Abstract
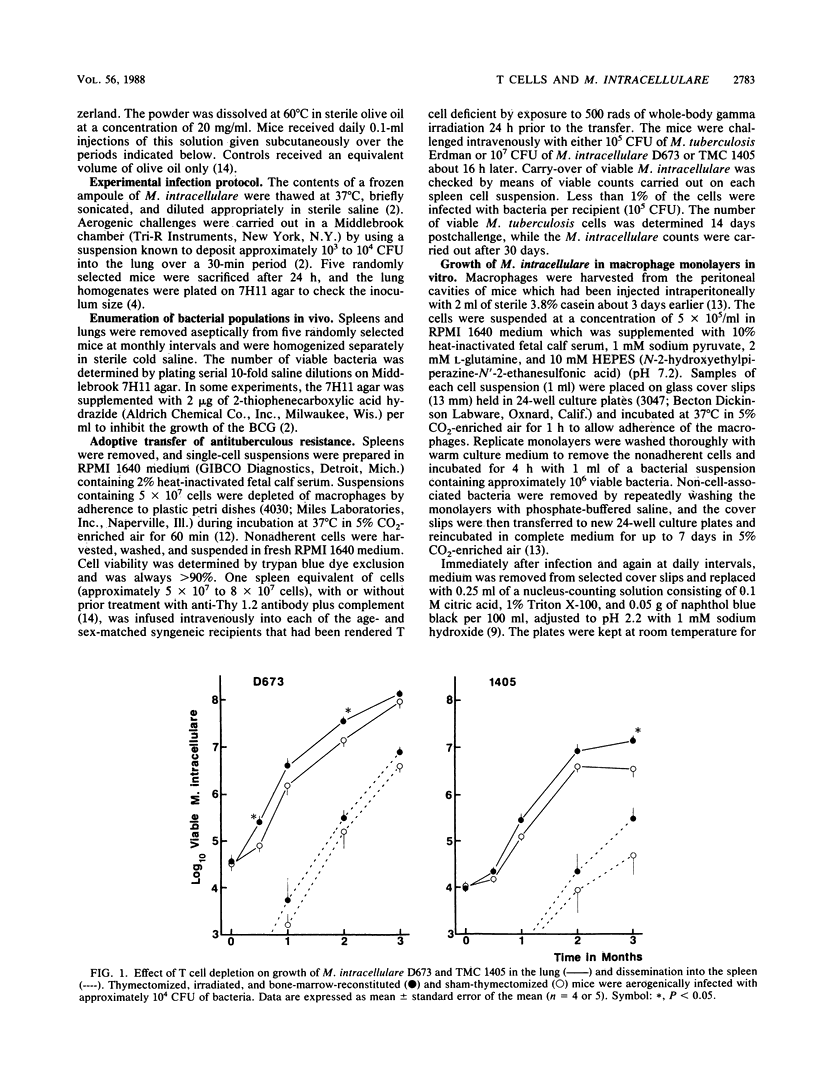
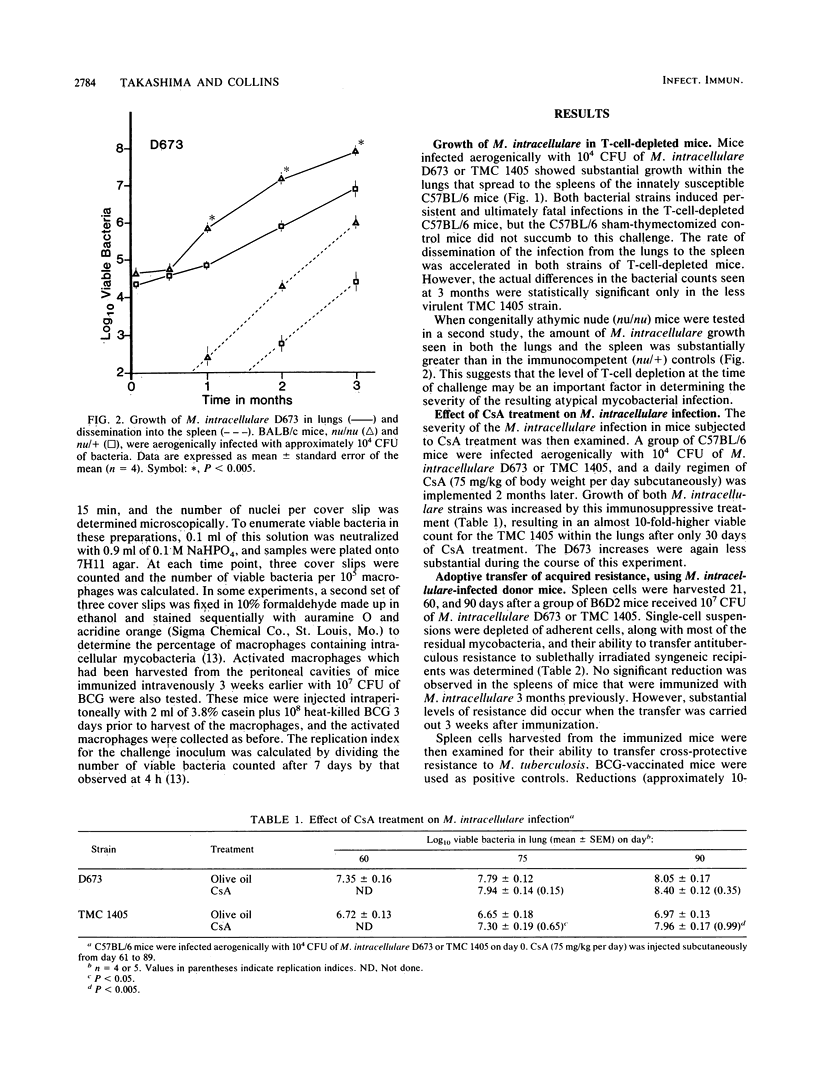
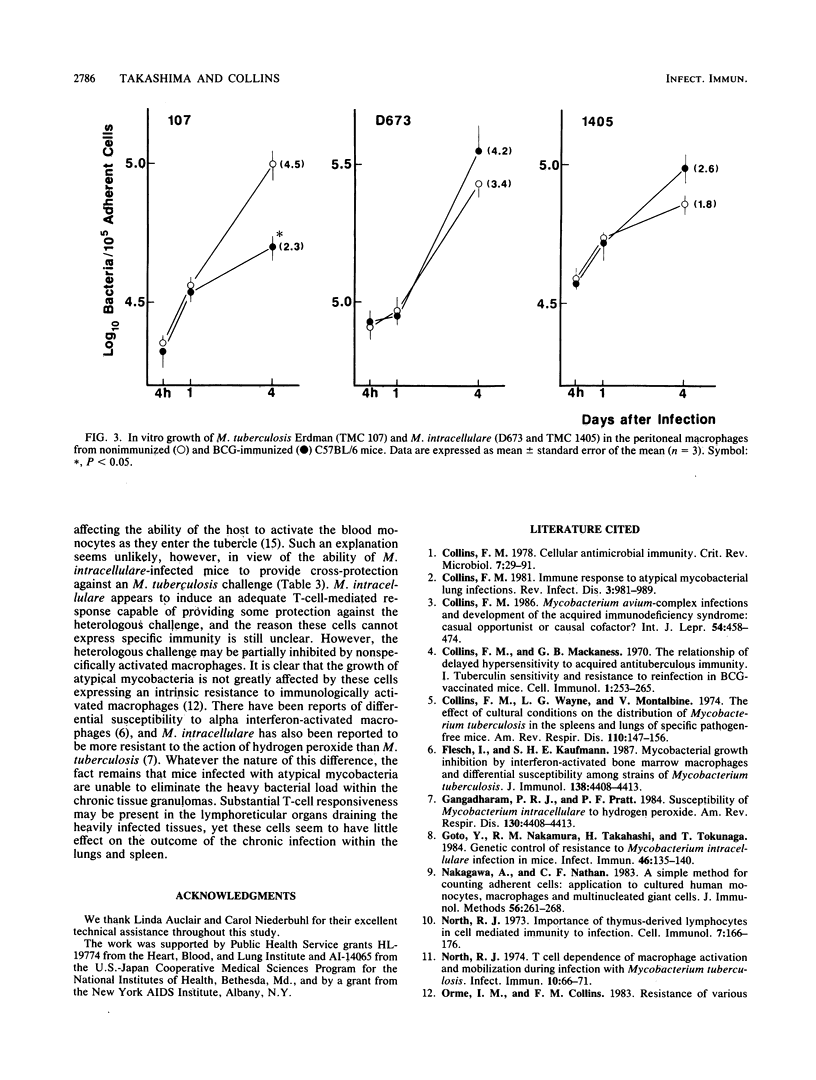
Growth of mouse-virulent Mycobacterium intracellulare D673 and TMC 1405 in the lung was affected by T-cell depletion in susceptible C57BL/6 mice. Significant differences also occurred between the growth patterns seen in congenitally athymic (nu/nu) mice and their nu/+ littermates. Treatment of the mice with an immunosuppressive regimen of cyclosporin A (75 mg/kg of body weight per day subcutaneously) provided further evidence of the importance of T cells in controlling growth of M. intracellulare in the normal host. Adoptive transfer experiments indicated the presence of a T-cell-mediated specific protective immunity against a subsequent M. intracellulare challenge when transfer was carried out 3 weeks after immunization of the donor host. At this time, cross-protective immunity was also observed against a virulent M. tuberculosis challenge. There was no difference in the rate of growth by M. intracellulare as challenge in Mycobacterium bovis BCG-activated or normal peritoneal macrophages from C57BL/6 mice tested in vitro during a 7-day period. However, M. tuberculosis growth rates were decreased substantially in the BCG-activated macrophages. These studies suggest that mice infected with M. intracellulare do not eliminate the infection, because this organism can resist the bactericidal activity of the T-cell-activated macrophage better than M. tuberculosis can.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Collins F. M. Cellular antimicrobial immunity. CRC Crit Rev Microbiol. 1978;7(1):27–91. doi: 10.3109/10408417909101177. