Abstract
BACKGROUND
Studies indicate a gap between evidence and clinical practice in osteoporosis management. Tools that facilitate clinical decision making at the point of care are promising strategies for closing these practice gaps.
OBJECTIVE
To systematically review the literature to identify and describe the effectiveness of tools that support clinical decision making in osteoporosis disease management.
DATA SOURCES
Medline, EMBASE, CINAHL, and EBM Reviews (CDSR, DARE, CCTR, and ACP J Club), and contact with experts in the field.
REVIEW METHODS
Randomized controlled trials (RCTs) in any language from 1966 to July 2006 investigating disease management interventions in patients at risk for osteoporosis. Outcomes included fractures and bone mineral density (BMD) testing. Two investigators independently assessed articles for relevance and study quality, and extracted data using standardized forms.
RESULTS
Of 1,246 citations that were screened for relevance, 13 RCTs met the inclusion criteria. Reported study quality was generally poor. Meta-analysis was not done because of methodological and clinical heterogeneity; 77% of studies included a reminder or education as a component of their intervention. Three studies of reminders plus education targeted to physicians and patients showed increased BMD testing (RR range 1.43 to 8.67) and osteoporosis medication use (RR range 1.60 to 8.67). A physician reminder plus a patient risk assessment strategy found reduced fractures [RR 0.58, 95% confidence interval (CI) 0.37 to 0.90] and increased osteoporosis therapy (RR 2.44, CI 1.43 to 4.17).
CONCLUSION
Multi-component tools that are targeted to physicians and patients may be effective for supporting clinical decision making in osteoporosis disease management.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-008-0812-9) contains supplementary material, which is available to authorized users.
Key Words: osteoporosis, disease management, decision making, randomized controlled trials
INTRODUCTION
Osteoporosis affects over 200 million people worldwide1, with an estimated 10 million people in the US2, 4 million people in the UK3, and 1.4 million people in Canada2,4. A first fragility fracture increases the risk of serious fractures of the hip and spine 20-fold 5. Vertebral fractures can cause pain, height loss, deformity, disability, and mortality6, but hip fractures have the most devastating prognosis7, with loss of physical function and social interaction, and need for long-term care8. The cost of treating osteoporosis and the fractures it can cause9–11 is compounded by the increasing number of people at risk in our aging population4,12.
There is evidence from clinical practice guidelines outlining how osteoporosis can be diagnosed and managed13–15. However, there is a gap between evidence and clinical practice16. Indeed, fewer than 40% of patients with this disorder receive appropriate therapy16. Many clinicians are uncertain about screening and management of osteoporosis17. The proportion of patients with fragility fractures who receive a diagnostic test for osteoporosis or a diagnosis from a physician is not optimal (range 1.7–50%)18. This gap highlights the need for effective knowledge translation and the finding that the provision of evidence is necessary but not sufficient to achieve knowledge translation19.
We systematically reviewed the literature to identify studies evaluating the effectiveness of tools that support clinical decision making in osteoporosis disease management for reducing fragility fractures and increasing bone mineral density (BMD) testing or osteoporosis therapy.
METHODS
Data Sources
Studies were identified by searching Medline (1966 to July 2006), EMBASE (1980 to 2006), CINAHL (1982 to July 2006), and Ovid EBM Reviews (Cochrane Database of Systematic Reviews, ACP Journal Club, Database of Abstracts of Reviews of Effects, and the Cochrane Clinical Controlled Trials Register). We also searched the grey literature: web sites of CIHR (Canadian Institutes of Health Research), US AHRQ (Agency for Healthcare Research and Quality), US CRISP (Computer Retrieval of Information on Scientific Projects), the US National Institutes of Health clinical trials register (ClinicalTrials.gov), bmjupdates+, Canadian Centre for Chronic Disease Prevention and Control, Osteoporosis Canada, and Digital Dissertations. We reviewed the reference lists of relevant articles, hand searched the current Canadian clinical practice guidelines for the diagnosis and management of osteoporosis13, and contacted experts in the field.
Search Strategy
To generate search terms, we conducted a preliminary search in Medline and EMBASE using known terms and synonyms suggested by clinicians, librarians, and experts in the field to capture all possible text words and MeSH terms that describe disease management, disease management tools, and clinical decision support systems (CDSSs). This list was supplemented by additional terms that were found in studies evaluating tools in other chronic diseases such as heart failure, diabetes, and asthma20–24. The final list of terms included clinical decision support systems/techniques/tools; disease management/tools; clinical decision aids/tools/guidelines, computer assisted; decision rules/trees/prediction guides; reminder systems; risk assessment instruments/tools; point of care system. We defined reminders as any system of communication that suggests, recommends, notifies, prompts, or alerts clinicians, other health-care professionals, or patients about osteoporosis risk, diagnosis, or treatment according to practice guidelines. We defined risk assessment as any strategy that assesses the risk for osteoporosis using measures of BMD [e.g., dual-energy x-ray absorptiometry (DXA) or quantitative ultrasound (QUS)], any risk assessment questionnaires such as the osteoporosis risk-assessment instrument (ORAI)25, or any other well-defined strategy. Using Ovid, the search terms were first combined using the Boolean “OR,” and the resulting sets were then “AND-ed” with “osteoporosis” in each database. The resulting retrieval yield was then limited using the most sensitive search strategy filter for treatment studies developed by Haynes et al.26.
Study Selection
Studies were included if they were randomized controlled trials (RCTs) in any language from 1966 to July 2006 and evaluated disease management or CDSS interventions in men or women at risk for osteoporosis (age ≥65 years of age, postmenopausal women, or >3 months systematic use of glucocorticoids) who had a confirmed diagnosis of osteoporosis or an existing or previous fragility fracture. Interventions could be in any format (e.g., electronic-, paper-, or program-based) as long as they incorporated an aspect of care coordination, were targeted to patients or health-care professionals involved in osteoporosis care, the intervention was characterized by at least one term that met our definitions for disease management or CDSS tools, evaluated any component of disease management (i.e., risk assessment, diagnosis, or treatment), and investigated living patients. Outcomes were fragility fractures (vertebral or nonvertebral), BMD investigations, initiation of any osteoporosis treatment (e.g. bisphosphonates), and fracture-related complications [e.g., quality of life (QOL), admission to long-term care, and fracture-related mortality]. We excluded studies evaluating pharmacological interventions for osteoporosis (e.g., bisphosphonates) unless they were a component of the osteoporosis tool, and studies investigating outcomes related to fractures from major trauma, the primary prevention of osteoporosis, and falls prevention.
Two investigators (MK and SS) independently reviewed the titles and abstracts of potentially relevant articles and applied the inclusion/exclusion criteria using a standardized form. The final list of articles was selected by the same two investigators who independently screened articles in full text using the inclusion/exclusion criteria. The agreement between the two reviewers for abstract review was excellent (κ = 0.91, 95% CI 0.80 to 1.00) and perfect for full-text review. Any disagreements were resolved through consensus.
