Abstract
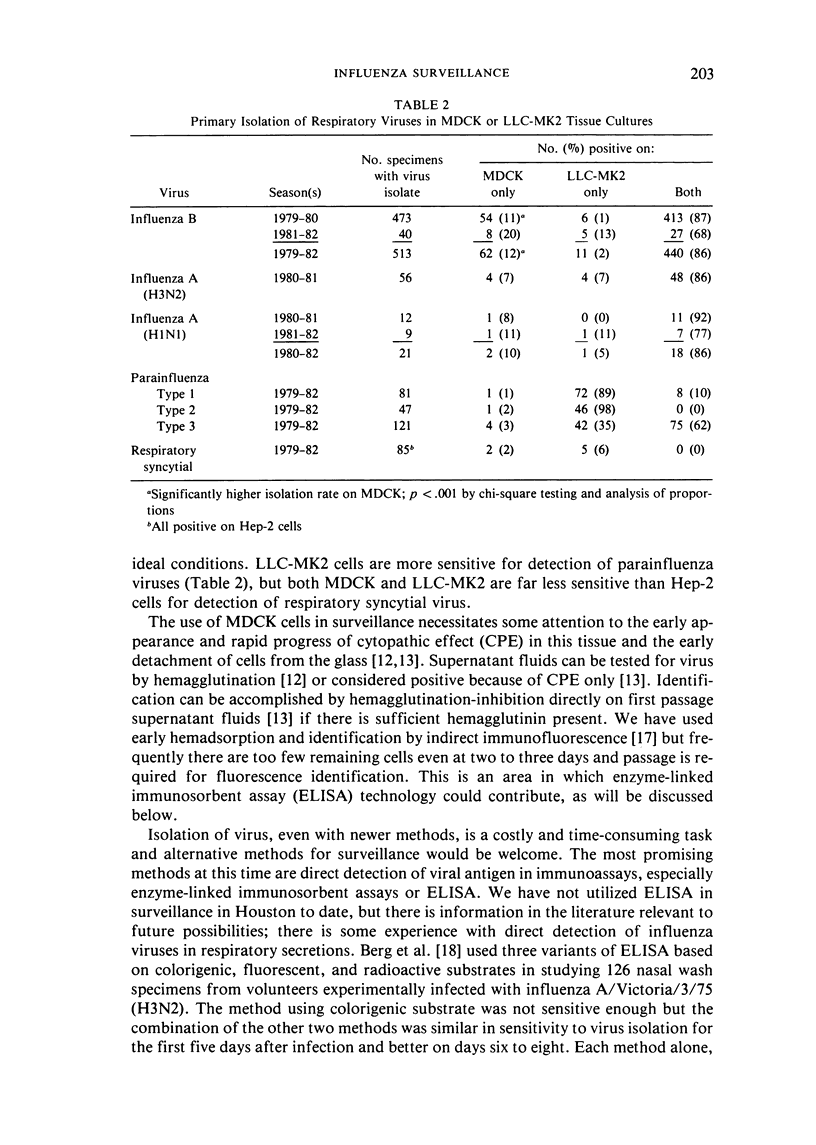
The importance of virologically documented infections in influenza surveillance is well recognized and has been reaffirmed in recent reviews. The large number of specimens tested in surveillance make efficiency and low cost of virologic methods important. Based on observations made by others and our work with reisolation of stored specimens we have used the continuous line tissue cultures MDCK and LLC-MK2 for virus isolation in large-scale influenza surveillance studies for three years. Both cell lines were equally successful in detecting influenza A viruses in 77 fresh, virus-positive specimens. However, during the influenza B outbreak of 1979--80, of 473 specimens positive in either or both tissue cultures, 54 were positive only in MDCK and just six in LLC-MK2 only. For parainfluenza viruses, LLC-MK2 was much superior to MDCK. The most promising alternative to tissue culture at this time, based on a review of the literature, appears to be enzyme immunoassay. Sensitivity sufficient for direct detection of viral antigen in routine specimens currently requires fluorescent or radioactive substrates. Identification of early virus growth in continuous cell line cultures by enzyme immunoassay is practical now and can be considered.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baxter B. D., Couch R. B., Greenberg S. B., Kasel J. A. Maintenance of viability and comparison of identification methods for influenza and other respiratory viruses of humans. J Clin Microbiol. 1977 Jul;6(1):19–22. doi: 10.1128/jcm.6.1.19-22.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg R. A., Yolken R. H., Rennard S. I., Dolin R., Murphy B. R., Straus S. E. New enzyme immunoassays for measurement of influenza A/Victoria/3/75 virus in nasal washes. Lancet. 1980 Apr 19;1(8173):851–853. doi: 10.1016/s0140-6736(80)91356-2. [DOI] [PubMed] [Google Scholar]
- Davies H. W., Appleyard G., Cunningham P., Pereira M. S. The use of a continuous cell line for the isolation of influenza viruses. Bull World Health Organ. 1978;56(6):991–993. [PMC free article] [PubMed] [Google Scholar]
- Frank A. L., Couch R. B., Griffis C. A., Baxter B. D. Comparison of different tissue cultures for isolation and quantitation of influenza and parainfluenza viruses. J Clin Microbiol. 1979 Jul;10(1):32–36. doi: 10.1128/jcm.10.1.32-36.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank A. L., Taber L. H., Glezen W. P., Paredes A., Couch R. B. Reinfection with influenza A (H3N2) virus in young children and their families. J Infect Dis. 1979 Dec;140(6):829–836. doi: 10.1093/infdis/140.6.829. [DOI] [PubMed] [Google Scholar]
- Glezen W. P., Couch R. B. Interpandemic influenza in the Houston area, 1974-76. N Engl J Med. 1978 Mar 16;298(11):587–592. doi: 10.1056/NEJM197803162981103. [DOI] [PubMed] [Google Scholar]
