Abstract
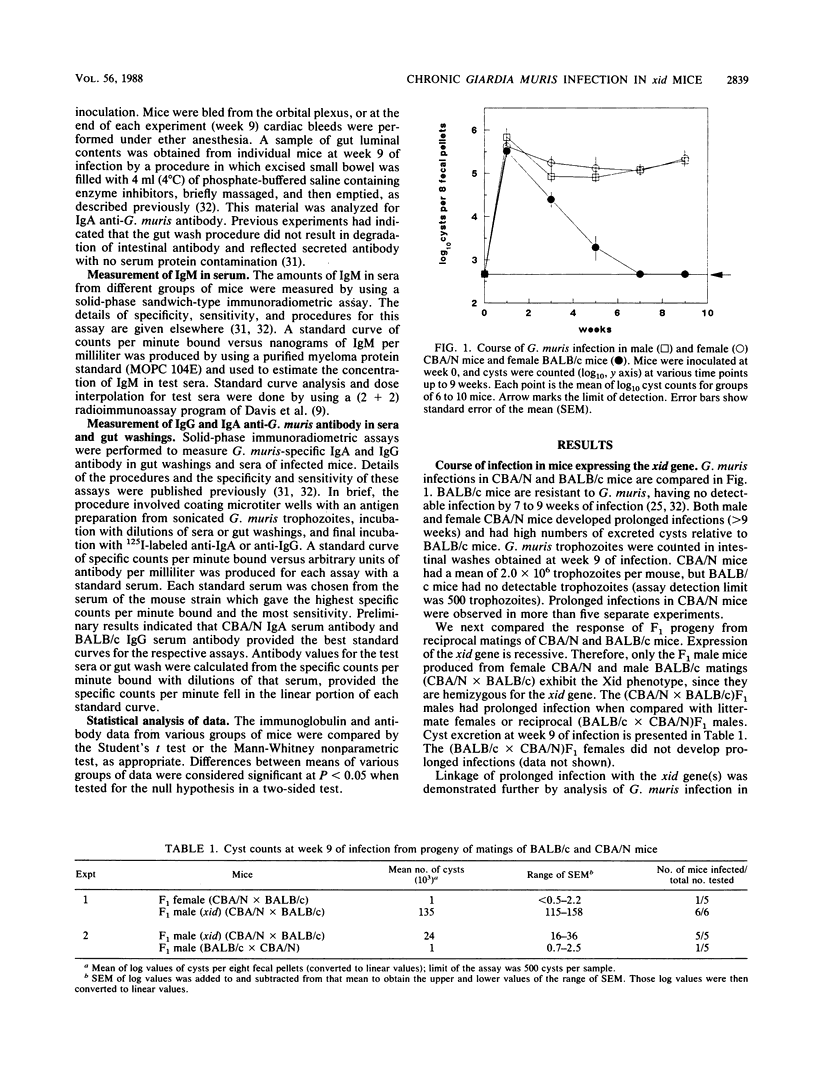
The role of antibody in immunity to Giardia muris infection was investigated by studying B-cell-deficient CBA/N mice expressing the xid gene. After gastric administration of infective G. muris cysts, CBA/N male and female mice developed prolonged G. muris infection, whereas BALB/c mice eliminated their infection in 6 to 8 weeks. Male F1 progeny obtained from matings between female CBA/N mice and male BALB/c mice expressed the xid gene and developed prolonged infections. In contrast, all other F1 progeny of CBA/N and BALB/c matings, which did express the xid gene, eliminated G. muris. The link between the xid gene and prolonged infection was confirmed by studies of C57BL/6 mice congenic for the xid gene. When compared with BALB/c or F1 mice, CBA/N mice produced large quantities of immunoglobulin A (IgA) anti-G. muris antibody in serum and gut secretions during prolonged infection. Serum IgG anti-G. muris antibody levels were reduced in CBA/N and F1 male mice that expressed the xid gene. The inability of xid mice to eliminate G. muris is consistent with the importance of antibody in the development of immunity to G. muris. We hypothesize that mice bearing the xid gene fail to produce IgA antibody of appropriate specificity to an antigen or antigens whose recognition by antibody is critical for successful elimination of the parasite.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amsbaugh D. F., Hansen C. T., Prescott B., Stashak P. W., Asofsky R., Baker P. J. Genetic control of the antibody response to type 3 pneumococcal polysaccharide in mice. II. Relationship between IgM immunoglobulin levels and the ability to give an IgM antibody response. J Exp Med. 1974 Jun 1;139(6):1499–1512. doi: 10.1084/jem.139.6.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsbaugh D. F., Hansen C. T., Prescott B., Stashak P. W., Barthold D. R., Baker P. J. Genetic control of the antibody response to type 3 pneumococcal polysaccharide in mice. I. Evidence that an X-linked gene plays a decisive role in determining responsiveness. J Exp Med. 1972 Oct 1;136(4):931–949. doi: 10.1084/jem.136.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belosevic M., Faubert G. M., Skamene E., MacLean J. D. Susceptibility and resistance of inbred mice to Giardia muris. Infect Immun. 1984 May;44(2):282–286. doi: 10.1128/iai.44.2.282-286.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett S. J., Cox F. E. Immunological aspects of Giardia muris and Spironucleus muris infections in inbred and outbred strains of laboratory mice: a comparative study. Parasitology. 1982 Aug;85(Pt 1):85–99. doi: 10.1017/s0031182000054172. [DOI] [PubMed] [Google Scholar]
- Briles D. E., Nahm M., Marion T. N., Perlmutter R. M., Davie J. M. Streptococcal group A carbohydrate has properties of both a thymus-independent (TI-2) and a thymus-dependent antigen. J Immunol. 1982 May;128(5):2032–2035. [PubMed] [Google Scholar]
- Brinkmann V., Remington J. S., Sharma S. D. Protective immunity in toxoplasmosis: correlation between antibody response, brain cyst formation, T-cell activation, and survival in normal and B-cell-deficient mice bearing the H-2k haplotype. Infect Immun. 1987 Apr;55(4):990–994. doi: 10.1128/iai.55.4.990-994.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrow E. W., Hector R. F., Domer J. E. Immunodeficient CBA/N mice respond effectively to Candida albicans. Clin Immunol Immunopathol. 1984 Dec;33(3):371–380. doi: 10.1016/0090-1229(84)90308-8. [DOI] [PubMed] [Google Scholar]
