Abstract
The metabolism of amyloid β peptide (Aβ) in the brain is crucial to the pathogenesis of Alzheimer disease. A body of evidence suggests that Aβ is actively transported from brain parenchyma to blood across the blood-brain barrier (BBB), although the precise mechanism remains unclear. To unravel the cellular and molecular mechanism of Aβ transport across the BBB, we established a new in vitro model of the initial internalization step of Aβ transport using TR-BBB cells, a conditionally immortalized endothelial cell line from rat brain. We show that TR-BBB cells rapidly internalize Aβ through a receptor-mediated mechanism. We also provide evidence that Aβ internalization is mediated by LRP1 (low density lipoprotein receptor-related protein 1), since administration of LRP1 antagonist, receptor-associated protein, neutralizing antibody, or small interference RNAs all reduced Aβ uptake. Despite the requirement of LRP1-dependent internalization, Aβ does not directly bind to LRP1 in an in vitro binding assay. Unlike TR-BBB cells, mouse embryonic fibroblasts endogenously expressing functional LRP1 and exhibiting the authentic LRP1-mediated endocytosis (e.g. of tissue plasminogen activator) did not show rapid Aβ uptake. Based on these data, we propose that the rapid LRP1-dependent internalization of Aβ occurs under the BBB-specific cellular context and that TR-BBB is a useful tool for analyzing the molecular mechanism of the rapid transport of Aβ across BBB.
Aggregation and deposition of amyloid β-peptide (Aβ)2 in the brain are crucial events in the pathogenesis of Alzheimer disease (AD) (1). Aβ is produced from β-amyloid precursor protein through sequential proteolytic cleavages by β- and γ-secretases. Missense mutations as well as duplication of the β-amyloid precursor protein gene have been identified in pedigrees of early onset familial AD (2–4), some of which have been shown to alter the β-amyloid precursor protein processing in such a way as to increase the level of Aβ, especially that of the more aggregable species Aβ42, leading to formation of Aβ fibrils (5–7). Cognitive deficits in transgenic mice overexpressing familial AD mutant form β-amyloid precursor protein, as well as alteration in synaptic plasticity and synapse loss induced by Aβ oligomers implicate aggregated species of Aβ in the neuronal dysfunction and death in AD brains (8–10).
Aβ, secreted from neurons in the brain, is thought to be catabolized by specific proteases (e.g. NEP and insulin-degrading enzyme) (11, 12); phagocytotic cells in brains (i.e. microglia and astrocytes) (13, 14) take up and clear soluble or aggregated Aβ. Notably, in vivo observations that Aβ injected into rodent brains is rapidly effluxed from brains (15, 16) suggest the presence of a novel pathway for Aβ clearance across the blood-brain barrier (BBB). The BBB is considered as a three-cell archetype composed of brain microvascular endothelial cells (BMECs), astrocytes, and supporting pericytes. It does not normally allow a free exchange of macromolecules between brain and blood, due to the presence of tight junctions formed by BMECs (17), which suggests a receptor-mediated transport mechanism for the efflux of Aβ across the BBB. It has been suggested that LRP1 (low density lipoprotein receptor-related protein 1) is involved in the Aβ efflux transport at the BBB in vivo (15, 18), although there is no direct evidence to support the role of LRP1 in BMECs, the latter bordering the outer limit of the BBB. Thus, there is a compelling need for an endothelial cell model that can recapitulate the transport of Aβ and other macromolecules across the BBB and with which a precise molecular mechanism of Aβ transport across the BBB could be elucidated.
To verify the molecular and cellular mechanisms of Aβ transport across the BBB, application of cell biological and physiological techniques at a single cell level is mandatory. In this study, we adopted the TR-BBB cells, a conditionally immortalized cell line derived from brain capillary endothelial cells of transgenic rats expressing temperature-sensitive large T antigen (19), whose inactivation upon incubation at 37 °C renders the cells into a nonimmortalized state similar to primary BMECs. TR-BBB cells have been shown to express a number of receptors and transporters expressed in endothelial cells comprising the BBB (e.g. GLUT-1, P-glycoprotein (P-gp), and other influx or efflux transporters, allowing for the characterization of their functions in vitro (20).
Using TR-BBB cells, we established an in vitro model of Aβ uptake and found that LRP1 is involved in the rapid and robust Aβ internalization in TR-BBB cells. In contrast, fibroblasts or neuroblastoma cells that express LRP1 did not internalize Aβ, suggesting that LRP1 is not sufficient for the rapid internalization of Aβ, implicating cell type specificity in the LRP1-dependent Aβ uptake. Our observations provide a new clue to the molecular mechanism of Aβ uptake and transport across the BBB.
EXPERIMENTAL PROCEDURES
Antibodies and Reagents—Synthetic Aβ-(1–40) and Aβ-(1–42) were purchased from Peptide Institute, Inc. (Osaka, Japan). 125I-radiolabeled Aβ-(1–40) (125I-Aβ) was purchased from PerkinElmer Life Sciences. Polyclonal anti-LRP1 antibody, RRR is a rabbit polyclonal antibody that recognizes full-length LRP1 (21). Anti-LRP1 monoclonal antibody 11H4 and human full-length LRP1 cDNA were provided by Dr. A. Kinoshita (22). Anti-LRP1 polyclonal antibody R488 recognizes both heavy chain and light chain of LRP1 (23). Anti-LRP1 polyclonal antibody LRP1-IC was raised against C-terminus of human LRP1 (LLGRGPEDEIGDPLA). Anti-Aβ monoclonal antibody 82E1 was purchased from Immuno-Biological Laboratories Co., Ltd. LRP1 was purified from human placenta as previously described (24). tPA was purchased from American Diagnostica, Inc. (Stamford, CT). Anti-tPA antibody was purchased from Oxford Biomed Research (Oxford, MS). Anti-receptor for advanced glycation end products (RAGE) antibody and control IgG were purchased from Sigma. A human full-length receptor-associated protein (RAP) cDNA was subcloned into pCold vector (TaKaRa) (25) between the XhoI site and HindIII site from human full-length RAP in pGEX-4T vector (26) using the following oligonucleotides: forward, 5′-CACGGCCTCGAGTACTCGCGGGAGAAGAACC-3′; reverse, 5′-TCCCCAAAGCTTTCAGAGTTCGTTGTGCC-3′. Recombinant LRP1 cluster II or IV fragments (sLRP2 or sLRP4, respectively) were subcloned into pCold vector between XhoI site and HindIII site from a cDNA encoding human full-length LRP1 (27). His-RAP fusion protein, sLRP2 and sLRP4 were expressed in Escherichia coli DH5α and purified according to the manufacturer's instructions. Astrocyte-secreted apoE4-containing lipoproteins were purified as described previously (28). Heparin was purchased from Sigma. IODO-GEN-precoated tubes and D-Salt polyacrylamide desalting columns, 6000 molecular weight cut-off were from Pierce.
Preparation of Aβ—125I-Aβ peptides were solubilized in distilled water at a concentration of 0.1 nm and stored at –80 °C until use. Synthetic Aβ peptides were solubilized in 1,1,1,3,3,3-hexafluoro-2-propanol (Kanto Chemical) at a concentration of 1 mg/ml, dried, and resolubilized in phosphate-buffered saline containing 3% (v/v) Me2SO (Kanto Chemical) upon use. We confirmed that 125I-Aβ migrates at 4 kDa on SDS-PAGE and does not show signs of aggregation at concentrations used in this study (see supplemental Fig. 1). In addition, synthetic Aβ utilized in this study showed no sign of fibrillization as examined by a thioflavin T fluorescence assay (data not shown).
