Abstract
Purpose:
Most lung cancers with activating epidermal growth factor receptor (EGFR) mutations respond to gefitinib, however resistance to this tyrosine kinase inhibitor (TKI) invariably ensues. The T790M mutation occurs in 50% and MET amplification in 20% of TKI-resistant tumors. Other secondary mutations (D761Y, L747S) are rare. Our goal was to determine the effects of erlotinib 150mg/day in EGFR mutated patients resistant to gefitinib 250mg/day, since the EGFR TKI erlotinib is given at a higher biologically active dose than gefitinib.
Experimental Design:
Retrospective review of 18 EGFR mutated (exon 19 deletions, L858R, L861Q) patients that were given gefitinib and subsequently erlotinib. 7 patients had tumor re-sampling after TKI therapy, and were analyzed for secondary EGFR mutations and MET amplification.
Results:
Most patients (14/18) responded to gefitinib with median progression-free survival (PFS) of 11 months (95%CI,4-16). After gefitinib resistance (de novo or acquired), 78% (14/18) of these patients displayed progressive disease while on erlotinib with PFS of 2 months (95%CI,2-3). 6/7 re-sampled patients acquired the T790M mutation, and 0/3 had MET amplification. Only 1 gefitinib-resistant patient with the acquired L858R-L747S EGFR, which in vitro is sensitive to achievable serum concentrations of erlotinib 150mg/day, achieved a partial response to erlotinib.
Conclusions:
In EGFR mutated tumors resistant to gefitinib 250mg/day, a switch to erlotinib 150mg/day does not lead to responses in most patients. These findings are consistent with pre-clinical models, since the common mechanisms of TKI-resistance (T790M and MET amplification) in vitro are not inhibited by clinically achievable doses of gefitinib or erlotinib. Alternative strategies to overcome TKI resistance must be evaluated.
Keywords: Epidermal growth factor receptor, EGFR, mutation, tyrosine kinase inhibitors, gefitinib, erlotinib, L858R, exon 19 deletions, T790M, lung cancer, non-small cell lung cancer
INTRODUCTION
In 2008, lung cancer continues to lead cancer-related deaths in the United States for both men and women (1). Non-small cell lung cancers (NSCLCs) comprise the majority of cases, and the prognosis of patients diagnosed with advanced NSCLC continues to be dismal (2). Use of palliative platinum-based chemotherapy has been the standard therapy for NSCLC (3). However, even the addition of the vascular endothelial growth factor monoclonal antibody bevacizumab (4) to chemotherapy can only achieve response rates (RRs) of 30%, progression-free survival (PFS) of less than 8 months and the median overall survival (OS) barely reaches 12 months. Despite three Food and Drug Administration (FDA)-approved second line therapies for platinum-progressive NSCLC, which are docetaxel (5), pemetrexed (6) and erlotinib (7), very few patients survive for longer than 2 years. Nonetheless, there is great heterogeneity between patients, their clinical course and response to different anti-cancer therapies.
The identification of somatic mutations in the tyrosine kinase domain of the epidermal growth factor receptor (EGFR) gene in patients with NSCLC provided one of the first examples of potential patient-tailored therapy in this disease (8-10). Large-scale sequencing efforts have consistently identified EGFR mutations in an enriched cohort of women, never smokers, adenocarcinomas and East Asians (11). The most prevalent EGFR mutations consist of small inframe deletions around the conserved LREA motif of exon 19 (residues 747-750), followed by a single point mutation (L858R) in exon 21 (12;13). Both cell line and mouse models of EGFR mutations demonstrate that tumor cells that harbor such mutations are exquisitely sensitive to EGFR inhibition (9;14;15). The aforementioned models have identified that EGFR-driven lung cancers are “addicted” to EGFR signaling for their survival and proliferation. More so, EGFR mutations are oncogenic and alter the tyrosine kinase pocket of EGFR to a degree that enhances the sensitivity to ATP-competitive EGFR inhibitors (16). Both these factors make EGFR mutated NSCLCs more sensitive to EGFR tyrosine kinase inhibitors (TKIs).
Retrospective studies of thousands of patients treated with the two currently available anilinoquinazoline small molecule EGFR TKIs, gefitinib and erlotinib, as 2nd or 3rd line therapies in NSCLC (17;18), demonstrated that a majority (close to 80%) of patients with classic EGFR mutant tumors attain radiographic and clinical responses to these oral agents. In some series, both PFS and OS were significantly better for EGFR TKI-treated patients with EGFR mutations when compared to wild-type cases (17). The evaluation of EGFR mutation as a prognostic and predictive marker is NSCLC is underway, with multiple phase II and III trials analyzing this biomarker. Seven prospective phase II trials have evaluated gefitinib monotherapy for patients selected based on their EGFR mutational status (19-21). These have confirmed that around 75% of patients with L858R or exon 19 deletion mutations achieve responses.