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Mackaness G. B. The relationship of delayed hypersensitivity to acquired antituberculous immunity. I. Tuberculin sensitivity and resistance to reinfection in BCG-vaccinated mice. Cell Immunol. 1970 Sep;1(3):253–265. doi: 10.1016/0008-8749(70)90047-x. [DOI] [PubMed] [Google Scholar]
- Collins F. M. Mycobacterium avium-complex infections and development of the acquired immunodeficiency syndrome: casual opportunist or causal cofactor? Int J Lepr Other Mycobact Dis. 1986 Sep;54(3):458–474. [PubMed] [Google Scholar]
- Collins F. M., Watson S. R. Immune responses to atypical mycobacterial lung infections. Rev Infect Dis. 1981 Sep-Oct;3(5):981–989. doi: 10.1093/clinids/3.5.981. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Wayne L. G., v Montalbine The effect of cultural conditions on the distribution of Mycobacterium tuberculosis in the spleens and lungs of specific pathogen-free mice. Am Rev Respir Dis. 1974 Aug;110(2):147–156. doi: 10.1164/arrd.1974.110.2.147. [DOI] [PubMed] [Google Scholar]
- Flesch I., Kaufmann S. H. Mycobacterial growth inhibition by interferon-gamma-activated bone marrow macrophages and differential susceptibility among strains of Mycobacterium tuberculosis. J Immunol. 1987 Jun 15;138(12):4408–4413. [PubMed] [Google Scholar]
- Goto Y., Nakamura R. M., Takahashi H., Tokunaga T. Genetic control of resistance to Mycobacterium intracellulare infection in mice. Infect Immun. 1984 Oct;46(1):135–140. doi: 10.1128/iai.46.1.135-140.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawara A., Nathan C. F. A simple method for counting adherent cells: application to cultured human monocytes, macrophages and multinucleated giant cells. J Immunol Methods. 1983 Jan 28;56(2):261–268. doi: 10.1016/0022-1759(83)90418-0. [DOI] [PubMed] [Google Scholar]
