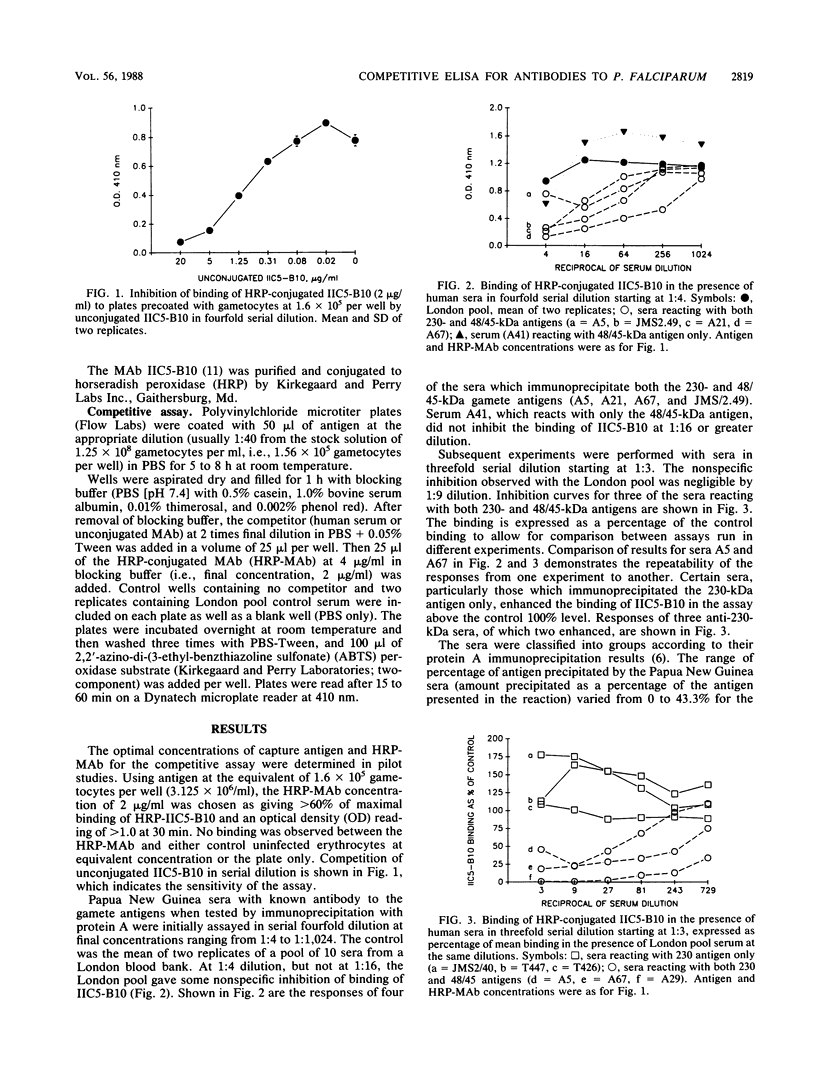
Abstract
The antibody response to an epitope on gamete antigens of Plasmodium falciparum in persons naturally exposed to malaria has been investigated by competitive enzyme-linked immunosorbent assay. The assay detects antibodies to an epitope on the 48/45-kilodalton (kDa) gamete surface antigen by competition with horseradish peroxidase-labeled monoclonal antibody IIC5-B10. Five sera previously shown to immunoprecipitate the 230- and 48/45-kDa antigens significantly inhibited IIC5-B10 binding to an average of 24.2% of control. The one serum which precipitated only the 48/45-kDa antigen did not inhibit IIC5-B10 binding. For 26 sera which were negative by immunoprecipitation, mean binding in the assay was 112.7% of control (pooled London nonimmune sera). Recognition of both 230-kDa and 48/45-kDa antigens was associated with a titer of 1:9 or greater (reciprocal geometric mean titer, 27.6) for inhibition to more than 2 standard deviations from the mean of the negative sera. The results show that the IIC5-B10 binding site is a naturally immunogenic epitope recognized by the majority of persons who had antibodies to the 48/45-kDa protein. An additional finding was enhancement of binding of IIC5-B10 to an average of 154.4% of control by five sera which recognized only the 230-kDa antigen, presumably due to conformational alteration of the gamete antigen complex.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Budkowska A., Dubreuil P., Pillot J. Detection of antibodies to pre-S2 encoded epitopes of hepatitis B virus by monoclonal antibody-enzyme immunoassay. J Immunol Methods. 1987 Aug 24;102(1):85–92. doi: 10.1016/s0022-1759(87)80013-3. [DOI] [PubMed] [Google Scholar]
- Burke D. S., Nisalak A., Gentry M. K. Detection of flavivirus antibodies in human serum by epitope-blocking immunoassay. J Med Virol. 1987 Oct;23(2):165–173. doi: 10.1002/jmv.1890230209. [DOI] [PubMed] [Google Scholar]
- Carter R., Bushell G., Saul A., Graves P. M., Kidson C. Two apparently nonrepeated epitopes on gametes of Plasmodium falciparum are targets of transmission-blocking antibodies. Infect Immun. 1985 Oct;50(1):102–106. doi: 10.1128/iai.50.1.102-106.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruise K. M., Mitchell G. F., Garcia E. G., Anders R. F. Hybridoma antibody immunoassays for the detection of parasitic infection: further studies on a monoclonal antibody with immunodiagnostic potential for Schistosomiasis japonica. Acta Trop. 1981 Dec;38(4):437–447. [PubMed] [Google Scholar]