- Cohen D. I., Hedrick S. M., Nielsen E. A., D'Eustachio P., Ruddle F., Steinberg A. D., Paul W. E., Davis M. M. Isolation of a cDNA clone corresponding to an X-linked gene family (XLR) closely linked to the murine immunodeficiency disorder xid. 1985 Mar 28-Apr 3Nature. 314(6009):369–372. doi: 10.1038/314369a0. [DOI] [PubMed] [Google Scholar]
- Davis S. E., Munson P. J., Jaffe M. L., Rodbard D. Radioimmunoassay data processing with a small programmable calculator. J Immunoassay. 1980;1(1):15–25. doi: 10.1080/01971528008055773. [DOI] [PubMed] [Google Scholar]
- Duran L. W., Metcalf E. S. Antibody-defective, genetically susceptible CBA/N mice have an altered Salmonella typhimurium-specific B cell repertoire. J Exp Med. 1987 Jan 1;165(1):29–46. doi: 10.1084/jem.165.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez C., Möller G. Immunological unresponsiveness to thymus-independent antigens: two fundamentally different genetic mechanisms of B-cell unresponsiveness to dextran. J Exp Med. 1977 Dec 1;146(6):1663–1677. doi: 10.1084/jem.146.6.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillon J., Al Thamery D., Ferguson A. Features of small intestinal pathology (epithelial cell kinetics, intraepithelial lymphocytes, disaccharidases) in a primary Giardia muris infection. Gut. 1982 Jun;23(6):498–506. doi: 10.1136/gut.23.6.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyworth M. F., Carlson J. R., Ermak T. H. Clearance of Giardia muris infection requires helper/inducer T lymphocytes. J Exp Med. 1987 Jun 1;165(6):1743–1748. doi: 10.1084/jem.165.6.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter K. W., Jr, Finkelman F. D., Strickland G. T., Sayles P. C., Scher I. Defective resistance to Plasmodium yoelii in CBA/N mice. J Immunol. 1979 Jul;123(1):133–137. [PubMed] [Google Scholar]
- Kiyono H., Mosteller L. M., Eldridge J. H., Michalek S. M., McGhee J. R. IgA responses in xid mice: oral antigen primes Peyer's patch cells for in vitro immune responses and secretory antibody production. J Immunol. 1983 Dec;131(6):2616–2622. [PubMed] [Google Scholar]
- Lucas S. J., Barry D. W., Kind P. Antibody production and protection against influenza virus in immunodeficient mice. Infect Immun. 1978 Apr;20(1):115–119. doi: 10.1128/iai.20.1.115-119.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosier D. E., Scher I., Paul W. E. In vitro responses of CBA/N mice: spleen cells of mice with an X-linked defect that precludes immune responses to several thymus-independent antigens can respond to TNP-lipopolysaccharide. J Immunol. 1976 Oct;117(4):1363–1369. [PubMed] [Google Scholar]
- O'Brien A. D., Scher I., Campbell G. H., MacDermott R. P., Formal S. B. Susceptibility of CBA/N mice to infection with Salmonella typhimurium: influence of the X-linked gene controlling B lymphocyte function. J Immunol. 1979 Aug;123(2):720–724. [PubMed] [Google Scholar]
