultures. TH+ neurons were selectively vulnerable to ziram-but not lactacystin-induced damage. Shown below are representative photomicrographs of TH-(red) and NeuN-stained (green) mesencephalic cultures. A, control. B, ziram (1 μm).
The 20 S proteasome inhibitor lactacystin is reported to cause selective DA cell death in VMC, but this has not been a universal finding (28–32). In the present study lactacystin was toxic to NeuN+ neurons (F4,110 = 13.12, p < 0.0001) and to the TH+ subset of such neurons (F4,110 = 4.26, p < 0.003), but the TH+/NeuN+ ratios revealed that the effects of lactacystin were not specific to dopaminergic neurons (F4,110 = 0.90, p > 0.05; Fig. 2). Because ziram caused preferential loss of TH+ neurons and lactacystin did not, they appear to act via different mechanisms, despite the fact that they are both UPS inhibitors.
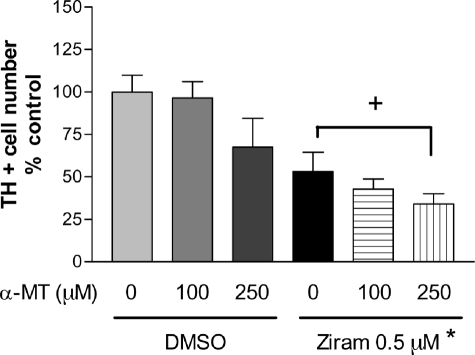
Ziram Toxicity Is Not Dopamine-dependent—One possible mechanism for the relative selective effect to TH+ neurons is that ziram interacts with dopamine metabolism to produce preferential toxicity. To test this hypothesis, ziram toxicity was measured in the presence of α-methyl l-tyrosine, an inhibitor of dopamine synthesis. Treatment of VMCs with α-methyl l-tyrosine (250 μm) for 48 h resulted in a decrease in dopamine content of 62 ± 6% compared with controls (p ≤ 0.05) but did not significantly alter the number of TH+ cells. Reducing dopamine content with α-methyl l-tyrosine was ineffective in attenuating the toxicity of ziram to TH+ neurons (Fig. 3).
FIGURE 3.
Inhibition of dopamine synthesis by α-methyl-l-tyrosine did not attenuate ziram-induced dopamine cell death (n = 14–44 wells per condition). *, p ≤ 0.05, ziram versus dimethyl sulfoxide (DMSO) control. +, not significant.
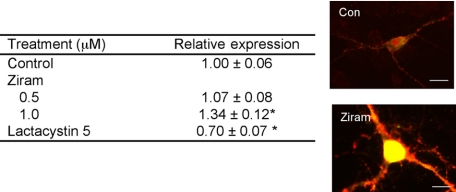
α-Synuclein Expression in VMCs—Several converging lines of evidence support a role for α-synuclein in the pathogenesis of PD. Mutations in the α-synuclein gene or increased expression of wild-type α-synuclein cause PD in familial PD, and α-synuclein is a major component of Lewy bodies in sporadic PD (22). To determine whether ziram alters the levels of α-synuclein in VMCs, the neurons were stained for both TH and α-synuclein, and relative fluorescence was measured. TH+ neurons showed robust and punctate α-synuclein staining, suggesting that they are mature and terminally differentiated (23, 24). Ziram at 0.5 μm resulted in increased α-synuclein expression, whereas lactacystin at 5 μm decreased expression (Fig. 4). The sizes of the regions of interest were the same among all of the conditions varied in all of the experiments (data not shown).
FIGURE 4.
Ziram increases and lactacystin decreases α-synuclein levels in TH+ neurons. The cells were exposed for 48 h prior to analysis. The relative intensities were measured in a blinded manner in cells that were selected randomly (n = 21–97 cells/condition). Representative α-synuclein-stained cells are shown on the right. Scale bars, 10 microns. **, p ≤ 0.01. Con, control.
An important pathological marker in PD is the formation of α-synuclein inclusions or aggregates. TH+ cells from ziram- and lactacystin-treated VMCs were determined to be either positive or negative for nuclear, perinuclear, or cytoplasmic α-synuclein inclusions by blinded raters. Aggregates were relatively common in both treated and untreated cells, but surprisingly no significant differences were found with ziram compared with controls. Interestingly, a decrease in nuclear and perinuclear aggregates was seen in the lactacystin-treated cultures (Table 2).
