Abstract
β-adrenergic receptor (βAR) blockade is standard therapy for cardiac failure and ischemia. G-protein coupled receptor kinases (GRKs) desensitize βAR, suggesting that genetic GRK variants might modify outcomes in these syndromes. Re-sequencing of GRK2 and GRK5 revealed a non-synonymous polymorphism of GRK5, common in African Americans (AA), substituting leucine (L) for glutamine (Q) at position 41. GRK5-L41 more effectively uncoupled isoproterenol-stimulated responses than GRK5-Q41 in transfected cells and transgenic mice, and like pharmacological βAR blockade, GRK5-L41 protected against experimental catecholamine-induced cardiomyopathy. Human association studies showed a pharmacogenomic interaction between GRK5-L41 and β-blocker treatment on mortality outcome in independent cohorts of AA cardiac failure (P=0.036) and ischemia (P=0.023). In 375 prospectively followed AA heart failure subjects, GRK5-L41 was protective against death/cardiac transplant (single allele: RR=0.28, 95% CI=0.12-0.66; two alleles: RR=0.08, 95% CI=0.04-0.19; P=0.004). The gain-of-function GRK5-L41 polymorphism facilitates βAR desensitization during catecholamine excess, imparting “genetic β-blockade” and improving survival in heart failure.
INTRODUCTION
Heart failure is an incurable syndrome of multiple causes that will affect one in five adults, conferring a ~25% chance of dying within a year of diagnosis and a mortality rate of ~50% at five years 1,2. Heart failure management is complicated by disease heterogeneity in both inherited genetic cardiomyopathies 3 and the more common non-familial dilated and ischemic cardiomyopathies 4–7. We and others have proposed that inter-individual differences in genetic polymorphisms involving catecholamine signaling pathways can modify heart failure risk, prognosis, or response to treatment. Especially relevant would be pharmacogenomic interactions between genetic variants of catecholamine receptors or their effectors and β-adrenergic receptor (βAR) antagonism (β-blockade), which is a standard therapy for heart failure and myocardial ischemia 8. Indeed, pharmacogenomics of β-blockade might have special importance as this therapy prolongs life and ameliorates symptoms, but concomitantly impairs a critical mechanism for acutely increasing cardiac output in response to physiological stress. Maintaining the proper balance between preservation of βAR signaling and avoidance of βAR-mediated catecholamine toxicity may therefore be critical to outcome.
An important mechanism downregulating βAR signaling in heart failure is increased expression of myocardial G-protein receptor kinase (GRK)-2, which phosphorylates cardiac βAR, leading to recruitment of β-arrestin and receptor uncoupling from G-proteins and downstream signaling effectors 9. While multiple studies have shown that expression of a GRK2-inhibiting mini-gene can improve cardiac function in experimental models of heart failure 10,11, cardiac-specific ablation of GRK2 in mice actually accelerates catecholamine-induced heart failure 12. Thus, the effects of GRK2 on heart function appear to depend both upon expression level and pathophysiological context. The function of the other dominant GRK in the heart, GRK5 13, has not been as well defined: Genetic GRK5 ablation was not associated with a cardiac phenotype in mice 14, but massive cardiac overexpression of bovine GRK5 depressed cardiac βAR responsiveness 15,16. Differences between GRK5 and GRK2 in sub-cellular localization, mechanism of activation, and receptor specificity suggest that these two cardiac GRKs may have non-redundant modulatory roles in the heart. Of particular interest is the rapid up- and down-regulation of GRK2 that correlates with ventricular function 17,18, which implies that the role of this GRK in the heart may be acute regulation. On the other hand, GRK5 expression appears to less dynamic and may therefore be more important in chronic regulation 19,20. As such, GRK5-mediated βAR desensitization could provide adaptive, beneficial effects during early ventricular decompensation, and prior to frank failure.
To examine the above notion, we searched for human genetic variants of cardiac-expressed GRK2 and GRK5 that might impart risk, modify the course, or alter the response to therapy of heart failure. We identified a non-synonymous polymorphism of GRK5 that changes amino acid 41 in the non-catalytic regulatory domain from glutamine (Q, the most common allele) to leucine (L). In levels of increasing complexity, we have defined the phenotype of this polymorphism in transfected cells, transgenic mice, and in two independent cohorts of cardiac disease among African Americans, in whom the polymorphism is common. We show that the GRK5-L41 variant augments βAR desensitization and represents a form of “genetic β-blockade” that diminishes βAR signaling, confers resistance to experimental catecholamine-induced cardiomyopathy, and protects against early death in African Americans with heart failure.
RESULTS
GRK5, but not GRK2, exhibits genetic variability
Of the seven human GRKs, GRK5 and GRK2 predominate in myocardium 10,12,13. Polymorphism discovery in the 16 exons of GRK5 and 21 exons of GRK2 was performed by resequencing 96 DNA samples (Human Variation Collection of the Coriell Institute [http://ccr.coriell.org/nigms/cells/humdiv.html]) from individuals of diverse ethnicity (40 Caucasians, 40 African American, 16 Asians), providing a 98% probability of detecting polymorphisms with allele frequencies as low as 0.02. Four non-synonymous polymorphisms were detected for GRK5, at cDNA nucleic acid positions 122 (A/T), 840 (G/A), 1274 (C/T) and 1624 (C/G), resulting in amino acid changes at residues 41 (Q to L) (rs17098707), 304 (R to H) (rs12718341), 425 (T to M) and 542 (P to A). The GRK5-Q41L variant was the only one with an allele frequency greater than 2% in any ethnic group, and was therefore studied further. In contrast to GRK5, we found no non-synonymous polymorphisms of the GRK2 coding exons, including the D457V and K465M variants reported in dbSNP (NCBI SNP Cluster ID # rs1977983 and rs1977982). To further confirm this, templates from the Whitehead Institute with the reported polymorphisms were sent to the University of Cincinnati and sequenced; these two SNPs were not confirmed.