- O'Brien A. D., Scher I., Metcalf E. S. Genetically conferred defect in anti-Salmonella antibody formation renders CBA/N mice innately susceptible to Salmonella typhimurium infection. J Immunol. 1981 Apr;126(4):1368–1372. [PubMed] [Google Scholar]
- Owen R. L., Nemanic P. C., Stevens D. P. Ultrastructural observations on giardiasis in a murine model. I. Intestinal distribution, attachment, and relationship to the immune system of Giardia muris. Gastroenterology. 1979 Apr;76(4):757–769. [PubMed] [Google Scholar]
- Perlmutter R. M., Hansburg D., Briles D. E., Nicolotti R. A., Davie J. M. Subclass restriction of murine anti-carbohydrate antibodies. J Immunol. 1978 Aug;121(2):566–572. [PubMed] [Google Scholar]
- Perlmutter R. M., Nahm M., Stein K. E., Slack J., Zitron I., Paul W. E., Davie J. M. Immunoglobulin subclass-specific immunodeficiency in mice with an X-linked B-lymphocyte defect. J Exp Med. 1979 Apr 1;149(4):993–998. doi: 10.1084/jem.149.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips N. E., Campbell P. A. IgG subclass distribution of anti-sheep red blood cell plaque-forming cells in mice with the CBA/N defect. J Immunol. 1982 May;128(5):2319–2321. [PubMed] [Google Scholar]
- Press J. L. The CBA/N defect defines two classes of T cell-dependent antigens. J Immunol. 1981 Apr;126(4):1234–1240. [PubMed] [Google Scholar]
- Roberts-Thomson I. C., Mitchell G. F. Giardiasis in mice. I. Prolonged infections in certain mouse strains and hypothymic (nude) mice. Gastroenterology. 1978 Jul;75(1):42–46. [PubMed] [Google Scholar]
- Roberts-Thomson I. C., Stevens D. P., Mahmoud A. A., Warren K. S. Giardiasis in the mouse: an animal model. Gastroenterology. 1976 Jul;71(1):57–61. [PubMed] [Google Scholar]
- Scher I., Berning A. K., Asofsky R. X-linked B lymphocyte defect in CBA/N mice. IV. Cellular and environmental influences on the thymus dependent IgG anti-sheep red blood cell response. J Immunol. 1979 Jul;123(1):477–486. [PubMed] [Google Scholar]
- Scher I., Steinberg A. D., Berning A. K., Paul W. E. X-linked B-lymphocyte immune defect in CBA/N mice. II. Studies of the mechanisms underlying the immune defect. J Exp Med. 1975 Sep 1;142(3):637–650. doi: 10.1084/jem.142.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher I. The CBA/N mouse strain: an experimental model illustrating the influence of the X-chromosome on immunity. Adv Immunol. 1982;33:1–71. doi: 10.1016/s0065-2776(08)60834-2. [DOI] [PubMed] [Google Scholar]
- Slack J., Der-Balian G. P., Nahm M., Davie J. M. Subclass restriction of murine antibodies. II. The IgG plaque-forming cell response to thymus-independent type 1 and type 2 antigens in normal mice and mice expressing an X-linked immunodeficiency. J Exp Med. 1980 Apr 1;151(4):853–862. doi: 10.1084/jem.151.4.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider D. P., Gordon J., McDermott M. R., Underdown B. J. Chronic Giardia muris infection in anti-IgM-treated mice. I. Analysis of immunoglobulin and parasite-specific antibody in normal and immunoglobulin-deficient animals. J Immunol. 1985 Jun;134(6):4153–4162. [PubMed] [Google Scholar]
- Snider D. P., Underdown B. J. Quantitative and temporal analyses of murine antibody response in serum and gut secretions to infection with Giardia muris. Infect Immun. 1986 Apr;52(1):271–278. doi: 10.1128/iai.52.1.271-278.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]