TABLE 2.
Ziram does not induce the formation of α-synuclein aggregates
n = 21–97 cells/condition.
|
Cells with inclusions |
|||
|---|---|---|---|
| Nuclear | Perinuclear | Cytoplasmic | |
| % | |||
|
Control |
32 ± 5 |
22 ± 4 |
4.3 ± 2.1 |
| Ziram | |||
| 0.5 μm | 38 ± 6 | 24 ± 5 | 6.6 ± 2.9 |
|
1.0 μm |
19 ± 9 |
14 ± 8 |
4.8 ± 4.8 |
| Lactacystin (5 μm) | 19 ± 4a | 8.6 ± 3.1b | 7.4 ± 2.9 |
p = 0.04.
p = 0.01.
To determine whether ziram treatment results in increased formation of detergent-soluble α-synuclein oligomer formation, we subjected VMCs lysates to Western blot analysis. Both monomeric and oligomeric forms of α-synuclein were apparent in detergent-soluble fractions as previously described (25). Ziram treatment resulted in a nonsignificant trend for an increase in oligomeric forms of α-synuclein compared with controls (170 ± 120% optical density units of controls, n = 8 for ziram and n = 5 for controls, p = 0.18). Oligomeric α-synuclein was unchanged in lactacystin-treated VMCs, and monomeric α-synuclein levels were similar in all three conditions (data not shown).
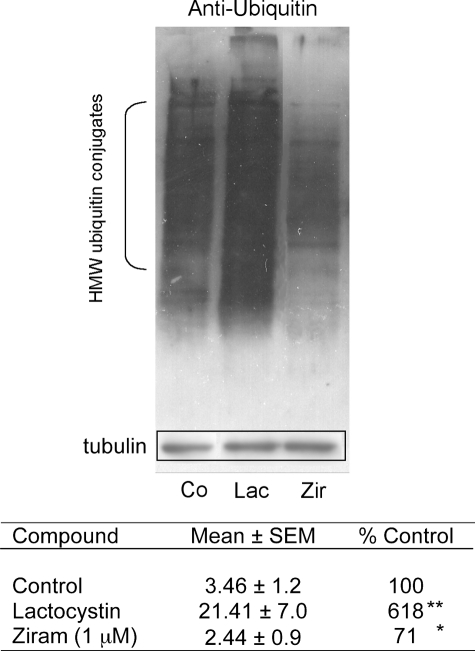
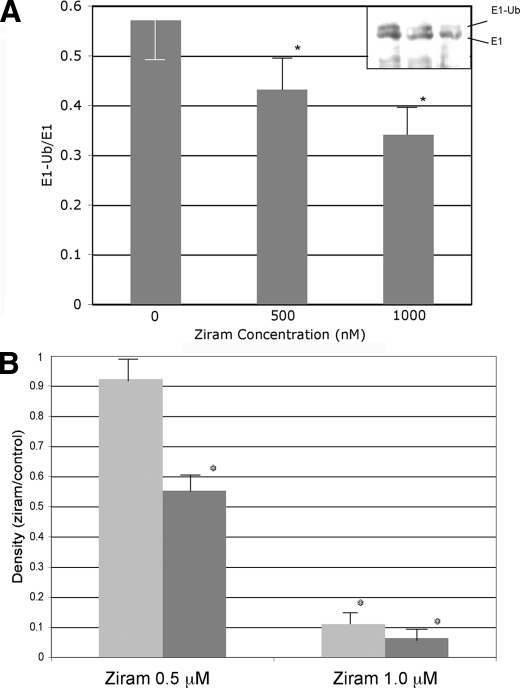
Ziram Inhibits E1 Ligase Activity—Ziram was found to inhibit the UPS using an assay that requires the substrate (i.e. degron) to be ubiquitinylated via ubiquitin ligases and recognized by the 26 S proteasome before it can be degraded by 20 S proteases (12). Disruption of any of these steps would be detected in the screen. It has been suggested that another dithiocarbamate fungicide, maneb, inhibits the 20 S component of the UPS (26). To determine whether ziram acted in this manner, HEK cells were treated with ziram (1 and 10 μm) for 24 h, but there was no change in 20 S chymotryptic activity (data not shown). The amount of α-subunit of the 20 S proteasome was also measured using Western blot analysis, and no differences were found (data not shown). Furthermore, our earlier study showed that ziram had no effect on 20 S UPS activity when added directly to cell lysate (11). Because 20 S proteolytic activity was not altered by ziram, Western blot analysis on lysates was performed from ziram-treated cells to determine whether ubiquitinated proteins accumulated. As expected, inhibition of the 20 S UPS by lactacystin and rotenone resulted in the accumulation of high molecular weight ubiquitin conjugates (Fig. 5). Conversely, ziram treatment resulted in a significant decrease in these proteins, suggesting that it inhibits the UPS by interfering with ubiquitin ligation. Polyubiquitin is added to proteins targeted for UPS degradation by a series of ligases (E1, E2, and E3 ligases). Dithiocarbamates can lead to GSH oxidation (27), and GSH depletion results in the loss of E1 ligase activity (14). Ziram does not appear to act by lowering GSH, because depleting cellular GSH using buthionine sulfoximine (1 mm) had no effect on UPS activity, and ziram did not alter the amount of reduced GSH in the cells at concentrations up