GRK5-Q41 and -L41 differentially affect β-adrenergic receptor desensitization in transfected CHO cells
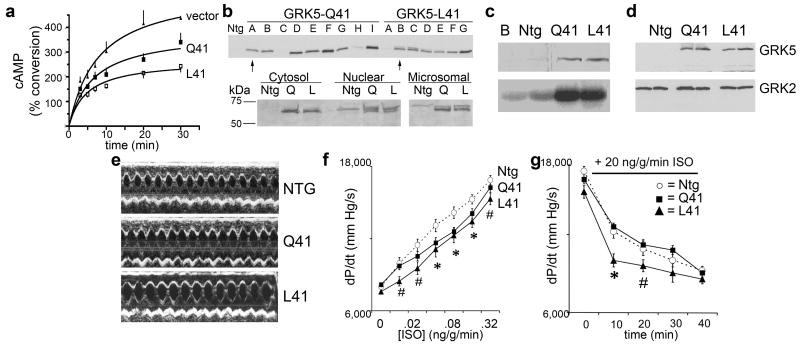
Amino acid 41 of GRK5 is adjacent to a lipid and calmodulin binding domain. To determine the significance of the Q to L substitution at this position on the canonical desensitization function of GRK5, the effects of recombinantly expressed GRK5-Q41 and -L41 on β1AR desensitization were examined during continuous agonist exposure. Chinese Hamster Ovary (CHO) cells were co-transfected with human β1AR (Arg389 variant, which is the most common) and either GRK5-Q41 or -L41. Receptor expression (determined by radioligand binding, data not shown) and GRK expression as determined by Western blotting were equivalent (not shown). The rate and maximal level of cAMP accumulation over time in response to 10 μM isoproterenol in these cells is a measure of receptor coupling to Gαs/adenylyl cyclase, and is inversely related to receptor desensitization. As shown in Figure 1a, GRK5-L41 cells had a different desensitization pattern compared to GRK-Q41 (P < 0.001 by ANOVA), with a ~25% decrease in the rate of cAMP accumulation (4.7±1.2 vs 6.4±1.9, P<0.05) and a ~33% decrease in maximal response (2.6±0.2 vs 3.8±0.4% conversion, P<0.05), compared to GRK5-Q41. Thus, the GRK5-L41 polymorphism decreases β1AR signaling by enhancing agonist-promoted desensitization.
Figure 1. Characteristics of mouse hearts expressing GRK5-Q41 and GRK5-L41.
a. Time dependent accumulation of cAMP in GRK5-transfected cells stimulated with isoproterenol, 10 μM. b. (top) Immunoblot analysis of cardiac GRK5 from multiple lines of GRK5-Q41 and GRK5-L41 transgenic mice. (bottom) Comparative subcellular localization of GRK5-Q41 and –L41. c. Rhodopsin phosphorylation (bottom panel) by buffer (b) and cardiac membranes from nontransgenic (ntg), Q41, and L41 hearts. Top panel shows immunoreactive GRK5 from same fractions. d. Comparative immunoblot analysis of GRK5 and GRK2 in mouse hearts. e. Representative M-mode echocardiograms. ef In vivo contractile dose-response (+dP/dt) to isoproterenol in GRK5-Q41 (black squares) and L41 (black triangles) mice. Nontransgenic (NTG, open circles) are shown for comparison. *P<0.05 vs ntg for Q41 and L41; #P<0.05 for L41. g. Mean data for time-dependent desensitization of dP/dt response to infused isoproterenol in closed chest in vivo catheterization studies. n=12/group. *P<0.05 vs ntg and Q41; # P=0.081 vs ntg and Q41.
Identification of a pharmacogenomic interaction between GRK5 –L41 and β-blockade in human cardiac ischemia and failure
Diminished isoproterenol-stimulated βAR signaling by GRK5-L41 resembles receptor antagonism by pharmacological β-blockers, suggesting that this polymorphism might interact with or mimic β-blockade in human cardiac syndromes wherein β-blockers are standard therapy. Accordingly, we genotyped for GRK5-Q41 or –L41 in two independent closely matched and highly phenotyped cohorts of cardiac disease subjects, 810 individuals (568 Caucasians and 242 African Americans) from Cincinnati with New York Heart Association class II – IV heart failure, and 822 individuals (580 Caucasians and 242 African Americans) from Kansas City and Atlanta with acute cardiac ischemia. Clinical characteristics of the two study groups are in Table 1. A non-affected control group consisted of 513 subjects (406 Caucasians and 107 African Americans) with negative histories and physical examinations for cardiac disease, normal ECGs, and normal echocardiograms. Among Caucasians, L41 allele frequencies were 0.013 (unaffected), 0.024 (heart failure), and 0.010 (acute ischemia), with no association between Q41L genotype and either cardiac disease. Among African Americans, L41 allele frequencies were ~10-fold higher than Caucasians, but again did not differ among unaffected (0.23), heart failure (0.24), and acute ischemia (0.28) subjects. Heterozygous L41 genotype frequency among African Americans was 0.35 and homozygous frequency was 0.062, consistent with predictions from Hardy-Weinberg equilibrium (P=0.57). Thus, the GRK5-L41 allele is rare in Caucasians, but common in African Americans, and is not disproportionately represented in cardiac disease.
Table 1.
Descriptive Statistics of Heart Failure and Coronary Ischemia Cohorts in Gene Association Studies.
| Variable | Heart Failure | Acute Coronary Ischemia | |||
|---|---|---|---|---|---|
| Caucasian n=568 | AA n=242 | Caucasian n=580 | AA n=242 | ||
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
| Age at enrollment (yrs) | 53 ± 13 | 51 ± 13 | 62 ± 12 | 55 ± 11 | |
| Males (%) | 69.3 | 55.0 | 65.7 | 57.4 | |
| Follow-up (yrs) | 2.3 ± 2.2 | 2.3 ± 2.1 | 3.8 ± 1.1 | 3.3 ± 1.4 | |
| Height (cm) | 172 ± 10 | 172 ± 10 | 171 ± 10 | 169 ± 10 | |
| Weight (kg) | 86 ± 21 | 90 ± 26 | 86 ± 18 | 83±21 | |
| Left Ventricular EF (%) | 28 ± 14 | 33 ± 15 | 48 ± 12 | 44 ± 16 | |
| Hypertension (%) | 45.1 | 80.2 | 62.8 | 81.0 | |
| βblocker use (%) | 69.2 | 80.2 | 82.2 | 73.4 | |
| Diagnosis (%) | |||||
| Heart Failure | Acute Coronary Ischemia | ||||
| Caucasian | AA | Caucasian | AA | ||
| Non-ischemic CHF | 54.8 | 71.1 | STEMI | 30.9 | 15.7 |
| Ischemic CHF | 43.1 | 26.8 | NSTEMI | 33.1 | 54.1 |
| Other | 2.2 | 1.5 | UA | 36.0 | 30.2 |
AA=African American; EF= left ventricular ejection fraction; CHF=congestive heart failure; STEMI=ST segment elevation myocardial infarction; NSTEMI=non-ST segment elevation myocardial infarction; UA=unstable angina.
