Abstract
Introduction
Intratympanic drug delivery has become widely used in the clinic but little is known about how clinically-utilized drug preparations affect round window membrane permeability or how much drug is actually delivered to the cochlea. This study evaluated the effect of clinically relevant carrier solutions and of suction near the round window membrane (RWM) on the permeability properties of the RWM.
Methods
RWM permeability was assessed by perfusion of the marker TMPA into the round window niche while monitoring entry into perilymph using TMPA-selective electrodes sealed into scala tympani.
Results
High osmolarity solution increased RWM permeability by a factor of 2 to 3, benzyl alcohol (a preservative used in some drug formulations) increased permeability by a factor of 3 to 5, and suctioning near the RWM increased permeability by a factor of 10 to 15.
Conclusions
Variations in available drug formulations can potentially alter RWM permeability properties and affect the amount of drug delivered to the inner ear. Drug solution osmolarity, benzyl alcohol content and possible drying of the round window membrane during suctioning the middle ear can all have a substantial influence of the perilymph levels of drug achieved.
Introduction
Treatment of cochlear disorders represents a promising future in otology but the underlying pathophysiology of common diseases such as Meniere’s syndrome and sensorineural hearing loss remain poorly understood. Treatment methods for inner ear disorders, where available, rely largely on delivery of medication to the round window at concentrations and through delivery mechanisms dictated mostly by convenience and anecdotal.1 Agents delivered to the human cochlea through the round window include gentamicin for the treatment of Meniere’s syndrome and steroids for the treatment of sudden sensorineural hearing loss, Meniere’s syndrome, and tinnitus.2–6 A variety of compounds delivered to the round window of animals have shown efficacy in prevention of ototoxicity and age related sensorineural hearing loss, but await application in humans.7–10 In the future, cochlear gene therapy and support of stem cell transplants will likely require delivery of drugs and growth factors to the fluid spaces of the inner ear.11–13
Unanswered questions of immediate clinical significance include establishment of the most efficacious round window delivery protocol: single bolus injection, repeated bolus injection, wick systems, or round window irrigation.14,15 The application of a drug and its carrying solution alters the appearance of the round window on histopathology16 and possibly affects the permeability of the round window to future drug application. Thus, the pharmacokinetics of the first bolus injection of drug to the middle ear may be significantly different from the pharmacokinetics of subsequent bolus injections despite identical drug concentrations and volumes. Benzyl alcohol, which is present in all three clinically used commercially available intratympanic steroid solutions (dexamethasone, methylprednisolone, triamcinolone) is known to be cytotoxic but its potential effects on the round window membrane have never been assessed. The possible effect of manipulations in and around the round window, such as advocated by some authors for clearance of adhesions from the round window niche, or contact of a wick against the round window4,17 has also never been evaluated. Understanding the influences of application protocols, background solution composition, and round window manipulations is essential for round window delivery regimens to be optimized. Studies indicate that RWM permeability may vary significantly between individual animals.18 Some of this variation may be a result of differences in round window membrane thickness.19,20 Substantial differences in RWM thickness also exist between humans 70 μm,20,21 and rodents, with thicknesses of 10 to 14 μm for the chinchilla,19 and 12 μm for rats22 and 10–30 μm for guinea pigs.23 If RWM permeability to drugs is related to thickness, these inter-species differences would affect the extrapolation of animal data to human drug delivery paradigms. Identification of a round window manipulation or carrying agent which decreases interanimal or interspecies variability would have significant clinical application.
Methods
In this study we have used the ionic marker trimethylphenylammonium (TMPA) to detect and quantify changes in RWM permeability. TMPA-selective microelectrodes sealed into scala tympani were used to monitor the rate of entry of TMPA into the scala continuously with time (Figure 1). TMPA entry rates were monitored before and after experimental treatments, allowing the effects of a treatment on RWM permeability to be assessed in individual animals and minimizing the influence of inter-animal variations. The TMPA marker was maintained at constant concentration on the RWM by continuous irrigation, simplifying the interpretation and modeling of the data. The methods have been previously detailed elsewhere.24
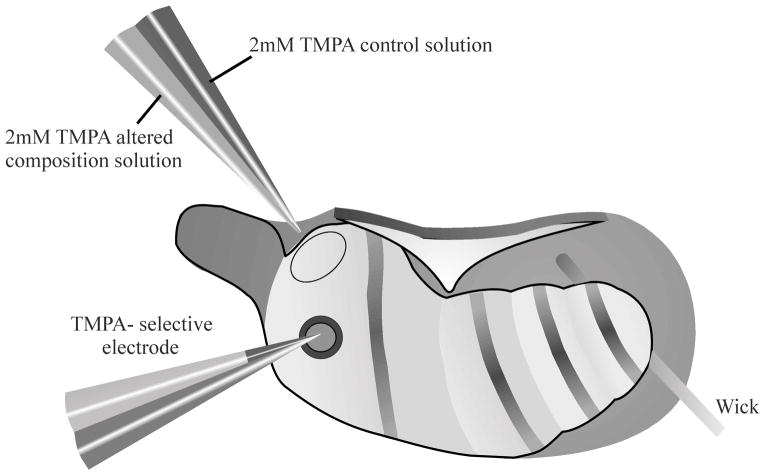
Figure 1.