to 50 μm (data not shown). Ubiquitin is activated by E1 ligase in an ATP-dependent manner forming an E1-ubiquitin adduct before the ubiquitin is transferred to E2 ligase. Because activated ubiquitin binds covalently to a cysteine of E1, the thiol group of ziram might interfere with this reaction. Indeed, ziram markedly reduced E1 ligase activity as measured by a reduction of endogenous E1-ubiquitin conjugates and the reduced formation of E1-ubiquitin conjugates in a purified preparation (Fig. 6).
FIGURE 5.
Anti-ubiquitin Western blot analysis of lysates from cells following treatment with 5 μm lactacystin, 0. 1 μm rotenone, or 1 μm ziram. The relative densities were determined using the NIH Image program, normalized to tubulin immuoreactivity in the same blot, and expressed as percentages of untreated controls (n = 4). 20 S inhibition using lactacystin resulted in an increase in high molecular weight ubiquitin conjugates, whereas ziram treatment reduced them. **, p ≤ 0.01; *, p ≤ 0.05.
FIGURE 6.
Effect of ziram on E1-ubiquitin conjugates and E1 ligase activity. A, endogenous E1-ubiquitin conjugates in lysates of ziram-treated HEK cells. Ziram resulted in a dose-dependent reduction in the E1-Ub/E1 ratio compared with untreated controls. The Western blot is shown in the inset. B, E1 ligase activity was inhibited by ziram in purified preparations after 5 and 10 min of incubation (n = 4, p ≤ 0.05).
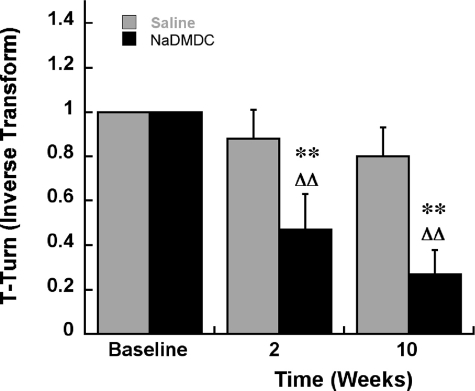
Motor Deficits in NaDMDC-treated Mice—NaDMDC was used for in vivo experiments because of its greater solubility than the zinc DMDC salt, ziram, with a similar effect on proteasomal function (Table 1). The treatment of male C57BL/6 mice for 2 weeks with NaDMDC resulted in increased time to turn in the pole test, a deficit also seen in mice with dysfunction of the nigrostriatal pathway (Fig. 7). The effect persisted 8 weeks after cessation of drug treatment, indicating that it was not because of an acute drug effect. Striatal TH fiber density was nonsignificantly reduced across striatal regions at 2 weeks, with a significant reduction in the ventrolateral quadrant 9 weeks after drug treatment in the NaDMDC-treated animals (Table 3). Stereological TH immunoreactive neuron counts in the SNc of treated mice were not changed (Table 3). Thus, 2 weeks of treatment of mice with NaDMDC, an E1-activating enzyme inhibitor, resulted in a motor deficit with a minor loss of nigrostriatal dopaminergic fibers.
FIGURE 7.
Motor deficits in mice determined by the pole test following 2 weeks of subcutaneous minipump treatment with NaDMDC at 50 mg/kg/day followed by an 8-week post-treatment period (10 weeks from the beginning of the experiment). Inverse Transform, reciprocal of the original data point. Baseline, 1 because untreated mice turn in less than 1 s. **, p < 0.01 compared with saline-treated mice at the same time point. ΔΔ represents p < 0.01 compared with a base line within the same treatment group (2 × 3 Mixed design ANOVA, Fisher's LSD).