Data Extraction and Quality Assessment
We assessed study quality using specific methodological criteria most relevant to the control of bias: randomization, allocation concealment, blinding, and completeness of follow-up. Authors were contacted to verify the accuracy of reporting of these criteria in their studies — we received verification from the authors of six studies (46%)31,34,35,40–42. We decided not to use a quality assessment scale because evidence exists that they lack empirically supported components27, and authors of scales can be influenced by their own perception of study quality28. The two authors (MK and SS) independently extracted data on the setting (location, enrollment dates), study design (method of randomization, allocation concealment, and blinding), population characteristics (inclusion/exclusion criteria, sample size, number of patients assessed for eligibility and the number who met inclusion criteria), interventions (components, format, aspect of osteoporosis disease management evaluated, and the target of the intervention), outcomes, results, and follow-up [duration of follow-up, intention to treat (ITT) analysis, withdrawals, and reasons for dropouts].
Data Synthesis and Analysis
We explored the potential sources of methodological and clinical heterogeneity according to differences in study quality, participants, interventions, and outcomes. Using the more conservative random effects model to account for extra between-trial variation29, relative risks (RRs) with their 95% confidence intervals (CIs) were calculated from event rates using Cochrane Review Manager 4.2.8. Authors of included studies were contacted to verify calculations, and we received verification from authors of six studies (46%)31,34,35,40–42.
RESULTS
Our search identified 39,953 potentially relevant citations, and none were identified from the grey literature. Using the most sensitive search filter by Haynes et al. for treatment studies26, this retrieval yield was then limited to 3,880 RCTs. Of these, 1,246 citations were screened for relevance, 42 articles were selected for full-text review, and 13 RCTs met the inclusion/exclusion criteria and were included in the systematic review30–42 (Fig. 1). Reasons for exclusion were that the study did not meet criteria for target intervention (21 studies)43,45–46,48–50,52,53,55–58,60–65,67–69, outcomes (7 studies)44,47,59,61,65–67, population (4 studies)44,54,64,70, or study design (3 studies)51, 53, 60.
Figure 1.
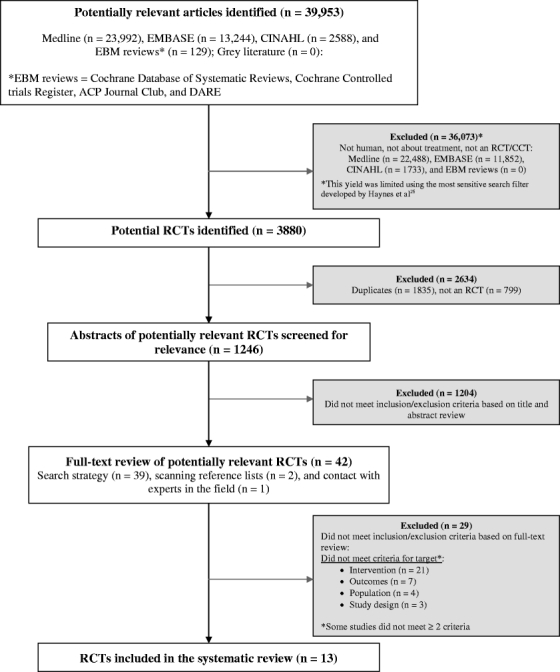
Study identification flowchart.
Sources of Heterogeneity
Meta-analysis was not done because studies were too heterogeneous to pool. Sources of methodological heterogeneity are shown in Tables 1 and 2. Reported study quality was generally poor—only four studies indicated all of sequence generation, concealment of randomization, blinding (Table 1)31,33–35, the inclusion of at least 80% of patients in the analysis, and a description of reasons for dropouts/withdrawals (Table 2)31,33–35. The follow-up period ranged between 2 and 28 months, and only four studies were analyzed according to ITT (Table 2)31,33,40,42.
Table 1.
Study Quality Assessment – Randomization and Blinding
| Study | Randomization* (done/not done/unclear) | Blinding† (done, not done, unclear) | ||
|---|---|---|---|---|
| Unit of randomization | Sequence generation | Concealment | ||
| Barr (2004) 30 | Patient | Done | Unclear | Not done |
| Bliuc (2006)31‡ | Patient | Done | Done | Done |
| Boyd (2002)32 | Practice | Unclear | Unclear | Unclear |
| Devereux (2005)33‡ | Patient | Done | Done | Done |
| Feldstein (2006)34‡ | Patient | Done | Done | Done |
| Gardner (2005)35‡ | Patient | Done | Done | Done |
| LaCroix (2005)36 | Patient | Unclear | Unclear | Not done |
| McDonough (2005)37 | Pharmacy | Unclear | Unclear | Not done |
| Morrison (2004)38 | Practice | Unclear | Unclear | Unclear |
| Rolnick (2000)39‡ | Patient | Done | Done | Not done |
| Solomon (2004)40‡ | Practice | Done | Unclear | Not done |
| Solomon (2006)41‡ | Practice | Done | Not done | Not done |
| Solomon (2007)42‡ | Practice | Done | Not done | Not done |
*Unclear = Authors did not report or provide a description for sequence generation or allocation concealment of randomization that allowed classification of “done” or “not done”
†Unclear = Authors did not report or provide an indication of who, if anyone, was unaware of group assignment that allowed classification of “done” or “not done”
‡Data verified by author
Table 2.
