Abstract
Macrophages play key roles in obesity-associated pathophysiology, including inflammation, atherosclerosis, and cancer, and processes that affect the survival-death balance of macrophages may have an important impact on obesity-related diseases. Adipocytes and other cells secrete a protein called extracellular nicotinamide phosphoribosyltransferase (eNampt; also known as pre-B cell colony enhancing factor or visfatin), and plasma levels of eNampt increase in obesity. Herein we tested the hypothesis that eNampt could promote cell survival in macrophages subjected to endoplasmic reticulum (ER) stress, a process associated with obesity and obesity-associated diseases. We show that eNampt potently blocks macrophage apoptosis induced by a number of ER stressors. The mechanism involves a two-step sequential process: rapid induction of interleukin 6 (IL-6) secretion, followed by IL-6-mediated autocrine/paracrine activation of the prosurvival signal transducer STAT3. The ability of eNampt to trigger this IL-6/STAT3 cell survival pathway did not depend on the presence of the Nampt enzymatic substrate nicotinamide in the medium, could not be mimicked by the Nampt enzymatic product nicotinamide mononucleotide (NMN), was not blocked by the Nampt enzyme inhibitor FK866, and showed no correlation with enzyme activity in a series of site-directed mutant Nampt proteins. Thus, eNampt protects macrophages from ER stress-induced apoptosis by activating an IL-6/STAT3 signaling pathway via a nonenzymatic mechanism. These data suggest a novel action and mechanism of eNampt that could affect the balance of macrophage survival and death in the setting of obesity, which in turn could play important roles in obesity-associated diseases.
Macrophage biology is intimately related to obesity-associated pathology. For example, inflammatory macrophages are numerous in adipose tissue of obese individuals (1, 2), and macrophage-mediated inflammation is a critical component of obesity-induced insulin resistance (2, 3). Macrophages also play a critical role in the progression of atherosclerosis, which is the process behind the leading cause of death in obese and insulin-resistant subjects, atherothrombotic vascular disease (4). Obesity is associated with an increased risk for certain cancers, and tumor-associated macrophages (TAMs)3 play a critical role in the development of these tumors (5–7). In all processes involving macrophages, a critical determinant of outcome is the survival-death balance of the cells. For example, macrophage apoptosis, when coupled with effective phagocytic clearance (efferocytosis) of the dead cells, suppresses inflammation (8). In atherosclerosis, macrophage apoptosis limits plaque progression in relatively early lesions, where efferocytosis is intact (9–12). In cancer, targeted apoptosis of TAMs suppresses tumor development (7). Thus, increased macrophage survival in obesity, perhaps mediated by circulating bioactive proteins whose levels change with increased body mass index (“adipocytokines”) (13, 14), may promote the aforementioned obesity-associated diseases.
An adipocytokine that has received much recent attention is a secreted form of an enzyme called nicotinamide phosphoribosyltransferase (Nampt; also known as pre-B cell colony enhancing factor (PBEF) or visfatin), which converts nicotinamide to nicotinamide mononucleotide (NMN) (15, 16). In vertebrates, intracellular Nampt plays an essential role in the biosynthesis of NAD from nicotinamide (17). However, adipocytes and other cells secrete Nampt through a nonclassical, brefeldin A-independent pathway into the extracellular medium in vitro and into the circulation in vivo (18–20). Recent studies have shown that plasma levels of circulating Nampt/PBEF/visfatin, referred to here as extracellular Nampt (eNampt), are elevated in obese humans, including those with type 2 diabetes (21–24).
eNampt has been implicated in a number of biological processes. For example, eNampt was initially identified as a cytokine, called PBEF, that can promote the maturation of B cell precursors and inhibit neutrophil apoptosis (19). Later work revealed proinflammatory actions of the protein in vitro and in mice, as well as a positive association among obesity, eNampt plasma levels, and inflammation in humans (14, 25). The issue as to whether these cytokine-like effects of eNampt depend on Nampt enzymatic activity has generally not been investigated, although a recent study has implicated Nampt enzymatic activity in enhancing the inflammatory response of macrophages to LPS (26).
In another set of studies in which eNampt was named “visfatin,” a group of investigators reported that the protein could activate insulin receptor signaling in cultured cells and in vivo through direct interaction with the insulin receptor (27). However, the authors have recently retracted the publication due to problems reproducing these results (28). More recently, Revollo et al. (20) showed that eNampt enhances glucose-stimulated insulin secretion in pancreatic β cells in vitro and in nonobese mice. This effect of the protein was shown not to be mediated through activation of insulin receptor signaling but rather involved the extracellular enzymatic conversion of nicotinamide to NMN by eNampt, leading to increased NAD biosynthesis in β cells (20).
Given that eNampt levels are increased in obesity and that macrophages play important roles in obesity-associated diseases, we questioned at a cell biological level whether eNampt might influence the survival-death balance of macrophages. In particular, increased macrophage survival might enhance the proposed inflammatory effects of eNampt (see above). We focused on an apoptosis inducer, endoplasmic reticulum (ER) stress, that occurs in obesity and is likely to be relevant to obesity-associated diseases. For example, ER stress is a critical process in obesity-associated insulin resistance and inflammation (29, 30), and evidence suggests that ER stress-induced macrophage apoptosis occurs in atherosclerotic lesions (31–35), particularly in the setting of insulin resistance (36). ER stress is also prominent in tumors, where it may affect TAMs (37). Using models of ER stress-induced apoptosis, we show here that eNampt potently blocks macrophage apoptosis. The cell survival pathway is mediated by STAT3, which is activated in an autocrine/paracrine manner by eNampt-induced IL-6. The ability of eNampt to activate STAT3 involves neither insulin receptor signaling nor the nicotinamide phosphoribosyltransferase activity of the protein, suggesting a nonenzymatic signaling role for the protein in this pathway. These findings have potential implications for how obesity might affect macrophage apoptosis-survival balance, with potential implications for macrophage-mediated obesity-associated diseases as well as for the mechanisms of action of eNampt.
EXPERIMENTAL PROCEDURES
Materials—Cell culture media and reagents were from Invitrogen, and biochemical reagents were from Sigma unless specified below. The acyl-CoA:cholesterol acyltransferase inhibitor 58035 (3-[decyldimethylsilyl]-N-[2-(4-methylphenyl)-1-phenethyl]propanamide) (SA 58-035; catalog number S9318) and 7-ketocholesterol were purchased from Sigma. A stock solution of 58035 was prepared at a concentration of 10 mg/ml in dimethyl sulfoxide and stored at -20 °C. Recombinant murine eNampt (His-tagged) was from Axxor, LLC (San Diego, CA). Low density lipoprotein (LDL; d = 1.020–1.063 g/ml) was isolated from fresh human plasma by preparative ultracentrifugation, as previously described (38). Acetyl-LDL was prepared by reaction of LDL with acetic anhydride (39). Recombinant murine IL-6 and IL-10; neutralizing antibodies against IL-6, IL-6R, IL-10, and IL-10R; and control IgG were from R&D Systems. JAK1/2 inhibitor AG490 was from EMD. Antibodies against STAT3 and phospho-STAT3 were from Cell Signaling Technology. Antibodies against Mcl-1 (rabbit polyclonal), Bcl-xL (mouse monoclonal), Bcl-2 (rabbit polyclonal), IAP (goat polyclonal), and β-actin were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). AIM/CD5L (rabbit polyclonal) was from AbD Serotec (Oxford, UK). Scrambled and Stat3 siRNA species were from Qiagen (Valencia, CA). siRNA 1 (SI01435287) and 2 (SI01435301) were designed from regions situated between base pairs 1050 and 1100 and base pairs 100 and 150, respectively, of Stat3 mRNA; the exact siRNA sequences are proprietary. Scrambled control siRNA (AllStars® negative control siRNA) from Qiagen was designed to have no homology to any known mammalian gene (see the Qiagen site on the World Wide Web). FK866, a noncompetitive allosteric inhibitor of Nampt, was a kind gift of Dr. L. Tong (Columbia University, New York).