Since GRKs exhibit activity only for ligand-occupied receptors, and functional differences between GRK5-Q41 and –L41 were observed only after catecholamine challenge (see Figure 1), it is not surprising that the GRK5 polymorphism does not alter the risk of developing either heart failure or ischemia. In these syndromes, catecholamine excess and chronic βAR stimulation occur during the course of the disease, and GRK5 effects (like protection afforded by β-blockade) may therefore only be detectable after the disease and accompanying catecholamine excess develop. Accordingly, we examined the heart failure and acute coronary ischemia cohorts for any interaction between GRK5-L41 and β-blocker usage that influenced survival.
Among African Americans either with heart failure or acute coronary ischemic syndromes, age- and gender-adjusted Cox proportional hazards modeling showed significant interactions between GRK5-L41 and β-blocker usage on the endpoint of death (Likelihood Ratio P=0.036 and 0.023, respectively). In Caucasians, wherein the GRK5-L41 variant is rare, there was no significant interaction in either the heart failure (P=0.46)or acute coronary ischemia (P=0.72) cohorts. These results reveal pharmacogenomic interactions between the GRK5-L41 allele and β-blocker therapy for heart failure and acute ischemic syndromes in African Americans only.
GRK5-L41 enhances β1-adrenergic receptor desensitization in transgenic mice
The above studies show that the GRK5-L41 variant decreases β1AR signaling in transfected CHO cells much like partial β-blockade, and that there is a significant interaction between GRK5-L41 and β-blocker use that affects long-term outcome in human heart failure and acute myocardial ischemia. As human gene-association studies do not address molecular mechanisms and are subject to epistatic effects from other polymorphisms, we further characterized the effects of GRK5-Q41 and -L41 using mice in which each allele was specifically expressed only in cardiac myocytes, so phenotypes would only reflect cardiac effects of the GRK5s. Since prior 30-fold overexpression of bovine GRK5 in transgenic mouse hearts significantly depressed basal and catecholamine-stimulated cardiac function 15,16, we created a large number of founder lines to identify pairs of human GRK5-Q41 and -L41 expressing mice with comparable, low levels of expression (Figure 1b, upper panel). The selected lines (Q41A and L41B) each exhibited 4 to 6-fold increases in myocardial GRK activity assessed by rhodopsin kinase assay (Figure 1c) and immunoblotting (Figures 1b-d), with no counter-regulation of GRK2 (Figure 1d). Importantly, no abnormalities of cardiac size, histological appearance, disease-related gene expression, or basal contractile function (M-mode echocardiography) were detected in GRK5-Q41 or -L41mice up to 6 months of age (Fig. 1e and data not shown), demonstrating that the presence of transgenic GRK5 at these levels does not cause dysfunction, and revealing no apparent effect of the L41 variant in normal hearts.
To determine the consequences of GRK5-Q41 and -L41 on βAR stimulation of cardiac contractility, mice underwent cardiac catheterization to measure left ventricular peak positive dP/dt at baseline and in response to increasing doses of intravenous isoproterenol. Both GRK5-Q41 and -L41 produced rightward shifts of the dose-response curves in comparison with nontransgenic mice (Figure 1f; EC50 for GRK5-Q41 transgenics =0.135±0.041 ng/g/min, and GRK5-L41 transgenics =0.086±0.025 ng/g/min, compared to 0.046±0.011 ng/g/min for nontransgenics, P=0.02), reproducing previous findings with transgenic GRK2 and GRK5 10,15,16. In contrast, desensitization of βAR-stimulated contraction, measured during continuous high-dose isoproterenol infusion, was greater (e.g., peak positive dP/dt response was lower) after 10 minutes in GRK5-L41 mice than either -Q41 mice or nontransgenic controls (P<0.001, n=12/group) (Figure 1g). This trend continued at 20 min (P=0.081), but after 30 minutes of continuous isoproterenol infusion desensitization in all groups had achieved similar levels (P=0.133). These data demonstrate that GRK5-L41 is more effective than -Q41 in desensitizingcardiac βAR under conditions of acute catecholamine excess.
GRK5-L41 protects against experimental heart failure caused by catecholamine excess
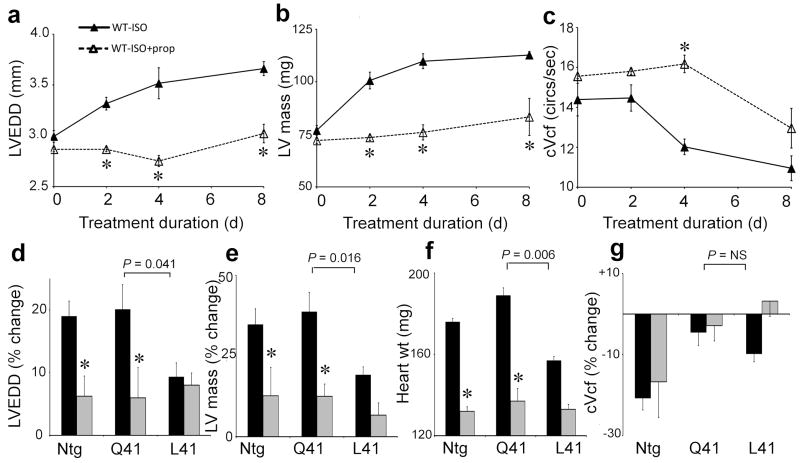
In experimental mouse models, overexpression of βARs 21 or their Gαs G-protein signaling transducer 22 causes cardiac dilation and failure. Conversely, genetic ablation of the βAR/Gαs downstream effector, adenylyl cyclase, preserves myocardial function after physiological stress 23. The finding that GRK5-L41 accelerates isoproterenol-promoted βAR desensitization suggested that, like beta-blockers 22, it might protect hearts from the effects of persistent βAR stimulation, i.e. cardiac dilation, ventricular hypertrophy, and heart failure (Fig. 2a-c)12. To directly examine this possibility we chronically administered isoproterenol to GRK5-Q41 and -L41 transgenic mice via implanted osmotic mini-pump. Whereas GRK5-Q41 did not protect against isoproterenol-mediated increases in left ventricular chamber size (LVEDD; Fig. 2d) and mass (LVM; Figs 2e and f), GRK5-L41 expression at the same levels was protective. Whereas β-blockade ameliorated the effects of catecholamine cardiomyopathy in NTG and GRK-Q41 mice (note LVEDD and LVM effects), in –L41 hearts β-blockade had no additional protective effect (Figs. 2d-f). Both GRK5-Q41 and -L41 blunted the isoproterenol-mediated deterioration in cardiac contractility (cVcf), while propranolol administration had relatively little effect on this parameter (Fig. 2g). These results demonstrate that enhanced βAR desensitization by GRK5-L41 can modulate catecholamine cardiotoxicity, protecting against left ventricular remodeling and cardiomyopathy development similar to pharmacological β-blockade.