Study Quality Assessment – Completeness of Follow-Up
| Study | Completeness of follow-up | ||||
|---|---|---|---|---|---|
| Follow-up period | Patients included in analysis | ITT (done, not done, unclear) | Patients lost to follow-up | Reason for drop-out described (yes, no, unclear) | |
| Barr (2004)30 | Mean 23.5–26.6 months | 74% | Not done | 34% | Yes |
| Bliuc (2006)31‡ | 6 months | 97% | Done | 3% | Yes |
| Boyd (2002)32 | 3–6 months | 62–76% | Not done | Unclear | No |
| Devereux (2005)33‡ | 10 weeks | 100% | Done | 6% | Yes |
| Feldstein (2006)34‡ | 6 months | 95% | Not done | 5% | Yes |
| Gardner (2005)35‡ | 6 months | 90% | Not done | 9% | Yes |
| LaCroix (2005)36 | Mean 28 months | 11% | Not done | Unclear | Unclear |
| McDonough (2005)37 | 9 months | 83% | Unclear | 17% | No |
| Morrison (2004)38 | 12 months | Unclear | Not done | Unclear | Unclear |
| Rolnick (2000)39‡ | 6 months | 70% | Not done | 22% | Yes |
| Solomon (2004)40‡ | 6 months | 100% | Done | None | Unclear |
| Solomon (2006)41‡ | 2 months | 53% | Not done | 47% | Yes |
| Solomon (2007)42‡ | 10 months | 100% | Done | None | Yes |
*Unclear = Authors did not report or provide a description for sequence generation or allocation concealment of randomization that allowed classification of “done” or “not done”
†Unclear = Authors did not report or provide an indication of who, if anyone, was unaware of group assignment that allowed classification of “done” or “not done”
‡Data verified by author
Table 3 shows the clinical heterogeneity across studies according to population and interventions, which also contributed to our decision not to combine results in a meta-analysis. Populations varied across studies for osteoporosis risk factors: age ≥65 years of age (six studies)30, 33, 35, 36, 41, 42, postmenopausal women (3 studies)34, 38, 39, previous fracture (six studies), and at least 6 months use of glucocorticoids (five studies)40–42. Differences were also found between studies for the components of interventions (i.e., different combinations of reminders, risk assessment, or education); format of the reminders (paper-based or electronic), and risk assessment [BMD testing or questionnaires, such as the SCORE (Simple Calculated Osteoporosis Risk Estimate) and SOF (Study of Osteoporotic Fracture)], and the target of the intervention: physicians (two studies)30, 40, patients (three studies)31, 33, 39, or both (six studies)32, 34, 35, 36, 37, 38, or physicians, patients, and pharmacists (two studies)37, 42. Seven studies (54%) incorporated all three aspects of osteoporosis disease management in their intervention30, 32, 34, 36, 38, 41, 42. Twelve of the 13 RCTs (92%) investigated outcomes related to BMD testing or initiation of osteoporosis therapy (e.g., bisphosphonates, and calcium and vitamin D)30–32, 34–42; and only two studies investigated outcomes of fracture or QOL30, 39.
Table 3.
Characteristics of Included Studies*
| Study (N) | Intervention | Population | |
|---|---|---|---|
| Description | Type (target) | ||
| Barr (2004)30 (N = 2,515) | Intervention: Surveyed patients were invited to attend their GPs for OP screening. GPs of women at increased risk for hip fracture were asked to prescribe calcium + vitamin D. GPs of women not at risk were sent screening results, but no treatment recommendation. Control: no screening | Reminder (Phys) + RA (Pts) | Age ≥70 years |
| Bliuc (2006)31 (N = 159) | Intervention: Personalized standard letter addressed to fracture patient about their risk factors for OP and recommendation to follow-up with their PCPs plus an offer of a free BMD test. Control: Letter only | Reminder (Pts) + RA (Pts) | Current fracture and no OP investigation in the last 3 months after fracture |
| Boyd (2002)32 (N = 258) | Intervention: An extended letter to recommend screening and treatment if BMD was low plus a treatment strategy for Pts with a T-score <-1.50 or <-2.00. If BMD was low, Pts were informed of the need to follow-up with their Phys. Control: Standard letter (treatment recommendation if BMD was low) | Reminder (Phys & Pts) | Low BMD |
| Devereux (2005)33 (N = 50) | Intervention: 10-week, water-based exercise program for 50 min and a 10-min self-management program on OP, medications, footwear, and physical activity Control: No interventions | Ed (Pts) + Exercise (Pts) | Community-dwelling patients age ≥65 years and a diagnosis of OP or osteopenia |
| Feldstein (2006)34 (N = 327) | Intervention 1: EMR reminder (patient-specific clinical guideline advice to PCPs delivered through an EMR about Pts’ OP risk based on age and prior fracture, and the need for evaluation and treatment); plus an advisory letter with Ed materials mailed to Pts (included guideline recommendation and request that Pt discuss management options with their PCP). PCPs also received this Pt letter. Intervention 2: EMR reminder to Phys but no letter to Pts. Controls: UC | Reminder (Phys & Pts) + Ed (Pts) | Postmenopausal women age 50–89 years who sustained a fracture and had not received a BMD test or OP medications |
| Gardner (2005)35 (N = 80) | Intervention: Prior to discharge from hospital, Pts received a 15-min visit by a researcher to discuss the role of OP in hip fractures, the importance of preventing future fractures, and effectiveness of current therapies; plus a printed copy of five questions to bring to their PCPs after discharge; plus a reminder to follow-up with their PCP and the need for OP management. Control: two-page pamphlet on falls prevention only | Reminder (Phys & Pts) + Ed (Pts) | Age ≥65 years, a low energy femoral neck or hip fracture, and no OP medications |
| LaCroix (2005)36 (N = 9,268) | Universal testing group: BMD testing offered to all women via letters of invitation. SCORE group: Completion of the SCORE and a BMD test if score was ≥7. SOF group: Completion of SOF and a BMD test if Pts had ≥5 risk factors. All groups: received personalized feedback identifying their risk factors for OP and suggestions for changing modifiable risks; plus printed Ed about OP, fracture, treatment, and prevention. Pts who had a BMD received enhanced personalized feedback on their hip and spine BMD and an individual estimate of their 10-year risk for hip fracture. Pts with ≥5 fracture risk factors and low BMD and all who reported a prior fracture at age >50 years were referred to their Phys for workup and treatment of possible OP – a copy of their feedback was also sent to their Phys | Reminder (Phys & Pts) + Ed (Pts) + RA (SCORE or SOF) (Pts) | Women age 60–80 years who have not taken HRT or OP medications within the past 12 months |
| McDonough (2005)37 (N = 15) | Intervention: Pts received Ed and a pamphlet about the risks of GIOP. Pharms monitored Pts’ drug therapy. Problems identified were discussed with the Pt or the prescribing Phys. A letter was sent to Phys explaining the program and to review Pharm’s letter about their Pt. Pharms also received 4 h of training on GIOP prior to Pt recruitment. Control: UC | Reminder (Phys) + Ed (Pts & Pharms) | Patients age ≥18 years at high risk for OP (taking ≥7.5 mg of prednisone for ≥6 months) |
| Morrison (2004)38 (N = 10,865) | Intervention: A case-finding strategy to offer open-access BMD to all Pts found to be at high risk for OP by their Phys. Referred Pts received an information sheet and an appointment for a DXA scan. Phys of Pts found to have OP were given explicit recommendation to begin bisphosphonate treatment. Control: No case-finding strategy and no DXA scan access | Reminder (Phys) + Ed (Pts) + RA (Pts) | Women age 45–75 years with a history of fracture, taking prednisone, or prematurely menopausal (i.e., at age <45 years) |