TABLE 3.
Effect of subcutaneous treatment with NaDMDC on TH immunoreactivity (IR) fiber density in striatal subregions and stereological estimates of TH-IR neurons in the SNc
DL, dorsolateral; DM, dorsomedial; VL, ventrolateral; VM, ventromedial.
|
Region |
Mean ± S.E. (n = 5) |
|||
|---|---|---|---|---|
|
2W |
2 + 9W |
|||
| Control | Treated | Control | Treated | |
| TH-IR fiber density | ||||
| DL striatum | 1515 ± 207 | 1456 ± 221 | 1934 ± 299 | 1951 ± 269 |
| DM striatum | 1048 ± 149 | 843 ± 117 | 1435 ± 199 | 1358 ± 130 |
| VL striatum | 1439 ± 142 | 1290 ± 140 | 1833 ± 254 | 1409 ± 166a |
|
VM striatum |
815 ± 103 |
696 ± 62 |
1073 ± 174 |
946 ± 152 |
| TH-IR-positive neurons | ||||
| SNc | 7980 ± 491 | 7832 ± 574 | 8251 ± 214 | 7897 ± 247 |
p ≤ 0.05 compared with corresponding region in control mice at the same time point; ANOVA followed by Fisher PLSD.
DISCUSSION
Pesticides, Ziram, and PD—The most consistent and strongest association between a group of environmental toxicants and the development of sporadic PD has been with chronic pesticide exposure, although no specific agents have been identified (3). Preliminary data from our population-based study in central California that determined pesticide exposure using a state application registry has revealed some intriguing results. Subjects living within 500 meters of where ziram was applied were at an over 3-fold higher risk of developing PD compared with those with lower exposure.3 Chronic inhibition of the UPS has been implicated in the pathogenesis of PD, and some pesticides might increase the risk of developing PD by causing UPS dysfunction. The widely used pesticide ziram is one of the most potent inhibitors of 26 S UPS (11). This study further demonstrates that ziram kills TH+ cells in a relatively selective manner, increases α-synuclein levels, and inhibits El ligase activity, thus interfering with the targeting of proteins destined for UPS degradation. If these preliminary epidemiology findings are confirmed, and taken together with the results of this study, chronic ziram exposure would be a strong candidate as a PD-associated toxicant. The results presented here add a biologically plausible mechanism (at relevant concentrations) by which ziram may increase the risk of developing the disease.
Mechanism of Ziram Effect on the UPS—Uncovering how ziram causes UPS dysfunction and cell death might provide important clues to the selective vulnerability of DA neurons. Some of the results in primary cultures were surprising. Although ziram damaged (and probably killed) TH+ cells in a selective manner, this was not the case for the 20 S proteasome inhibitor lactacystin. Even though TH staining was used as a marker of DA cell survival, it is possible that the cells were still alive but simply lost their TH phenotype. However, this is unlikely because TH levels were actually increased in the remaining cells after ziram exposure (but not for lactacystin). Conflicting results on proteasome inhibitor-induced selective DA cell death may be due to differences in culturing techniques and conditions (28–32). Cultures in the current study contained DA neurons of the ventral tegmental region to increase the number of TH+ cells/well and power analysis. Because ventral tegmental region neurons are believed to be more resistant to stressors, the results likely underestimate the effects of ziram on SNc DA neurons. Indeed, pilot experiments using predominantly nigral cultures yielded similar results. The fact that different effects were found with ziram compared with lactacystin suggests that they act through different mechanisms. As opposed to lactacystin, a 20 S protease inhibitor, we show that ziram acts upstream by interfering with ubiquitin ligation. Because ubiquitinylation is also important in many cellular processes in addition to the UPS, including modification of protein function, facilitation of cell surface receptor turnover, and control of gene transcription, it is possible that some of the actions of ziram may be via alternative pathways (33).