Schematic diagram showing experimental protocol utilizing ventral approach to the right ear of a guinea pig. TMPA was irrigated across the RWM by a double barreled pipette, allowing remote switching of solution perfused, with excess fluid removed by a wick. The rate of entry of TMPA into scala tympani was measured by sealing a calibrated double-barreled TMPA-selective microelectrode into the basal turn of scala tympani. Cyanoacrylate and silicon glues were used to seal the electrode in place.
Animal preparation
Experiments were performed on pigmented NIH-strain guinea-pigs (Cavia porcellus) of both sexes and weighing 250 – 500 grams supplied from an in-house breeding colony. All recordings were performed as acute procedures on animals surgically anesthetized with Inactin (sodium thiobutabarbital, dose: 100 mg/kg IP). Animals were artificially respired via a tracheal cannula, setting tidal volume to maintain 4–5% end-tidal CO2. Rectal temperature was maintained at 38°C with a DC-powered, thermistor-controlled heating pad. Oxygen was monitored with a pulse-ox unit and heart rate was monitored acoustically. The left jugular vein was cannulated for the administration of supplemental anesthetic doses. The right cochlea was exposed by a ventrolateral approach, leaving middle ear function intact. During electrophysiologic recordings, pancuronium bromide was given to reduce myogenic recording artifacts. At the end of the experiments animals were euthanized with a lethal dose of potassium chloride (0.5 ml, 2 M KCl), given while deeply anesthetized. Protocols used in this study were approved by the Animal Studies Committee of Washington University (Approval number 20060031).
Ion electrode techniques
Measurements of TMPA concentration in perilymph were made with double-barreled TMPA-selective electrodes. TMPA allows concentration versus time to be measured, as opposed to single concentration measurements achieved at one time point with sampling techniques. TMPA can be detected at lower, non-toxic concentrations than other quarternary ammonium markers and passes through the round window membrane more readily, allowing it to be detected in scala tympani. Electrodes were made from glass, cleaned and drawn into a capillary in our own laboratory to ensure uniformity of properties. After the tip was pulled, one barrel of the electrode was silanized by exposure to dimethyldichlorosilane vapor followed by baking for 30 minutes at 120 °C. The silanized (ion) side was filled with 0.5 M KCl and the non-silanized (reference) side was filled with 0.5 M NaCl. The electrode tip was beveled to a diameter of 2 – 4 μm, after which a 200–400 μm column of ion exchanger was drawn into the ion side. Electrodes were connected to a dual channel electrometer with input impedance > 1014 Ohms and a concentration dependent potential was recorded differentially between the ion and reference barrels of the electrode. Electrodes were individually calibrated using 5 standard solutions of (0, 2, 20, 200, and 2000 μM) in a background of artificial perilymph (see below) maintained at 39 °C in a water-jacketed calibration chamber.
Electrode sealing procedures
Perforations of the bony otic capsule can result in perilymph being displaced from the cochlea by CSF entering through the cochlear aqueduct. Therefore, accurate perilymphatic measurements required that electrodes be sealed into the scala in a manner that avoids all perilymph leakage. We used a very reliable method for sealing electrodes into the scalae in which the otic capsule was first thinned over the basal turn of scala tympani. After the bone had dried a layer of thin cyanoacrylate glue was applied to the intact bone, followed by a layer of 2-part silicone adhesive (WPI Kwik-cast) which formed a “donut” around the area of thinned bone. A small (30 – 50 μm diameter) fenestra was made in the thinned, hydrophobic area using a 30 degree, 1/3 mm House oval window pick (Storz). Fluid, temporarily escaping from the fenestra forms a bead and does not wet the hydrophobic surface. After the pipette is inserted into the scala, the fluid droplet is wicked away and a small drop of cyanoacrylate is applied. This sets immediately on contact with perilymph and seals the electrode to the silicone. With this method, we were confident that no fluid leakage could occur at the insertion site.
Solutions
The solutions irrigated across the RWM contained 2 mM TMPA in an artificial perilymph with final composition (mM) NaCl (127.5), KCl (3.5), NaHCO3 (25), CaCl2 (1.3), MgCl2 (1.2), NaH2PO4 (0.75), Glucose (11). The artificial perilymph was initially made up “double-strength” so that a pair of control and altered-composition solutions with identical TMPA content could readily be made. In each case, a fixed volume (2 ml) of 100 mM TMPA chloride was added to the double strength artificial perilymph. Benzyl alcohol or mannitol optionally added as required for the altered composition solution and then water added to make up the final volume. A low-osmolarity solution was made by diluting the “double-strength” artificial perilymph 6.1:3.9 with water, before adding TMPA to ensure an unchanged TMPA level. Altered composition solutions included:1) High osmolarity solution of 620 mOsm made by adding 320mM Mannitol; 2) Low osmolarity solution of 190 mOsm; 3) solution containing 10 mg/ml benzyl alcohol. A Harvard model 55-2226 programmable pump was used to irrigate solutions across the RWM at a rate of 5 μL/min. A double barreled perfusion pipette was utilized, allowing change of solution to occur from outside the electrically insulated testing chamber. Absorbent wicks were placed to prevent fluid accumulation in the bulla.