Preparation of Mouse Peritoneal Macrophages and Bone Marrow-derived Macrophages—Unless otherwise noted, the mice used in this study were female C57BL6/J mice, 8–10 weeks of age. Peritoneal macrophages were elicited by intraperitoneal injection of methyl-bovine serum albumin in mice previously immunized with this antigen as previously described (40). The cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated fetal bovine serum, 100 units/ml penicillin/streptomycin, and 20% L-cell-conditioned medium for 24–48 h, at which time the cells were ∼80–90% confluent. Mouse bone marrow cells were collected by flushing femurs with Dulbecco's modified Eagle's medium. The suspension was centrifuged at 1000 × g for 5 min, and the cell pellet was resuspended in macrophage medium (above) and cultured for 9–11 days to allow differentiation into mature macrophages.
Apoptosis Induction and Assays—Apoptosis was induced by simultaneous or separate activation of the unfolded protein response (UPR) and pattern recognition receptors (PRRs) (31, 32, 41, 42) or, in the case of Fig. 1B, with 5 μg/ml staurosporine. For simultaneous UPR/PRR activation, macrophages were incubated for 20 h with 50 μg/ml acetyl-LDL plus 10 μg/ml acyl-CoA:cholesterol acyltransferase inhibitor, SA 58-035. For separate UPR/PRR activation, macrophages were incubated for 24 h with 5 μg/ml tunicamycin plus 25 μg/ml fucoidan. At the end of the incubation period, macrophages were assayed for externalization of phosphatidylserine, a marker of early to mid-stage apoptosis, by staining with Alexa-488-labeled annexin V. Late stage apoptosis, as indicated by increased membrane permeability, was assayed by staining with propidium iodide (43). For Figs. 3 and 4F, the annexin data were verified by terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) analysis, using the in situ cell death detection kit, TMR red, from Roche Applied Science. Cells were viewed immediately with an Olympus IX-70 inverted fluorescence microscope, and four representative fields (∼1,000 cells) for each condition were counted. The data are expressed as the percentage of apoptotic cells per total cells.
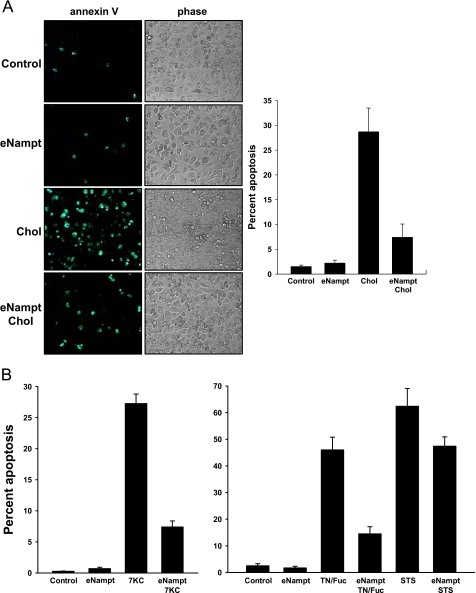
FIGURE 1.
eNampt protects macrophages from ER stress-induced apoptosis. A, macrophages were preincubated in the absence or presence of 100 ng/ml eNampt for 24 h and then incubated with medium alone (Control) or medium containing eNampt, 50 μg/ml acetyl-LDL + 10 μg/ml 58035 (Chol), or eNampt plus acetyl-LDL/58035 for 20 h. Apoptosis was assayed by Alexa-488 annexin V staining (green). One representative image per condition is shown. The graph to the right of the images shows quantification of the apoptosis data (mean ± S.E., n = 4 fields of cells). p < 0.001 for Chol versus eNampt/Chol. B, macrophages were preincubated in the absence or presence of 100 ng/ml eNampt for 24 h and then incubated with medium alone (Control) or medium containing eNampt, 20 μg/ml 7-ketocholesterol (7KC), eNampt plus 7-ketocholesterol, 5 μg/ml tunicamycin plus 25 μg/ml fucoidan (TN/Fuc), eNampt plus tunicamycin/fucoidan, 5 μg/ml staurosporine (STS), or eNampt plus staurosporine. The incubation times were 18 h for the 7KC experiment, 24 h for the TN/Fuc experiment, and 16 h for the STS experiment. Apoptosis was assayed and quantified as described above. p < 0.001 for 7KC versus eNampt/7KC, TN/Fuc versus eNampt/TN/Fuc, and STS versus eNampt/STS.
FIGURE 3.
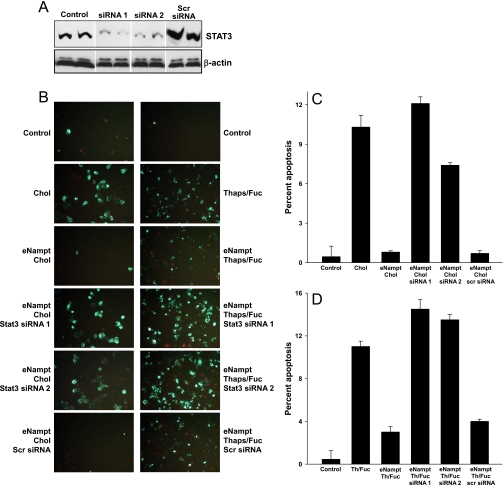
eNampt-induced survival of ER-stressed macrophages requires STAT3. A, immunoblot of STAT3 in control macrophages and macrophages incubated for 60 h with two different Stat3 siRNA species or scrambled siRNA. B, annexin V fluorescence images are shown for macrophages that were first incubated for 60 h in the absence or presence of the indicated siRNA species and then preincubated in the absence or presence of 100 ng/ml eNampt for 24 h. The cells shown in the left column of images were incubated with medium alone or medium containing 50 μg/ml acetyl-LDL + 10 μg/ml 58035 (Chol) for 20 h, and those in the right column of images were incubated in medium alone or medium containing 0.25 μm thapsigargin + 50 μg/ml fucoidan (Thaps/Fuc) for 24 h. Apoptosis was assayed by Alexa-488 annexin V staining (green). C and D, quantified apoptosis data for both cholesterol-induced and thapsigargin/fucoidan-induced apoptosis (means ± S.E., n = 4 fields of cells). For both sets of apoptosis data, p < 0.001 for the Nampt samples treated with the two Stat3 siRNA species versus no siRNA or scrambled siRNA. When cholesterol loading or thapsigargin/fucoidan was omitted from the incubations, none of the siRNA species induced apoptosis (data not shown).
FIGURE 4.