Figure 2. Cardiac expression of GRK5-L41, but not -Q41, confers resistance to catecholamine-induced cardiomyopathy.
a–c. Time course for development of catecholamine cardiomyopathy in normal mice (closed triangles) and effect of β-blockade with propanolol (open triangles). LVEDD, left ventricular end diastolic dimension; cVcf, velocity of circumferential shortening corrected for heart rate. (n=6/group, *P<0.05 vs no propanolol). d–g. % change in cardiac parameters before and after 8 days of chronic isoproterenol treatment in vehicle (black, n=12/group) and propanolol (grey, n=6/group) treated mice.. P values compare GRK5-Q41 and –L41 responses. *P<0.05 for propanolol vs vehicle at day eight. ntg is shown for comparison.
GRK5-L41 prolongs survival in β-blocker naive human heart failure
In our human case-control gene association studies and transgenic mouse experiments we found noevidence that the GRK5-L41 polymorphism could cause heart disease. However, we identified a pharmacogenomic interaction between GRK5-L41 and β-blockers in African American heart failure and coronary ischemia, and enhanced β-adrenergic receptor desensitization in GRK5-L41 transgenic mice likewise altered the response to β-blocker treatment under conditions of catecholamine excess. To further define the magnitude of the clinical benefit of the GRK5 polymorphism, we undertook a prospective analysis of 375 African American heart failure subjects, comparing time to death or cardiac transplantation as a function of GRK5-Q41L genotype and β-blocker treatment status. Characteristics of the prospective study group, classified by GRK5 genotype and β-blocker use, are in Table 2. Kaplan-Meyer analysis and Cox proportional modeling were utilized to assess potential effects on outcomes. In addition, race-specific genotyping at short-tandem repeats was undertaken to address potential population stratification. Validation techniques were then employed to show internal reproducibility and predictiveness of the model.
Table 2.
Descriptive Statistics of AA Heart Failure Subjects in the Prospective Study
| Only Q Alleles | ≥ 1 L Allele | |||
|---|---|---|---|---|
| Variable | No BB Use (N=34) | BB Use (N=182) | No BB Use (N=27) | BB Use (N=132) |
| Fractional Shortening (%) | 26±12 | 22±11 | 24±10 | 23±12 |
| LV Ejection Fraction (%) | 34±25 | 34±14 | 31±14 | 34±14 |
| Non-ischemic Cardiomyopathy (%) | 76 | 66 | 69 | 71 |
| Ischemic Cardiomyopathy (%) | 21 | 31 | 27 | 29 |
| LV Mass Indexed to BSA (g) | 160±51 | 178±58 | 180±72 | 186±69 |
| Percent Predicted LV Mass (%) | 175±55 | 178±56 | 179±55 | 198±69 |
| Hypertension (%) | 85 | 80 | 67 | 81 |
| Female (%) | 56 | 43 | 48 | 46 |
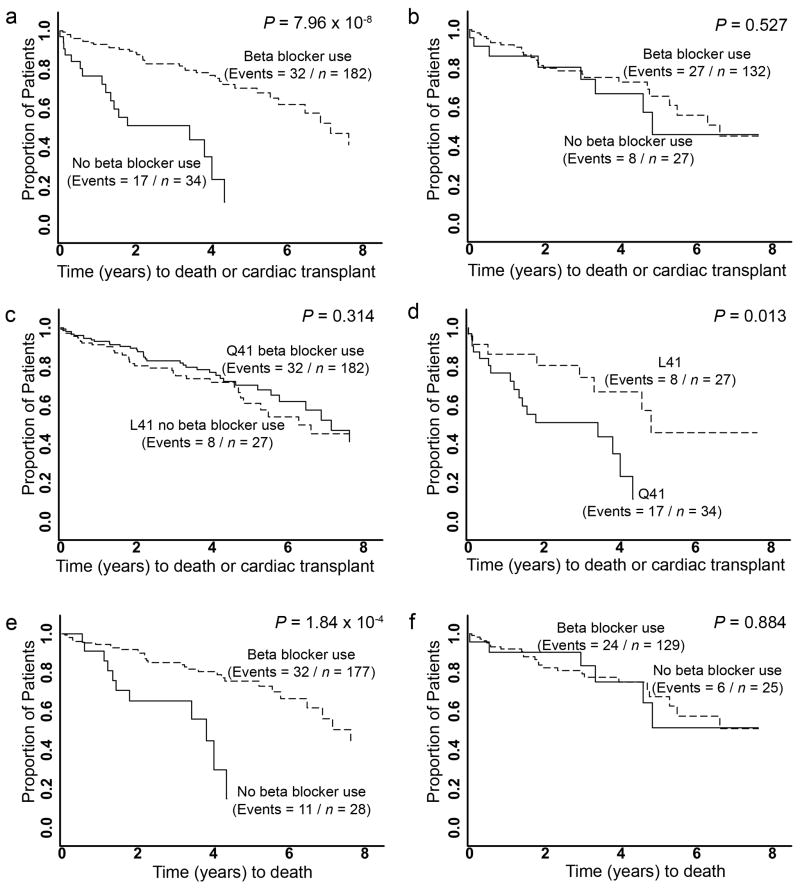
Consistent with therapeutic benefits of β-blocker treatment in heart failure 4,24–26, individuals homozygous for “wild-type” GRK5-Q41 who received β-blockers had longer transplant-free survival times than those of the same genotype who were β-blocker naïve (controls) (HR=0.22, 95% CI=0.12 – 0.40, P<0.001; Figure 3a). In contrast, there was no difference in outcome among L41 heart failure subjects regardless of β-blocker treatment (HR=0.78, 95% CI= 0.35 – 1.7, P=0.53; Figure 3b). To formally evaluate the interaction effect of β-blockers and GRK5-L41 on time to death/transplant, we compared Cox Proportional Hazards models that included age, sex, β-blocker usage, and genotype status, with and without an interaction term between β-blocker usage and Q41L status. The model with the interaction term was significantly better than the reduced model (likelihood ratio test P=0.005). The β-blocker-L41 carrier status interaction term was significant at P=0.004, demonstrating an interaction between GRK5 genotype and β-blocker usage for the endpoint of death/transplant. β-blocker naïve L41 subjects had transplant-free survival times significantly greater than β-blocker naïve Q41 subjects (Figure 3c), but β-blocker treatment fully mimicked the survival advantage of L41 (Figure 3d). Similar results were obtained when all-cause mortality alone was considered (Figures 3e, f). When the homozygous Q41 β-blocker naïve group was set as the reference to derive age- and sex-adjusted hazard ratios for genotype and β-blocker treatment, β-blocker naïve L41 carriers were protected against death/transplantation (Table 3) and death alone. In β-blocker naïve subjects with two L41 alleles, the hazard ratio was 0.081 (95% CI 0.035 to 0.19), suggesting a gene-dose response.