In each experiment, the baseline entry rate of TMPA entry into ST was monitored from the TMPA-selective microelectrode sealed into the scala during a 40 minute irrigation period with TMPA in normal artificial perilymph. The irrigation solution was then changed to one with identical TMPA concentration, but of altered background composition. Under this protocol a total of 48 animals were tested, including technical failures. Successful experiments with complete data sets include 10 mg/ml benzyl alcohol (n=7), hypertonic (620 mOsm) (n=7), hypotonic (190 mOsm) (n=2), calcium-free (0.2mg/mL EDTA, n=5) and controls (normal artificial perilymph in both pipettes, n=3).
Data interpretation
Results were interpreted by simulating the experimental protocol with the finite-element cochlear fluids simulation program, available at http://oto.wustl.edu/cochlea/. The diffusion coefficient used for TMPA was 1.01 × 10−9 m2/s. The entry of TMPA into ST was simulated using a RW baseline TMPA permeability of 5 × 10−8 m/s, based on permeability values obtained previously by using similar surgical protocols.1,25
Results
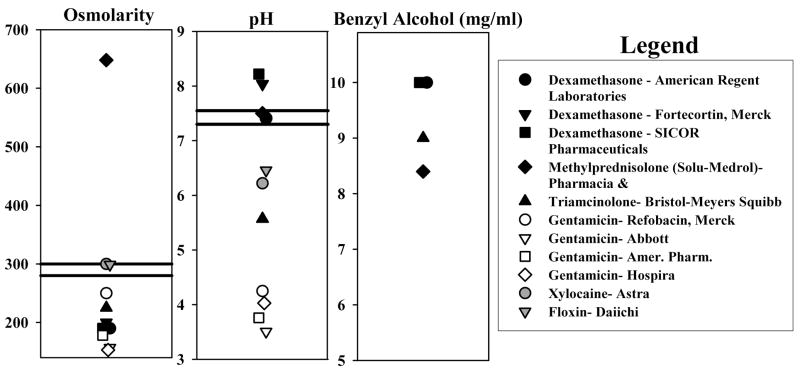
The composition of drug preparations that are commonly used for local application to the middle ear of patients was surveyed. We measured pH and osmolarity, and determined whether the preparation contained benzyl alcohol (which is used as a preservative) based on what was stated in the packaging. Figure 2 shows the variations in composition in a number of drug formulations. Gentamicin (open symbols) was typically at very acid pH and low osmolarity. Glucocorticoids (solid symbols) were mostly found to be near-normal pH but sometimes at non-physiologic osmolarity. The most commonly used methylprednisolone preparation had an osmolarity of over 650 mOsm. The majority of glucocorticoid preparations contained benzyl alcohol at a concentration of up to 10 mg/ml.
Figure 2.
Composition of drug preparations that are widely used for application to the middle ears of patients. Osmolarity and pH were measured while benzyl alcohol content (used as a preservative) was based on what was stated in the packaging.
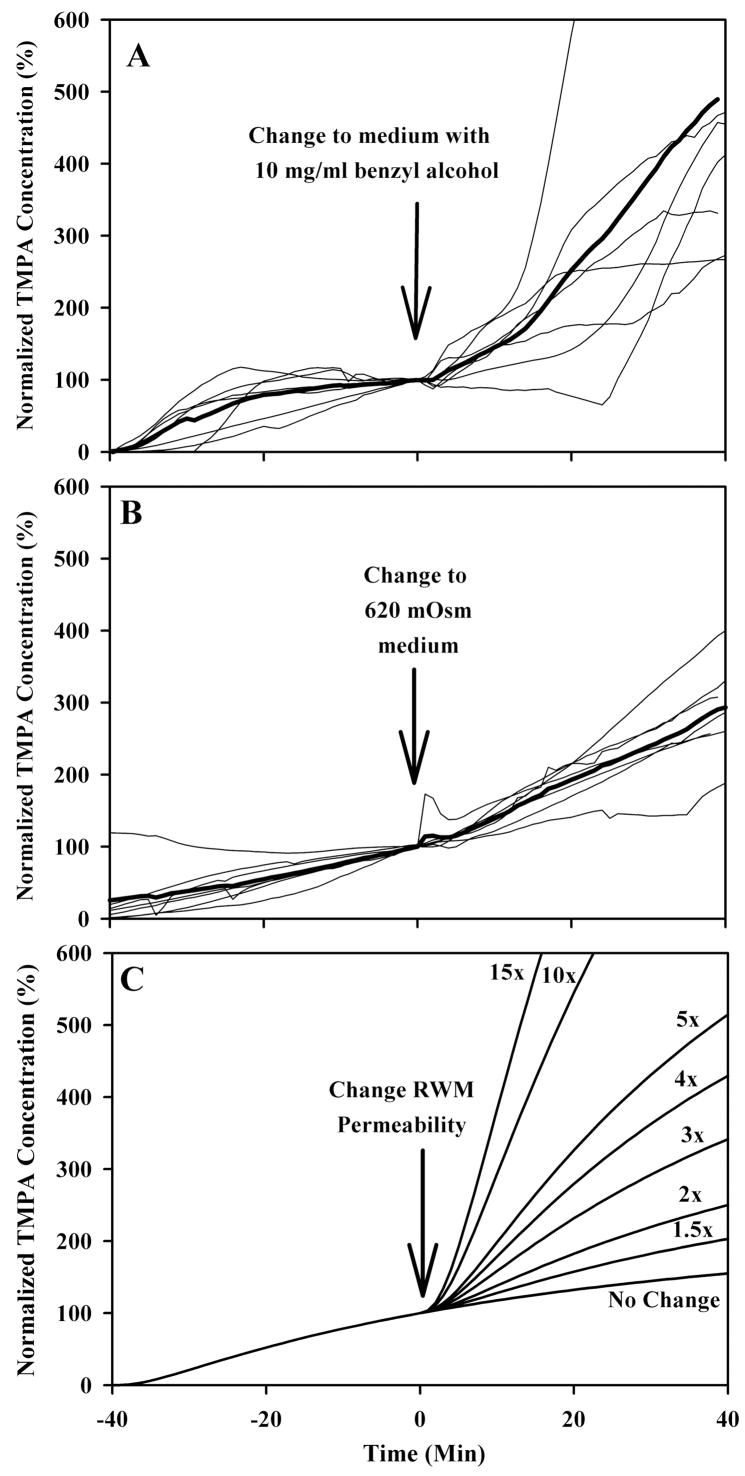
