Abstract
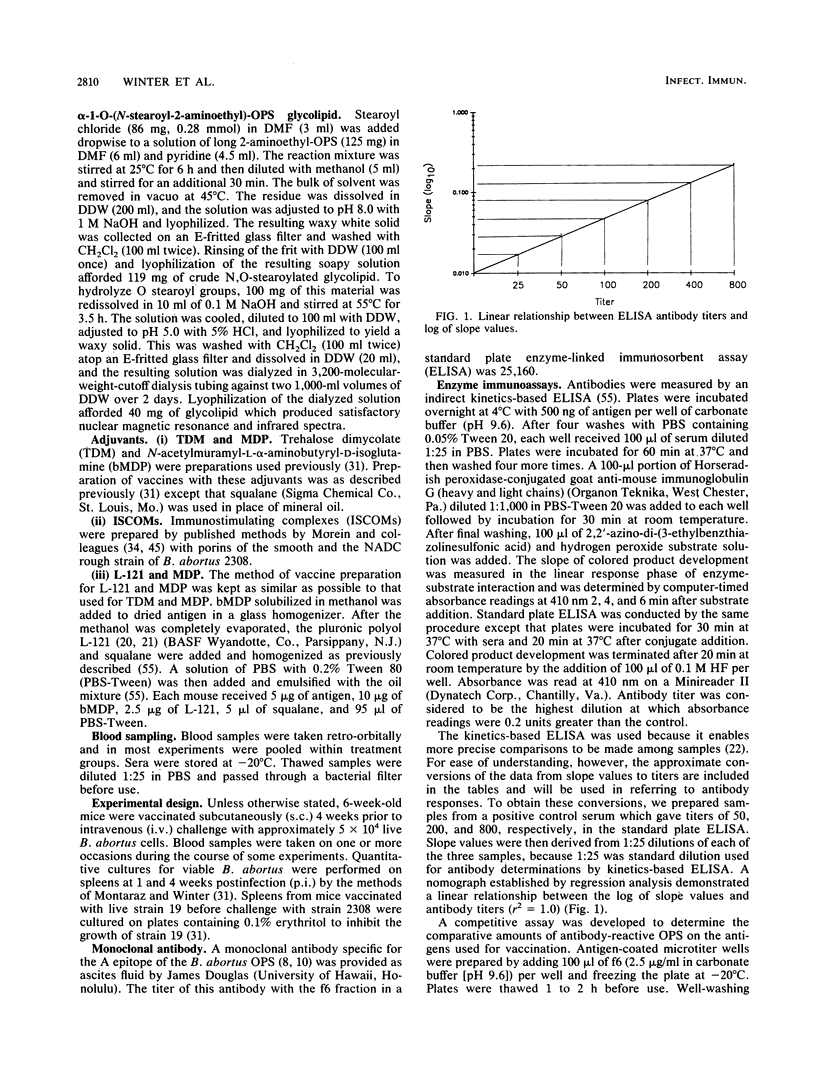
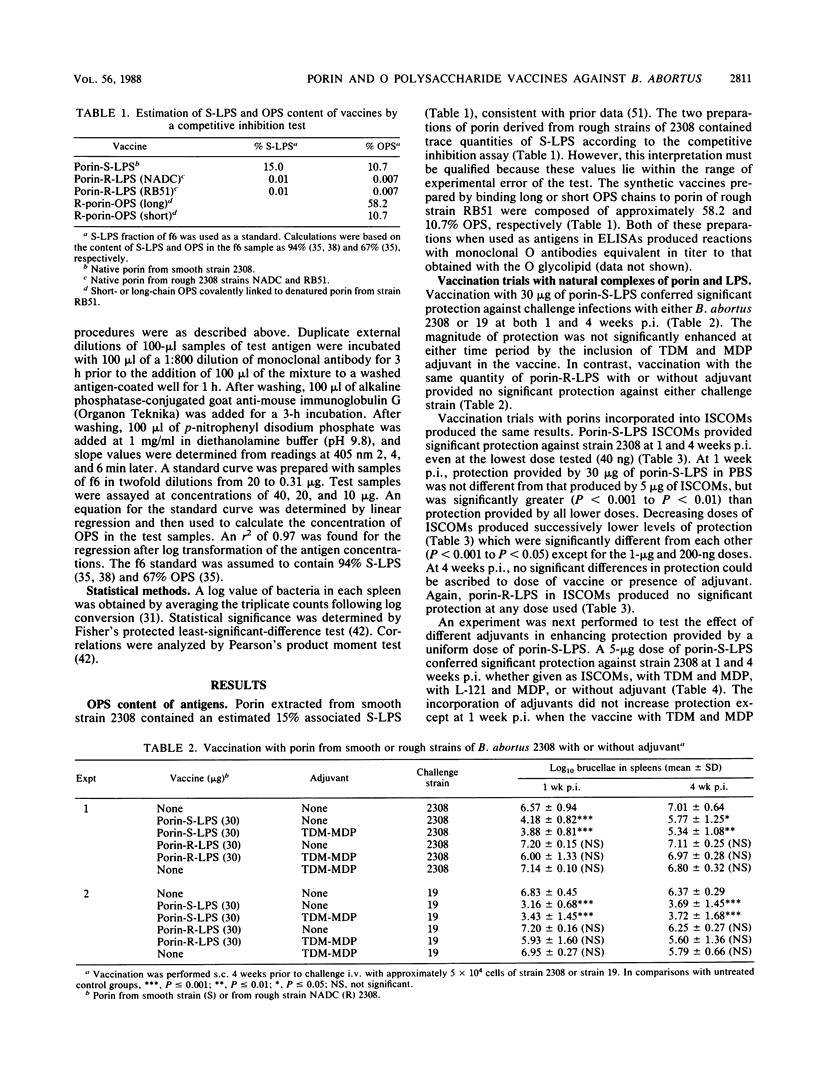
A single vaccination of mice with a complex of porin and smooth lipopolysaccharide (porin-S-LPS) extracted from virulent Brucella abortus 2308 provided significant protection (P less than 0.01 to P less than 0.001) against challenge with the same strain, equivalent to that achieved by vaccination with living attenuated B. abortus 19. The porin-S-LPS vaccine given without adjuvant or in several adjuvants (trehalose dimycolate and muramyl dipeptide; the pluronic polymer L-121 and muramyl dipeptide; or complexed with Quil A in immunostimulating complexes) provided equivalent protection. In contrast, one vaccination with porin complexed with rough LPS (porin-R-LPS) from a rough mutant of strain 2308 provided no protection with any adjuvant tested. In one experiment, two inoculations with the porin-R-LPS resulted in a low level of protection, probably owing to priming of the animals for production of O-polysaccharide-specific antibodies. However, one vaccination with rough-strain porin covalently bound to purified O polysaccharide conferred protection equal to that obtained with natural complexes of porin-S-LPS or with living strain 19. A synthetic vaccine containing long chains of O polysaccharide was more effective than one prepared with short chains. Protective vaccines caused the formation of increased concentrations of circulating O-polysaccharide-specific antibodies, although there were individual exceptions to the quantitative association between O-polysaccharide-specific antibodies and protection. Antibodies specific for porin or R-LPS were found in negligible quantities in vaccinated mice. These results provide additional evidence that the O polysaccharide will constitute an essential component of an effective subcellular vaccine against B. abortus and that O-polysaccharide-specific antibodies play an important role in protective immunity in brucellosis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam A., Petit J. F., Lefrancier P., Lederer E. Muramyl peptides. Chemical structure, biological activity and mechanism of action. Mol Cell Biochem. 1981 Dec 4;41:27–47. doi: 10.1007/BF00225295. [DOI] [PubMed] [Google Scholar]
- Baldwin C. L., Verstreate D. R., Winter A. J. Immune response of cattle to Brucella abortus outer membrane proteins measured by lymphocyte blastogenesis. Vet Immunol Immunopathol. 1985 Aug;9(4):383–396. doi: 10.1016/0165-2427(85)90067-4. [DOI] [PubMed] [Google Scholar]
- Bascoul S., Cannat A., Huguet M. F., Serre A. Studies on the immune protection to murine experimental brucellosis conferred by Brucella fractions. I. Positive role of immune serum. Immunology. 1978 Aug;35(2):213–221. [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar N., Müller W., Schlecht S. Proteins from Salmonella R-mutants mediating protection against Salmonella typhimurium infection in mice I. Preparation of proteins free from lipopolysaccharide using various chromatographic methods. Zentralbl Bakteriol Mikrobiol Hyg A. 1982 Oct;253(1):88–101. [PubMed] [Google Scholar]