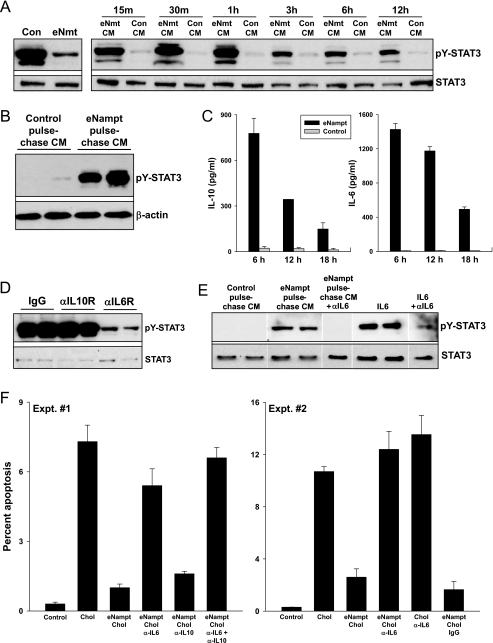
eNampt induces STAT3 phosphorylation by promoting the secretion of an autocrine/paracrine STAT3 activator, interleukin-6. A, left blot, macrophages were incubated in the absence (Con) or presence of 100 ng/ml eNampt (eNmt) for 24 h, and cell lysates were immunoblotted for tyrosine-phosphorylated and total STAT3. Right blot, for these experiments, CM from control or eNampt-treated macrophages was transferred to fresh macrophages. The cells were then incubated in this medium for the indicated periods of time, after which cell lysates were immunoblotted for tyrosine-phosphorylated and total STAT3. B, macrophages were incubated in the absence or presence of 100 ng/ml eNampt for 5 h. The cells were washed and then incubated in fresh medium for 12 h. This “chase” conditioned medium (CM) was then transferred onto fresh macrophages. After 30 min of incubation, cell lysates were immunoblotted for tyrosine-phosphorylated STAT3 (pY-STAT3) and β-actin. C, macrophages were incubated in the absence or presence of 100 ng/ml eNampt for the indicated times. The culture media were collected and cleared of cell debris by centrifugation, and concentrations of IL-6 and IL-10 were measured by an enzyme-linked immunosorbent assay. D, eNampt-conditioned media were prepared as in A and then transferred to recipient macrophages that had been pretreated for 30 min with 1 μg/ml anti-IL-10 receptor IgG, anti-IL-6 receptor IgG, or nonimmune IgG control. The recipient cells were incubated for 30 min with the conditioned medium plus the respective IgGs. Cells were lysed, and the extracts were immunoblotted for tyrosine-phosphorylated and total STAT3. E, eNampt or control “chase” CM were prepared as in B. The CM was incubated with or without 1 μg/ml anti-IL-6 antibody for 30 min. The media were then added to recipient macrophages and incubated for 30 min. 100 pg/ml recombinant IL-6 and IL-6 plus anti-IL-6 antibody were used as positive and negative controls, respectively. Cells were lysed, and the extracts were immunoblotted for tyrosine-phosphorylated STAT3 and β-actin. F, in both experiments shown, cholesterol-induced apoptosis was assayed in control or eNampt-pretreated macrophages exactly as in Fig. 1A, except some of the cells were incubated with 1 μg/ml anti-IL-6 antibody, anti-IL-10 antibody, or both throughout both the 24-h eNampt pretreatment period and the 20-h cholesterol-loading period. In experiment 1, p < 0.01 for cholesterol versus control and for the eNampt groups treated with anti-IL-6 or anti-IL-6 + anti-IL-10 antibody versus no antibody or anti-IL-10 alone. In Experiment 1, p < 0.01 for cholesterol versus control and for the eNampt groups treated with anti-IL-6 versus control IgG. There was no statistically significant difference among the cholesterol, eNampt/cholesterol/anti-IL-6, and cholesterol/anti-IL-6 groups.
Immunoblotting—Whole cell lysates were prepared by lysing cells in radioimmune precipitation buffer (25 mm Tris, pH 7.4, 150 mm KCl, 5 mm EDTA, 1% Nonidet P-40, 0.5% sodium deoxycholate, and 0.1% SDS) at the end of treatments. The lysates were electrophoresed on 4–20% gradient SDS-polyacrylamide gels and transferred to 0.22-μm nitrocellulose membranes. The membrane was blocked for 1 h at room temperature in Tris-buffered saline, 0.1% Tween 20 (TBST) containing 5% (w/v) nonfat milk and then incubated for 18 h with the primary antibody in TBST containing 5% (w/v) nonfat milk or 5% bovine serum albumin at 4 °C. The blots were then incubated with the appropriate secondary antibody coupled to horseradish peroxidase, and proteins were detected by ECL chemiluminescence (Pierce).
Stat3 siRNA Experiments—Scrambled and Stat3 siRNA (50 pg/well) were transfected into 50% confluent monolayers of peritoneal macrophages using Lipofectamine (Invitrogen) according to the manufacturer's instructions. After 4 h of transfection, the media were replaced, and 60 h later the indicated experiments were conducted.
In Vivo Administration of eNampt—Male C57BL/6J mice were injected intraperitoneally with 1 ml of 3% thioglycollate to elicit peritoneal macrophages. Four days later, the mice were injected intravenously with 5 μg of eNampt in 0.5 ml of phosphate-buffered saline or with phosphate-buffered saline alone as control. Peritoneal macrophages were harvested 12 h after the eNampt injection and then immediately assayed for phospho-STAT3 by immunoblotting.
Mutant Nampt Proteins and Kinetic Analysis—His-tagged wild-type and mutant Nampt proteins were expressed as previously described (44). The proteins were purified to at least 95% pure using one-step Histrap purification. The nicotinamide phosphoribosyltransferase activity of Nampt was measured as previously described (16, 20, 44). For kinetics studies on His-tagged wild-type and mutant Nampt proteins, each reaction consists of 100 μl of 20 mm Tris, pH 8.0, 50 mm NaCl, 2 mm dithiothreitol, 12.5 mm MgCl2, 2.5 mm ATP, 0.03% bovine serum albumin, 1.5% ethanol, 13 μg/ml His-tagged Nampt, 100 μg/ml alcohol dehydrogenase, 500 μm phosphoribosyl pyrophosphate, and varying concentrations of nicotinamide. Depending on different catalytic abilities, different amounts of His-Nampt were used for the wild type and the mutants. Catalytic constants Km and kcat were determined for His-tagged mutant and wild-type proteins by analyzing plots of initial rate measurements at 25 °C under varying nicotinamide concentrations using the KaleidaGraph version 3.6 program (Synergy Software), which calculates constants Km and kcat automatically.
Statistics—Data are presented as mean ± S.E. Unless otherwise noted, the number of samples for each group (n) = 4. Absent error bars in the bar graphs signify S.E. values smaller than the graphic symbols. Analysis of variance followed by Tukey post-test (GraphPad Prism 4 version 4.03) was used to determine statistical significance among all groups.
RESULTS
Extracellular Nampt Suppresses ER-Stress-induced Macrophage Apoptosis via Activation of STAT3—ER stress-induced macrophage apoptosis requires activation of the UPR-CHOP pathway plus a second “hit” involving PRR activation (32, 42). In one example of this model that may be relevant to atherosclerosis, macrophages are incubated with modified lipoproteins that carry out both hits; the lipoproteins bind a PRR called the type A scavenger receptor, and, in the absence of cholesterol re-esterification, lipoprotein-derived unesterified cholesterol accumulates in the ER, alters the ER membrane, and triggers the UPR-CHOP pathway (31, 32, 41). Using this model, we found that preincubation of the macrophages with recombinant eNampt markedly suppressed apoptosis (Fig. 1A).
There is evidence that the oxidized sterol 7-ketocholesterol is present in atherosclerotic lesions, and 7-ketocholesterol activates the UPR and is proapoptotic (35, 45). As shown in the left graph in Fig. 1B, 7-ketocholesterol induced apoptosis in macrophages, and the apoptotic response was markedly suppressed by eNampt. A more broadly applicable, sterol-independent example of this apoptosis model involves incubating macrophages with the combination of a low dose UPR activator (tunicamycin) and a PRR ligand (fucoidan) (32). eNampt was also very effective at blocking apoptosis in this model (right graph in Fig. 1B). Interestingly, eNampt also had some survival effect in a non-UPR-based model of apoptosis, incubation with the phosphatase inhibitor staurosporine. Although the decrease in staurosporine-induced apoptosis was statistically significant (p < 0.01), the effect was considerably less than that observed with UPR-induced apoptosis (Fig. 1B).