Immunoblot Analysis—SDS-PAGE was performed as described previously (29). The immunoblots were visualized using Immunostar reagents (Wako Pure Chemical) and visualized using LAS-1000plus (FUJIFILM) as described (29).
PICUP Experiment—0.1 nm 125I-Aβ or 20 μm of unlabeled Aβ-(1–40) was subjected to a PICUP reaction. The resulting samples were separated by SDS-PAGE using 10–20% Tris-glycine gradient gels and analyzed by immunoblotting or autoradiography as described (30, 31).
Cell Culture—TR-BBB cells were grown in collagen type I-coated 75-cm2 tissue flasks (BD Biosciences) at 33 °C under 5% CO2/air. Dulbecco's modified Eagle's medium supplemented with 1.5 mg/ml sodium bicarbonate, 15 μg/ml bovine endothelial cell growth factor (Roche Applied Science), 70 μg/ml benzyl-penicillin potassium, 100 μg/ml streptomycin sulfate, and 10% fetal bovine serum was used as culture medium.
Mouse embryonic fibroblasts (MEFs) genetically deficient in LRP1 (PEA13 cells), LRP1 (+/–) MEFs (PEA10 cells), and normal MEFs (MEF-1 cells) derived from the same mouse strain were obtained from the American Type Culture Collection (Manassas, VA) and cultured in Dulbecco's modified Eagle's medium with 10% fetal bovine serum, as described previously (32). Chinese hamster ovary cells (from ATCC), Neuro2a cells (from ATCC), and McARH7777 cells (provided by Dr. J. Aoki) were cultured in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum. SH-SY5Y cells (from ATCC) were cultured in Dulbecco's modified Eagle's medium/F-12 containing 10% fetal bovine serum. HUVEC cells (purchased from Sanko Junyaku) were cultured in the basal medium containing SingleQuotos (Sanko Junyaku).
In Vitro Uptake Assays of 125I-Labeled Proteins in TR-BBB Cells—The uptake of 125I-Aβ or 125I-tPA into TR-BBB cells was examined as reported previously (33). Briefly, TR-BBB cells cultured onto collagen I coated 24-well dishes were grown to 90–100% confluence. Prior to experiments, cells were washed three times with 1 ml of ECF buffer (138 mm NaCl, 5.0 mm KCl, 1.3 mm CaCl2, 0.8 mm MgCl2, 0.3 mm KH2PO4, 0.3 mm Na2HPO4, 5.6 mm d-glucose, 10 mm HEPES, pH 7.4) and incubated at 37 °C with 125I-labeled ligand proteins for a predetermined time period. After incubation, 125I-labeled ligand proteins were removed, and the cells were washed three times with 1 ml of ice-cold ECF buffer and an additional three times with acid wash buffer (28 mm CH3COONa, 120 mm NaCl, 20 mm sodium barbital, pH 3.0). In some experiments, cells were further treated with Pronase (1 mg/ml; Sigma) for 1 h at 4 °C to completely remove cell surface-attached radioligands. The amount of surface-bound labeled ligands was calculated as the amount of ligands released by this treatment, and the amount of internalized ligands was defined as the amount of ligands that remained associated with the cell pellet following this treatment. Cells were solubilized with 200 μl of 5 m NaOH for 12 h, and the protein amount in the cells was measured by the Lowry method. The amount of the cell-associated ligands was expressed as the cell/medium ratio (see below).
The cell/medium ratio (μl/mg of protein) is equal to 125I counts in the cells (cpm/mg protein)/125I counts in the incubation medium (cpm/μl). When the effects of various inhibitors or siRNA against LRP1 were studied, TR-BBB cells were incubated with 125I-Aβ and 125I-tPA for 5 and 10 min, respectively.
In the efflux assay, TR-BBB cells were first incubated with 125I-Aβ for 10 min (first incubation). After incubation, the media were replaced with the ECF buffer (incubation medium), and cells were further incubated at 37 °C for a various time period to allow efflux of Aβ (second incubation). Degradation of 125I-Aβ was studied by a trichloroacetic acid precipitation assay (15). Incubation medium was mixed with trichloroacetic acid (final concentration, 10%) and bovine serum albumin (final concentration, 1.25%) and centrifuged for 20 min, and the radioactivities in the precipitate and supernatant were quantitated by a γ counter. We confirmed that ∼85–90% of total 125I-Aβ in medium prior to the first incubation was trichloroacetic acid-precipitable. Thus, we quantitated the extent of trichloroacetic acid precipitability of Aβ prior to the first incubation upon each experiment and normalized the level of trichloroacetic acid-precipitable Aβ in incubation medium at each time point upon the second incubation by the precipitation extent and indicated the ratio of trichloroacetic acid-precipitable Aβ as the percentage of that at time 0 of the first incubation. For the uptake assay of Aβ·apoE complex, Aβ was preincubated with apoE4-containing lipoproteins (2 μg/ml) for 30 min at room temperature.
To examine the involvement of heparan sulfate proteoglycans or chondroitin sulfate proteoglycans in 125I-Aβ uptake, TR-BBB cells were preincubated in medium containing heprinase I (Seikagaku), heparinase II (Sigma), heparinase III (Sigma), or condroitinase ABC (Seikagaku) at a concentration of 1 sigma unit/ml for 4 h at 37 °C, followed by three washes with ECF buffer.
Transfection of Small Interference RNA (siRNA) against Rat LRP1—Control siRNA (Stealth™ RNAi negative control medium GC duplex 2) and two Stealth RNAis against rat LRP1 (siRNA 1, 5′-UUGACAUUCGGAUCACAGACGUGGG-3′; siRNA 2, 5′-AUGAAUGCCCGUUUGAUGGCUUGGG-3′) were purchased from Invitrogen. TR-BBB cells cultured in 24-well dishes were transfected with 42 pmol of siRNA duplex using Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer's instructions. The Lipofectamine RNAiMAX and siRNA mixture were incubated with TR-BBB cells for 30 h. Transfected TR-BBB cells were collected for immunoblotting or uptake assays as described above.
Binding of Aβ to Immobilized LRP1 in Vitro—An in vitro binding assay was performed as described previously (29). Briefly, microtiter wells were coated with purified full-length human LRP1 or recombinant sLRP2, sLRP4, or bovine serum albumin (negative control) at 4 °C. Unoccupied sites were blocked with Block Ace (Snow Brand, Sapporo, Japan) and washed with phosphate-buffered saline containing 0.05% Tween 20. Wells were then incubated with 100 nm Aβ or tPA in the presence or absence of 1 μm RAP for 12 h, washed with phosphate-buffered saline containing 0.05% Tween 20, and then reacted with an anti-Aβ antibody (BA27; gift of Takeda Pharmaceutical Co., Ltd.) (34) or anti-tPA (Oxford Biomedical Research) antibodies for 3 h. After incubation with a horseradish peroxidase-tagged secondary antibody (GE Healthcare), the levels of ligands bound to LRP1 were quantitated by development using a TMB microwell system (KPL, Inc.).
Preparation of Radiolabeled tPA—Human tPA was iodinated, using IODO-GEN-precoated tubes, according to the manufacturer's instructions. The unincorporated 125I was removed using a D-salt polyacrylamide desalting column.