When a 2 mM TMPA solution was irrigated across the RWM, the measured TMPA concentration in ST increased with time as TMPA entered the scala through the RWM to reach a steady-state concentration. The fluid manipulation that produced the most dramatic change in TMPA entry was the addition of 10 mg/ml benzyl alcohol to the medium, as shown in Figure 3. In this figure, the TMPA concentrations have each been normalized relative to the concentration present just prior to changing solutions (and designated as time 0). The rate of marker entry, which is stable or slowly increasing prior to the solution change, increased when benzyl alcohol was in the medium, usually with a delay of 10–20 minutes. Similar, but less dramatic, results occurred when the applied solution osmolarity was increased to 620 mOsm, as shown in Figure 3B. These data have been similarly amplitude normalized and are shown with identical time and amplitude scaling. An indication of the likely magnitude of permeability changes is given by calculating time courses resulting from RWM permeability changes of a specific magnitude using a finite element computer model (oto.wustl.edu/cochlea/), as shown in Figure 3C. From these curves we estimate that benzyl alcohol increased RWM permeability by a factor of 3 to 5 and elevating osmolarity to 620 mOsm increased permeability by a factor of 2 to 3.
Figure 3.
Light traces in panel A and B show individual experiments in which TMPA concentration was measured in the basal turn of scala tympani. For 40 minutes prior to time zero, TMPA in artificial perilymph was irrigated across the round window membrane. At time zero, the solution irrigated across the round window membrane was changed to a different composition, as indicated, but containing an identical TMPA concentration. Measured TMPA concentrations were normalized with respect to the concentration in scala tympani prior to the solution change. The mean curve of the group is shown as a heavy line. In panel C, a finite-element model was used to calculate the change of TMPA entry rate resulting from varying amounts of round window membrane permeability change. By comparison with the measured curves in panels A and B, it is estimated that benzyl alcohol increases round window membrane permeability by a factor of 3 to 5 times and high osmolarity by a factor of 2 to 3 times.
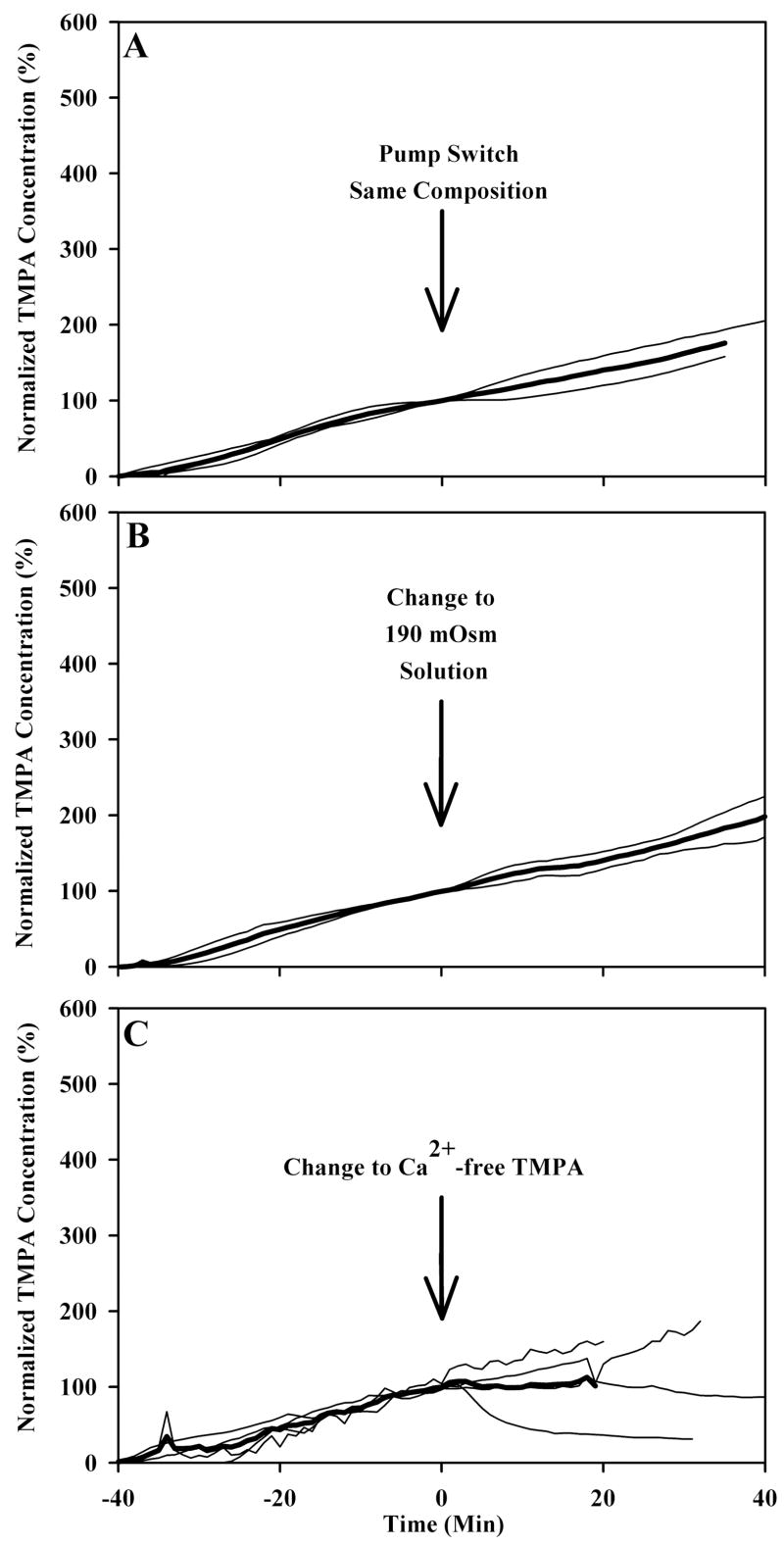
Several manipulations did not significantly alter TMPA movement across the RWM. Figure 4A shows that switching irrigation from one to the other barrel of a double barreled perfusion pipette resulted in no change in TMPA entry rate. Figure 4B and 4C show that changing to a low osmolarity solution and calcium free solution, respectively, also had minimal effect on TMPA entry rate. A low calcium solution was investigated because such solutions are known to impair intercellular junctions and are widely used to dissociate tissues for in vitro preparations. If the permeability of the round window membrane depends on the maintenance of tight junctions between adjacent cells, we hypothesized that reduced calcium solutions, by disrupting intercellular tight junctions, would allow greater paracellular solute movements and allow greater drug entry into the scala tympani.