Figure 3. Prospective analysis of GRK5 polymorphism interaction with β-blockade as a determinant of heart failure outcome in African Americans.
Kaplan-Meier curves for time from diagnosis of heart failure to death or cardiac transplantation (a–d) or death alone (e,f). a. GRK5-Q41 by β-blocker usage. b. GRK5-L41 by β-blocker usage. c. GRK5-Q41 no β-blocker use vs. GRK5-L41 no β-blocker use. d. GRK5-Q41 β-blocker users vs. GRK5-L41 β-blocker users. e. GRK5-Q41 by β-blocker usage. f. GRK5-L41 by β-blocker usage.
Table 3.
Cox proportional hazards of GRK5-Q41L polymorphism, β-blocker use, and death/transplant, adjusted for age at heart failure diagnosis and sex (n=375).
| Group | Hazard ratio | 95% CI | P-value | ||
|---|---|---|---|---|---|
| Genotype† | β-blocker | N (# of Events) | |||
| Without adjustment for % African ancestry * | |||||
| Q41 | − | 34 (17) | 1.0 (reference) | -- | -- |
| Q41 | + | 182 (37) | 0.19 | 0.10 to 0.34 | <0.001 |
| L41 | − | 27 (8) | 0.28 | 0.12 to 0.66 | 0.004 |
| L41 | + | 132 (27) | 0.20 | 0.05 to 0.80 | 0.02 |
| With adjustment for % African ancestry ** | |||||
| Q41 | − | 34 (17) | 1.0 (reference) | -- | -- |
| Q41 | + | 182 (37) | 0.19 | 0.10 to 0.37 | <0.001 |
| L41 | − | 27 (8) | 0.31 | 0.13 to 0.73 | 0.007 |
| L41 | + | 132 (27) | 0.20 | 0.05 to 0.84 | 0.03 |
Likelihood ratio test P-value (GRK5-Q41 status and β-blocker usage interaction) = 0.005.
Likelihood ratio test P-value = 0.01.
L41 represents heterozygotes; see text for effects of homozygotes.
Although not a prospective study, a cohort analysis within the case-control study of acute coronary ischemia also showed an interaction between GRK5 genotype and β-blocker use. The specific effect of genotype was most evident in those receiving β-blockers, where GRK5-L41 carriers exhibited improvement in survival (HR = 0.45, 95% CI = 0.238 – 0.853, P = 0.01).
Given the reported associations between the β1AR Arg389 polymorphism and the response to β-blocker treatment in heart failure 27, and between β1AR Arg-389 and α2c del 322-325 polymorphisms and the risk of having heart failure 28, we examined whether an interaction between these functionally-related polymorphisms and GRK5-L41 might provide for a more predictive model. We observed no significant interaction affecting time to death or cardiac transplant between GRK5-L41 and β1AR Arg389 (P=0.46), between GRK5-L41 and α2c del322-325 (P=0.21), or between all three polymorphisms (P=0.87).
Population stratification and significant differences in admixture within the African American cohort have the potential to result in spurious associations. Therefore, we estimated % African ancestry based on genotyping of 13 race-informative short tandem repeats 29,30. No difference in racial admixture was found between the β-blocker treated (African ancestry = 73.4%) and β-blocker naïve groups (African ancestry = 76.8 %; P=0.22). We then utilized the estimated African ancestry as an additional term to adjust the Cox Proportional Hazards model, which did not significantly change the hazard ratios and P-values (Table 3).
Internal reproducibility of the β-blocker-GRK5 interaction was assessed using a sequential analysis procedure 31. We rejected the null hypothesis of no interaction at P<0.05 using 50 randomly chosen subjects (Stage I) and found that the remaining sample had a P=0.01 (Stage II). When randomization and re-analysis were repeated 100 times, in 86% of the instances it was rejected at least twice, considered a very high degree of internal reproducibility 32. To assess the interaction’s predictive utility, we performed a leave-one-out cross-validation, demonstrating that relative risks calculated from the β-blocker-GRK5 allele interaction Cox Proportional Hazards model were significant predictors of transplant-free survival (P<0.001) with an R2 of 0.039 (Supplemental Table S1). The predictive ability of the GRK5-L41 allele compares favorably to clinical predictors of heart failure outcome (age, sex, hypertension), which had an R2 of 0.023. The R2 increased to 0.05 when these clinical predictors were included in the β-blocker-GRK5 allele interaction model.
DISCUSSION
Multiple polymorphisms within the βAR signaling pathway have been proposed as modifiers of heart failure risk 7,8,27,33. Given the morbidity, mortality and health-care costs of heart failure 34,35, efforts are underway to identify additional genetic markers that will indicate prognosis and guide patient management. Here, we examined the genes encoding GRK5 and GRK2 because they constitute a critical regulatory node for the pathophysiologically important cardiac βAR signaling pathway that has not previously been explored for genotype-phenotype interactions in human heart disease. Thesecandidate genes were particularly attractive since their role in signaling is to modify receptor coupling to G-proteins and downstream adenylyl cyclase, i.e., the important parameters of βAR function that are perturbed in heart failure. Furthermore, GRK5 and GRK2 have the potential to modify signaling through both β1- and β2AR receptor subtypes, as well as other critical receptors in heart failure 16,36. Compared to the highly polymorphic receptors they regulate, we found these two human GRKs to be highly conserved, with only one common non-synonymous polymorphism in GRK5 identified by screening the complete coding sequences of both genes in 192 chromosomes. Interestingly, there is also striking cross-species similarity in amino acid sequence for GRKs among mammals, with 96% identity between human and mouse GRK5, and absolute conservation of Q at position 41 or its analog from humans to zebrafish (Supplemental Figure 1). The rarity of polymorphic variations in human GRK2 and GRK5 and the high degree of sequence conservation between species for GRK5 suggest that minor changes can have significant functional and physiological consequences, as we found for GRK5-L41.