RESULTS
Aβ Is Internalized by TR-BBB Cells in a Specific Manner—We reasoned that if Aβ is transported across the BBB, Aβ should be internalized by BMECs. To test this, we used TR-BBB cells, an in vitro cellular model in which the internalization in BBB endothelial cells can be analyzed (19). We first evaluated the uptake of 125I-Aβ in monolayer cultures of TR-BBB cells.
First, we checked whether 125I-Aβ-(1–40) does not form oligomers at the concentration used in this study (0.1 nm) by a light-induced chemical cross-linking experiment, PICUP, an efficient method for detecting SDS-sensitive Aβ assemblies by forming covalently cross-linked Aβ oligomers (30, 31). The oligomerization state of each peptide after cross-linking was determined by immunoblot analysis or autoradiography. When 20 μm of unlabeled Aβ was subjected to PICUP reaction, several covalently cross-linked oligomer bands (i.e. dimer, trimer, and tetramer) appeared in an irradiation-dependent manner, suggesting that a fraction of Aβ formed SDS-sensitive oligomers in the solution (supplemental Fig. 1). In contrast, 0.1 nm 125I-Aβ-(1–40) did not exhibit any cross-linked oligomers, and only a single band migrating at 4 kDa was detected even after the PICUP reaction (supplemental Fig. 1). This suggested that the concentration of 125I-Aβ-(1–40) used in this experiment was low enough to eliminate formation of oligomers.
Cells grown in collagen-coated dishes were incubated with 125I-Aβ-(1–40). After incubation, cells were extensively washed with acid wash buffer to remove 125I-Aβ that remained on cell surface. The remaining radioactivities in TR-BBB cells that represent 125I-Aβ were calculated as cell/medium ratio (μl/mg) (see “Experimental Procedures”). Incubation with 125I-Aβ at 37 °C elicited a rapid and robust uptake of 125I-Aβ by TR-BBB cells with total uptake reaching a plateau within 8 min (Fig. 1A). This rapid uptake of 125I-Aβ was not observed at 4 °C, suggesting that the Aβ uptake is a receptor-mediated event. The uptake was competed by the addition of excess unlabeled synthetic Aβ-(1–40) in a concentration-dependent manner, with an IC50 of ∼600 nm (Fig. 1B). Because the uptake assays were performed in serum-free medium, it is unlikely that Aβ uptake requires a prior complex formation of Aβ with serum proteins (e.g. apoE or α2-macroglobulin) (35–38).
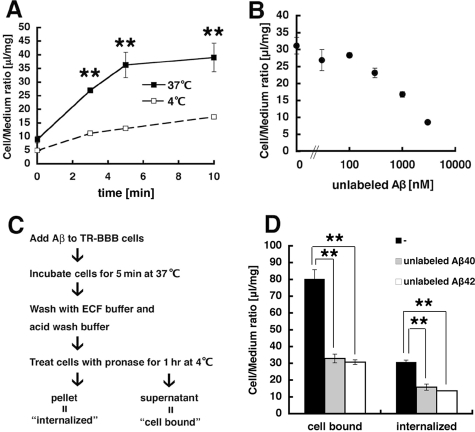
FIGURE 1.
Rapid internalization of Aβ by TR-BBB cells. A, TR-BBB cells were incubated with 125I-Aβ at 37 °C (filled squares) or 4 °C (open squares) for 0, 3, 5, and 10 min, and the amount of 125I-Aβ uptake was quantitated as cell/medium ratio. The mean ± S.E. in three independent assays is shown. **, p < 0.001. B, the uptake assay of 125I-Aβ was performed in the presence of 0, 30, 100, 300, 1000, and 3000 nm unlabeled Aβ as a competitor. The mean ± S.E. in three independent assays is shown. C, a schematic diagram depicting the uptake assays using Pronase treatment in TR-BBB cells. “Cell-bound Aβ” was defined as radioactivities released by this treatment and “internalized Aβ”as radioactivities associated with cell pellet after the treatment. D, the levels of cell-bound and internalized Aβ were measured in the presence or absence of 3 μm unlabeled Aβ-(1–40) or 3 μm unlabeled Aβ-(1–42), respectively. The mean ± S.E. in three independent assays is shown. **, p < 0.01.
To more rigorously estimate the amount of internalized Aβ into TR-BBB cells, we further treated TR-BBB cells with Pronase after incubation with 125I-Aβ at 37 °C to remove surface-bound Aβ. We defined “cell-bound” Aβ as the sum of surface-bound Aβ that includes the binding to specific receptor(s) as well as nonspecific binding and defined “internalized” Aβ as the amount of Aβ taken up into cells and resistant to Pronase treatment (Fig. 1C). TR-BBB cells retained certain levels of 125I-Aβ radioactivity even after Pronase treatment, which was reduced by co-incubation with unlabeled Aβ-(1–40), confirming that Aβ is actually internalized into cells (Fig. 1D). We also found that unlabeled Aβ-(1–42) blocked internalization of 125I-Aβ-(1–40) with an efficacy similar to that of Aβ-(1–40) (Fig. 1D). Taken together, our data show that TR-BBB cells rapidly internalize Aβ possibly through a receptor-mediated mechanism and that Aβ-(1–40) and Aβ-(1–42) probably share the same receptor.
TR-BBB Cells Partially Degrade Internalized Aβ and Release Fragments and Intact Aβ into Culture Medium—To examine the fate of internalized Aβ, we performed an efflux assay. TR-BBB cells were first incubated with 125I-Aβ for 10 min, and the media were then replaced with the ECF buffer. Cells were further incubated at 37 °C for various periods of time to allow efflux of Aβ. We found that the cell/medium ratio of Aβ internalized into TR-BBB cells was rapidly decreased over the incubation time (shown as cell in Fig. 2A). Concurrently, the radioactivity in the incubation medium was increased (shown as medium in Fig. 2A), suggesting that the internalized Aβ is rapidly effluxed into the culture medium (Fig. 2A). We next precipitated Aβ released into culture medium by trichloroacetic acid and found that ∼80% of 125I-Aβ in the culture medium escaped trichloroacetic acid precipitation and that the ratio of 125I-Aβ precipitated by trichloroacetic acid was constant for 15 min (Fig. 2B). However, SDS-PAGE analysis of culture medium revealed that a portion of effluxed Aβ remained intact and migrated at 4 kDa, which increased in a time-dependent manner (Fig. 2C). These data suggest that a significant proportion of internalized Aβ was degraded within TR-BBB cells before being released into the extracellular space.
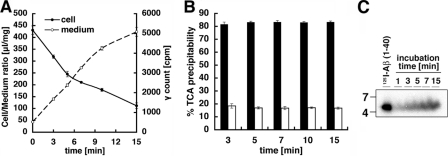
FIGURE 2.
Degradation and release of Aβ from TR-BBB cells. A, TR-BBB cells were preincubated for 10 min at 37 °C with 125I-Aβ. The incubation medium was changed to the ECF buffer without 125I-Aβ, and the cells were then incubated at 37 °C for 0, 3, 5, 7, 10, and 15 min to allow efflux of internalized 125I-Aβ into the culture medium. The amount of internalized 125I-Aβ (drawn on the left-hand y axis (Cell/Medium ratio) as filled circles) and the radioactivity in the incubation buffer in chase period (drawn on the right-hand y axis (γ count) as open circles) are shown. The mean ± S.E. in three independent assays is shown. B, the levels of trichloroacetic acid-precipitable Aβ in the culture medium incubated for 3, 5, 7, 10, and 15 min. Trichloroacetic acid (TCA)-precipitable (open bars) and non-trichloroacetic acid-precipitable (filled bars) radioactivities are expressed as a percentage of total radioactivities in medium. The mean ± S.E. in three independent assays is shown. C, SDS-PAGE analysis of 125I-Aβ effluxed into the culture medium.