- Bosseray N. Immunity to Brucella in mice vaccinated with a fraction (F8) or a killed vaccine (H38) with or without adjuvant. Level and duration of immunity in relation to dose of vaccine, recall injection and age of mice. Br J Exp Pathol. 1978 Aug;59(4):354–365. [PMC free article] [PubMed] [Google Scholar]
- Bosseray N., Plommet A. M., Plommet M. Theoretical, practical and statistical basis for a general control method of activity for anti-Brucella vaccines. Dev Biol Stand. 1984;56:257–270. [PubMed] [Google Scholar]
- Brodeur B. R., Larose Y., Tsang P., Hamel J., Ashton F., Ryan A. Protection against infection with Neisseria meningitidis group B serotype 2b by passive immunization with serotype-specific monoclonal antibody. Infect Immun. 1985 Nov;50(2):510–516. doi: 10.1128/iai.50.2.510-516.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundle D. R., Gidney M. A., Perry M. B., Duncan J. R., Cherwonogrodzky J. W. Serological confirmation of Brucella abortus and Yersinia enterocolitica O:9 O-antigens by monoclonal antibodies. Infect Immun. 1984 Nov;46(2):389–393. doi: 10.1128/iai.46.2.389-393.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byars N. E., Allison A. C. Adjuvant formulation for use in vaccines to elicit both cell-mediated and humoral immunity. Vaccine. 1987 Sep;5(3):223–228. doi: 10.1016/0264-410x(87)90105-8. [DOI] [PubMed] [Google Scholar]
- Caroff M., Bundle D. R., Perry M. B., Cherwonogrodzky J. W., Duncan J. R. Antigenic S-type lipopolysaccharide of Brucella abortus 1119-3. Infect Immun. 1984 Nov;46(2):384–388. doi: 10.1128/iai.46.2.384-388.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheers C. Pathogenesis and cellular immunity in experimental murine brucellosis. Dev Biol Stand. 1984;56:237–246. [PubMed] [Google Scholar]
- Colwell D. E., Michalek S. M., Briles D. E., Jirillo E., McGhee J. R. Monoclonal antibodies to Salmonella lipopolysaccharide: anti-O-polysaccharide antibodies protect C3H mice against challenge with virulent Salmonella typhimurium. J Immunol. 1984 Aug;133(2):950–957. [PubMed] [Google Scholar]
- Confer A. W., Tabatabai L. B., Deyoe B. L., Oltjen S. L., Hall S. M., Oltjen J. W., Morton R. J., Fulnechek D. L., Smith R. E., Smith R. A. Vaccination of cattle with chemically modified and unmodified salt-extractable proteins from Brucella abortus. Vet Microbiol. 1987 Dec;15(4):325–339. doi: 10.1016/0378-1135(87)90020-4. [DOI] [PubMed] [Google Scholar]
- Corner L. A., Alton G. G. Persistence of Brucella abortus strain 19 infection in adult cattle vaccinated with reduced doses. Res Vet Sci. 1981 Nov;31(3):342–344. [PubMed] [Google Scholar]