- Graves P. M., Burkot T. R., Carter R., Cattani J. A., Lagog M., Parker J., Brabin B. J., Gibson F. D., Bradley D. J., Alpers M. P. Measurement of malarial infectivity of human populations to mosquitoes in the Madang area, Papua, New Guinea. Parasitology. 1988 Apr;96(Pt 2):251–263. doi: 10.1017/s003118200005825x. [DOI] [PubMed] [Google Scholar]
- Graves P. M., Carter R., Burkot T. R., Quakyi I. A., Kumar N. Antibodies to Plasmodium falciparum gamete surface antigens in Papua New Guinea sera. Parasite Immunol. 1988 Mar;10(2):209–218. doi: 10.1111/j.1365-3024.1988.tb00215.x. [DOI] [PubMed] [Google Scholar]
- Graves P. M., Carter R., Burkot T. R., Rener J., Kaushal D. C., Williams J. L. Effects of transmission-blocking monoclonal antibodies on different isolates of Plasmodium falciparum. Infect Immun. 1985 Jun;48(3):611–616. doi: 10.1128/iai.48.3.611-616.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N., Carter R. Biosynthesis of the target antigens of antibodies blocking transmission of Plasmodium falciparum. Mol Biochem Parasitol. 1984 Nov;13(3):333–342. doi: 10.1016/0166-6851(84)90124-5. [DOI] [PubMed] [Google Scholar]
- Kumar N. Target antigens of malaria transmission blocking immunity exist as a stable membrane bound complex. Parasite Immunol. 1987 May;9(3):321–335. doi: 10.1111/j.1365-3024.1987.tb00511.x. [DOI] [PubMed] [Google Scholar]
- Quakyi I. A., Carter R., Rener J., Kumar N., Good M. F., Miller L. H. The 230-kDa gamete surface protein of Plasmodium falciparum is also a target for transmission-blocking antibodies. J Immunol. 1987 Dec 15;139(12):4213–4217. [PubMed] [Google Scholar]
- Rener J., Graves P. M., Carter R., Williams J. L., Burkot T. R. Target antigens of transmission-blocking immunity on gametes of plasmodium falciparum. J Exp Med. 1983 Sep 1;158(3):976–981. doi: 10.1084/jem.158.3.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen A. N., Ponnudurai T., Beckers P. J., Verhave J. P., Smits M. A., Meuwissen J. H. Sequential expression of antigens on sexual stages of Plasmodium falciparum accessible to transmission-blocking antibodies in the mosquito. J Exp Med. 1985 Nov 1;162(5):1460–1476. doi: 10.1084/jem.162.5.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen A. N., Roeffen W. F., Henderik J. B., Ponnudurai T., Beckers P. J., Meuwissen J. H. Plasmodium falciparum transmission blocking monoclonal antibodies recognize monovalently expressed epitopes. Dev Biol Stand. 1985;62:91–97. [PubMed] [Google Scholar]
- Vermeulen A. N., van Deursen J., Brakenhoff R. H., Lensen T. H., Ponnudurai T., Meuwissen J. H. Characterization of Plasmodium falciparum sexual stage antigens and their biosynthesis in synchronised gametocyte cultures. Mol Biochem Parasitol. 1986 Aug;20(2):155–163. doi: 10.1016/0166-6851(86)90027-7. [DOI] [PubMed] [Google Scholar]