Aβ Internalization Is Inhibited by RAP or Anti-LRP1 Antibody—We next examined whether LRP1 plays a major role in the internalization of 125I-Aβ into TR-BBB cells. We conducted the experiments in the presence of RAP, an antagonist of low density lipoprotein receptor family proteins, including LRP1 (39). When TR-BBB cells were incubated with 125I-Aβ in the presence of RAP, the uptake of 125I-Aβ was significantly inhibited (Fig. 3A). The strong inhibitory effect of Aβ uptake by RAP was observed even after Pronase treatment. The internalization of 125I-Aβ by TR-BBB cells was significantly inhibited by RAP, at an extent of ∼90% of that inhibited by unlabeled Aβ-(1–40) (Fig. 3B). Aβ binding to the cell surface was also blocked by RAP, at an extent of ∼95% of that by unlabeled Aβ-(1–40), supporting the notion that the major proportion of Aβ internalization is a RAP-sensitive process.
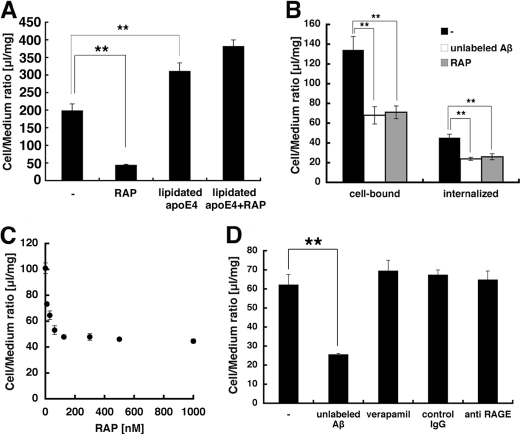
FIGURE 3.
Inhibition the Aβ internalization by RAP in TR-BBB cells. A, the uptake assay of 125I-Aβ or 125I-Aβ·apoE complex in the absence or presence of 500 nm RAP. The mean ± S.E. in four independent assays is shown. **, p < 0.01; ANOVA. B, the internalized and cell-bound 125I-Aβ in TR-BBB cells were measured in the absence or presence of 500 nm RAP and 3μm unlabeled Aβ-(1–40). The mean ± S.E. in six independent assays is shown. **, p < 0.01; ANOVA. B, the uptake assay of 125I-Aβ was performed with 0, 10, 30, 62.5, 125, 300, 500, and 1000 nm RAP in the uptake medium. The mean ± S.E. in 3–7 independent assays is shown. D, the uptake assays in the presence of 3 μm unlabeled Aβ-(1–40), 10 μg/ml anti-RAGE antibody, 10 μg/ml control IgG, or 100 μm verapamil. The mean ± S.E. in four independent assays is shown.
It has been suggested that apoE is involved in Aβ clearance (36, 40). To examine the effect of apoE on 125I-Aβ internalization, we prepared an 125I-Aβ·apoE complex by preincubating 125I-Aβ with astrocyte-derived human apoE4 for 30 min and added it to TR-BBB cells. 125I-Aβ uptake was increased following incubation with apoE, compared with that by 125I-Aβ alone, suggesting that 125I-Aβ·apoE complex formation enhanced 125I-Aβ uptake (Fig. 3A). However, the uptake of 125I-Aβ·apoE complex was not inhibited by RAP (Fig. 3A), suggesting that the uptake of free Aβ and that of Aβ·apoE complex are mediated by different pathways in TR-BBB cells. We also confirmed that the effect of RAP on Aβ uptake was concentration-dependent, with an IC50 of ∼1 nm (Fig. 3C), the latter being almost identical to those previously reported for LRP1 (39, 41).
We further examined the possibility of involvement of other Aβ receptor candidates. RAGE and P-gp were reported to mediate the Aβ transport across the BBB from blood to brain (42) and brain to blood (43), respectively. Neither anti-RAGE antibody, which is known to inhibit RAGE function, nor verapamil, a major substrate of P-gp that works as a competitor, affected the internalization of Aβ, excluding an involvement of RAGE and P-gp in the Aβ uptake by TR-BBB cells (Fig. 3D).
We further performed Aβ uptake experiments in the presence of a neutralizing antibody that specifically inhibits LRP1 function. Western blot analysis revealed that an antibody RRR, generated against LRP1 holoprotein purified from human placenta, specifically recognized the heavy chain of endogenous LRP1 in TR-BBB cells (data not shown). Coincubation with RRR robustly inhibited Aβ uptake by TR-BBB cells at an extent of ∼69% of that inhibited by RAP, in a concentration-dependent manner (Fig. 4, A and B). Aβ binding to the cell surface was also inhibited by RRR at ∼32% compared with that in the presence of control immunoglobulin (data not shown). These data strongly support the notion that Aβ internalization into TR-BBB cells is mediated by LRP1.
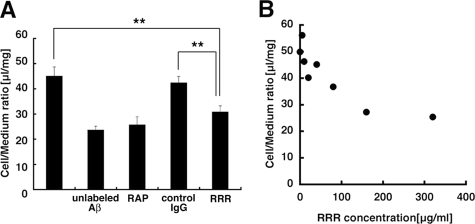
FIGURE 4.
Anti-LRP1 antibody reduced Aβ internalization in TR-BBB cells. A, the internalized 125I-Aβ in TR-BBB cells was measured in the presence of anti-LRP1 antibody (RRR) or control IgG (each 160 μg/ml). The mean ± S.E. in 5–6 independent assays is shown. **, p < 0.01; ANOVA. B, the uptake assay of 125I-Aβ was performed in the presence of 0, 5, 10, 20, 40, 80, 160, and 320 μg/ml RRR in the uptake medium. The mean ± S.E. in three independent assays is shown.
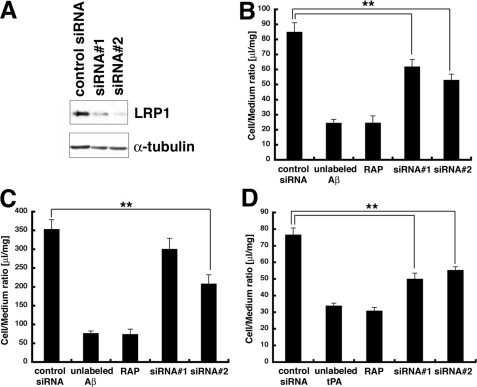
RNAi Knockdown of Endogenous LRP1 Reduced Aβ Internalization in TR-BBB Cells—To further confirm the involvement of LRP1 in the uptake of Aβ into TR-BBB cells, we knocked down LRP1 in TR-BBB cells by siRNA treatment using two stealth RNAis, a chemically modified RNA molecule that eliminates the induction of interferon pathway, against LRP1 and a stealth negative control RNA. TR-BBB cells were incubated with siRNAs against LRP1 for 30 h. The expression levels of LRP1 in TR-BBB cells were significantly reduced by the two LRP1-specific siRNAs to ∼34 and ∼29% of the levels by a control siRNA (Fig. 5A). We incubated the siRNA-treated and control cells with 125I-Aβ and found that the internalization of Aβ by TR-BBB cells was reduced in siRNA-treated cells by ∼38% (siRNA 1) and ∼52% (siRNA 2) of that inhibited by RAP (Fig. 5B). Cell-bound Aβ was also reduced by siRNA treatment for LRP1 (Fig. 5C). These data strongly suggest that LRP1 is involved in the uptake of Aβ by TR-BBB cells. We also confirmed that siRNAs against LRP1 inhibited internalization of 125I-tPA, one of the well known ligands of LRP1, in TR-BBB cells (Fig. 5D).
