Abstract
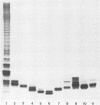
Lipooligosaccharides (LOSs) from four strains of "Haemophilus somnus" were purified and their electrophoretic profile, composition, endotoxic activity, and antigenic properties were analyzed. The LOSs were most efficiently purified by enzyme digestion, hot aqueous phenol extraction, and ultracentrifugation. Each LOS could be separated into two to six distinct bands with apparent Mrs of 3280 to 4960, following electrophoresis in polyacrylamide gels. Each LOS contained dodecanoic, tetradecanoic, and 3-hydroxytetradecanoic fatty acids; a high proportion of hexose, 3-deoxy-D-manno-octulosonic acid, and phosphate; and a small amount of heptose; glucosamine was present in both the oligosaccharide and the lipid A. Each "H. somnus" LOS demonstrated endotoxic activity, as determined by gelation of Limulus ameobocyte lysate, the dermal Schwartzman reaction, and mouse lethality. Antiserum to purified "H. somnus" LOS cross-reacted with all strains of "H. somnus" tested by enzyme-linked immunosorbent assay (ELISA), but not to any Enterobacteriaceae, Pseudomonas, or Pasteurella species tested. "H. somnus" LOS was a poor immunogen, but inhibition, dot blot, and sandwich ELISA data indicated that antibodies made to LOS were predominantly, though not exclusively, to lipid A. Monoclonal antibodies directed to "H. somnus" LOS confirmed that lipid A and non-lipid A determinants were present.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumgartner J. D., O'Brien T. X., Kirkland T. N., Glauser M. P., Ziegler E. J. Demonstration of cross-reactive antibodies to smooth gram-negative bacteria in antiserum to Escherichia coli J5. J Infect Dis. 1987 Jul;156(1):136–143. doi: 10.1093/infdis/156.1.136. [DOI] [PubMed] [Google Scholar]
- Canto G. J., Biberstein E. L. Serological diversity in Haemophilus somnus. J Clin Microbiol. 1982 Jun;15(6):1009–1015. doi: 10.1128/jcm.15.6.1009-1015.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbeil L. B., Arthur J. E., Widders P. R., Smith J. W., Barbet A. F. Antigenic specificity of convalescent serum from cattle with haemophilus somnus-induced experimental abortion. Infect Immun. 1987 Jun;55(6):1381–1386. doi: 10.1128/iai.55.6.1381-1386.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbeil L. B., Blau K., Prieur D. J., Ward A. C. Serum susceptibility of Haemophilus somnus from bovine clinical cases and carriers. J Clin Microbiol. 1985 Aug;22(2):192–198. doi: 10.1128/jcm.22.2.192-198.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbeil L. B., Widders P. R., Gogolewski R., Arthur J., Inzana T. J., Ward A. C. Haemophilus somnus: Bovine Reproductive and Respiratory Disease. Can Vet J. 1986 Feb;27(2):90–93. [PMC free article] [PubMed] [Google Scholar]
- Darveau R. P., Hancock R. E. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J Bacteriol. 1983 Aug;155(2):831–838. doi: 10.1128/jb.155.2.831-838.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. Preparation and properties of antisera against the lipid-A component of bacterial lipopolysaccharides. Eur J Biochem. 1971 Dec 22;24(1):116–122. doi: 10.1111/j.1432-1033.1971.tb19661.x. [DOI] [PubMed] [Google Scholar]
- Gogolewski R. P., Kania S. A., Inzana T. J., Widders P. R., Liggitt H. D., Corbeil L. B. Protective ability and specificity of convalescent serum from calves with Haemophilus somnus pneumonia. Infect Immun. 1987 Jun;55(6):1403–1411. doi: 10.1128/iai.55.6.1403-1411.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel R. A., Anderson P., Loeb M. R., Smith D. H. A polysaccharide-protein complex from Haemophilus influenzae type b. II. Human antibodies to its somatic components. J Infect Dis. 1981 Dec;144(6):521–529. doi: 10.1093/infdis/144.6.521. [DOI] [PubMed] [Google Scholar]
- Inzana T. J., Anderson P. Serum factor-dependent resistance of Haemophilus influenzae type b to antibody to lipopolysaccharide. J Infect Dis. 1985 May;151(5):869–877. doi: 10.1093/infdis/151.5.869. [DOI] [PubMed] [Google Scholar]
- Inzana T. J., Corbeil L. B. Development of a defined medium for Haemophilus somnus isolated from cattle. Am J Vet Res. 1987 Mar;48(3):366–369. [PubMed] [Google Scholar]
- Inzana T. J. Electrophoretic heterogeneity and interstrain variation of the lipopolysaccharide of Haemophilus influenzae. J Infect Dis. 1983 Sep;148(3):492–499. doi: 10.1093/infdis/148.3.492. [DOI] [PubMed] [Google Scholar]
- Inzana T. J., Mathison B. Serotype specificity and immunogenicity of the capsular polymer of Haemophilus pleuropneumoniae serotype 5. Infect Immun. 1987 Jul;55(7):1580–1587. doi: 10.1128/iai.55.7.1580-1587.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzana T. J., Seifert W. E., Jr, Williams R. P. Composition and antigenic activity of the oligosaccharide moiety of Haemophilus influenzae type b lipooligosaccharide. Infect Immun. 1985 May;48(2):324–330. doi: 10.1128/iai.48.2.324-330.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J. A., Andrews J. J., Hargis J. W. Experimental Haemophilus somnus pneumonia in calves. Vet Pathol. 1987 Mar;24(2):129–134. doi: 10.1177/030098588702400205. [DOI] [PubMed] [Google Scholar]