To pursue the mechanism, we first examined the effect of eNampt on PRR expression, which is essential for ER stress-induced apoptosis (32). We found that treatment of macrophages with eNampt had no effect on the expression of the type A scavenger receptor or another PRR, CD36, both of which serve as second hits in UPR-induced apoptosis (32, 42) (data not shown). Next, in view of the previously postulated, and since retracted, role of eNampt (visfatin) as an insulin receptor agonist (27, 28), we determined whether eNampt could block apoptosis in macrophages harvested from insulin receptor-null mice (36). We found that thapsigargin/fucoidan-induced apoptosis was blocked by eNampt to the same degree in these macrophages as in wild-type macrophages (data not shown).
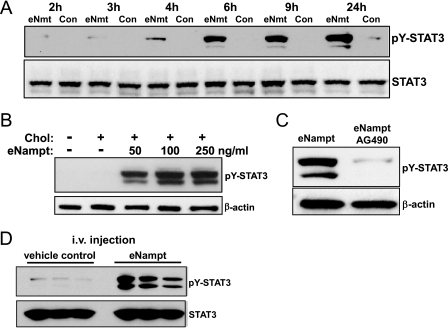
We next considered another possible mechanism for the cell survival effect of eNampt, namely activation of one or more cell survival signaling pathways. Activated STAT3 can act as a survival signal in a number of cell types (46). As shown in Fig. 2A, eNampt is a potent inducer of STAT3 tyrosine phosphorylation, a marker of STAT3 activation, and the effect was seen with an eNampt concentration as low as 50 ng/ml (Fig. 2B). The ability of eNampt to activate STAT3 was not dependent on macrophage insulin signaling, because the response was the same in insulin receptor-null versus wild-type macrophages (data not shown). However, STAT3 activation was inhibited by the Janus kinase inhibitor AG490 (tyrphostin) (Fig. 2C), consistent with the role of Janus kinases in activation of STAT3 (47). Importantly, STAT3 activation occurred in vivo; macrophages isolated from mice 12 h after intravenous injection with eNampt showed robust phosphorylation of STAT3 (Fig. 2D).
FIGURE 2.
eNampt activates tyrosine phosphorylation of STAT3. A, macrophages were incubated in the absence (Con) or presence of 100 ng/ml eNampt for the indicated times. Whole cell lysates were immunoblotted for tyrosine-phosphorylated STAT3 (pY-STAT3) and total STAT3. B, macrophages were treated with the indicated concentrations of eNampt or control medium for 24 h and then incubated under control or cholesterol (Chol) loading conditions for 5 h. Whole cell lysates were immunoblotted for tyrosine-phosphorylated STAT3 and β-actin. C, macrophages were incubated for 18 h with 100 ng/ml eNampt with or without 10 μg/ml AG490. Whole cell lysates were immunoblotted for tyrosine-phosphorylated STAT3 and β-actin. D, male C57/B6L mice (three per group) were injected intravenously (i.v.) with 5 μg of eNampt in 0.5 ml of phosphate-buffered saline or phosphate-buffered saline alone (vehicle control). 12 h after this injection, peritoneal macrophages were collected and lysed in radioimmune precipitation buffer. Equal amounts of cell lysates were blotted for tyrosine-phosphorylated STAT3 and total STAT3.
To establish causation for apoptosis, we knocked down STAT3 expression in macrophages using siRNA. As shown by the immunoblot in Fig. 3A, STAT3 knockdown was achieved using two separate Stat3 siRNA species, whereas scrambled siRNA did not decrease STAT3 expression. As in Fig. 1, lipoprotein-cholesterol-induced apoptosis was markedly suppressed by pretreatment of the cells with eNampt (Fig. 3B, left column of images; quantified data in Fig. 3C). However, suppression of apoptosis by eNampt was largely abrogated in cells treated with the two active Stat3 siRNA species. With regard to additional controls for this experiment, scrambled siRNA did not reverse the survival effect of eNampt; none of the siRNA species induced apoptosis in non-cholesterol-loaded macrophages, either alone or plus eNampt, and the siRNA did not significantly alter cholesterol-induced apoptosis (data not shown). Almost identical results were obtained when apoptosis was induced by the UPR activator thapsigargin plus the SRA ligand fucoidan (Fig. 3B, right column of images; quantified data in Fig. 3D) and when the TUNEL assay was used for apoptosis instead of annexin V staining (data not shown).
Extracellular Nampt Induces STAT3 Phosphorylation by Promoting the Secretion of an Autocrine/Paracrine Stat3 Activator, Interleukin-6—eNampt could activate STAT3 through a direct effect on macrophages or by inducing the secretion of an autocrine/paracrine activator, which would then secondarily activate STAT3. We considered the latter possibility, because when eNampt was added directly to macrophages, the onset of STAT3 phosphorylation was delayed for at least 4 h (see Fig. 2A). To test the autocrine/paracrine hypothesis, conditioned medium (CM) was collected from control and eNampt-treated macrophages (“donor” macrophages) and then added to “recipient” macrophages for various periods of time (Fig. 4A). The data show that CM from eNampt-treated donor macrophages (eNampt-CM) induced STAT3 tyrosine phosphorylation in recipient macrophages after as little as 15 min of incubation. This result could not be explained by a direct effect on the recipient cells of residual eNampt in the CM, because, as mentioned above, eNampt takes at least 4 h to activate STAT3. This point was proven in a subsequent experiment in which the donor macrophages were incubated with eNampt for 5 h and then chased in eNampt-free medium for 12 h. The 12-h chase CM was also able to activate STAT3 in recipient macrophages after only 30 min of incubation (Fig. 4B). The ability of eNampt to induce the secretion of the STAT3-activating factor was dependent on new protein synthesis in the donor macrophages, because CM from macrophages treated with eNampt in the presence of the protein synthesis inhibitor cycloheximide did not induce STAT3 phosphorylation in recipient macrophages (data not shown).
We undertook a candidate approach to identify the active factor(s) in eNampt CM. Two STAT3-activating cytokines known to be secreted by macrophages are IL-10 and IL-6 (48), and IL-6 can be induced by Nampt (25). We found that eNampt induced the secretion of both of these cytokines (Fig. 4C). We then tested the ability of neutralizing antibodies against the IL-10 receptor and IL-6 receptor to block the ability of eNampt-CM to activate STAT3 in recipient macrophages. As shown by the data in Fig. 4D, the IL-10R antibody had no effect, whereas the IL-6R antibody markedly suppressed STAT3 phosphorylation. In a control experiment, we showed that the IL-10R antibody was effective at blocking STAT3 phosphorylation in macrophages incubated with recombinant IL-10 (data not shown). To further prove the role of IL-6 as the critical STAT3-activating factor in eNampt CM, we showed that anti-IL-6 antibody was also able to block STAT3 phosphorylation in recipient macrophages incubated with eNampt-CM (Fig. 4E).