FIGURE 5.
siRNA against LRP1 significantly reduced both internalization and cell surface binding of Aβ in TR-BBB cells. A, TR-BBB cells transfected with siRNAs were analyzed by immunoblotting with an anti-LRP1 monoclonal antibody 11H4 (upper panel) and with anti-α-tubulin (lower panel). B, the internalization of 125I-Aβ after Pronase treatment in TR-BBB cells. The mean ± S.E. in 12 independent assays is shown. **, p < 0.01; ANOVA. C, the cell surface binding of 125I-Aβ after Pronase treatment in TR-BBB cells. The mean ± S.E. in 12 independent assays is shown. **, p < 0.01; ANOVA. D, the internalization of 125I-tPA after Pronase treatment in TR-BBB cells. The mean ± S.E. in 12 independent assays is shown. **, p < 0.01; ANOVA.
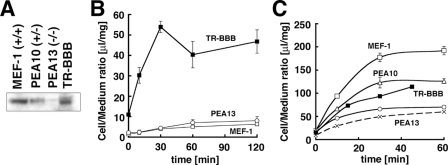
Lack of Rapid Uptake of Aβ by MEF Cells That Express Endogenous LRP1—To further examine the role of LRP1 in the cellular internalization of Aβ, we performed Aβ uptake assays in MEFs derived from LRP1-deficient and wild-type mice (32). An anti-LRP1 antibody, R488, revealed the expression of light chain of LRP1 in MEF-1 cells derived from wild-type mice, whereas PEA10 cells derived from LRP1 heterodeficient mice exhibited ∼50% levels of LRP1 compared with that of wild-type mice, and PEA13 cells derived from LRP1 homodeficient mice completely lacked the expression of LRP1 (Fig. 6A). Unexpectedly, however, neither the MEF-1 (wild-type), PEA10, nor PEA13 cells exhibited any significant uptake of Aβ that was observed in TR-BBB cells (Fig. 6B). Furthermore, we confirmed that the levels of cell surface binding of Aβ were at similar levels in either of the MEF cell lines (data not shown), suggesting that MEF cells fail to bind and internalize Aβ regardless of the expression levels of LRP1.
FIGURE 6.
Lack of Aβ internalization in fibroblasts endogenously expressing LRP1. A, immunoblot analysis of TR-BBB, MEF-1 (wild-type), PEA10 (LRP1 heterozygous-deficient), and PEA13 (LRP1 homozygous-deficient) cells using a polyclonal anti-LRP1 antibody R488. B, Aβ uptake assay in MEF cells. TR-BBB (filled squares), MEF-1 (open squares), and PEA13 (open circles) cells were incubated with 125I-Aβ for 0, 10, 30, 60, and 120 min. The mean ± S.E. in three independent assays is shown. C, the uptake assay of tPA in TR-BBB (filled squares), MEF-1 (open squares), PEA10 (open triangles), and PEA13 (open circles at 37 °C, crosses at 4 °C) cells for 0, 10, 30, and 60 min (MEF1, PEA10, and PEA13 cells) or for 0, 15, 30, and 45 min (TR-BBB cells). The mean ± S.E. in three independent assays is shown.
To see whether this inability of Aβ internalization was due to the low expression levels of functional LRP1 in these MEF cells, we performed a tPA internalization assay. As shown in Fig. 6C, three MEF cell lines took up 125I-tPA at levels proportional to their LRP1 expression, suggesting that LRP1 is present and functional at the surface of these MEF cells (Fig. 6C). We also conducted Aβ internalization assays using a range of cell lines (i.e. Neuro2a, Chinese hamster ovary, SH-SY5Y, McARH7777, and HUVEC) but failed to detect Aβ internalization in any of the cell lines, regardless of the levels of expression of endogenous LRP1 (supplemental Fig. 2). These data suggested that LRP1-mediated Aβ uptake depends on some specific cellular context in TR-BBB cells.
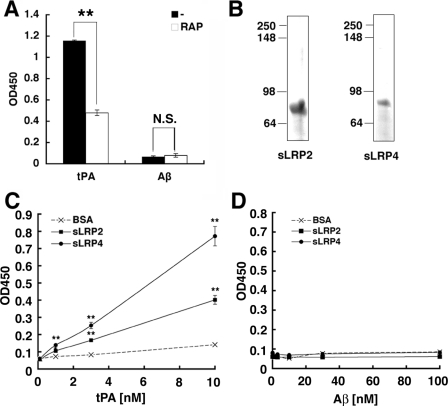
Aβ Does Not Directly Bind LRP1 in Vitro—Based on these data, we reasoned that the expression of functional LRP1 per se is not sufficient for the binding and internalization of Aβ. We thus reexamined the binding of Aβ to LRP1 in an in vitro assay. LRP1 holoprotein purified from human placenta was immobilized to microtiter plates, and the binding of Aβ to LRP1 was evaluated by an overlay assay. tPA and apoE, both being authentic ligands of LRP1, exhibited robust binding to immobilized LRP1 holoprotein, which was significantly blocked by RAP. Unexpectedly, however, Aβ did not show any significant binding to LRP1 either in the presence or in the absence of RAP (Fig. 7A). We also performed similar sets of binding experiments using recombinant fragments of ligand-binding clusters of LRP1 (i.e. sLRP2 and sLRP4) (Fig. 7B). tPA bound to immobilized sLRP2 and sLRP4 in a dose-dependent manner (Fig. 7C), whereas no binding of Aβ to sLRP2 and sLRP4 was detected (Fig. 7D). Altogether, these data argue against the notion that Aβ directly interacts with LRP1.
FIGURE 7.
Lack of direct binding of Aβ to LRP1 in vitro. A, the binding of full-length human LRP1 purified from placenta and coated onto microtiter plates to human Aβ-(1–40) and tPA in the absence or presence of 1 μm RAP, respectively. The mean ± S.E. of optical densities (450 nm) in four independent assays is shown. **, p < 0.01. B, the CBB staining of purified recombinant sLRP2 and sLRP4. C, the binding of increasing concentrations of tPA to immobilized sLRP2, sLRP4, or bovine serum albumin. The mean ± S.E. of optical densities (450 nm) in four independent assays is shown. *, p < 0.05; **, p < 0.01. D, the binding of increasing concentrations of Aβ to immobilized sLRP2, sLRP4, or bovine serum albumin. The mean ± S.E. of optical densities (450 nm) in four independent assays is shown. N.S., not significant; BSA, bovine serum albumin.
DISCUSSION
To gain insights into the molecular and cellular mechanism of Aβ transport across the BBB, we established a novel cellular model using an immortalized brain endothelial cell line, TR-BBB cells, that recapitulates the robust and rapid uptake of Aβ and found the following: 1) TR-BBB cells rapidly internalize chaperone-free Aβ by a receptor-mediated mechanism; 2) the internalized Aβ is rapidly degraded and released into the medium; 3) the internalization of Aβ into TR-BBB cells is mediated by LRP1, although Aβ may not directly bind LRP1 on the cell-surface. Based on these findings, we hypothesize that BMECs that delineate BBB rapidly internalize chaperone-free Aβ peptides in the brain parenchyma and efflux them into blood by LRP1-dependent transcytosis.