- North R. J. Importance of thymus-derived lymphocytes in cell-mediated immunity to infection. Cell Immunol. 1973 Apr;7(1):166–176. doi: 10.1016/0008-8749(73)90193-7. [DOI] [PubMed] [Google Scholar]
- North R. J. T cell dependence of macrophage activation and mobilization during infection with Mycobacterium tuberculosis. Infect Immun. 1974 Jul;10(1):66–71. doi: 10.1128/iai.10.1.66-71.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Resistance of various strains of mycobacteria to killing by activated macrophages in vivo. J Immunol. 1983 Sep;131(3):1452–1454. [PubMed] [Google Scholar]
- Stokes R. W., Orme I. M., Collins F. M. Role of mononuclear phagocytes in expression of resistance and susceptibility to Mycobacterium avium infections in mice. Infect Immun. 1986 Dec;54(3):811–819. doi: 10.1128/iai.54.3.811-819.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima T., Collins F. M. Immunosuppressive effect of cyclosporin A on Mycobacterium bovis BCG infections in mice. Infect Immun. 1987 Jul;55(7):1701–1706. doi: 10.1128/iai.55.7.1701-1706.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S. R., Collins F. M. Development of suppressor T cells in mice heavily infected with mycobacteria. Immunology. 1980 Mar;39(3):367–373. [PMC free article] [PubMed] [Google Scholar]
- Wolinsky E. Nontuberculous mycobacteria and associated diseases. Am Rev Respir Dis. 1979 Jan;119(1):107–159. doi: 10.1164/arrd.1979.119.1.107. [DOI] [PubMed] [Google Scholar]