A major function of GRKs is uncoupling of ligand-occupied receptors from signaling effectors, resulting in decreased cellular responsiveness (desensitization) and a time-dependent loss of agonist promoted function at the organ level 9. In human heart failure, increases in myocardial GRK2 activity 17,18 and marked desensitization of cardiac β1- and β2-AR 37 represent endogenous processes that may be acting to protect the heart from high circulating catecholamine levels 38,39. Here, we found a gain-of-function genetic polymorphism of GRK5 that augments βAR desensitization. As expected for a kinase that specifically modulates the agonist-occupied form of G-protein coupled receptors, the GRK5-Q41 and –L41 variants could not be distinguished in the absence of agonist. Indeed, within the African American population where it is fairly common (~40% carry at least one allele), the GRK5-L41 polymorphism also did not alter the risk for developing heart failure. However, GRK5-L41 was markedly more effective than –Q41 in promoting isoproterenol-mediated β-receptor desensitization in transfected cells and transgenic mice, and was associated with prolonged survival in clinical heart failure. The transgenic mouse studies with GRK5-Q41 and -L41, which also showed a β-blocker-like protective effect in the context of chronic catecholamine excess, indicate a major role for direct cardioprotection by this polymorphism since expression was targeted specifically to cardiac myocytes. Taken together, these data suggest that GRK5-L41 acts to attenuate β1AR signaling in a manner similar to partial β1AR antagonism with β-blockers, favoring protection against remodeling and improving survival.
An important feature of the current work is the mechanistic experiments in transfected cells and transgenic mice. These experiments revealed a phenotype that aided in the design of our human studies. Indeed, had analysis been restricted to conventional assessments of risk or survival, the important pharmacogenomic phenotype might have been overlooked. Such mechanistic studies are also crucial in making the case that the polymorphism is the basis of the human phenotype, rather than another locus that is in linkage disequilibrium with GRK5-L41 8. Our findings with this polymorphism in heart failure and acute ischemia, and the observation that its actions are consistent with genetic β-blockade, provide additional insight into inter-individual variation in outcome in these diseases, and suggest alternate monitoring and treatment strategies in individuals with GRK5-L41. For example, in African-Americans with heart failure who carry the GRK5-L41 allele it may be more prudent to maximize treatment focused on other pathways rather than continue to treat aggressively with drugs that may provide less additional benefit in these individuals. Since ~40% of African-Americans carry GRK5-L41, personalization of medical care could impact a large number of individuals suffering from cardiac disease.
METHODS
Study Subjects
Human study protocols were approved by the Institutional Review Boards of the University of Cincinnati and Washington University. Subjects provided written informed consent. Enrollment criteria for the heart failure observational study were: age of 18 to 80 years, left ventricular ejection fraction of less than 40%, and New York Heart Association heart failure class II–IV. Enrollment criteria for the acute coronary ischemia observational study were: age of 18 to 80 years and hospital admission with the confirmed diagnosis of acute myocardial infarction or unstable coronary syndrome. Non-affected controls were recruited from the greater Cincinnati area. Racial classification as Caucasian or African American was self-reported.
In the prospective GRK5-β-blocker interaction study, 402 African American heart failure subjects were consented between May 1, 2000 and June 1, 2006, 242 of whom were also in the observational study. Of these, 383 subjects completed input studies and had blood draw for DNA. GRK5 genotypes were not obtained on five subjects (success rate of 98.7%), and three subjects (0.8%) were lost to follow-up. Genotypes of these three subjects were Q/Q. The primary study endpoint was death or transplantation, with secondary endpoints of death or cardiac transplantation only, with an average follow-up period of 30 months. β-blocker use was defined as continuous therapy for at least 6 months. Decisions regarding β-blocker treatment (73%-carvedilol, 22%-metoprolol, and 5%-others) were made by the subjects’ physicians.
Sequencing and Genotyping
Based on sequences of the human genes (accession numbers NC_000011 and NC_000010) the exons for GRK2 and GRK5 were amplified from genomic DNA using PCR. (primers for GRK5 coding exons are in Supplemental Table S2). Polymorphism discovery and GRK5 genotyping used bi-directional automated sequencing and outputs were aligned with reference sequence using SeqScape v2.5, and variants individually verified by an investigator (RRP). African American heart failure subjects were further genotyped at 13 race-informative short tandem repeat loci 29.
In vitro βAR desensitization studies
Chinese Hamster Ovary (CHO) cells were transfected with cDNAs encoding the human β1AR, and either empty vector, GRK5-Q41 or GRK5-L41. Cell monolayers were treated with isoproterenol for the indicated times at 37°, and cAMP quantitated as previously described 40.
Experimental Heart Failure
Transgenic mice (FVB/N background) were generated using the α–myosin heavy chain (MHC) promoter to express human GRK5-Q41 and L41 using methods similar to those previously described 41. Multiple founders were identified by genomic Southern analysis of tail clip DNA. F1 or F2 mice were sacrificed and myocardial GRK5 content analyzed by immunoblotting with GRK5-specific antisera (Santa Cruz). Mouse lines with equivalent cardiac GRK5-Q41 and GRK5-L41 protein expression were propagated for study. Animals were treated in accordance with approved University of Cincinnati Animal Care and Use Committee protocols.
To assess βAR responsiveness and desensitization, mice underwent left ventricular catheterization 41 during graded infusions of the nonselective β-agonist isoproterenol, 0.01 to 0.32 ng/g/min., and sustained 30 minute infusion of 20 ng/g/min to evoke desensitization 42. Heart failure was induced by chronic isoproterenol infusion by osmotic mini-pump 12. Cardiac remodeling was assessed by transthoracic echocardiography.