- Glezen W. P., Couch R. B., Taber L. H., Paredes A., Allison J. E., Frank A. L., Aldridge C. Epidemiologic observations of influenza B virus infections in Houston, Texas, 1976-1977. Am J Epidemiol. 1980 Jan;111(1):13–22. doi: 10.1093/oxfordjournals.aje.a112865. [DOI] [PubMed] [Google Scholar]
- Glezen W. P. Serious morbidity and mortality associated with influenza epidemics. Epidemiol Rev. 1982;4:25–44. doi: 10.1093/oxfordjournals.epirev.a036250. [DOI] [PubMed] [Google Scholar]
- Harmon M. W., Pawlik K. M. Enzyme immunoassay for direct detection of influenza type A and adenovirus antigens in clinical specimens. J Clin Microbiol. 1982 Jan;15(1):5–11. doi: 10.1128/jcm.15.1.5-11.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meguro H., Bryant J. D., Torrence A. E., Wright P. F. Canine kidney cell line for isolation of respiratory viruses. J Clin Microbiol. 1979 Feb;9(2):175–179. doi: 10.1128/jcm.9.2.175-179.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D. J., Galland G. G., Reimer C. B., Kendal A. P. Evaluation of a solid-phase immunoassay with fluorescein isothiocyanate-conjugated heterogeneous or monoclonal antibodies for identification of virus isolates, with influenza virus as a model. J Clin Microbiol. 1982 May;15(5):931–937. doi: 10.1128/jcm.15.5.931-937.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkkinen H. K., Halonen P. E., Salmi A. A. Detection of influenza A virus by radioimmunoassay and enzyme-immunoassay from nasopharyngeal specimens. J Med Virol. 1981;7(3):213–220. doi: 10.1002/jmv.1890070305. [DOI] [PubMed] [Google Scholar]
- Silver S. M., Scheid A., Choppin P. W. Loss on serial passage of rhesus monkey kidney cells of proteolytic activity required for Sendai virus activation. Infect Immun. 1978 Apr;20(1):235–241. doi: 10.1128/iai.20.1.235-241.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita K., Maru M., Sato K. A sensitive plaque assay for Sendai virus in an established line of monkey kidney cells. Jpn J Microbiol. 1974 May;18(3):262–264. doi: 10.1111/j.1348-0421.1974.tb00955.x. [DOI] [PubMed] [Google Scholar]
- Taber L. H., Paredes A., Glezen W. P., Couch R. B. Infection with influenza A/Victoria virus in Houston families, 1976. J Hyg (Lond) 1981 Jun;86(3):303–313. doi: 10.1017/s0022172400069059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobita K. Permanent canine kidney (MDCK) cells for isolation and plaque assay of influenza B viruses. Med Microbiol Immunol. 1975 Dec 30;162(1):23–27. doi: 10.1007/BF02123574. [DOI] [PubMed] [Google Scholar]
- Tobita K., Sugiura A., Enomote C., Furuyama M. Plaque assay and primary isolation of influenza A viruses in an established line of canine kidney cells (MDCK) in the presence of trypsin. Med Microbiol Immunol. 1975 Dec 30;162(1):9–14. doi: 10.1007/BF02123572. [DOI] [PubMed] [Google Scholar]