| Rolnick (2000)39 (N = 508) | Intervention: Ed program about OP (conducted by a nurse practitioner), completion of the SCORE questionnaire, and BMD of the forearm. Control: All interventions but no BMD | Ed (Pts) + RA (Pts) | Women age 54–65 years from a large health care organization and not taking HRT or OP therapy |
| Solomon (2004)40 (N = 373) | Intervention: A 90-min lecture to Phys on GIOP and a review of baseline data on GIOP from their practice followed by discussion of methods for improving GIOP therapy; plus provision of a list of their Pts who were taking oral glucocorticoids indicating whether Pts had BMD and if OP medications were listed in their record; plus a one-page reminder to guide Phys’ GIOP management Control: No interventions | Reminder (Phys) + Ed (Phys) | Pts with rheumatoid arthritis who were taking oral glucocorticoids |
| Solomon (2006)41 (N = 1,200) | Intervention: Three, one-page personalized Ed mailings sent 3 weeks apart, which differed for men and women, and included information about OP risk and future fractures, the consequences of fractures, suggestions to prevent falls in the home, appropriate calcium and vitamin D intake, the importance of assessing BMD, the role of OP medications, and strategies to discuss OP and fall prevention with a PCP. A simple version of OP management guidelines was included for Pts to discuss with Phys. Control: No interventions | Reminder (Pts) + Ed (Pts) | Women age ≥65 years or men with prior fracture or taking glucocorticoids for ≥90 consecutive days |
| Solomon (2007)42 (N = 1,973) | Intervention: A CME program conducted during the Phys visit by a Pharm educator, who received 1-day training on OP. CME included a summary of OP, an algorithm for OP diagnosis and treatment, and a guide to OP therapy. Phys were also offered “tear sheets” that resembled prescription pads with check boxes for fall prevention, calcium and vitamin D use, BMD testing, and treatment that could be given to Pts. Phys also received a list of their Pts at risk for OP, which was used by educators during the one-on-one visit as examples of Pts that should be considered for BMD testing or treatment. Pts received a letter and then an automated telephone call (tailored Ed) inviting them to have a BMD test, which they could schedule immediately after the call. Control: No interventions | Reminder (Phys & Pts) + Ed (Phys, Pharms & Pts) | Women age ≥65 years, women or men age ≥45 years with a prior fracture, or women or men taking glcuocorticoids for ≥90 days |
*GP = general practitioner; OP = osteoporosis; RA = risk assessment; Phys = physicians; Pts = patients; PCP = primary care physician; UC = usual care; BMD = bone mineral density; Ed = education; EMR = electronic medical record; SCORE = simple calculated osteoporosis risk estimation; SOF = study of osteoporotic fracture; HRT = hormone replacement therapy; GIOP = glucocorticoid-induced osteoporosis; Pharms = pharmacists; DXA = dual-energy X-ray absorptiometry; CME = continuing medical education
Eleven of the 13 studies (85%) included a reminder system or education as a component of their intervention30–32, 34–38, 40–42. Of these, three studies included a reminder with or without a risk assessment strategy (Table 4)30–32, and 8 studies included a reminder system plus education with or without a risk assessment strategy (Table 5)34–38, 40–42. Two studies investigated interventions that included education with exercise or risk assessment (Table 6)33, 39. We also summarized how the grouping of different baseline risk factors by intervention might impact on the interpretation of its effectiveness (Tables 7, 8, 9, 10 and 11; available as a web appendix).
Table 4.
Reminder With or Without Risk Assessment*
| Study | Intervention (target of intervention) | Outcomes | Event rates (intervention vs. control) | Relative risk† (95% CI) |
|---|---|---|---|---|
| Barr (2004)30 | Reminder (Phys) + RA (Pts) | Fracture at any site | 4% vs. 7% | 0.58 (0.37 to 0.90)§ |
| Any osteoporosis medication | 24% vs. 1% | 2.44 (1.43 to 4.17)§ | ||
| Bliuc‡ (2006)31 | Reminder (Pts) + RA (Pts) | Investigation for osteoporosis | 38% vs. 7% | 5.70 (2.33 to 13.91)§∥ |
| Antiresorptive therapy | 5% vs. 7% | 0.76 (0.21 to 2.72) | ||
| Vitamin D therapy | 5% vs. 1% | 3.80 (0.43 to 33.21) | ||
| Boyd (2002)32 | Reminder (Phys & Pts) | Bone mineral density testing | 37% vs. 30% | 1.23 (0.80 to 1.91) |
| Initiation of osteoporosis therapy | 11% vs. 15% | 0.70 (0.34 to 1.47) |
*CI = confidence interval; Phys = physicians; Pts = patients; RA = risk assessment
†Using random effects model, relative risk with 95% confidence intervals was calculated from event rates using Cochrane Review Manager 4.2.8
‡Study indicated all of: method of randomization sequence and concealment, blinding, follow-up, and ≥80% follow-up
§Significant
∥Data verified by author
Table 5.
Reminder Plus Education With or Without Risk Assessment*
| Study | Intervention component (target of intervention) | Outcomes | Event rates (intervention vs. control) | Relative risk† (95% CI) |
|---|---|---|---|---|
| Feldstein‡ (2006)34 | Reminder (Phys & Pts) + Ed (Pts) | BMD testing or initiation of OP medication | 51% vs. 6% | 8.67 (3.90 to 19.26)§∥ |
| 43% vs. 6% | 7.26 (3.24 to 16.24)§∥ | |||
| Gardner‡ (2005)35 | Reminder (Phys & Pts) + Ed (Pts) | OP addressed (BMD or OP therapy) | 42% vs. 19% | 2.14 (1.00 to 4.62)§∥ |
| Solomon (2007)42 | Reminder (Phys & Pts) + Ed (Phys, Pharmacists, & Pts) | BMD testing | 13% vs. 9% | 1.43 (1.11 to 1.86)§∥ |
| OP medication | 6% vs. 4% | 1.60 (1.07 to 2.41)§∥ | ||
| McDonough (2005)37 | Reminder (Phys) + Ed (Pharmacists & Pts) | BMD testing | 72% vs. 63% | 1.14 (0.78 to 1.67) |
| Bisphosphonate therapy | 26% vs. 10% | 2.49 (0.63 to 9.87) | ||
| Calcium intake | 56% vs. 32% | 1.77 (0.88 to 3.55) | ||
| Solomon (2004)40 | Reminder (Phys) + Ed (Phys) | BMD testing | 32% vs. 38% | 0.84 (0.64 to 1.12)§ |
| OP medications | 8% vs. 8% | 0.99 (0.49 to 2.00)§∥ | ||
| Solomon (2006)41 | Reminder (Pts) + Ed (Pts) | BMD testing | 46% vs. 42% | 1.09 (0.91 to 1.30)∥ |
| Calcium intake | 68% vs. 69% | 0.98 (0.89 to 1.09)∥ | ||
| LaCroix (2005)36 | Reminder (Phys & Pts) + Ed (Pts) + RA (SCORE) (Pts) | Any fracture | 9% vs. 12% | 0.77 (0.52 to 1.12) |
| Initiation of any therapy | 21% vs. 20% | 1.05 (0.82 to 1.35) | ||
| Reminder (Phys & Pts) + Ed (Pts) + RA (SOF) (Pts) | Any fracture | 9% vs. 9% | 0.96 (0.69 to 1.34) | |
| Initiation of any therapy | 21 vs. 17% | 1.27 (1.03 to 1.56)§ | ||
| Morrison (2004)38 | Reminder (Phys) + Ed (Pts) + RA (Pts) | Prescription of bisphosphonates | Both groups increased in the prescription of bisphosphonates by 50% (p < 0.001), but no difference between groups for outcome | |
*CI = confidence interval; Phys = physician; Pts = patients; Ed = education; BMD = bone mineral density; OP = osteoporosis; RA = risk assessment; SCORE = simple calculated osteoporosis risk estimation; SOF = study of osteoporotic fracture
†Using random effects model, relative risk with 95% confidence intervals was calculated from event rates using Cochrane Review Manager 4.2.8
‡Study indicated all of: method of randomization sequence and concealment, blinding, follow-up, and ≥80% follow-up
§Significant
∥Data verified by author
Table 6.