- Dubray G., Bézard G. Isolation of three Brucella abortus cell-wall antigens protective in murine experimental brucellosis. Ann Rech Vet. 1980;11(4):367–373. [PubMed] [Google Scholar]
- Eisenstein T. K., Killar L. M., Sultzer B. M. Immunity to infection with Salmonella typhimurium: mouse-strain differences in vaccine- and serum-mediated protection. J Infect Dis. 1984 Sep;150(3):425–435. doi: 10.1093/infdis/150.3.425. [DOI] [PubMed] [Google Scholar]
- Frasch C. E. Status of a group B Neisseria meningitidis vaccine. Eur J Clin Microbiol. 1985 Dec;4(6):533–536. doi: 10.1007/BF02013388. [DOI] [PubMed] [Google Scholar]
- Gilleland H. E., Jr, Parker M. G., Matthews J. M., Berg R. D. Use of a purified outer membrane protein F (porin) preparation of Pseudomonas aeruginosa as a protective vaccine in mice. Infect Immun. 1984 Apr;44(1):49–54. doi: 10.1128/iai.44.1.49-54.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulig P. A., McCracken G. H., Jr, Frisch C. F., Johnston K. H., Hansen E. J. Antibody response of infants to cell surface-exposed outer membrane proteins of Haemophilus influenzae type b after systemic Haemophilus disease. Infect Immun. 1982 Jul;37(1):82–88. doi: 10.1128/iai.37.1.82-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter R. L., Bennett B. The adjuvant activity of nonionic block polymer surfactants. II. Antibody formation and inflammation related to the structure of triblock and octablock copolymers. J Immunol. 1984 Dec;133(6):3167–3175. [PubMed] [Google Scholar]
- Hunter R., Strickland F., Kézdy F. The adjuvant activity of nonionic block polymer surfactants. I. The role of hydrophile-lipophile balance. J Immunol. 1981 Sep;127(3):1244–1250. [PubMed] [Google Scholar]
- Jacobson R. H., Downing D. R., Lynch T. J. Computer-assisted enzyme immunoassays and simplified immunofluorescence assays: applications for the diagnostic laboratory and the veterinarian's office. J Am Vet Med Assoc. 1982 Nov 15;181(10):1166–1168. [PubMed] [Google Scholar]
- Kuusi N., Nurminen M., Saxen H., Valtonen M., Mäkelä P. H. Immunization with major outer membrane proteins in experimental salmonellosis of mice. Infect Immun. 1979 Sep;25(3):857–862. doi: 10.1128/iai.25.3.857-862.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer E. Synthetic immunostimulants derived from the bacterial cell wall. J Med Chem. 1980 Aug;23(8):819–825. doi: 10.1021/jm00182a001. [DOI] [PubMed] [Google Scholar]
- Limet J., Plommet A. M., Dubray G., Plommet M. Immunity conferred upon mice by anti-LPS monoclonal antibodies in murine brucellosis. Ann Inst Pasteur Immunol. 1987 May-Jun;138(3):417–424. doi: 10.1016/s0769-2625(87)80052-1. [DOI] [PubMed] [Google Scholar]