Statistical Analysis
Student’s t-tests and chi-square tests were used to assess significant differences in variables between ethnic groups and between genotype classes within ethnic groups. Hardy-Weinberg Equilibrium (HWE) was assessed in each ethnic group separately. The primary clinical endpoint was combined all-cause mortality or cardiac transplant; secondary endpoints were all-cause mortality and cardiac transplantation. Differences in time from diagnosis to endpoint were assessed using Kaplan-Meier curves and Log Rank tests 43. Hazards ratios were obtained by Cox Proportional Hazards modeling 44 using an additive genetic model 45 after adjustment for age at diagnosis, β-blocker usage, hypertension status, and sex. To assess internal reproducibility of the association between GRK5 allele and survival we used an analytical strategy that reduces the probability of type I errors through sequential hypothesis testing 31. The smallest possible sample sizes were used to reject the null hypothesis of no GRK5-β-blocker interaction, and the remaining samples were used to confirm those findings 46. The type I error (α), type II error (β), and effect size D were preset to α=0.05, β =0.8, and D=0.5 to create stopping rules for the procedure where the sample size N is considered a random variable. Starting with n=50, the null hypothesis of no GRK5-β-blocker interaction was tested using the Wald test and then sequentially another individual was added and retested.
To assess the predictive value of Cox proportional hazards models, we utilized leave-one-out cross-validation. Each individual was sequentially left out and a Cox proportional hazards model for time to death or transplant were fitted. Using the coefficients estimated with the n-1 individuals, an overall relative risk was calculated for the individual left out. These relative risks were then used as the predictor in a new Cox proportional hazards model. Because each individual is omitted from the model used to calculate his relative risk, the performance of a model using these relative risks as predictors approximates the predictive ability of the association in an independent sample drawn from the same population. The performance of each model was assessed using overall model significance and Cox Snell R2, a measure of the model’s predictive ability 47. All analyses were carried out using the R Statistical Language 32. Two-tailed tests and an alpha level of 0.05 was used to assess significance. Percent African ancestry was estimated with the program Structure 30.
Acknowledgments
Supported by NHLBI Special Clinical Centers of Research in Heart Failure, P50 HL77101 and HL77113, and by HL87871.
Footnotes
The authors declare that they have no competing interests.
References
- 1.Levy D, et al. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1397–1402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 2.Roger VL, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 3.Franz WM, Muller OJ, Katus HA. Cardiomyopathies: from genetics to the prospect of treatment. Lancet. 2001;358:1627–1637. doi: 10.1016/S0140-6736(01)06657-0. [DOI] [PubMed] [Google Scholar]
- 4.The Merit HF Investigators. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF) Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- 5.BEST Trial Investigators. A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med. 2001;344:1659–1667. doi: 10.1056/NEJM200105313442202. [DOI] [PubMed] [Google Scholar]
- 6.van Campen LC, Visser FC, Visser CA. Ejection fraction improvement by beta-blocker treatment in patients with heart failure: an analysis of studies published in the literature. J Cardiovasc Pharmacol. 1998;32(Suppl 1):S31–S35. doi: 10.1097/00005344-199800003-00006. [DOI] [PubMed] [Google Scholar]
- 7.Wagoner LE, et al. Polymorphisms of the beta(2)-adrenergic receptor determine exercise capacity in patients with heart failure. Circ Res. 2000;86:834–840. doi: 10.1161/01.res.86.8.834. [DOI] [PubMed] [Google Scholar]
- 8.Liggett SB. Pharmacogenetic applications of the Human Genome project. Nat Med. 2001;7:281–283. doi: 10.1038/85411. [DOI] [PubMed] [Google Scholar]
- 9.Kohout TA, Lefkowitz RJ. Regulation of G protein-coupled receptor kinases and arrestins during receptor desensitization. Mol Pharmacol. 2003;63:9–18. doi: 10.1124/mol.63.1.9. [DOI] [PubMed] [Google Scholar]
- 10.Koch WJ, et al. Cardiac function in mice overexpressing the beta-adrenergic receptor kinase or a beta ARK inhibitor. Science. 1995;268:1350–1353. doi: 10.1126/science.7761854. [DOI] [PubMed] [Google Scholar]
- 11.Koch WJ. Genetic and phenotypic targeting of beta-adrenergic signaling in heart failure. Mol Cell Biochem. 2004;263:5–9. doi: 10.1023/B:MCBI.0000041843.64809.48. [DOI] [PubMed] [Google Scholar]
- 12.Matkovich SJ, et al. Cardiac-specific ablation of G-protein receptor kinase 2 redefines its roles in heart development and beta-adrenergic signaling. Circ Res. 2006;99:996–1003. doi: 10.1161/01.RES.0000247932.71270.2c. [DOI] [PubMed] [Google Scholar]
- 13.Premont RT, Koch WJ, Inglese J, Lefkowitz RJ. Identification, purification, and characterization of GRK5, a member of the family of G protein-coupled receptor kinases. J Biol Chem. 1994;269:6832–6841. [PubMed] [Google Scholar]
- 14.Gainetdinov RR, et al. Muscarinic supersensitivity and impaired receptor desensitization in G protein-coupled receptor kinase 5-deficient mice. Neuron. 1999;24:1029–1036. doi: 10.1016/s0896-6273(00)81048-x. [DOI] [PubMed] [Google Scholar]
- 15.Chen EP, Bittner HB, Akhter SA, Koch WJ, Davis RD. Myocardial function in hearts with transgenic overexpression of the G protein-coupled receptor kinase 5. Ann Thorac Surg. 2001;71:1320–1324. doi: 10.1016/s0003-4975(00)01754-9. [DOI] [PubMed] [Google Scholar]
- 16.Rockman HA, et al. Receptor-specific in vivo desensitization by the G protein-coupled receptor kinase-5 in transgenic mice. Proc Natl Acad Sci U S A. 1996;93:9954–9959. doi: 10.1073/pnas.93.18.9954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ungerer M, Bohm M, Elce JS, Erdmann E, Lohse MJ. Altered expression of beta-adrenergic receptor kinase and beta 1-adrenergic receptors in the failing human heart. Circulation. 1993;87:454–463. doi: 10.1161/01.cir.87.2.454. [DOI] [PubMed] [Google Scholar]