To establish that this eNampt/IL-6 pathway is important for eNampt-mediated survival of ER-stressed macrophages, the effect of eNampt on macrophage apoptosis was tested in the absence or presence of neutralizing IL-6 antibodies, IL-10 antibodies, or both. As shown in Fig. 4F (Expt. #1), the survival effect of eNampt was substantially abrogated by the anti-IL-6 antibody but not by the anti-IL-10 antibody. Very similar results were obtained when apoptosis was assayed using the TUNEL assay instead of the annexin V assay (data not shown). A repeat experiment with two additional controls is shown in experiment 2 (Expt. #2) in Fig. 4F. Note that anti-IL-6 had no significant effect on apoptosis when added to cholesterol-loaded cells in the absence of eNampt and that nonimmune IgG had no effect on eNampt-treated, cholesterol-loaded cells. In a separate experiment, we found that conditioned medium from eNampt-treated donor cells did not decrease expression of the proapoptotic UPR effector CHOP in recipient macrophages (data not shown), indicating that eNampt-induced survival was not mediated through CHOP suppression. In summary, eNampt activates STAT3 in macrophages by first inducing the secretion of IL-6, which then secondarily activates STAT3 through interaction with the IL-6 receptor. This pathway is critical for the ability of eNampt to block UPR-induced apoptosis.
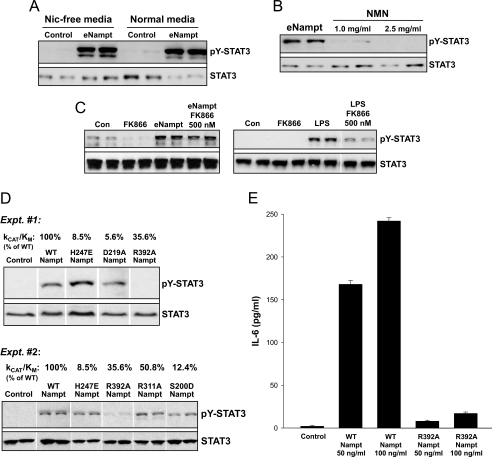
Extracellular Nampt-induced STAT3 Phosphorylation Does Not Require Nampt Enzymatic Activity—Two recent reports have shown that signaling/cytokine-like activities of eNampt require its enzymatic activity (20, 26), but most other studies in this area have not addressed this critical issue. To explore this point in the context of eNampt-induced STAT3 activation in macrophages, we first determined whether depletion of the Nampt substrate nicotinamide in the medium could block eNampt-induced STAT3 phosphorylation. We found that eNampt induced STAT3 phosphorylation similarly in macrophages incubated in control versus nicotinamide-free medium (Fig. 5A). Moreover, STAT3 phosphorylation was not induced by the product of the nicotinamide phosphoribosyltransferase, NMN (Fig. 5B). Note that NMN can mimic the effect of eNampt on glucose-stimulated insulin secretion, a scenario in which enzymatic activity of eNampt is required (20). Third, the nicotinamide phosphoribosyltransferase inhibitor FK866 did not block the ability of eNampt to stimulate the phosphorylation of STAT3 (Fig. 5C, left blot). As a positive control for the inhibitory action of FK866, we took advantage of the finding of Busso et al. (26) that LPS-induced IL-6 requires endogenous Nampt and is thus inhibited by FK866 (see “Discussion”). As expected from this finding, LPS also activated STAT3, and this effect was markedly blocked by FK866 (Fig. 5C, right blot). These data also highlight another important point, namely that the ability of eNampt to trigger IL-6/STAT3 signaling in the macrophages in the current study cannot be explained by contaminating endotoxin. This point is further substantiated by the following observations: (a) no other molecules were detected by mass spectrometric analysis of our Nampt preparation; (b) endotoxin levels were below the limit of detection by the Limulus amebocyte lysate assay, and similar results in terms of STAT3 activation and macrophage survival were found with recombinant eNampt made in HEK-293 cells instead of in Escherichia coli; (c) eNampt led to STAT3 phosphorylation equally well in wild-type versus LPS-resistant Myd88-/- macrophages; and (d) treatment of macrophages with LPS did not mimic the prosurvival action of eNampt in ER-stressed macrophages (data not shown).
FIGURE 5.
Induction of STAT3 phosphorylation by eNampt does not depend upon nicotinamide phosphoribosyltransferase enzymatic activity. A, macrophages were cultured in nicotinamide-free or normal medium for 24 h. The cells were then incubated in the absence (Con) or presence of 100 ng/ml eNampt (eNmt) for 24 h, and cell lysates were immunoblotted for tyrosine-phosphorylated STAT3 (pY-STAT3) and total STAT3. B, macrophages were incubated for 18 h with 100 ng/ml eNampt or 1.0 or 2.5 mg/ml NMN, and cell lysates were immunoblotted for tyrosine-phosphorylated and total STAT3. C, left blot, macrophages were incubated for 18 h with 500 nm FK866 alone, 100 ng/ml eNampt alone, or 100 ng/ml eNampt + 500 nm FK866. Whole cell lysates were immunoblotted for tyrosine-phosphorylated and total STAT3. Right blot, a similar experiment was conducted, except 100 ng/ml LPS was used instead of eNampt, based on the protocol of Busso et al. (26). D, in the two experiments shown, macrophages were incubated for 18 h in the absence or presence of 100 ng/ml wild-type Nampt (WT) or the indicated Nampt mutants. Whole cell lysates were immunoblotted for tyrosine-phosphorylated and total STAT3. kcat/Km data for each mutant, expressed as the percentage of kcat/Km for wild-type Nampt, is indicated above each lane. E, macrophages were incubated for 9 h in the absence or presence 50 or 100 ng/ml wild-type Nampt or the R392A Nampt mutant. Cell culture supernatants were analyzed for the presence of IL-6 by an enzyme-linked immunosorbent assay (n = 6 per condition).
As another test of the idea that the eNampt-induced STAT3 pathway described here does not involve Nampt enzymatic activity, we used a series of Nampt mutants that have varying degrees of enzymatic activity (44). The data in Fig. 5D show that there was no correlation between eNampt enzymatic activity and its ability to activate STAT3. In particular, R392A Nampt, which retains approximately a third of Nampt enzymatic activity, was almost completely unable to activate STAT3 or to stimulate the secretion of IL-6 (Fig. 5, D and E). Conversely, H247E, D219A, and S200D Nampt, which have very low enzymatic activity, were potent stimulators of STAT3 phosphorylation (Fig. 5D). In summary, the combined data in Fig. 5 indicate that the nicotinamide phosphoribosyltransferase activity of eNampt is not needed to activate the IL-6/STAT3 pathway in macrophages.
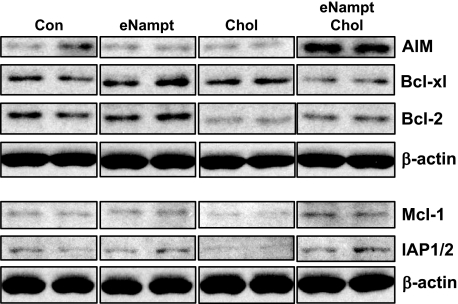
Extracellular Nampt Is Associated with Increased Levels of Specific Cell Survival Proteins in Cholesterol-loaded Macrophages—STAT3 activation has been linked to cell survival and the induction of cell survival proteins in certain models of apoptosis (46). We therefore hypothesized that eNampt pretreatment of cholesterol-loaded macrophages would increase the level of one or more cell survival proteins, which could explain the cell survival action of eNampt in these cells. In this context, we assayed the levels of five well known macrophage survival proteins, apoptosis inhibitor in macrophages (AIM; SPα), Bcl-xL, Bcl-2, Mcl-1, and inhibitor of apoptosis proteins-1/2 (IAP1/2) (Fig. 6). The most important analysis involved comparing cholesterol-loaded macrophages with or without eNampt to determine which proteins were increased by eNampt under conditions that eventually lead to apoptosis and protection by eNampt (third and fourth lanes of the immunoblots). Of the five proteins analyzed, AIM was markedly increased by eNampt under cholesterol-loading conditions, and Mcl-1 and IAP1/2 were modestly increased. The basal level of Bcl-2 was actually decreased by cholesterol loading, and there was a slight restoration of Bcl-2 expression by eNampt treatment of cholesterol-loaded cells. Bcl-xL was not increased by eNampt under these conditions. In non-cholesterol-loaded cells, none of the cell survival proteins were increased by eNampt. These data are consistent with the hypothesis that eNampt promotes the survival of cholesterol-loaded macrophages by increasing the expression of certain cell survival proteins, particularly AIM.