- Loeb M. R., Smith D. H. Human antibody response to individual outer membrane proteins of Haemophilus influenzae type b. Infect Immun. 1982 Sep;37(3):1032–1036. doi: 10.1128/iai.37.3.1032-1036.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. THE IMMUNOLOGICAL BASIS OF ACQUIRED CELLULAR RESISTANCE. J Exp Med. 1964 Jul 1;120:105–120. doi: 10.1084/jem.120.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madraso E. D., Cheers C. Polyadenylic acid-polyuridylic acid (poly A : U) and experimental murine brucellosis. II. Macrophages as target cells of poly A : U in experimental brucellosis. Immunology. 1978 Jul;35(1):77–84. [PMC free article] [PubMed] [Google Scholar]
- Matthews-Greer J. M., Gilleland H. E., Jr Outer membrane protein F (porin) preparation of Pseudomonas aeruginosa as a protective vaccine against heterologous immunotype strains in a burned mouse model. J Infect Dis. 1987 Jun;155(6):1282–1291. doi: 10.1093/infdis/155.6.1282. [DOI] [PubMed] [Google Scholar]
- Montaraz J. A., Winter A. J. Comparison of living and nonliving vaccines for Brucella abortus in BALB/c mice. Infect Immun. 1986 Aug;53(2):245–251. doi: 10.1128/iai.53.2.245-251.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaraz J. A., Winter A. J., Hunter D. M., Sowa B. A., Wu A. M., Adams L. G. Protection against Brucella abortus in mice with O-polysaccharide-specific monoclonal antibodies. Infect Immun. 1986 Mar;51(3):961–963. doi: 10.1128/iai.51.3.961-963.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morein B., Sundquist B., Höglund S., Dalsgaard K., Osterhaus A. Iscom, a novel structure for antigenic presentation of membrane proteins from enveloped viruses. 1984 Mar 29-Apr 4Nature. 308(5958):457–460. doi: 10.1038/308457a0. [DOI] [PubMed] [Google Scholar]
- Moreno E., Pitt M. W., Jones L. M., Schurig G. G., Berman D. T. Purification and characterization of smooth and rough lipopolysaccharides from Brucella abortus. J Bacteriol. 1979 May;138(2):361–369. doi: 10.1128/jb.138.2.361-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardon P. Resistance against a subcutaneous Brucella challenge of mice immunized with living or dead Brucella or by transfer of immune serum. Ann Immunol (Paris) 1977 Nov-Dec;128(6):1025–1037. [PubMed] [Google Scholar]
- Pavlov H., Hogarth M., McKenzie I. F., Cheers C. In vivo and in vitro effects of monoclonal antibody to Ly antigens on immunity to infection. Cell Immunol. 1982 Jul 15;71(1):127–138. doi: 10.1016/0008-8749(82)90502-0. [DOI] [PubMed] [Google Scholar]
- Plommet M., Plommet A. M. Immune serum-mediated effects on brucellosis evolution in mice. Infect Immun. 1983 Jul;41(1):97–105. doi: 10.1128/iai.41.1.97-105.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SULITZEANU D. Passive protection experiments with Brucella antisera. J Hyg (Lond) 1955 Jun;53(2):133–142. doi: 10.1017/s0022172400000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxén H., Nurminen M., Kuusi N., Svenson S. B., Mäkelä P. H. Evidence for the importance of O antigen specific antibodies in mouse-protective Salmonella outer membrane protein (porin) antisera. Microb Pathog. 1986 Oct;1(5):433–441. doi: 10.1016/0882-4010(86)90005-7. [DOI] [PubMed] [Google Scholar]