- 18.Ungerer M, et al. Expression of beta-arrestins and beta-adrenergic receptor kinases in the failing human heart. Circ Res. 1994;74:206–213. doi: 10.1161/01.res.74.2.206. [DOI] [PubMed] [Google Scholar]
- 19.Oyama N, et al. Angiotensin converting enzyme inhibitors attenuated the expression of G-protein coupled receptor kinases in heart failure patients. Circ J. 2006;70:362–363. doi: 10.1253/circj.70.362. [DOI] [PubMed] [Google Scholar]
- 20.Dzimiri N, Basco C, Moorji A, Afrane B, Al Halees Z. Characterization of lymphocyte beta 2-adrenoceptor signalling in patients with left ventricular volume overload disease. Clin Exp Pharmacol Physiol. 2002;29:181–188. doi: 10.1046/j.1440-1681.2002.03625.x. [DOI] [PubMed] [Google Scholar]
- 21.Liggett SB, et al. Early and delayed consequences of beta(2)-adrenergic receptor overexpression in mouse hearts: critical role for expression level. Circulation. 2000;101:1707–1714. doi: 10.1161/01.cir.101.14.1707. [DOI] [PubMed] [Google Scholar]
- 22.Asai K, et al. Beta-adrenergic receptor blockade arrests myocyte damage and preserves cardiac function in the transgenic G(salpha) mouse. J Clin Invest. 1999;104:551–558. doi: 10.1172/JCI7418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okumura S, et al. Disruption of type 5 adenylyl cyclase gene preserves cardiac function against pressure overload. Proc Natl Acad Sci U S A. 2003;100:9986–9990. doi: 10.1073/pnas.1733772100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bristow MR. beta-adrenergic receptor blockade in chronic heart failure. Circulation. 2000;101:558–569. doi: 10.1161/01.cir.101.5.558. [DOI] [PubMed] [Google Scholar]
- 25.Packer M. Current role of beta-adrenergic blockers in the management of chronic heart failure. Am J Med. 2001;110(Suppl 7A):81S–94S. doi: 10.1016/s0002-9343(01)00676-3. [DOI] [PubMed] [Google Scholar]
- 26.Waagstein F, et al. Beneficial effects of metoprolol in idiopathic dilated cardiomyopathy. Metoprolol in Dilated Cardiomyopathy (MDC) Trial Study Group. Lancet. 1993;342:1441–1446. doi: 10.1016/0140-6736(93)92930-r. [DOI] [PubMed] [Google Scholar]
- 27.Liggett SB, et al. A polymorphism within a conserved beta(1)-adrenergic receptor motif alters cardiac function and beta-blocker response in human heart failure. Proc Natl Acad Sci U S A. 2006;103:11288–11293. doi: 10.1073/pnas.0509937103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Small KM, Wagoner LE, Levin AM, Kardia SL, Liggett SB. Synergistic polymorphisms of beta1- and alpha2C-adrenergic receptors and the risk of congestive heart failure. N Engl J Med. 2002;347:1135–1142. doi: 10.1056/NEJMoa020803. [DOI] [PubMed] [Google Scholar]
- 29.Barnholtz-Sloan JS, Chakraborty R, Sellers TA, Schwartz AG. Examining population stratification via individual ancestry estimates versus self-reported race. Cancer Epidemiol Biomarkers Prev. 2005;14:1545–1551. doi: 10.1158/1055-9965.EPI-04-0832. [DOI] [PubMed] [Google Scholar]
- 30.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bechhoffer RE, Kiefer J, Sobel M. Sequential Identification and Ranking Proceedures. University of Chicago Press; Chicago: 1986. [Google Scholar]
- 32.R Develpoment Core Team. R: A language and environment for statistical computing. (2.3.0). 2005. R foundation for statistical computing. Ref Type: Computer Program
- 33.Liggett SB. Beta-adrenergic receptors in the failing heart: the good, the bad, and the unknown. J Clin Invest. 2001;107:947–948. doi: 10.1172/JCI12774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lloyd-Jones DM, et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068–3072. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 35.Thom T, et al. Heart disease and stroke statistics--2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 36.Kim J, et al. Functional antagonism of different G protein-coupled receptor kinases for beta-arrestin-mediated angiotensin II receptor signaling. Proc Natl Acad Sci U S A. 2005;102:1442–1447. doi: 10.1073/pnas.0409532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Port JD, Bristow MR. Altered beta-adrenergic receptor gene regulation and signaling in chronic heart failure. J Mol Cell Cardiol. 2001;33:887–905. doi: 10.1006/jmcc.2001.1358. [DOI] [PubMed] [Google Scholar]
- 38.Cohn JN, et al. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med. 1984;311:819–823. doi: 10.1056/NEJM198409273111303. [DOI] [PubMed] [Google Scholar]
- 39.Bristow MR. Why does the myocardium fail? Insights from basic science. Lancet. 1998;352(Suppl 1):SI8–14. doi: 10.1016/s0140-6736(98)90311-7. [DOI] [PubMed] [Google Scholar]
- 40.Liggett SB, et al. Altered patterns of agonist-stimulated cAMP accumulation in cells expressing mutant beta 2-adrenergic receptors lacking phosphorylation sites. Mol Pharmacol. 1989;36:641–646. [PubMed] [Google Scholar]
- 41.D’Angelo DD, et al. Transgenic Galphaq overexpression induces cardiac contractile failure in mice. Proc Natl Acad Sci U S A. 1997;94:8121–8126. doi: 10.1073/pnas.94.15.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Odley A, et al. Regulation of cardiac contractility by Rab4-modulated beta2-adrenergic receptor recycling. Proc Natl Acad Sci U S A. 2004;101:7082–7087. doi: 10.1073/pnas.0308335101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaplan EL, Meier Paul. Nonparametric Estimation from Incomplete Observations. Journal of the American Statistical Association. 2006;53:457–481. [Google Scholar]
- 44.Parmar M, Machin D. Survival Analysis: A practical approach. John Wiley & Sons; 1995. [Google Scholar]
- 45.Lynch M, Walsh B. Genetics and Analysis of Quantative Traits. Sinauer Associates; 1998. [Google Scholar]
- 46.Province MA. A single, sequential, genome-wide test to identify simultaneously all promising areas in a linkage scan. Genet Epidemiol. 2000;19:301–322. doi: 10.1002/1098-2272(200012)19:4<301::AID-GEPI3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 47.Cox DR, snell EJ. Analysis of Binary Data. Chapman & Hall; 1989. [Google Scholar]