Internalization of soluble or fibrillar forms of Aβ has been documented in several types of cells (13, 35–38, 40, 45, 46, 48). However, the most striking difference between the previous reports and the present observation consists in the time course of Aβ uptake; Aβ internalization took place over a long incubation time of hours to days in previous experiments, whereas TR-BBB cells take up Aβ quite rapidly with the time course of <10 min (Fig. 1A). Recently, Nazer et al. (46) reported an Aβ transport model using polarized Madin-Darby canine kidney cells expressing LRP1, although the Aβ transport took place over several hours. The rapid time course in our TR-BBB cells is in good agreement with the in vivo observation that Aβ is effluxed from the brain with a half-life of ∼30 min (15, 16), underscoring the relevance of our cellular model in the study of Aβ clearance from brain.
We showed that RAP or an anti-LRP1 antibody efficiently inhibited Aβ internalization by TR-BBB cells (Figs. 3A and 4A). RNAi knockdown of LRP1 also decreased Aβ uptake (Fig. 5B). In contrast, the involvement of other known Aβ receptors (i.e. RAGE and P-gp) was not detected (Fig. 3D). Taken together, it is likely that the Aβ internalization in TR-BBB cells is mediated chiefly by LRP1. However, because the inhibitory effect of siRNA against LRP1 was incomplete, we cannot exclude the possibility that other RAP-inhibitable receptors are involved in this process.
We also found that Aβ complexed with lipidated form of apoE was efficiently internalized (Fig. 3A), although this uptake was not inhibited by RAP, suggesting that the uptake of Aβ complexed with lipidated apoE was mediated by an LRP1-independent mechanism. Although the precise mechanism of the uptake of Aβ·apoE complex observed here is uncertain, it is possible that other apoE receptors (e.g. low density lipoprotein receptor, which is known as the major receptor for apoE) (49) were involved; RAP might have failed to efficiently block the uptake of Aβ·lipidated apoE complex because of the relatively low affinity of other apoE receptors for RAP compared with that of LRP1 (50).
Despite the requirement of LRP1 in Aβ uptake by TR-BBB cells, Aβ uptake was not observed in the MEF cells, although the expression of functional LRP1 that can mediate tPA internalization was demonstrated (Fig. 6B). We also tested Aβ uptake in a range of cell lines but failed to observe the rapid LRP1-dependent Aβ internalization in any cells other than TR-BBB (supplemental Fig. 2). These data suggest that LRP1 is required, but not sufficient, to cause a rapid internalization of Aβ and that cell type specificity may significantly affect the LRP1-dependent Aβ internalization. Because we could not detect the direct binding of Aβ to LRP1 in a series of carefully controlled in vitro binding assays using full-length LRP1 or ligand-binding cluster fragments thereof (Fig. 7), we speculate that this specificity of LRP1-dependent internalization of Aβ may best be explained by the intercalation of an Aβ-binding molecule (including cell surface LRP1 ligands) that is specifically expressed on the surface of TR-BBB cells and cooperates with LRP1 to internalize Aβ into cells.
LRP1 is known to function as a multifunctional receptor that recognizes at least 30 different ligands, although the mechanism regarding how LRP1 can recognize structurally unrelated ligands remains unknown (51, 52). It is noteworthy that LRP1 directly binds to a few of these molecules and that many of the ligands interact with their “co-receptor” and are subsequently internalized via LRP1. For example, urokinase-type plasminogen activator and PAI-1 initially bind urokinase-type plasminogen activator receptor, and then the heterotrimeric complex is rapidly internalized by LRP1 and catabolized (53). Also, factor VIII initially adheres to heparan sulfate proteoglycans on the surface of the hepatocytes, and the complex is then scavenged by LRP1 (54). Based on these previous observations, it is plausible that an as yet unidentified Aβ-binding molecule that acts as a co-receptor of LRP1 may best explain the unique feature of TR-BBB cells, which robustly internalize chaperone-free Aβ (supplemental Fig. 3). Among the known LRP1 co-receptors, heparan sulfate proteoglycans have been shown to bind Aβ (55), although treatment of TR-BBB cells with heparin or heparinase or condroitinase did not affect the Aβ uptake, ruling out the possibility that heparan sulfate proteoglycans or chondroitin sulfate proteoglycans represent the cell surface co-receptor for Aβ in TR-BBB cells (supplemental Fig. 4). Notably, HUVEC cells derived from umbilical veins failed to show Aβ uptake, and the expression level of LRP1 in HUVEC cells was extremely low (supplemental Fig. 2). This may support the crucial role of LRP1 in the rapid Aβ internalization in brain endothelial cells. However, it is still possible that some cellular environments, other than an Aβ binding co-receptor, are the major determinants for Aβ internalization in TR-BBB cells. The nature of the cell type specificity for Aβ uptake should further be addressed.
In contrast to our results, previous studies showed that recombinant LRP1 polypeptides, especially the cluster II and IV domains, directly bind Aβ in vitro by the surface plasmon resonance analysis (18, 47). Although the reason for this discrepancy is not clear, we believe that our present data based on the plate binding assays that have been extensively used for the characterization of ligand-LRP1 (27) as well as ligand-Aβ (44) interactions, using purified LRP1 and appropriate controls, are quite reliable so far as in vitro interactions are concerned.
Because of the lack of polarity as well as formation of tight junctions in TR-BBB cells, we cannot fully replicate the “transport” across the BBB; in this regard, what we have analyzed here represents the “uptake” of Aβ by endocytosis in brain-derived endothelial cells. However, previous studies showed that the Km constant obtained from the uptake assay in TR-BBB cells is similar to that of an in vivo “transport” experiment (e.g. using 3-O-methyl-d-glucose as a substrate for GLUT-1) (20). Thus, the Aβ uptake in TR-BBB cells may well recapitulate the initial internalization step of the Aβ transport in vivo.
Another problem regarding the role of LRP1 in Aβ clearance is whether LRP1 mediates transcytosis or degradation of Aβ, as Nazer et al. (46) studied in polarized Madin-Darby canine kidney cells. Indeed, our trichloroacetic acid precipitation assay suggested that ∼80% of Aβ released from TR-BBB cells underwent degradation as in the Madin-Darby canine kidney cells (46). However, further study is required for the characterization of transcytosis versus degradation of Aβ in relation to the time course and cell type specificity.
In summary, we established a new in vitro model of rapid Aβ internalization using TR-BBB cells. We believe that Aβ internalization by TR-BBB may recapitulate some aspects of the initial internalization step of Aβ transport across the BBB. Stimulation of the Aβ efflux from brain to blood may be a useful strategy to inhibit Aβ accumulation and prevent the development of pathological changes in AD. TR-BBB cells may serve as a useful tool for the screening of modulation of proteins or genes participating in Aβ transport at the BBB as well as small molecule compounds that facilitate the Aβ clearance. The identification of the mechanism of the cell type specificity in Aβ internalization may be crucial to the understanding of the precise mechanism of Aβ transport across the BBB.