Figure 4.
Several manipulations resulted in no alteration of TMPA entry across the round window. Light traces in panel A, B, and C show individual experiments in which TMPA concentration was measured in the basal turn of scala tympani. For 40 minutes prior to time zero, TMPA in artificial perilymph was irrigated across the round window membrane. At time zero, in panel A, the identical solution was perfused through the second barrel in the double barreled pipette to serve as a control. In panels B and C, at time zero the solution irrigated across the round window membrane was changed to a different composition, as indicated, but containing an identical TMPA concentration. Measured TMPA concentrations were normalized with respect to the concentration in scala tympani prior to the solution change. The mean curve of the group is shown as a heavy line.
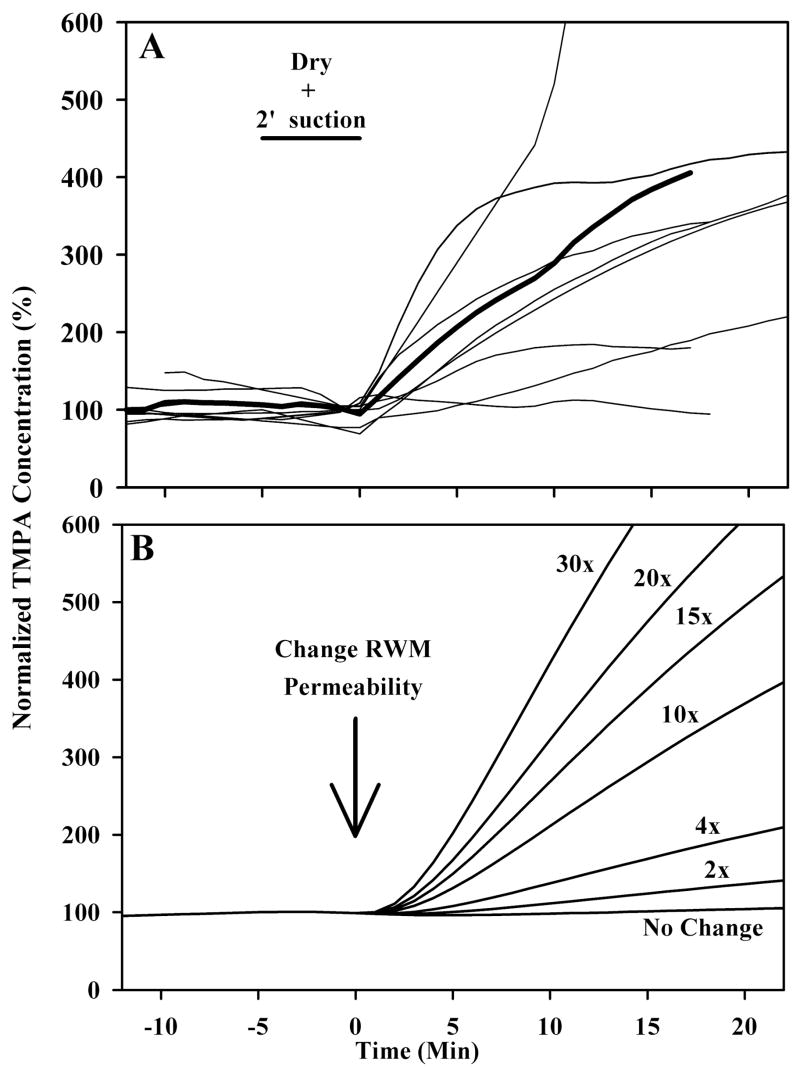
At the end of selected experiments, we also investigated the influence of applying suction in the middle ear on RWM permeability as shown in Figure 5. After the irrigating solution was turned off, a #3 French suction was placed near the RW niche, avoiding direct trauma to the RW membrane, and suction was applied for a period of 2 minutes. The resulting passage of air over the round window resulted in a drying effect. The irrigating solution was then restored, designated in the figures as time zero. It is apparent that the passage of air near the RWM produced by the suction had a substantial influence on the rate of TMPA entry, with large increases of entry rate seen in most animals. It should be emphasized that most of these animals had previously been used in the above solution-change experiments, so the baseline prior to suctioning includes animals with elevated permeability due to solution changes. Nevertheless, in all but one case, there is an immediate, substantial increase in the rate of TMPA entry after suctioning the middle ear. A comparison of the measured data with time courses was calculated using the computer model to demonstrate specific permeability changes is shown in Figure 5B. Based on the magnitude of the changes measured experimentally, it can be estimated that suctioning typically increased RWM permeability by a factor of 10 to 15.