FIGURE 6.
eNampt treatment of cholesterol-loaded macrophages increases the expression of specific cell survival proteins. Macrophages were preincubated in the absence or presence of 100 ng/ml eNampt for 24 h and then incubated with medium alone (Con) or medium containing eNampt, 50 μg/ml acetyl-LDL + 10 μg/ml 58035 (Chol), or eNampt plus acetyl-LDL/58035 for 6 h. Cell extracts were then assayed by immunoblot for the indicated cell survival protein and β-actin as a loading control.
DISCUSSION
A critical area of obesity research is centered on circulating proteins whose levels are altered by changes in body mass index. Understanding how these proteins affect obesity-associated diseases may provide invaluable clues to help stem the devastating consequences of the current obesity epidemic (14). One such protein, eNampt/PBEF/visfatin, has received much attention lately, but the biological effects of this molecule and its mechanisms of action are complex and incompletely understood. The biological effect studied here, promotion of macrophage survival, could represent an important process by which elevated levels of eNampt in obesity might influence obesity-associated disease (see Introduction and below). With regard to the mechanism, we have shown that the key signaling pathway that mediates the cell-survival effect of eNampt, autocrine/paracrine activation of STAT3, does not involve eNampt enzymatic activity.
We provide four independent pieces of evidence, including the use of inactive eNampt mutants, to support the conclusion that nicotinamide phosphoribosyltransferase activity is not needed to activate the STAT3 pathway. In theory, the IL-6/STAT3/cell survival pathway shown here could have been caused by a contaminating molecule in our preparations of eNampt. However, we subjected our preparation of eNampt to mass spectrometric analysis, and only one molecule, eNampt, was detected (data not shown). Moreover, the IL-6 effect could not be explained by contaminating endotoxin, as described under “Results.” Rather, we hypothesize that eNampt stimulates secretion of IL-6 by interacting with a yet to be identified signaling receptor, although not the insulin receptor (data not shown; cf. Ref. 28). In a preliminary experiment, we have shown that macrophages subjected to Proteinase K-mediated cell surface proteolysis no longer respond to eNampt in terms STAT3 activation (data not shown). However, identification of an “eNampt receptor,” if it does exist, will require a major new undertaking.
Although it may seem implausible that cells secrete an enzyme that acts nonenzymatically in triggering cell signaling, previous work has shown that the extracellular form of phosphoglucose isomerase induces cellular migration through interaction with a seven-transmembrane domain receptor (49). Intriguingly, the only Nampt mutant that lacked STAT3-activating activity was R392A, suggesting that Arg329 somehow plays a role in either cell interaction or its downstream effects. The fact that the S200D mutant retained activity may also be instructive. This mutant cannot form dimers, which is the predominant form of enzymatically active wild-type Nampt (44). Thus, although the dimer form is necessary for enzymatic activity, the monomer form may retain cytokine-stimulating activity.
The two mechanistic areas that will require further study include how eNampt triggers IL-6 secretion and how STAT3 activation promotes survival in ER-stressed macrophages. A previous study showed that an inhibitor of the mitogen-activated protein kinase p38 could block eNampt-induced IL-6 mRNA and IL-6 protein secretion from human monocytes (25). However, there were no data in this study addressing the involvement or lack thereof of eNampt enzymatic activity. In terms of the cell survival effect of STAT3, there is evidence of increased apoptosis in Stat3-/- mice, and STAT3-induced survival in cultured cells has been associated with induction of a number of cell survival molecules, including Bcl-2, Bcl-xL, Mcl-1, and survivin (46). The data in Fig. 6 show that several cell survival proteins, particularly AIM, are increased in eNampt-pretreated cholesterol-loaded macrophages. Future experiments in which STAT3 and the cell survival proteins are silenced will be needed to definitively establish the hypothesized pathway, namely eNampt → STAT3 → AIM/Mcl-1/IAP → cell survival. Beyond the cell survival effect of STAT3 signaling, Nowell et al. (50) suggested that IL-6/STAT3 signaling can actually induce Nampt itself, which, if applicable to macrophages, might represent an amplifying positive feedback loop.
In terms of the biological end point investigated here, how might enhancement of macrophage survival by eNampt have an impact on obesity-related processes? Macrophage apoptosis has been proposed to be one mechanism of curbing inflammation after an initial host defense response (51). Prolonged survival of these cells, therefore, may bolster the inflammatory response known to be important in obesity (1–3), particularly in the setting of ER stress, where the inflammatory response is enhanced (52–55). In addition, the autocrine/paracrine factor that initiates the STAT3-mediated cell survival pathway, IL-6, directly promotes inflammation (56). As mentioned above, an enzymatic Nampt pathway enhances IL-6 secretion in LPS-activated macrophages (26), and this process could complement the nonenzymatic pathway shown.
In another scenario, prolonged macrophage survival has been shown to promote the early progression of macrophage-rich atherosclerotic lesions (9, 10), another hallmark of obesity. This concept is not to be confused with the pronecrotic effect of macrophage apoptosis in advanced plaques, where efferocytosis is defective (11, 12). Even in advanced lesions, however, simply promoting macrophage survival may result in detrimental effects associated with living macrophages in these plaques, such as secretion of inflammatory cytokines, matrix proteases, and tissue factor (57).
A third possible link is related to the known role of IL-6/STAT3 signaling, including antiapoptotic pathways, in promoting cancer (58), another obesity-associated disease (5). In particular, an eNampt-mediated survival effect on tumor cells or, apropos to the current study, TAMs might promote tumor development (6). Interestingly, TAMs have a particularly important role in prostate cancer, one of the major cancers associated with obesity (5, 59). If so, these effects of eNampt may complement an NAD-mediated survival effect of enzymatically active Nampt on tumor cells themselves (60).
Whether or not macrophage survival and the hypothesized consequences are increased in obesity in an eNampt-dependent manner must await the creation of conditional eNampt-null mice, because Nampt-/- mice die in utero (20). However, the concentration of eNampt used in our cell culture studies is in the range of eNampt measured in the circulation of obese mice and humans (21–24). Indeed, studies in humans have shown positive correlations among obesity, plasma eNampt levels, and inflammatory markers, including IL-6 (14, 25). Moreover, we showed that intravenous injection of mice with eNampt activates STAT3 in peritoneal macrophages (Fig. 2D). In addition, locally synthesized eNampt, including that in adipose tissue (20) and atherosclerotic lesions (61), may result in high local concentrations of the protein.
In summary, eNampt stimulates an IL-6/STAT3-mediated cell survival pathway in macrophages through a nonenzymatic mechanism. These actions may account for alterations in macrophage physiology in the setting of obesity, where eNampt levels are elevated. The consequences of such macrophage alterations could mediate the effects of obesity in inflammation, atherogenesis, and tumorigenesis. Future genetic manipulations of eNampt in mice will be needed to test these ideas in vivo.