- Sundquist B., Lövgren K., Höglund S., Morein B. Influenza virus ISCOMs: biochemical characterization. Vaccine. 1988 Feb;6(1):44–48. doi: 10.1016/0264-410x(88)90013-8. [DOI] [PubMed] [Google Scholar]
- Svenson S. B., Nurminen M., Lindberg A. A. Artificial Salmonella vaccines: O-antigenic oligosaccharide-protein conjugates induce protection against infection with Salmonella typhimurium. Infect Immun. 1979 Sep;25(3):863–872. doi: 10.1128/iai.25.3.863-872.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabatabai L. B., Deyoe B. L. Biochemical and biological properties of soluble protein preparations from Brucella abortus. Dev Biol Stand. 1984;56:199–211. [PubMed] [Google Scholar]
- Tabatabai L. B., Deyoe B. L., Ritchie A. E. Isolation and characterization of toxic fractions from Brucella abortus. Infect Immun. 1979 Nov;26(2):668–679. doi: 10.1128/iai.26.2.668-679.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udhayakumar V., Muthukkaruppan V. R. Protective immunity induced by outer membrane proteins of Salmonella typhimurium in mice. Infect Immun. 1987 Mar;55(3):816–821. doi: 10.1128/iai.55.3.816-821.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstreate D. R., Creasy M. T., Caveney N. T., Baldwin C. L., Blab M. W., Winter A. J. Outer membrane proteins of Brucella abortus: isolation and characterization. Infect Immun. 1982 Mar;35(3):979–989. doi: 10.1128/iai.35.3.979-989.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter A. J., Rowe G. E. Comparative immune responses to native cell envelope antigens and the hot sodium dodecyl sulfate insoluble fraction (PG) of Brucella abortus in cattle and mice. Vet Immunol Immunopathol. 1988 Mar;18(2):149–163. doi: 10.1016/0165-2427(88)90057-8. [DOI] [PubMed] [Google Scholar]
- Winter A. J., Verstreate D. R., Hall C. E., Jacobson R. H., Castleman W. L., Meredith M. P., McLaughlin C. A. Immune response to porin in cattle immunized with whole cell, outer membrane, and outer membrane protein antigens of Brucella abortus combined with trehalose dimycolate and muramyl dipeptide adjuvants. Infect Immun. 1983 Dec;42(3):1159–1167. doi: 10.1128/iai.42.3.1159-1167.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young E. J. Human brucellosis. Rev Infect Dis. 1983 Sep-Oct;5(5):821–842. doi: 10.1093/clinids/5.5.821. [DOI] [PubMed] [Google Scholar]
- van der Ley P., Kuipers O., Tommassen J., Lugtenberg B. O-antigenic chains of lipopolysaccharide prevent binding of antibody molecules to an outer membrane pore protein in Enterobacteriaceae. Microb Pathog. 1986 Feb;1(1):43–49. doi: 10.1016/0882-4010(86)90030-6. [DOI] [PubMed] [Google Scholar]