Education With Exercise or Risk Assessment*
| Study | Intervention (target of intervention) | Outcome | Intervention (mean CFB SD]) | Control [mean CFB (SD)] | Difference between groups in mean CFB (95% CI) |
|---|---|---|---|---|---|
| Devereux† (2005)33 | Ed (Pts) + Exercise (Pts) | QOL (SF36): Physical function | 3.9 (14.1) | -4.6 (13.5) | 8.6 (0.4 to 16.8)‡ |
| QOL (SF36): Vitality | 10.1 (18.2) | -1.9 (14.4) | 12.0 (2.3 to 21.8)‡ | ||
| QOL (SF36): Social function | 15.2 (29.7) | 1.1 (11.2) | 14.1 (0.6 to 27.7)‡ | ||
| QOL (SF36): Mental health | 9.8 (16.2) | -0.4 (10.9) | 10.2 (2.0 to 18.4)‡ | ||
| Study | Intervention (target of intervention) | Outcome | Event rates(intervention vs. control) | Relative risk§(95% CI) | |
| Rolnick (2000)39 | Ed (Pts) + RA (Pts) | Calcium intake | 64% vs. 63% | 1.02 (0.88 to 1.17) | |
*CFB = mean change from baseline; SD = standard deviation; CI = confidence interval; Ed = education; Pts = patients; HRQOL = health-related quality of life; QOL = quality of life; RA = risk assessment
†Study indicated all of: method of randomization sequence and concealment, blinding, follow-up, and ≥80% follow-up
‡Significant
§Using random effects model, relative risk with 95% confidence intervals was calculated from event rates using Cochrane Review Manager 4.2.8
Reminder With or Without Risk Assessment (Table 4; three studies)30–32
The study by Barr et al. targeted the reminder component of the intervention to physicians, which recommended calcium and vitamin D for patients at increased risk for hip fracture following osteoporosis screening. Results showed that fewer patients who received the intervention had a fracture at any site than those in the control group (RR 0.58, CI 0.37 to 0.90), and more patients received any osteoporosis medication (RR 2.44, CI 1.43 to 4.17)30. The second study targeted the reminder to patients with previous fracture who were prompted to follow up with their physician about their increased risk for osteoporosis. The intervention group was investigated more for osteoporosis than the control group (RR 5.70, CI 2.33 to 13.91), but no difference was found between groups for antiresorptive (RR 0.76, CI 0.21 to 2.72) or vitamin D use (RR 3.80, CI 0.43 to 33.21)31. In the third study, which targeted a reminder strategy to physicians and patients, no difference was found between groups for BMD testing (RR 1.23, CI 0.80 to 1.91) or initiation of osteoporosis therapy (RR 0.70, CI 0.34 to 1.47)32.
Reminder Plus Education With or Without Risk Assessment (Table 5; eight studies)34–38, 40–42
Six RCTs included a reminder plus education as part of the intervention. Of these, three studies that showed an improvement in outcomes targeted the reminder component of the intervention to both physicians and patients34–35, 42. The study by Feldstein et al. compared usual care with an EMR reminder to alert physicians of their patients at risk for osteoporosis and the need for evaluation and treatment plus a letter to patients to discuss management options with their physician. The intervention increased BMD testing (RR 8.67, CI 3.90 to 19.26) or the initiation of osteoporosis medication (RR 7.26, CI 3.24 to 16.24)34. Another study provided fracture patients with a 15-minute visit prior to discharge about fracture prevention and current therapies plus a printed copy of five questions to discuss with their physician35. This study showed that the intervention group had osteoporosis addressed more than in the control group (RR 2.14, CI 1.00 to 4.62)35. The third study by Solomon et al. targeted all components of the intervention to physicians, patients, and pharmacists42. The intervention consisted of a continuing medical education program delivered by a pharmacist educator trained in osteoporosis and included a summary of osteoporosis, an algorithm for osteoporosis diagnosis and treatment, and a guide to pharmacotherapy. Another component of the intervention involved an automated telephone call inviting patients to have a BMD test, which they could schedule immediately after the call42. Results showed that compared with controls, the multi-faceted intervention increased BMD testing (RR 1.43, CI 1.11 to 1.86) and osteoporosis medication use (RR 1.60, CI 1.07 to 2.41)42.
Three studies targeted reminders and education to physicians and patients at risk for glucocorticoid-induced osteoporosis37, 40–41. None of these studies found a difference between groups for BMD testing (RR range 0.84 to 1.14)37, 40–41, bisphosphonate therapy (RR 2.49, 0.63 to 9.87)37, or calcium intake (RR range 0.98 to 1.77)37, 41.
Two studies combined a reminder with education plus a risk assessment strategy36, 38. Of these, LaCroix et al. targeted reminders to both physicians and patients, and found that more patients received any osteoporosis therapy than controls (RR 1.27, CI 1.03 to 1.56), but found no difference between groups for any fracture (0.96, CI 0.69 to 1.34)36. The second study showed increased rates of prescription for bisphosphonates from baseline in all groups (50% increase, p < 0.001), but the groups did not differ38.
Education With Exercise or Risk Assessment (Table 6: two studies)33, 39
Devereux et al. investigated a patient education intervention within a self-assessment program combined with aquatic exercise in patients ≥65 years of age with osteoporosis or osteopenia, and found greater improvement than controls in QOL (difference between groups in mean change from baseline 8.6, CI 0.4 to 16.8; 12.0, CI 2.3 to 21.8; 14.1, CI 0.6 to 27.7; 10.2, CI 2.0 to 18.4, respectively)33. Rolnick et al. evaluated an intervention that combined patient education with a risk assessment strategy (the SCORE questionnaire and BMD testing) in a population of postmenopausal women and showed no difference between groups for initiating calcium or vitamin D (RR 1.02, CI 0.88 to 1.17)39.