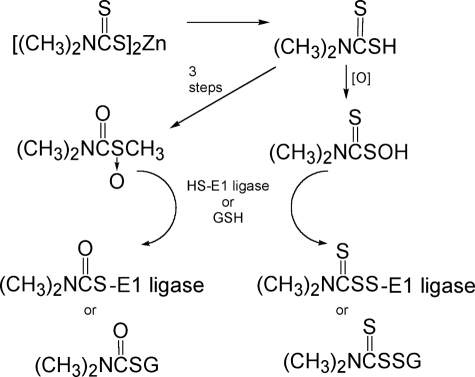
The molecular basis of the ability of ziram to inhibit E1 ligase activity was studied by determining the relative potencies of several of its analogs. The most potent 26 S UPS inhibitors were dimethyl- and diethyldithiocarbamates and their salts and disulfides. These compounds may act by copper or iron chelation (34) or undergo oxidative activation to the S-oxides of the dithiocarbamic acids (35) or of the S-methyl thiocarbamates (36), which are reactive with GSH and potentially with a thiol group of the UPS E1 ligase (Fig. 8).
FIGURE 8.
Alternative mechanisms for ziram oxidative activation and reaction with 26 S E1 ligase or GSH.
Maneb and Ethylenebisdithiocarbamate Fungicides—The effect of ziram on UPS activity has not been observed by others, but the structurally related fungicide maneb is reported to inhibit 20 S protease activity (26). Although ziram and maneb have many structural features in common, our results with ziram differ significantly from those of Zhou et al. (26) using maneb. In our studies, ziram did not inhibit the 20 S proteasome at concentrations up to 10 μm, did not cause oxidative stress as measured by 6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate fluorescence (11), and did not induce α-synuclein aggregates in primary cultures. These observations may be due to differences in the compounds and/or cell assays used. Maneb is an ethylenebis(dithiocarbamate) and contains manganese, which possibly could contribute to some of its effects, but manganese by itself did not alter 26 S UPS activity (data not shown).
Another difference between the effects of maneb and ziram is that maneb is reported to induce α-synuclein aggregates in a rat embryonic mesencephalon murine neuroblastoma-glioma hybrid cell line (26). The present study did not find an increase in aggregate formation in primary mesencephalic cultures but did not evaluate aggregates in immortalized cell lines because neither HEK or SK-N-MC cells expressed significant levels. Several lines of evidence support a mechanism for ziram upstream of the 20 S: inhibition of the formation of high molecular weight ubiquitin conjugates, reduction of the E1-ubquitin/E1 ratio, and direct inhibition of purified E1 ligase activity. Ziram effects on E2 or E3 ligase activities were not studied because they are dependent on E1 ligase, but because they transfer ubiquitin in a manner similar to that of E1, it is possible that ziram would have similar inhibitory effects on these enzymes.
Animal Studies on Dithiocarbamate Fungicides—Further support for the potential role dithiocarbamates in PD comes from animal studies. Chronic maneb exposure given with paraquat recapitulates many of the behavioral and pathological hallmarks of PD (37). Two week subcutaneous minipump treatment with NaDMDC at 50 mg/kg/day resulted in persistent motor abnormalities typically seen in mice with dysfunction of the nigrostriatal pathway (38). Abnormal motor behavior in our study was associated with a mild, delayed dopamine nerve terminal damage in the ventrolateral region of the striatum but not with nigral cell death. Motor dysfunction without dopamine cell loss may indicate functional damage to these neurons, but we cannot exclude the possibility that the behavioral tests were detecting abnormalities in other areas involved in motor control.
In summary, we show that ziram and structurally related dithiocarbamates induce dysfunction of the UPS by inhibiting E1 ligase. We demonstrate that ziram exposure increases α-synuclein levels and selectively damages dopaminergic neurons in primary cultures. Furthermore, chronic systemic exposure to NaDMDC causes persistent motor deficits and mild injury to the nigrostriatal pathway. This study, along with emerging epidemiological investigations, suggests that chronic exposure to dithiocarbamate fungicides can contribute to the pathogenesis of PD.
Acknowledgments
We thank Dr. Sheila Fleming for help with the statistical analysis.
This work was supported, in whole or in part, by National Institutes of Health Grant 5 U54 ESO12078. This work was also supported by Veterans Administration Grant SW PADRREC, the National Institutes of Health Medical Scientist Training Program (to A. P. C.), a postdoctoral fellowship from the Swedish Research Council (to R. K.), and funds from the Michael J. Fox Foundation (to F. M.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: PD, Parkinson disease; E1, ubiquitin-activating enzyme; E2, ubiquitin-conjugating enzyme; E3, ubiquitin ligase; UPS, ubiquitin proteasome system; VMC, ventral mesencephalic cultures; TH, tyrosine hydroxylase; DA, dopaminergic; DMDC, dimethyldithiocarbamate; NaDMDC, sodium DMDC; HEK, human embryonic kidney; GFP, green fluorescent protein; SNc, substantia nigra pars compacta; ANOVA, analysis of variance.