Acknowledgments
We thank Dr. Liang Tong for providing FK866 and for helpful discussions.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 HL075662 and P01 HL087123 (to I. T.) and National Institutes of Health, NIA, Grant AG024150. This work was also supported by United States Army Medical Research and Materiel Command (USAMRMC) Grant W81XWH-06-1-0212 (to I. T.); grants from the Glenn Foundation, Ellison Medical Foundation, American Diabetes Association, and Juvenile Diabetes Research Foundation (to S. I.); and Deutsche Forschungsgemeinschaft Grant DO1289/2-1 (to B. D.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: TAM, tumor-associated macrophage; CHOP, C/EBP-homologous protein; CM, conditioned medium; eNampt, extracellular Nampt; ER, endoplasmic reticulum; IL, interleukin; LDL, low density lipoprotein; LPS, lipopolysaccharide; PBEF, pre-B cell colony-enhancing factor; PRR, pattern recognition receptor; UPR, unfolded protein response; siRNA, small interfering RNA; TUNEL, terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling.
References
- 1.Weisberg, S. P., McCann, D., Desai, M., Rosenbaum, M., Leibel, R. L., and Ferrante, A. W., Jr. (2003) J. Clin. Invest. 112 1796-1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu, H., Barnes, G. T., Yang, Q., Tan, G., Yang, D., Chou, C. J., Sole, J., Nichols, A., Ross, J. S., Tartaglia, L. A., and Chen, H. (2003) J. Clin. Invest. 112 1821-1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wellen, K. E., and Hotamisligil, G. S. (2005) J. Clin. Invest. 115 1111-1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson, P. W., D'Agostino, R. B., Parise, H., Sullivan, L., and Meigs, J. B. (2005) Circulation 112 3066-3072 [DOI] [PubMed] [Google Scholar]
- 5.Irigaray, P., Newby, J. A., Lacomme, S., and Belpomme, D. (2007) Biomed. Pharmacother. 61 665-678 [DOI] [PubMed] [Google Scholar]
- 6.Porta, C., Subhra, K. B., Larghi, P., Rubino, L., Mancino, A., and Sica, A. (2007) Adv. Exp. Med. Biol. 604 67-86 [DOI] [PubMed] [Google Scholar]
- 7.Miselis, N. R., Wu, Z. J., Van, R. N., and Kane, A. B. (2008) Mol. Cancer Ther. 7 788-799 [DOI] [PubMed] [Google Scholar]
- 8.Vandivier, R. W., Henson, P. M., and Douglas, I. S. (2006) Chest 129 1673-1682 [DOI] [PubMed] [Google Scholar]
- 9.Liu, J., Thewke, D. P., Su, Y. R., Linton, M. F., Fazio, S., and Sinensky, M. S. (2005) Arterioscler. Thromb. Vasc. Biol. 25 174-179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arai, S., Shelton, J. M., Chen, M., Bradley, M. N., Castrillo, A., Bookout, A. L., Mak, P. A., Edwards, P. A., Mangelsdorf, D. J., Tontonoz, P., and Miyazaki, T. (2005) Cell Metab. 1 201-213 [DOI] [PubMed] [Google Scholar]
- 11.Tabas, I. (2005) Arterioscler. Thromb. Vasc. Biol. 25 2255-2264 [DOI] [PubMed] [Google Scholar]
- 12.Schrijvers, D. M., De Meyer, G. R., Herman, A. G., and Martinet, W. (2007) Cardiovasc. Res. 73 470-480 [DOI] [PubMed] [Google Scholar]
- 13.Matarese, G., Mantzoros, C., and La, C. A. (2007) Curr. Pharm. Des. 13 3676-3680 [DOI] [PubMed] [Google Scholar]
- 14.Tilg, H., and Moschen, A. R. (2008) Clin. Sci. (Lond.) 114 275-288 [DOI] [PubMed] [Google Scholar]
- 15.Luk, T., Malam, Z., and Marshall, J. C. (2008) J. Leukocyte Biol. 83 804-816 [DOI] [PubMed] [Google Scholar]
- 16.Revollo, J. R., Grimm, A. A., and Imai, S. (2004) J. Biol. Chem. 279 50754-50763 [DOI] [PubMed] [Google Scholar]
- 17.Revollo, J. R., Grimm, A. A., and Imai, S. (2007) Curr. Opin. Gastroenterol. 23 164-170 [DOI] [PubMed] [Google Scholar]
- 18.Samal, B., Sun, Y., Stearns, G., Xie, C., Suggs, S., and McNiece, I. (1994) Mol. Cell. Biol. 14 1431-1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jia, S. H., Li, Y., Parodo, J., Kapus, A., Fan, L., Rotstein, O. D., and Marshall, J. C. (2004) J. Clin. Invest. 113 1318-1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Revollo, J. R., Korner, A., Mills, K. F., Satoh, A., Wang, T., Garten, A., Dasgupta, B., Sasaki, Y., Wolberger, C., Townsend, R. R., Milbrandt, J., Kiess, W., and Imai, S. (2007) Cell Metab. 6 363-375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berndt, J., Kloting, N., Kralisch, S., Kovacs, P., Fasshauer, M., Schon, M. R., Stumvoll, M., and Bluher, M. (2005) Diabetes 54 2911-2916 [DOI] [PubMed] [Google Scholar]
- 22.Haider, D. G., Holzer, G., Schaller, G., Weghuber, D., Widhalm, K., Wagner, O., Kapiotis, S., and Wolzt, M. (2006) J. Pediatr. Gastroenterol. Nutr. 43 548-549 [DOI] [PubMed] [Google Scholar]
- 23.Sandeep, S., Velmurugan, K., Deepa, R., and Mohan, V. (2007) Metabolism 56 565-570 [DOI] [PubMed] [Google Scholar]
- 24.Chen, M. P., Chung, F. M., Chang, D. M., Tsai, J. C., Huang, H. F., Shin, S. J., and Lee, Y. J. (2006) J. Clin. Endocrinol. Metab. 91 295-299 [DOI] [PubMed] [Google Scholar]
- 25.Moschen, A. R., Kaser, A., Enrich, B., Mosheimer, B., Theurl, M., Niederegger, H., and Tilg, H. (2007) J. Immunol. 178 1748-1758 [DOI] [PubMed] [Google Scholar]
- 26.Busso, N., Karababa, M., Nobile, M., Rolaz, A., Van, G. F., Galli, M., Leo, O., So, A., and De, S. T. (2008) PLoS ONE 3 e2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fukuhara, A., Matsuda, M., Nishizawa, M., Segawa, K., Tanaka, M., Kishimoto, K., Matsuki, Y., Murakami, M., Ichisaka, T., Murakami, H., Watanabe, E., Takagi, T., Akiyoshi, M., Ohtsubo, T., Kihara, S., Yamashita, S., Makishima, M., Funahashi, T., Yamanaka, S., Hiramatsu, R., Matsuzawa, Y., and Shimomura, I. (2005) Science 307 426-430 [DOI] [PubMed] [Google Scholar]
- 28.Fukuhara, A., Matsuda, M., Nishizawa, M., Segawa, K., Tanaka, M., Kishimoto, K., Matsuki, Y., Murakami, M., Ichisaka, T., Murakami, H., Watanabe, E., Takagi, T., Akiyoshi, M., Ohtsubo, T., Kihara, S., Yamashita, S., Makishima, M., Funahashi, T., Yamanaka, S., Hiramatsu, R., Matsuzawa, Y., and Shimomura, I. (2007) Science 318 565. [DOI] [PubMed] [Google Scholar]