DISCUSSION
This systematic review suggests that some tools that support clinical decision making in osteoporosis disease management may reduce fracture rates, and increase BMD investigations and the initiation of osteoporosis therapy. In particular, interventions that target both physicians and patients, and those that consist of multiple components such as reminders and education were associated with greater improvement in outcomes than single-target interventions with fewer components. Five of the six studies (83%) that showed significant improvement in outcomes were targeted to both physicians and patients30, 34, 35, 42, 36. Furthermore, 6 of the 11 studies (55%) that incorporated a reminder30, 31, 34–36, 42 and 5 of the 10 studies (50%) that included education33–36, 42 as part of the intervention showed improvement in outcomes. These results are not surprising, as they confirm the multidisciplinary nature of osteoporosis disease management. It also suggests the need to consider multiple components and targets in the development of any future interventions.
Although several studies did show improved outcomes, these results have to be interpreted with caution for several reasons. First, very few RCTs evaluate osteoporosis disease management tools that support clinical decision making. Second, quality assessments of the 13 included studies indicated that trial reporting was not optimal. The lack of reporting of appropriately used methods of randomization and its concealment, blinding, and follow-up in the studies largely limits the internal validity of their findings. The four studies that were found to be the most methodologically rigorous (i.e., indicated randomization and concealment, blinding, follow-up, and at least 80% included in the analysis)31,33–35 were among the six studies that showed improved outcomes. Third, it was not possible to combine the results in a meta-analysis because the studies were too heterogeneous to pool. Differences between studies for study quality and interventions were the main sources of variability. Generalizability of the interventions is also limited because only half of the studies included all three components of osteoporosis disease management as part of their intervention, and studies varied substantially for the components and targets of the interventions.
To reduce the effects of bias, we ensured that a rigorous methodology was used for our systematic review by limiting our selection of studies to RCTs71, conducting a comprehensive literature search with well-defined terms and inclusion/exclusion criteria, screening articles independently at each level of article selection using standardized data abstraction forms, and measuring inter-rater reliability using kappa statistics. We also addressed potential sources of variability between relevant studies (in addition to random error) by the rigorous study quality assessment and differences between studies for populations, interventions, and outcomes.
Of the disease management tools that we found in our systematic review, about half addressed all dimensions of osteoporosis disease management, and few utilized a computerized format that could be used by health-care professionals at the point of care. This gap highlights an area of future work since evidence has shown that CDSSs can facilitate disease management24; provide and automatically generate evidence-based recommendations for the screening, diagnosis, or treatment of specific patients; improve the clarity of guidelines; send reminders; and provide accessible references72, 73. CDSSs can also investigate outcomes related to improving practitioner performance and look at clinically meaningful patient outcomes74, 75. Furthermore, there is evidence from studies in other chronic diseases20–24 that integration of electronic technology into such systems can facilitate disease management and positively impact patient outcomes.
In summary, interventions that incorporate reminders and education, and those that target both physicians and patients are promising strategies for improving outcomes in osteoporosis disease management. The low number of studies found in this systematic review highlights the gaps that currently exist for optimal disease management in osteoporosis for reducing fractures, performing BMD investigations, and initiating osteoporosis treatment. The lack of rigorously evaluated interventions suggests the need for the development and evaluation of comprehensive tools to bridge the gap between evidence and practice.
ELECTRONIC SUPPLEMENTARY MATERIAL
Below is the link to the electronic supplementary material.
(DOC 141 kb)
Acknowledgments
Source of Funding We received no external support for this study.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-008-0812-9) contains supplementary material, which is available to authorized users.
An erratum to this article can be found at http://dx.doi.org/10.1007/s11606-008-0871-y
References
- 1.Gullberg B, Johnell O, Kanis JA. World-wide projections for hip fracture. Osteoporosis Int. 1997;7:407–13. [DOI] [PubMed]
- 2.Lane NE. Epidemiology, etiology, and diagnosis of osteoporosis. Am J Obst Gynecol. 2006;194:S3–11. [DOI] [PubMed]
- 3.National Osteoporosis Society. Available at: http://www.nos.org.uk/: Accessed September, 2008.
- 4.Osteoporosis Canada. Available at: http://www.osteoporosis.ca/english/home/: Accessed September, 2008.
- 5.Hajcsar EE, Hawker G, Bogoch ER. Investigation and treatment of ostoporosis in patients with fragility fractures. CMAJ. 2000;163:819–22. [PMC free article] [PubMed]
- 6.Johnell O, Kanis JA, Oden A, et al. Mortality after osteoporotic fractures. Osteoporosis Int. 2004;15:38–42. [DOI] [PubMed]
- 7.Reginster J, Burlet N. Osteoporosis: A still increasing prevalence. Bone. 2006;38:S4–9. [DOI] [PubMed]
- 8.Lips P, van Schoor NM. Quality of life in patients with osteoporosis. Osteoporosis Int. 2005;16:447–55. [DOI] [PubMed]
- 9.Goeree ROB, Pettitt DB, Cuddy L, et al. An assessment of the burden of illness due to osteoporosis in Canada. J Soc Obstet Gynaecol Can. 1996;18(Suppl):15–24.
- 10.Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. The Lancet. 2002;359:1761–7. [DOI] [PubMed]
- 11.Poole KES, Compston JE. Clinical review: Osteoporosis and its management. BMJ. 2006;333:1251–6. [DOI] [PMC free article] [PubMed]
- 12.International Osteoporosis Foundation. Available at: http://www.iofbonehealth.org/facts-and-statistics.html: Accessed September 2008.
- 13.Brown JP, Josse RG, for the Scientific Advisory Council of the Osteoporosis Society of Canada. 2002 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada (revised, August 26, 2004). CMAJ. 2002;167(10):S1–34. [PMC free article] [PubMed]
- 14.Osteoporosis, Clinical Guidelines for Prevention and Treatment: Update on pharmacological interventions and algorithm for management. Royal College of Physicians, Bone and Tooth Society of Great Britain, 2003. Available at: http://www.rcplondon.ac.uk: Accessed September 2008.