Figure 5.
In panel A, light traces show individual experiments in which the round window membrane was exposed to either control or permeability-altering treatments. Solution was removed from the bulla and suction was applied to the round window niche for 2 minutes, and then TMPA containing solution was reapplied to the bulla. The mean is shown by the dark line. In panel B, a finite-element model was used to calculate entry rates with varying degrees of round window membrane permeability change. By comparison with the measured curves in panel A, it is estimated that drying due to suction increases round window membrane permeability by a factor of 3 to 5 times and high osmolarity by a factor of 10 to 15 times.
Discussion
Drugs applied to the middle ear with the intention of delivering them to the inner ear are typically used as “off-label” applications of the drug, with formulations that are not specifically intended for intratympanic use. Their compositions vary in pH and osmolarity and may contain substantial amounts of benzyl alcohol as a preservative. When given intravenously, these drugs rapidly mix with a larger blood volume, but when given intratympanically the non-physiologic solution may remain in contact with the RWM and other tissues of the middle ear for a prolonged period. Our findings demonstrate that RWM permeability is highly sensitive to physiologic insults, with resultant alteration of its permeability. Drying, non-physiologic solutions and preservatives increased permeability and resulted in higher amounts of marker ion entering the inner ear. Based on the results of the current study, we can appreciate that the amount of locally-applied drug entering the cochlea may depend on the drug formulation, presence of preservatives and on specific aspects of the surgical protocol, such as the use of suction in the middle ear. In some applications, such as with glucocorticoids, the use of protocols or formulations that permeabilize the RWM may allow higher drug levels and perhaps more efficacious results to be achieved. In other applications, such as the use of gentamicin to treat Meniere’s disease, there is the potential for specific aspects of the protocol to cause higher doses and increased cochleotoxicity. Thus, a careful definition of both the application formulation and delivery protocol should be established. We cannot yet conclude whether more consistent results will be obtained by avoiding trauma to the RWM or by intentionally permeabilizing it by some protocol such as the drying by suction scheme used here. In animals given intratympanic gentamicin either as the commercial solution or made up at the same concentration in an artificial perilymph solution, morphological changes were reported to be greater in animals receiving the drug as the commercial solution,26 suggesting a higher drug entry rate with the non-physiologic solution. Our data support the conclusion that the background composition of a drug formulation may play a significant role in the drug level achieved and in the results of the drug treatment. It is important to note that significant inter-subject variability exists (Figures 3 to 5) and has been seen previously in studies quantifying drug entry with RWM applications18. Large variation remained even after experimental manipulations. The variability is likely caused by inherent variations in round window permeability, possibly associated with variations in thickness20 or cellular permeability properties between individuals. Subtle differences in gas composition or humidity caused by opening the bulla, with different degrees of drying of the RWM from one animal to the next, could also contribute to the variation.
In the present study we have no measurements of the time course of induced permeability changes and how quickly the changes recover. It has been suggested that manipulations of the RWM which initially increase permeability subsequently result in thickening and scarring21 which could reduce permeability for a second or third attempt at inner ear drug delivery some days later. Also, increased round window permeability may make the ear more susceptible to toxins that are present in a non-sterile middle ear space.