Supplementary Material
Acknowledgments
We thank Y. Hori for kind help in PICUP analysis and Drs. T. Yamasaki for providing GST-RAP cDNA, A. Kinoshita for anti-LRP1 monoclonal antibody 11H4 and human full-length LRP1 cDNA, J. Aoki for McARH7777 cells, Takeda Pharmaceutical Company for anti-Aβ antibody and S. Ito, C. Tamaki, and S. Hori for helpful comments and discussions.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 AG027924 (to G. B.) and AG13956 (to D. H.). This work was also supported by Core Research for Evolutional Science and Technology of Japan Science and Technology agency, by the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation, and by grants-in-aid from the Japan Society for the Promotion of Science (to T. H. and K. Y.) and from the Takeda Science Foundation (to K. Y.), Japan. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–4.
Footnotes
The abbreviations used are: Aβ, amyloidβ peptide; AD, Alzheimer disease; BBB, blood-brain barrier; BMEC, brain microvascular endothelial cell; P-gp, P-glycoprotein; tPA, tissue-type plasminogen activator; RAP, receptor-associated protein; PICUP, photo-induced cross-linking of unmodified proteins; MEF, mouse embryo fibroblast; siRNA, small interference RNA; RNAi, RNA interference; RAGE, receptor for advanced glycation end products; HUVEC, human umbilical vein endothelial cell; ANOVA, analysis of variance.
References
- 1.Selkoe, D. J. (2001) Physiol. Rev. 81 741–766 [DOI] [PubMed] [Google Scholar]
- 2.Goate, A., Chartier-Harlin, M. C., Mullan, M., Brown, J., Crawford, F., Fidani, L., Giuffra, L., Haynes, A., Irving, N., James, L., Mant, R., Newton, P., Rooke, K., Rogues, P., Talbot, C., Pericak-Vance, M., Roses, A., Williamson, R., Rossor, M., Owen, M., and Hardy, J. (1991) Nature 349 704–706 [DOI] [PubMed] [Google Scholar]
- 3.Mullan, M., Crawford, F., Axelman, K., Houlden, H., Lilius, L., Winblad, B., and Lannfelt, L. (1992) Nat. Genet. 1 345–347 [DOI] [PubMed] [Google Scholar]
- 4.Rovelet-Lecrux, A., Hannequin, D., Raux, G., Le Meur, N., Laquerrière, A., Vital, A., Dumanchin, C., Feuillette, S., Brice, A., Vercelletto, M., Dubas, F., Frebourg, T., and Campion, D. (2006) Nat. Genet. 38 24–26 [DOI] [PubMed] [Google Scholar]
- 5.Citron, M., Oltersdorf, T., Haass, C., McConlogue, L., Hung, A. Y., Seubert, P., Vigo-Pelfrey, C., Lieberburg, I., and Selkoe, D. J. (1992) Nature 360 672–674 [DOI] [PubMed] [Google Scholar]
- 6.Suzuki, N., Cheung, T. T., Cai, X. D., Odaka, A., Otvos, L., Jr., Eckman, C., Golde, T. E., and Younkin, S. G. (1994) Science 264 1336–1340 [DOI] [PubMed] [Google Scholar]
- 7.Iwatsubo, T., Odaka, A., Suzuki, N., Mizusawa, H., Nukina, N., and Ihara, Y. (1994) Neuron 13 45–53 [DOI] [PubMed] [Google Scholar]
- 8.Hsiao, K., Chapman, P., Nilsen, S., Eckman, C., Harigaya, Y., Younkin, S., Yang, F., and Cole, G. (1996) Science 274 99–102 [DOI] [PubMed] [Google Scholar]
- 9.Walsh, D. M., Klyubin, I., Fadeeva, J. V., Cullen, W. K., Anwyl, R., Wolfe, M. S., Rowan, M. J., and Selkoe, D. J. (2002) Nature 416 535–539 [DOI] [PubMed] [Google Scholar]
- 10.Shankar, G. M., Bloodgood, B. L., Townsend, M., Walsh, D. M., Selkoe, D. J., and Sabatini, B. L. (2007) J. Neurosci. 27 2866–2875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwata, N., Tsubuki, S., Takaki, Y., Watanabe, K., Sekiguchi, M., Hosoki, E., Kawashima-Morishima, M., Lee, H. J., Hama, E., Sekine-Aizawa, Y., and Saido, T. C. (2000) Nat. Med. 6 143–150 [DOI] [PubMed] [Google Scholar]
- 12.Qiu, W. Q., Walsh, D. M., Ye, Z., Vekrellis, K., Zhang, J., Podlisny, M. B., Rosner, M. R., Safavi, A., Hersh, L. B., and Selkoe, D. J. (1998) J. Biol. Chem. 273 32730–32738 [DOI] [PubMed] [Google Scholar]
- 13.Paresce, D. M., Ghosh, R. N., and Maxfield, F. R. (1996) Neuron 17 553–565 [DOI] [PubMed] [Google Scholar]
- 14.Wyss-Coray, T., Loike, J. D., Brionne, T. C., Lu, E., Anankov, R., Yan, F., Silverstein, S. C., and Husemann, J. (2003) Nat. Med. 9 453–457 [DOI] [PubMed] [Google Scholar]
- 15.Shibata, M., Yamada, S., Kumar, S. R., Calero, M., Bading, J., Frangione, B., Holtzman, D. M., Miller, C. A., Strickland, D. K., Ghiso, J., and Zlokovic, B. V. (2000) J. Clin. Invest. 106 1489–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shiiki, T., Ohtsuki, S., Kurihara, A., Naganuma, H., Nishimura, K., Tachikawa, M., Hosoya, K., and Terasaki, T. (2004) J. Neurosci. 24 9632–9637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zlokovic, B. V. (1995) Pharm. Res. 12 1395–1406 [DOI] [PubMed] [Google Scholar]
- 18.Deane, R., Wu, Z., Sagare, A., Davis, J., Yan, S. D., Hamm, K., Xu, F., Parisi, M., LaRue, B., Hu, H. W., Spijkers, P., Guo, H., Song, X., Lenting, P. J., Van Nostrand, W. E., and Zlokovic, B. V. (2004) Neuron 43 333–344 [DOI] [PubMed] [Google Scholar]
- 19.Terasaki, T., and Hosoya, K. (2001) Biol. Pharm. Bull. 24 111–118 [DOI] [PubMed] [Google Scholar]
- 20.Hosoya, K. I., Takashima, T., Tetsuka, K., Nagura, T., Ohtsuki, S., Takanaga, H., Ueda, M., Yanai, N., Obinata, M., and Terasaki, T. (2000) J. Drug Target. 8 357–370 [DOI] [PubMed] [Google Scholar]
- 21.Bu, G., Geuze, H. J., Strous, G. J., and Schwartz, A. L. (1995) EMBO J. 14 2269–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kinoshita, A., Shah, T., Tangredi, M. M., Strickland, D. K., and Hyman, B. T. (2003) J. Biol. Chem. 278 41182–41188 [DOI] [PubMed] [Google Scholar]
- 23.Lee, S. H., Takahashi, M., Honke, K., Miyoshi, E., Osumi, D., Sakiyama, H., Ekuni, A., Wang, X., Inoue, S., Gu, J., Kadomatsu, K., and Taniguchi, N. (2006) J. Biochem. (Tokyo) 139 391–398 [DOI] [PubMed] [Google Scholar]