- 15.ACOG Committee on Practice Bulletins. ACOG practice bulletin. Clinical management guidelines for obstetrician-gynecologists. Number 50, January 2004. Osteoporosis Obstet Gynecol. 2004;103(1):203–16. [PubMed]
- 16.Jaglal SB, McIsaac WJ, Hawker G, et al. Information needs in the management of osteoporosis in family practice: an illustration of the failure of the current guideline implementation process. Osteoporosis Int. 2003;14:672–6. [DOI] [PubMed]
- 17.Jaglal SB, Carroll J, Hawker G, et al. How are family physicians managing osteoporosis? Qualitative study of their experiences and educational needs. Can Fam Phys. 2003;49:462–8. [PMC free article] [PubMed]
- 18.Papaioannou A, Giangregorio L, Kvern B, Boulos P, Ioannidis G, Adachi JD. The osteoporosis care gap in Canada. BMC Musculoskelet Disord. 2004;6:5–11. [DOI] [PMC free article] [PubMed]
- 19.Grimshaw JM, Thomas RE, MacLennan G, et al. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol Assessment. 2004;(86):iii–iv, 1–72. [DOI] [PubMed]
- 20.Smith SA, Murphy ME, Huschka TR, et al. Impact of a diabetes electronic management system on the care of patients seen in a subspecialty diabetes clinic. Diabetes Care. 1998;21:972–6. [DOI] [PubMed]
- 21.Baker AM, Lafatat JE, Ward RE, Whitehouse F, Divine G. A web-based diabetes care management support system. J Qual Improv. 2001;27(4):179–90. [DOI] [PubMed]
- 22.Finkelstein J, O’connor G, Friedmann RH. Development and implementation of the home asthma telemonitoring (HAT) system to facilitate asthma self-care. Medinfo. 2001;10(Pt 1):810–4. [PubMed]
- 23.Roccaforte R, Demers C, Baldassarre F, Teo KK, Yusuf S. Effectiveness of comprehensive disease management programmes in improving clinical outcomes in heart failure patients. A meta-analysis. Eur J Heart Fail. 2005;7(7):1133–44. [DOI] [PubMed]
- 24.Gonseth J, Guallar-Castillon P, Banegas JR, Rodriguez-Artalejo F. The effectiveness of disease management programmes in reducing hospital re-admission in older patients with heart failure: a systematic review and meta-analysis of published reports. Eur Heart J. 2004;25:1570–95. [DOI] [PubMed]
- 25.Cadarette SM, Jaglal SB, Kreiger N, McIsaac WJ, Darlington GA, Tu JV. Development and validation of the Osteoporosis Risk Assessment Instrument to facilitate selection of women for bone densitometry. CMAJ. 2000;162(9):1289–94. [PMC free article] [PubMed]
- 26.Haynes RB, McKibbon KA, Wilczynski NL, Walter SD, Werre SR, Hedges Team. Optimal search strategies for retrieving scientifically strong studies of treatment from Medline: analytical survey. BMJ. 2005;330(7501):1179. [DOI] [PMC free article] [PubMed]
- 27.Jüni P, Witschi A, Bloch R, Egger M. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA. 1999;282(11):1054–60. [DOI] [PubMed]
- 28.Moyer A, Finney JW. Rating methodologic quality: toward improved assessment and investigation. Account Res. 2005;12(4):299–313. [DOI] [PubMed]
- 29.Egger M, Smith GD, Altman DG. Systematic reviews in health care: Meta-analysis in context. London: BMJ Publishing Group; 2001:309–10;395–6.
- 30.Barr RJ, Stewart A, Torgerson DJ, Seymour DG, Reid DM. Screening elderly women for risk of future fractures—Participation rates and impact on incidence of falls and fractures. Calcif Tissue Int. 2005;76:243–8. [DOI] [PubMed]
- 31.Bliuc D, Eisman JA, Center JR. A randomized study of two different information-based interventions on the management of osteoporosis in minimal and moderate trauma fractures. Osteoporos Int. 2006;17(9):1309–17. [DOI] [PubMed]
- 32.Boyd JL, Holcomb JP, Rothenberg RJ. Physician treatment of osteoporosis in response to heel ultrasound bone mineral density reports. J Clin Densitometry. 2002;5(4):375–81. [DOI] [PubMed]
- 33.Devereux K, Robertson D, Briffa NK. Effects of a water-based program on women 65 years and over: a randomized controlled trial. Aust J of Physiother. 2005;51:102–8. [DOI] [PubMed]
- 34.Feldstein A, Elmer PJ, Smith DH, et al. Electronic medical record reminder improves osteoporosis management after a fracture: a randomized, controlled trail. J Am Geriatr Soc. 2006;54(3):450–7. [DOI] [PubMed]
- 35.Gardner MJ, Brophy RH, Demetrakopoulos D, et al. Interventions to improve osteoporosis treatment following hip fracture. J Bone and Joint Surg. 2005;87:3–7. [DOI] [PubMed]
- 36.LaCroix AZ, Buist DSM, Brenneman SK, Abbott TA. Evaluation of three population-based strategies for fracture prevention. Medical Care. 2005;43:293–302. [DOI] [PubMed]
- 37.McDonough RP, Doucette WR, Kumbera P, Klepser DG. An evaluation of managing and educating patients on the risk of glucocorticoid-induced osteoporosis. Value in Health. 2005;8(1):24–31. [DOI] [PubMed]
- 38.Morrison LS, Tobias JH. Effect of a case-finding strategy for osteoporosis on bisphosphonate prescribing in primary care. Osteoporosis Int. 2005;16(1):71–7. [DOI] [PubMed]
- 39.Rolnick SJ, Kopher R. What is the impact of osteoporosis education and bone mineral density testing for postmenopausal women in a managed care setting? Menopause. 2001;8(2):141–8. [DOI] [PubMed]
- 40.Solomon DH, Katz JN, La Tourette AM, Coblyn JS. Multifaceted intervention to improve reumatologists’ management of glucocorticoid-induced osteoporosis: A randomized controlled trial. Arthritis & Rheumatism. 2004;51:383–7. [DOI] [PubMed]
- 41.Solomon DH, Finkelsteing JS, Polinski JM, et al. A randomized controlled trial of mailed osteoporosis education to older adults. Osteoporosis Int. 2006;17:760–7. [DOI] [PubMed]
- 42.Solomon DH, Polinski JM, Stedman M, et al. Improving care of patients at-risk for osteoporosis: A randomized controlled trial. J Gen Intern Med. 2007;22(3):362–7. [DOI] [PMC free article] [PubMed]
- 43.Allen ML, Wyatt LE. Guidelines for the diagnosis, screening, and treatment of osteoporosis in women. Adv Studies Med. 2005;5(10):518–23.
- 44.Ashworth NL, Chad KE, Harrison EL, Reeder BA, Marshall SC. Home versus center based physical activity programs in older adults. Cochrane Database Syst Rev. 2005;25(1):CD004017, Jan. [DOI] [PMC free article] [PubMed]
- 45.Bai B, Wang KZ, Liu WK, Song JH, Chen JC. Comprehensive treatment for old patients with hip fractures. Chin J Traumatol. 2003;6(5):297–301. [PubMed]
- 46.Berard A, Bravo G, Gauthier P. Meta-analysis of the effectiveness of physical activity for the prevention of bone loss in postmenopausal women. Osteoporosis Int. 1997;7(4):331–7. [DOI] [PubMed]
- 47.Berarducci A. The effects of an osteoporosis preventive cognitive/behavioural intervention on knowledge, self-efficacy, role strain, and intention in midlife women. University of South Florida PhD dissertation.