- 29.Ozcan, U., Cao, Q., Yilmaz, E., Lee, A. H., Iwakoshi, N. N., Ozdelen, E., Tuncman, G., Gorgun, C., Glimcher, L. H., and Hotamisligil, G. S. (2004) Science 306 457-461 [DOI] [PubMed] [Google Scholar]
- 30.Boden, G., Duan, X., Homko, C., Molina, E. J., Song, W., Perez, O., Cheung, P., and Merali, S. (2008) Diabetes 57 2438-2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feng, B., Yao, P. M., Li, Y., Devlin, C. M., Zhang, D., Harding, H. P., Sweeney, M., Rong, J. X., Kuriakose, G., Fisher, E. A., Marks, A. R., Ron, D., and Tabas, I. (2003) Nat. Cell Biol. 5 781-792 [DOI] [PubMed] [Google Scholar]
- 32.DeVries-Seimon, T., Li, Y., Yao, P. M., Stone, E., Wang, Y., Davis, R. J., Flavell, R., and Tabas, I. (2005) J. Cell Biol. 171 61-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou, J., Lhotak, S., Hilditch, B. A., and Austin, R. C. (2005) Circulation 111 1814-1821 [DOI] [PubMed] [Google Scholar]
- 34.Gargalovic, P. S., Gharavi, N. M., Clark, M. J., Pagnon, J., Yang, W. P., He, A., Truong, A., Baruch-Oren, T., Berliner, J. A., Kirchgessner, T. G., and Lusis, A. J. (2006) Arterioscler. Thromb. Vasc. Biol. 26 2490-2496 [DOI] [PubMed] [Google Scholar]
- 35.Myoishi, M., Hao, H., Minamino, T., Watanabe, K., Nishihira, K., Hatakeyama, K., Asada, Y., Okada, K., Ishibashi-Ueda, H., Gabbiani, G., Bochaton-Piallat, M. L., Mochizuki, N., and Kitakaze, M. (2007) Circulation 116 1226-1233 [DOI] [PubMed] [Google Scholar]
- 36.Han, S., Liang, C. P., DeVries-Seimon, T., Ranalletta, M., Welch, C. L., Collins-Fletcher, K., Accili, D., Tabas, I., and Tall, A. R. (2006) Cell Metab. 3 257-266 [DOI] [PubMed] [Google Scholar]
- 37.Lee, A. S., and Hendershot, L. M. (2006) Cancer Biol. Ther. 5 721-722 [DOI] [PubMed] [Google Scholar]
- 38.Havel, R. J., Eder, H., and Bragdon, J. (1955) J. Clin. Invest. 34 1345-1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Basu, S. K., Goldstein, J. L., Anderson, R. G. W., and Brown, M. S. (1976) Proc. Natl. Acad. Sci. U. S. A 73 3178-3182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cook, A. D., Braine, E. L., and Hamilton, J. A. (2003) J. Immunol. 171 4816-4823 [DOI] [PubMed] [Google Scholar]
- 41.Li, Y., Ge, M., Ciani, L., Kuriakose, G., Westover, E., Dura, M., Covey, D., Freed, J. H., Maxfield, F. R., Lytton, J., and Tabas, I. (2004) J. Biol. Chem. 279 37030-37039 [DOI] [PubMed] [Google Scholar]
- 42.Seimon, T. A., Obstfeld, A., Moore, K. J., Golenbock, D. T., and Tabas, I. (2006) Proc. Natl. Acad. Sci. U. S. A 103 19794-19799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yao, P. M., and Tabas, I. (2000) J. Biol. Chem. 275 23807-23813 [DOI] [PubMed] [Google Scholar]
- 44.Wang, T., Zhang, X., Bheda, P., Revollo, J. R., Imai, S., and Wolberger, C. (2006) Nat. Struct. Mol. Biol. 13 661-662 [DOI] [PubMed] [Google Scholar]
- 45.Brown, A. J., and Jessup, W. (1999) Atherosclerosis 142 1-28 [DOI] [PubMed] [Google Scholar]
- 46.Hirano, T., Ishihara, K., and Hibi, M. (2000) Oncogene 19 2548-2556 [DOI] [PubMed] [Google Scholar]
- 47.Nielsen, M., Kaltoft, K., Nordahl, M., Ropke, C., Geisler, C., Mustelin, T., Dobson, P., Svejgaard, A., and Odum, N. (1997) Proc. Natl. Acad. Sci. U. S. A 94 6764-6769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lang, R. (2005) Immunobiology 210 63-76 [DOI] [PubMed] [Google Scholar]
- 49.Tsutsumi, S., Gupta, S. K., Hogan, V., Tanaka, N., Nakamura, K. T., Nabi, I. R., and Raz, A. (2003) FEBS Lett. 534 49-53 [DOI] [PubMed] [Google Scholar]
- 50.Nowell, M. A., Richards, P. J., Fielding, C. A., Ognjanovic, S., Topley, N., Williams, A. S., Bryant-Greenwood, G., and Jones, S. A. (2006) Arthritis Rheum. 54 2084-2095 [DOI] [PubMed] [Google Scholar]
- 51.Marriott, H. M., Hellewell, P. G., Cross, S. S., Ince, P. G., Whyte, M. K., and Dockrell, D. H. (2006) J. Immunol. 177 6480-6488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deng, J., Lu, P. D., Zhang, Y., Scheuner, D., Kaufman, R. J., Sonenberg, N., Harding, H. P., and Ron, D. (2004) Mol. Cell. Biol. 24 10161-10168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Endo, M., Mori, M., Akira, S., and Gotoh, T. (2006) J. Immunol. 176 6245-6253 [DOI] [PubMed] [Google Scholar]
- 54.Knorre, A., Wagner, M., Schaefer, H. E., Colledge, W. H., and Pahl, H. L. (2002) Biol. Chem. 383 271-282 [DOI] [PubMed] [Google Scholar]
- 55.Li, Y., Schwabe, R. F., DeVries-Seimon, T., Yao, P. M., Gerbod-Giannone, M. C., Tall, A. R., Davis, R. J., Flavell, R., Brenner, D. A., and Tabas, I. (2005) J. Biol. Chem. 280 21763-21772 [DOI] [PubMed] [Google Scholar]
- 56.Naugler, W. E., and Karin, M. (2008) Trends Mol. Med. 14 109-119 [DOI] [PubMed] [Google Scholar]
- 57.Libby, P., and Clinton, S. K. (1993) Curr. Opin. Lipidol. 4 355-363 [Google Scholar]
- 58.Hodge, D. R., Hurt, E. M., and Farrar, W. L. (2005) Eur. J. Cancer 41 2502-2512 [DOI] [PubMed] [Google Scholar]
- 59.Zhu, P., Baek, S. H., Bourk, E. M., Ohgi, K. A., Garcia-Bassets, I., Sanjo, H., Akira, S., Kotol, P. F., Glass, C. K., Rosenfeld, M. G., and Rose, D. W. (2006) Cell 124 615-629 [DOI] [PubMed] [Google Scholar]
- 60.Olesen, U. H., Christensen, M. K., Bjorkling, F., Jaattela, M., Jensen, P. B., Sehested, M., and Nielsen, S. J. (2008) Biochem. Biophys. Res. Commun. 367 799-804 [DOI] [PubMed] [Google Scholar]
- 61.Dahl, T. B., Yndestad, A., Skjelland, M., Oie, E., Dahl, A., Michelsen, A., Damas, J. K., Tunheim, S. H., Ueland, T., Smith, C., Bendz, B., Tonstad, S., Gullestad, L., Froland, S. S., Krohg-Sorensen, K., Russell, D., Aukrust, P., and Halvorsen, B. (2007) Circulation 115 972-980 [DOI] [PubMed] [Google Scholar]