Current inner ear drug delivery mechanisms include transtympanic injection by the physician, placement of a tympanostomy tube, with or without a wick, allowing patient self treatment, and a variety of catheter devices which enable steady infusion of drug but can require a trip to the operating room.2,6,17,27 The most commonly employed method of drug delivery to the round window appears to be transtympanic injection, likely due to a low incidence of complications such as tympanic membrane perforation and the inherent simplicity of the procedure for patient and physician. However, the kinetics of drug delivery through the round window and cochlear fluids remain poorly defined. Recent advances in cochlear fluids computer modeling and new in vivo cochlear sampling techniques have helped elucidate basic mechanisms such as drug diffusion and clearance from the cochlear fluids.6,28 A large multicenter trial is currently being conducted to determine the role of bolus injections of transtympanic methylprednisolone in the treatment of sudden sensorineural hearing loss (Rauch SD, personal communication). Methylprednisolone, dexamethasone, and trimacinolone are the most commonly delivered steroids to the inner ear. Methylprednisolone and dexamethasone are popular in the United States, while triamcinolone has been used in Europe during hearing preservation cochlear implantation. The efficacy of the various agents remains disputed as does the appropriate concentration for delivery to the round window.1,29,30 Triamcinolone is a suspension with particles in the 1–5 micrometer size range (freshpatents.com), which are too large to cross the round window membrane. However, deposition of some of these particles onto the round window may allow for a time-release type mechanism of steroid delivery as the particles slowly dissolve.
In this study, a constant composition was maintained in the RW niche by perfusion because results from such a delivery paradigm can be quantified in terms of RWM permeability changes. However, it differs from the most used clinical protocol of intratympanic injection of a drug bolus. With intratympanic injections, a constant drug level on the RWM cannot be assumed, as drug is progressively cleared from the middle ear space. Experiments with bolus application of marker are currently under way and will establish whether comparable RWM permeability changes occur in a clinically-relevant protocol, and will provide an initial assessment of middle ear kinetics of an applied substance under varying delivery conditions. This is an important issue that is not just relevant to our experiments but is important for the field of local drug delivery to the cochlea as a whole. If the drug level at the RWM can be maintained more consistently by a given protocol, then more quantitative drug delivery to the ear would be possible.
Our conclusions assume that treatments that increase TMPA entry would increase the entry rate of other substances. Hahn et al18 used microdialysis to quantify the entry of two substances simultaneously (dexamethasone and fluorescein). There was a correlation between entry rates for the two substances, with animals showing the highest rate of entry of dexamethasone, also showing the highest rate of fluorescein entry.
Conclusions
The delivery of drug across the round window membrane into the cochlea is dependent not only on applied drug concentration and duration of drug application, but also on the composition of the carrier solution and pretreatment of the round window. It may be possible to selectively alter round window membrane permeability to enhance the efficacy of inner ear drug delivery.
Acknowledgments
American Otological Society (AAM), R01 DC01368 (ANS) from NIH/NIDCD
This work was supported by a grant from the American Otological Society to AAM, and R01 DC01368 (ANS) from NIH/NIDCD.
References
- 1.Salt AN, Plontke SK. Local inner-ear drug delivery and pharmacokinetics. Drug Discov Today. 2005;10:1299–306. doi: 10.1016/S1359-6446(05)03574-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jackson LE, Silverstein H. Chemical perfusion of the inner ear. Otolaryngol Clin North Am. 2002;35:639–53. doi: 10.1016/s0030-6665(02)00023-3. [DOI] [PubMed] [Google Scholar]
- 3.Light JP, Silverstein H, Jackson LE. Gentamicin perfusion vestibular response and hearing loss. Otol Neurotol. 2003;24:294–8. doi: 10.1097/00129492-200303000-00027. [DOI] [PubMed] [Google Scholar]
- 4.Silverstein H, Lewis WB, Jackson LE, Rosenberg SI, Thompson JH, Hoffmann KK. Changing trends in the surgical treatment of Meniere’s disease: results of a 10-year survey. Ear Nose Throat J. 2003;82:185–7. 191–4. [PubMed] [Google Scholar]
- 5.Rauch SD. Intratympanic steroids for sensorineural hearing loss. Otolaryngol Clin North Am. 2004;37:1061–74. doi: 10.1016/j.otc.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Plontke S, Lowenheim H, Preyer S, Leins P, Dietz Koitschev A, Zimmermann R, Zenner HP. Outcomes research analysis of continuous intratympanic glucocorticoid delivery in patients with acute severe to profound hearing loss: basis for planning randomized controlled trials. Acta Otolaryngol. 2005;125:830–9. doi: 10.1080/00016480510037898. [DOI] [PubMed] [Google Scholar]
- 7.Feghali JG, Liu W, Van De Water TR. L-n-acetyl-cysteine protection against cisplatin-induced auditory neuronal and hair cell toxicity. Laryngoscope. 2001;111:1147–55. doi: 10.1097/00005537-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Li G, Frenz DA, Brahmblatt S, Feghali JG, Ruben RJ, Berggren D, Arezzo J, Van De Water TR. Round window membrane delivery of l-methionine provides protection from cisplatin ototoxicity without compromising chemotherapeutic efficacy. Neurotoxicol. 2001;22:163–176. doi: 10.1016/s0161-813x(00)00010-3. [DOI] [PubMed] [Google Scholar]