- 48.Blalock SJ, Currey SS, DeVellis RF, et al. Effects of educational materials concerning osteoporosis on women’s knowledge, beliefs, and behaviour. Am J Health Prom. 2000;14(3):161–9. [DOI] [PubMed]
- 49.Bonaiuti D, Cranney A, Iovine R, et al. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev. 2002;(3):CD000333. [DOI] [PubMed]
- 50.Boulos P, Adachi JD. Guidelines for the prevention and therapy of glucocorticoid-induced osteoporosis. Clin Exp Rheumatol. 2000;18(Suppl 21):S79–86.
- 51.Brenneman SK, Lacroix AZ, Buist DSM, Chen Y, Abbott TA III. Evaluation of decision rules to identify postmenopausal women for inervention related to osteoporosis. Dis Manage. 2003;6:159–68. [DOI] [PubMed]
- 52.Buist DSM, LaCroix AZ, Manfredonia D, Abbott T. Identifying postmenopausal women at high risk of fracture in populations: a comparison of three strategies. J Am Geriatrics Soc. 2002;50(6):1031–8. [DOI] [PubMed]
- 53.Colon-Emeric CS, Caminis J, Suh TT, et al. The HORIZON Recruitment Fracture Trial: Design of a clinical trial in the prevention of subsequent fractures after low trauma hip fracture repair. Curr Med Res Opin. 2004;20(6):903–10. [DOI] [PubMed]
- 54.Delafuente JC, Weakley DF. Can an educational program for residents of assisted living facilities improve drug utilization for osteoporosis? Consultant Pharmacist. 2005;20(2):137–40. [DOI] [PubMed]
- 55.Devogelaer J-P, Goemaere S, Boonen S, et al. Evidence-based guidelines for the prevention and treatment of glucocorticoid-induced osteoporosis: A consensus document of the Belgian Bone Club. Osteoporosis Int. 2006;17(1):8–19. [DOI] [PubMed]
- 56.Dhillon V, Creiger J, Hannan J, Hurst N, Nuki G. The effect of DXA scanning on clinical decision making by general practitioners: A randomized, prospective trial of direct access versus referral to a hospital consultant. Osteoporosis Int. 2003;14:326–33. [DOI] [PubMed]
- 57.Edwards BJ, Brooks ER, Langman CB. Osteoporosis screening of postmenopausal women in the primary care setting: A case-based approach. Gender Med. 2004;1(2):70–85. [DOI] [PubMed]
- 58.Gold DT, Shipp KM, Pieper CF, Duncan PW, Martinez S, Lyles KW. Group treatment improves trunk strength and psychological status in older women with vertebral fractures: results osf a randomized, clinical trial. J Am Geriatr Soc. 2004;52:1471–8. [DOI] [PubMed]
- 59.Hunskaar S, Hannestad YS, Backe B, Matheson I. Direct, mailing of consensus recommendations did not alter GPs’ knowledge and prescription of oestrogen in the menopause. Scand J Prim Health Care. 1996;14(4):203–8. [DOI] [PubMed]
- 60.Josse R, Tenengouse AM, Hanley DA, et al. Clinical practice guidelines for the diagnosis and management of osteoporosis. CMAJ. 1996;155(8):1113–29. [PMC free article] [PubMed]
- 61.Lock CA, Lecouturier J, Mason JM, Dickinson HO. Lifestyle interventions to prevent osteoporotic fractures: A systematic review. Osteoporosis Int. 2006;17(1):20–8. [DOI] [PubMed]
- 62.McGinley AM. Effect of Web-based computer-tailoring on women’s intention to continue or begin to use hormone replacement therapy to lower their risk for osteoporosis. University of Pennsylvania PhD Dissertation 2002.
- 63.Nelson ME, Fiatarone MA, Morganit CM, Trice I, Greenberg RA, Evans WJ. Effects of high-intensity strength training on multiple risk factors for osteoporotic fractures: a randomized controlled trial. JAMA. 1994;272:1909–14. [DOI] [PubMed]
- 64.O’Connor AM, Stacey D, Entwistle V, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2003;(2):CD001431. [DOI] [PubMed]
- 65.Polinski JM, Brookhart MA, Katz JN, et al. Educational outreach (academic detailing) regarding osteoporosis in primary care. Pharmacoepidemiol Drug Safety. 2005;14:843–50. [DOI] [PubMed]
- 66.Feder G, Cryer D, Donovan S, Carter Y. Guidelines for the prevention of falls in people over 65. BMJ. 2000;321:1007–11. [DOI] [PMC free article] [PubMed]
- 67.Rothert ML, Holmes-Rovner M, Rovner D, et al. An educational intervention as decision support for menopausal women. Res Nurs Health. 1997;20(5):377–87. [DOI] [PubMed]
- 68.Silverman SL, Greenwald M, Klein RA, Drinkwater BL. Effect of bone density information on decisions about hormone replacement therapy: a randomized trail. Obstetrics Gynecol. 1997;89(3):321–5. [DOI] [PubMed]
- 69.Stock JL, Waud CE, Coderre JA, et al. Clinical reporting to primary care physicians leads to increased use and understanding of bone densitometry and affects the management of osteoporosis. A randomized trial. Ann Intern Med. 1998;128(12 Pt 1):996–9. [DOI] [PubMed]
- 70.Winzenberg T, Oldenburg B, Frendin S, De Wit L, Riley M, Jones G. The effect on behaviour and bone mineral density of individualized bone mineral density feedback and educational interventions in premenopausal women: a randomized controlled trial. BMC Pub Health. 2006;6:12. [DOI] [PMC free article] [PubMed]
- 71.Cook DJ, Sackett DL, Spitzer WO. Methodologic guidelines for systematic reviews of randomized control trials in health care from the Potsdam consultation on meta-analysis. J Clin Epidemiol. 1995;48:167–71. [DOI] [PubMed]
- 72.Sullivan F, Wyatt JC. How decision support tools help define clinical problems. BMJ. 2005;331:831–3. [DOI] [PMC free article] [PubMed]
- 73.Randolph AG, Haynes RB, Wyatt JC, Cook DJ, Guyatt GH. Users’ guides to the medical literature XVII. How to use an article evaluating the clinical impact of a computer-based clinical decision support system. JAMA. 1999;281:67–74. [DOI] [PubMed]
- 74.Garg AX, Adhikari NKJ, McDonald H, et al. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review. JAMA. 2005;293:1223–38. [DOI] [PubMed]
- 75.Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ. 2005;330:765–73. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.
(DOC 141 kb)