- 24.Ashcom, J. D., Tiller, S. E., Dickerson, K., Cravens, J. L., Argraves, W. S., and Strickland, D. K. (1990) J. Cell Biol. 110 1041–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qing, G., Ma, L. C., Khorchid, A., Swapna, G. V., Mal, T. K., Takayama, M. M., Xia, B., Phadtare, S., Ke, H., Acton, T., Montelione, G. T., Ikura, M., and Inouye, M. (2004) Nat. Biotechnol. 22 877–882 [DOI] [PubMed] [Google Scholar]
- 26.Herz, J., Goldstein, J. L., Strickland, D. K., Ho, Y. K., and Brown, M. S. (1991) J. Biol. Chem. 266 21232–21238 [PubMed] [Google Scholar]
- 27.Mikhailenko, I., Battey, F. D., Migliorini, M., Ruiz, J. F., Argraves, K., Moayeri, M., and Strickland, D. K. (2001) J. Biol. Chem. 276 39484–39491 [DOI] [PubMed] [Google Scholar]
- 28.Morikawa, M., Fryer, J. D., Sullivan, P. M., Christopher, E. A., Wahrle, S. E., DeMattos, R. B., O'Dell, M. A., Fagan, A. M., Lashuel, H. A., Walz, T., Asai, K., and Holtzman, D. M. (2005) Neurobiol. Dis. 19 66–76 [DOI] [PubMed] [Google Scholar]
- 29.Hashimoto, T., Wakabayashi, T., Watanabe, A., Kowa, H., Hosoda, R., Nakamura, A., Kanazawa, I., Arai, T., Takio, K., Mann, D. M., and Iwatsubo, T. (2002) EMBO J. 21 1524–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bitan, G., Lomakin, A., and Teplow, D. B. (2001) J. Biol. Chem. 276 35176–35184 [DOI] [PubMed] [Google Scholar]
- 31.Bitan, G., Kirkitadze, M. D., Lomakin, A., Vollers, S. S., Benedek, G. B., and Teplow, D. B. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 330–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willnow, T. E., and Herz, J. (1994) J. Cell Sci. 107 719–726 [PubMed] [Google Scholar]
- 33.Tanabe, S., Shimohigashi, Y., Nakayama, Y., Makino, T., Fujita, T., Nose, T., Tsujimoto, G., Yokokura, T., Naito, M., Tsuruo, T., and Terasaki, T. (1999) J. Pharmacol. Exp. Ther. 290 561–568 [PubMed] [Google Scholar]
- 34.Asami-Odaka, A., Ishibashi, Y., Kikuchi, T., Kitada, C., and Suzuki, N. (1995) Biochemistry 34 10272–10278 [DOI] [PubMed] [Google Scholar]
- 35.Qiu, Z., Strickland, D. K., Hyman, B. T., and Rebeck, G. W. (1999) J. Neurochem. 73 1393–1398 [DOI] [PubMed] [Google Scholar]
- 36.Gylys, K. H., Fein, J. A., Tan, A. M., and Cole, G. M. (2003) J. Neurochem. 84 1442–1451 [DOI] [PubMed] [Google Scholar]
- 37.Narita, M., Holtzman, D. M., Schwartz, A. L., and Bu, G. (1997) J. Neurochem. 69 1904–1911 [DOI] [PubMed] [Google Scholar]
- 38.Kang, D. E., Pietrzik, C. U., Baum, L., Chevallier, N., Merriam, D. E., Kounnas, M. Z., Wagner, S. L., Troncoso, J. C., Kawas, C. H., Katzman, R., and Koo, E. H. (2000) J. Clin. Invest. 106 1159–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bu, G., Maksymovitch, E. A., and Schwartz, A. L. (1993) J. Biol. Chem. 268 13002–13009 [PubMed] [Google Scholar]
- 40.Koistinaho, M., Lin, S., Wu, X., Esterman, M., Koger, D., Hanson, J., Higgs, R., Liu, F., Malkani, S., Bales, K. R., and Paul, S. M. (2004) Nat. Med. 10 719–726 [DOI] [PubMed] [Google Scholar]
- 41.Horn, I. R., van den Berg, B. M., van der Meijden, P. Z., Pannekoek, H., and van Zonneveld, A. J. (1997) J. Biol. Chem. 272 13608–13613 [DOI] [PubMed] [Google Scholar]
- 42.Deane, R., Yan, S. D., Submamaryan, R. K., LaRue, B., Jovanovic, S., Hogg, E., Welch, D., Manness, L., Lin, C., Yu, J., Zhu, H., Ghiso, J., Frangione, B., Stern, A., Schmidt, A. M., Armstrong, D. L., Arnold, B., Liliensiek, B., Nawroth, P., Hofman, F., Kindy, M., Stern, D., and Zlokovic, B. (2003) Nat. Med. 9 907–913 [DOI] [PubMed] [Google Scholar]
- 43.Lam, F. C., Liu, R., Lu, P., Shapiro, A. B., Renoir, J. M., Sharom, F. J., and Reiner, P. B. (2001) J. Neurochem. 76 1121–1128 [DOI] [PubMed] [Google Scholar]
- 44.Tokuda, T., Calero, M., Matsubara, E., Vidal, R., Kumar, A., Permanne, B., Zlokovic, B., Smith, J. D., Ladu, M. J., Rostagno, A., Frangione, B., and Ghiso, J. (2000) Biochem. J. 348 359–365 [PMC free article] [PubMed] [Google Scholar]
- 45.Yazawa, H., Yu, Z. X., Takeda Le, Y., Gong, W., Ferrans, V. J., Oppenheim, J. J., Li, C. C., and Wang, J. M. (2001) FASEB J. 15 2454–2462 [DOI] [PubMed] [Google Scholar]
- 46.Nazer, B., Hong, S., and Selkoe, D. J. (2008) Neurobiol. Dis. 30 94–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sagare, A., Deane, R., Bell, R. D., Johnson, B., Hamm, K., Pendu, R., Marky, A., Lenting, P. J., Wu, Z., Zarcone, T., Goate, A., Mayo, K., Perlmutter, D., Coma, M., Zhong, Z., and Zlokovic, B. V. (2007) Nat. Med. 13 1029–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koenigsknecht, J., and Landreth, G. (2004) J. Neurosci. 4 9838–9846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fryer, J. D., Demattos, R. B., McCormick, L. M., O'Dell, M. A., Spinner, M. L., Bales, K. R., Paul, S. M., Sullivan, P. M., Parsadanian, M., Bu, G., and Holtzman, D. M. (2005) J. Biol. Chem. 280 25754–25759 [DOI] [PubMed] [Google Scholar]
- 50.Ziere, G. J., van der Kaaden, M. E., Vogelezang, C. J., Boers, W., Bihain, B. E., Kuiper, J., Kruijt, J. K., and van Berkel, T. J. (1996) Eur. J. Biochem. 242 703–711 [DOI] [PubMed] [Google Scholar]
- 51.Herz, J., and Strickland, D. K. (2001) J. Clin. Invest. 108 779–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nykjaer, A., and Willnow, T. E. (2002) Trends Cell Biol. 12 273–280 [DOI] [PubMed] [Google Scholar]
- 53.Nykjaer, A., Petersen, C. M., Møller, B., Jensen, P. H., Moestrup, S. K., Holtet, T. L., Etzerodt, M., Thøgersen, H. C., Munch, M., Andreasen, P. A., and Gliemann, J. (1992) J. Biol. Chem. 267 14543–14546 [PubMed] [Google Scholar]
- 54.Sarafanov, A. G., Ananyeva, N. M., Shima, M., and Saenko, E. L. (2001) J. Biol. Chem. 276 11970–11979 [DOI] [PubMed] [Google Scholar]
- 55.Leveugle, B., Scanameo, A., Ding, W., and Fillit, H. (1994) Neuroreport 5 1389–1392 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.