- 9.Light JP, Silverstein H. Transtympanic perfusion: indications and limitations. Curr Opin Otolaryngol Head Neck Surg. 2004;12:378–83. doi: 10.1097/01.moo.0000134438.91734.38. [DOI] [PubMed] [Google Scholar]
- 10.Seidman MD, Vivek P. Intratympanic treatment of hearing lows with novel and traditional agents. Otolaryngol Clin North Am. 2004;37:973–00. doi: 10.1016/j.otc.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Van de Water TR, Staecker H, Halterman MW, Federoff HJ. Gene therapy in the inner ear. Mechanisms and clinical implications. Ann N Y Acad Sci. 1999;884:345–60. doi: 10.1111/j.1749-6632.1999.tb08653.x. [DOI] [PubMed] [Google Scholar]
- 12.Maiorana CR, Staecker H. Advances in inner ear gene therapy: exploring cochlear protection and regeneration. Curr Opin Otolarygol Head Neck Surg. 2005;13:308–12. doi: 10.1097/01.moo.0000179248.51476.11. [DOI] [PubMed] [Google Scholar]
- 13.Chen Z, Mikulec AA, McKenna MJ, Sewell WF, Kujawa SG. A method for intracochlear drug delivery in the mouse. J Neurosci Methods. 2006;150:67–73. doi: 10.1016/j.jneumeth.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 14.Hoffer ME, Allen K, Kpoke RD, Weisskopf P, Gottshall K, Wester D. Transtympanic versus sustained-release administration of gentaminic: kinetics, morphology, and function. Laryngoscope. 2001;111:1343–57. doi: 10.1097/00005537-200108000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Hoffer ME, Kopke RD, Weisskopf P, Gottshall K, Allen K, Wester D, Balaban C. Use of the round window microcathether in the treatment of Meniere’s disease. Laryngoscope. 2001;111:2046–9. doi: 10.1097/00005537-200111000-00033. [DOI] [PubMed] [Google Scholar]
- 16.Nordang L, Linder B, Anniko M. Morphologic changes in the round window membrane after topical hydrocortisone and dexamethasone treatment. Otol Neurotol. 2003 Mar24;(2):33–43. doi: 10.1097/00129492-200303000-00034. [DOI] [PubMed] [Google Scholar]
- 17.Silverstein H, Thompson J, Rosenberg SI, Brown N, Light J. Silverstein MicroWick. Otolaryngol Clin North Am. 2004;37:1019–34. doi: 10.1016/j.otc.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Hahn H, Kammerer B, DiMauro A, Salt AN, Plontke SK. Cochlear microdialysis for quantification of dexamethasone and fluoroscein entry into scala tympani during round window administration. Hear Res. 2006;212:236–44. doi: 10.1016/j.heares.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goycoolea MV, Carpenter AM, Muchow D. Ultrastructural studies of the round-window membrane. Arch Otolaryngol Head Neck Surg. 1987;113:617–624. doi: 10.1001/archotol.1987.01860060043013. [DOI] [PubMed] [Google Scholar]
- 20.Sahni RS, Paparella MM, Schachern PA, Goycoolea MV, Le CT. Thickness of the human round window membrane in different forms of otitis media. Arch Otolaryngol Head Neck Surg. 1987;113:630–4. doi: 10.1001/archotol.1987.01860060056015. [DOI] [PubMed] [Google Scholar]
- 21.Goycoolea MV. Clinical aspects of round window membrane permeability under normal and pathologic conditions. Acta Otolaryngol. 2001;121:437–47. doi: 10.1080/000164801300366552. [DOI] [PubMed] [Google Scholar]
- 22.Nordang L, Linder Birgitta Anniko M. Morphologic changes in the round window memebrane after topical hydrocortisone and dexamethasone treatment. Otology and Neurotology. 2003;24:339–43. doi: 10.1097/00129492-200303000-00034. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka K, Motomura S. Permeability of the labyrinthine window in guinea pigs. Arch Otorhinolaryngol. 1981;233:67–75. doi: 10.1007/BF00464276. [DOI] [PubMed] [Google Scholar]
- 24.Salt AN, Ma Y. Quantification of solute entry into cochlear perilymph through the round window membrane. Hear Res. 2001;154:88–97. doi: 10.1016/s0378-5955(01)00223-4. [DOI] [PubMed] [Google Scholar]
- 25.Mynatt R, Hale SA, Gill RM, Plontke SKR, Salt AN. Demonstration of a longitudinal concentration gradient along scala tympani by sequential sampling of perilymph from the cochlear apex. JARO. 2006 doi: 10.1007/s10162-006-0034-y. Accepted with minor revisions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imamura S, Adams JC. Distribution of gentamicin in the guinea pig inner ear after local or systemic application. J Assoc Res Otolaryngol. 2003;4:176–195. doi: 10.1007/s10162-002-2036-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffer ME, Balough BJ, Gottshall KR, Allen K, Weisskopf P, Wester D, Balaban C. Sustained-release devices in inner ear medical therapy. Otolaryngol Clin North Am. 2004;37:1053–60. doi: 10.1016/j.otc.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Salt AN, Kellner C, Hale S. Contamination of perilymph sampled from the basal cochlear turn with cerebrospinal fluid. Hear Res. 2003;182:24–33. doi: 10.1016/s0378-5955(03)00137-0. [DOI] [PubMed] [Google Scholar]
- 29.Parnes LS, Sun AH, Freeman DJ. Corticosteroid pharmacokinetics in the inner ear fluids: An animal study followed by clinical application. Laryngoscope. 1999;109:1–17. doi: 10.1097/00005537-199907001-00001. [DOI] [PubMed] [Google Scholar]
- 30.Banerjee A, Parnes LS. The biology of intratympanic drug administration and pharmacodynamics of round window drug absorption. Otolaryngol Clin North Am. 2004;(5):1035–51. doi: 10.1016/j.otc.2004.04.003. [DOI] [PubMed] [Google Scholar]