B. Ritz, J. M. Bronstein, and S. Costello, unpublished data.
References
- 1.Ascherio, A., Chen, H., Weisskopf, M. G., O'Reilly, E., McCullough, M. L., Calle, E. E., Schwarzschild, M. A., and Thun, M. J. (2006) Ann. Neurol. 60197 –203 [DOI] [PubMed] [Google Scholar]
- 2.Kamel, F., Tanner, C., Umbach, D., Hoppin, J., Alavanja, M., Blair, A., Comyns, K., Goldman, S., Korell, M., Langston, J., Ross, G., and Sandler, D. (2007) Am. J. Epidemiol. 165364 –374 [DOI] [PubMed] [Google Scholar]
- 3.Brown, T. P., Rumsby, P. C., Capleton, A. C., Rushton, L., and Levy, L. S. (2006) Environ. Health Perspect. 114156 –164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McNaught, K. S., and Jenner, P. (2001) Neurosci. Lett. 297191 –194 [DOI] [PubMed] [Google Scholar]
- 5.Manning-Bog, A. B., Reaney, S. H., Chou, V. P., Johnston, L. C., McCormack, A. L., Johnston, J., Langston, J. W., and Di Monte, D. A. (2006) Ann. Neurol. 60256 –260 [DOI] [PubMed] [Google Scholar]
- 6.Kordower, J. H., Kanaan, N. M., Chu, Y., Suresh Babu, R., Stansell, J., 3rd, Terpstra, B. T., Sortwell, C. E., Steece-Collier, K., and Collier, T. J. (2006) Ann. Neurol. 60264 –268 [DOI] [PubMed] [Google Scholar]
- 7.Bove, J., Zhou, C., Jackson-Lewis, V., Taylor, J., Chu, Y., Rideout, H. J., Wu, D. C., Kordower, J. H., Petrucelli, L., and Przedborski, S. (2006) Ann. Neurol. 60260 –264 [DOI] [PubMed] [Google Scholar]
- 8.McNaught, K. S., and Olanow, C. W. (2006) Ann. Neurol. 60243 –247 [DOI] [PubMed] [Google Scholar]
- 9.Schapira, A. H., Cleeter, M. W., Muddle, J. R., Workman, J. M., Cooper, J. M., and King, R. H. (2006) Ann. Neurol. 60253 –255 [DOI] [PubMed] [Google Scholar]
- 10.McNaught, K. S., Perl, D. P., Brownell, A. L., and Olanow, C. W. (2004) Ann. Neurol. 56149 –162 [DOI] [PubMed] [Google Scholar]
- 11.Wang, X. F., Li, S., Chou, A. P., and Bronstein, J. M. (2006) Neurobiol. Dis. 23198 –205 [DOI] [PubMed] [Google Scholar]
- 12.Bence, N. F., Sampat, R. M., and Kopito, R. R. (2001) Science 2921552 –1555 [DOI] [PubMed] [Google Scholar]
- 13.Rayport, S., Sulzer, D., Shi, W. X., Sawasdikosol, S., Monaco, J., Batson, D., and Rajendran, G. (1992) J. Neurosci. 124264 –4280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jha, N., Kumar, M. J., Boonplueang, R., and Andersen, J. K. (2002) J. Neurochem. 80555 –561 [DOI] [PubMed] [Google Scholar]
- 15.Laemmli, U. K. (1970) Nature 227680 –685 [DOI] [PubMed] [Google Scholar]
- 16.Chow, E., Mottahedeh, J., Prins, M., Ridder, W., Nusinowitz, S., and Bronstein, J. M. (2005) Mol. Cell Neurosci. 29405 –413 [DOI] [PubMed] [Google Scholar]
- 17.Fleming, S. M., Salcedo, J., Fernagut, P. O., Rockenstein, E., Masliah, E., Levine, M. S., and Chesselet, M. F. (2004) J. Neurosci. 249434 –9440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bordelon, Y. M., and Chesselet, M. F. (1999) Neuroscience 93843 –853 [DOI] [PubMed] [Google Scholar]