- Johnson K. G., Perry M. B. Improved techniques for the preparation of bacterial lipopolysaccharides. Can J Microbiol. 1976 Jan;22(1):29–34. doi: 10.1139/m76-004. [DOI] [PubMed] [Google Scholar]
- Karkhanis Y. D., Zeltner J. Y., Jackson J. J., Carlo D. J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978 Apr;85(2):595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee C., Robbins J. B. Enzymatic measurement of glucose and galactose content pneumococcal capsular polysaccharides. J Biol Stand. 1978;6(2):97–95. doi: 10.1016/s0092-1157(78)80038-9. [DOI] [PubMed] [Google Scholar]
- Lüderitz O., Galanos C., Lehmann V., Rietschel E. T. Recent findings on the chemical structure and biological activity of bacterial lipopolysaccharides. J Hyg Epidemiol Microbiol Immunol. 1974;18(4):381–390. [PubMed] [Google Scholar]
- Morrison D. C., Ulevitch R. J. The effects of bacterial endotoxins on host mediation systems. A review. Am J Pathol. 1978 Nov;93(2):526–618. [PMC free article] [PubMed] [Google Scholar]
- Novitsky T. J., Roslansky P. F., Siber G. R., Warren H. S. Turbidimetric method for quantifying serum inhibition of Limulus amoebocyte lysate. J Clin Microbiol. 1985 Feb;21(2):211–216. doi: 10.1128/jcm.21.2.211-216.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieroni R. E., Broderick E. J., Bundeally A., Levine L. A simple method for the quantitation of submicrogram amounts of bacterial endotoxin. Proc Soc Exp Biol Med. 1970 Mar;133(3):790–794. doi: 10.3181/00379727-133-34565. [DOI] [PubMed] [Google Scholar]
- Radvany R., Neale N. L., Nowotny A. Relation of structure to function in bacterial O-antigens. VI. Neutralization of endotoxic O-antigens by homologous O-antibody. Ann N Y Acad Sci. 1966 Jun 30;133(2):763–786. doi: 10.1111/j.1749-6632.1966.tb52404.x. [DOI] [PubMed] [Google Scholar]
- Rurangirwa F. R., McGuire T. C., Magnuson N. S., Kibor A., Chema S. Composition of a polysaccharide from mycoplasma (F-38) recognised by antibodies from goats with contagious pleuropneumonia. Res Vet Sci. 1987 Mar;42(2):175–178. [PubMed] [Google Scholar]
- Saunders J. R., Janzen E. D. Haemophilus somnus infections. II. A Canadian field trial of a commercial bacterin: clinical and serological results. Can Vet J. 1980 Aug;21(8):219–224. [PMC free article] [PubMed] [Google Scholar]
- Schneider H., Hale T. L., Zollinger W. D., Seid R. C., Jr, Hammack C. A., Griffiss J. M. Heterogeneity of molecular size and antigenic expression within lipooligosaccharides of individual strains of Neisseria gonorrhoeae and Neisseria meningitidis. Infect Immun. 1984 Sep;45(3):544–549. doi: 10.1128/iai.45.3.544-549.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. L., Gilkerson E. Quantitation of glycosaminoglycan hexosamine using 3-methyl-2-benzothiazolone hydrazone hydrochloride. Anal Biochem. 1979 Oct 1;98(2):478–480. doi: 10.1016/0003-2697(79)90170-2. [DOI] [PubMed] [Google Scholar]
- Stephens L. R., Little P. B., Humphrey J. D., Wilkie B. N., Barnum D. A. Vaccination of cattle against experimentally induced thromboembolic meningoencephalitis with a Haemophilus somnus bacterin. Am J Vet Res. 1982 Aug;43(8):1339–1342. [PubMed] [Google Scholar]
- Stephens L. R., Little P. B. Ultrastructure of Haemophilus somnus, causative agent of bovine infectious thromboembolic meningoencephalitis. Am J Vet Res. 1981 Sep;42(9):1638–1640. [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Teng N. N., Kaplan H. S., Hebert J. M., Moore C., Douglas H., Wunderlich A., Braude A. I. Protection against gram-negative bacteremia and endotoxemia with human monoclonal IgM antibodies. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1790–1794. doi: 10.1073/pnas.82.6.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Boykins R., Frasch C. E. Heterogeneity and variation among Neisseria meningitidis lipopolysaccharides. J Bacteriol. 1983 Aug;155(2):498–504. doi: 10.1128/jb.155.2.498-504.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- WOLFROM M. L., PATIN D. L., DELEDERKREMER R. M. THIN-LAYER CHROMATOGRAPHY ON MICROCRYSTALLINE CELLULOSE. J Chromatogr. 1965 Mar;17:488–494. doi: 10.1016/s0021-9673(00)99899-6. [DOI] [PubMed] [Google Scholar]
- Widders P. R., Paisley L. G., Gogolewski R. P., Evermann J. F., Smith J. W., Corbeil L. B. Experimental abortion and the systemic immune response to "Haemophilus somnus" in cattle. Infect Immun. 1986 Nov;54(2):555–560. doi: 10.1128/iai.54.2.555-560.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright B. G., Rebers P. A. Procedure for determining heptose and hexose in lipopolysaccharides. Modification of the cysteine-sulfuric acid method. Anal Biochem. 1972 Oct;49(2):307–319. doi: 10.1016/0003-2697(72)90433-2. [DOI] [PubMed] [Google Scholar]