- 19.McCormack, A. L., Thiruchelvam, M., Manning-Bog, A. B., Thiffault, C., Langston, J. W., Cory-Slechta, D. A., and Di Monte, D. A. (2002) Neurobiol. Dis. 10119 –127 [DOI] [PubMed] [Google Scholar]
- 20.West, M. J., Slomianka, L., and Gundersen, H. J. (1991) Anat. Rec. 231482 –497 [DOI] [PubMed] [Google Scholar]
- 21.Cohen, J., and Cohen, P. (1983) Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences, 2nd Ed., Lawrence Erlbaum Associates, Hillsdale, NJ
- 22.Singleton, A. B. (2005) Trends Neurosci. 28416 –421 [DOI] [PubMed] [Google Scholar]
- 23.Quilty, M. C., Gai, W. P., Pountney, D. L., West, A. K., and Vickers, J. C. (2003) Exp. Neurol. 182195 –207 [DOI] [PubMed] [Google Scholar]
- 24.Lesuisse, C., and Martin, L. J. (2002) J. Neurobiol. 519 –23 [DOI] [PubMed] [Google Scholar]
- 25.Rideout, H. J., Dietrich, P., Wang, Q., Dauer, W. T., and Stefanis, L. (2004) J. Biol. Chem. 27946915 –46920 [DOI] [PubMed] [Google Scholar]
- 26.Zhou, Y., Shie, F. S., Piccardo, P., Montine, T. J., and Zhang, J. (2004) Neuroscience 128281 –291 [DOI] [PubMed] [Google Scholar]
- 27.Burkitt, M. J., Bishop, H. S., Milne, L., Tsang, S. Y., Provan, G. J., Nobel, C. S., Orrenius, S., and Slater, A. F. (1998) Arch. Biochem. Biophys. 35373 –84 [DOI] [PubMed] [Google Scholar]
- 28.Rideout, H. J., Lang-Rollin, I. C., Savalle, M., and Stefanis, L. (2005) J. Neurochem. 931304 –1313 [DOI] [PubMed] [Google Scholar]
- 29.Kikuchi, S., Shinpo, K., Tsuji, S., Takeuchi, M., Yamagishi, S., Makita, Z., Niino, M., Yabe, I., and Tashiro, K. (2003) Brain Res. 964228 –236 [DOI] [PubMed] [Google Scholar]
- 30.Hoglinger, G. U., Carrard, G., Michel, P. P., Medja, F., Lombes, A., Ruberg, M., Friguet, B., and Hirsch, E. C. (2003) J. Neurochem. 861297 –1307 [DOI] [PubMed] [Google Scholar]
- 31.McNaught, K. S., Mytilineou, C., Jnobaptiste, R., Yabut, J., Shashidharan, P., Jennert, P., and Olanow, C. W. (2002) J. Neurochem. 81301 –306 [DOI] [PubMed] [Google Scholar]
- 32.Petrucelli, L., O'Farrell, C., Lockhart, P. J., Baptista, M., Kehoe, K., Vink, L., Choi, P., Wolozin, B., Farrer, M., Hardy, J., and Cookson, M. R. (2002) Neuron 361007 –1019 [DOI] [PubMed] [Google Scholar]
- 33.Liu, Y. C. (2004) Annu. Rev. Immunol. 2281 –127 [DOI] [PubMed] [Google Scholar]
- 34.Montine, T. J., Underhill, T. M., Valentine, W. M., and Graham, D. G. (1995) Neurodegeneration 4283 –290 [DOI] [PubMed] [Google Scholar]
- 35.Schmitt, A., and Neibergall, H. (1989) Deutsche Lebensmittel-Rundschau 85111 –115 [Google Scholar]
- 36.Staub, R. E., Sparks, S. E., Quistad, G. B., and Casida, J. E. (1995) Chem. Res. Toxicol. 81063 –1069 [DOI] [PubMed] [Google Scholar]
- 37.Thiruchelvam, M., Richfield, E. K., Baggs, R. B., Tank, A. W., and Cory-Slechta, D. A. (2000) J. Neurosci. 209207 –9214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hwang, D. Y., Fleming, S. M., Ardayfio, P., Moran-Gates, T., Kim, H., Tarazi, F. I., Chesselet, M. F., and Kim, K. S. (2005) J. Neurosci. 252132 –2137 [DOI] [PMC free article] [PubMed] [Google Scholar]