Despite the efficacy of gefitinib monotherapy for EGFR-mutant NSCLC, acquired resistance to EGFR TKI therapy is seen in most patients. In almost all prospective trials the PFS did not exceed 12 months (19). The secondary resistant T790M mutation (22;23) arises most often in cis to L858R or exon 19 deletions in around 50% of patients with radiographic progression (24;25). The acquired amplification of the MET oncogene occurs in around 20% of gefitinib/erlotinib-resistant patients and in half of these cases in conjunction with T790M (26;27). The mechanisms of resistance in the remaining tumors have not been completely clarified and very few other secondary mutations, such as L858R-D761Y (24) and L858R-L747S (28;29), identified in gefitinib-progressive specimens.
The management of this growing population of EGFR TKI-resistant NSCLC is not established, but the success of any approach will likely be dependent on the mechanism of acquired resistance of the tumor. In other “oncogene addicted” tumors, such as chronic myeloid leukemia (CML) and gastro-intestinal stromal tumor (GIST), where the BCR-ABL translocation or c-KIT mutations, respectively, make these cancers sensitive to imatinib, it seems the dose of the TKI matters (30). In both disorders, one clinical step when resistance emerges is to increase the dose of imatinib from 400 mg to 600 mg/day or higher (31-33). This dose escalation maneuver is only effective in some patients, possibly by inhibiting secondary mutations with borderline resistance to imatinib or by affecting non-mutation dependent mechanisms, with short periods of disease control (31;33). Second generation ABL and KIT inhibitors have gained momentum and recently received FDA approval as alternative therapies (34;35).
In EGFR mutated tumors, it is unknown if EGFR TKI dose escalations, in the face of acquired or de novo resistance, changes the course of TKI-progressive tumors. To evaluate the efficacy of such approach, we retrospectively studied the course of EGFR mutated patients that first received gefitinib 250 mg/day and upon becoming gefitinib-resistant were exposed to erlotinib 150 mg/day. This gefitinib to erlotinib switch is predicted to expose patients to almost double the biologically active dose of an EGFR TKI (36;37). Since EGFR-T790M and MET amplification lead to high level of in vitro resistance to both gefitinib and erlotinib (22;27), we hypothesized that erlotinib should only alter the response of acquired borderline resistant clones carrying the rare L858R-D761Y or L858R-L747S gefitinib-resistant mutations.
MATERIAL AND METHODS
Patient selection
Patients were identified from the databases of five academic medical centers: 1) Beth Israel Deaconess Medical Center, 2) Dana-Farber Cancer Institute, 3) Massachusetts General Hospital, 4) Memorial Sloan-Kettering Cancer Center, and 5) Yonsei University College of Medicine. Inclusion criteria to use the patient's data included signed informed consent for EGFR mutation analysis, an institutional approved protocol for human studies and genomic analysis of stored tumor tissue, a diagnosis of stage IV metastatic non-small cell lung cancer with a proven EGFR mutation, and the exposure to both gefitinib and erlotinib. Gefitinib at an initial dose of 250 mg/day had to be given as the first EGFR TKI therapy and erlotinib at a starting dose of 150 mg/day subsequently to progression on gefitinib. We did not exclude patients that had received investigational compounds between gefitinib and erlotinib in order to maximize the number of patients identified. Data was collected from the patient's medical records for baseline clinical, demographic and pathologic characteristics. Radiographic data was reviewed by each center. Portions of the clinical characterisics and response to gefitinib and erlotinib monotherapy in some of these patients has been reported previously by our academic groups (21;28;29;38-41).
EGFR genotype in the identified patients
Each institution performed EGFR genotypes using their own protocols, as described previously (10;13;21;22;24;28;39;41). The methods of DNA and RNA isolation from fresh tissue or paraffin-embedded tissue, and the technique used to enhance tumor-derived DNA, which included either micro-dissection or use of more sensitive polymerase chain reaction (PCR) amplification techniques, was left to the discretion of each institution. All protocols either sequenced exons 18 to 21 of the EGFR gene or identified L858R and deletions in exon 19.
In patients who had tumor re-sampling after progression on gefitinib or erlotinib, DNA or RNA was isolated from the tumor tissue and the EGFR gene was sequenced as above. Specific attention was made to compare results to the original biopsy and identify the exon 20 T790M mutation (22).
MET amplification analysis
In the tumor specimens that were obtained after progression on TKIs, we attempted to identify the amplification of MET when enough material for studies was available. Levels of MET and endogenous control were evaluated using quantitative genomic PCR methods described previously (26;27) in DNA samples. Fluorescence in situ hybridization (FISH) was employed, as described previously (27), in tumor samples that had paraffin-embedded tissue available for analysis.
Treatment schedules, response, progression-free survival assessment and statistical analysis in the identified patients
All the identified patients had the same initial treatment schedule for gefitinib. This medication was given orally at a dose of 250 mg/day, and gefitinib was used until tumor progression and afterwards continued at the physician's discretion. Erlotinib was given orally at a dose of 150 mg/day and continued until radiographic tumor progression or overt clinical progression. Need for EGFR TKI dose reduction was determined by each treating physician based on the patient's tolerance and side-effect profile.
The objective tumor response was determined by RECIST criteria (Response Evaluation Criteria in Solid Tumors) (42). It was left at the discretion of each institution and physician to determine when to obtain re-imaging radiographs. PFS and OS were calculated from the date of starting the EGFR TKI until the date of radiographic tumor progression or overt clinical progression (for PFS), and death (for OS). PFS and OS estimates were made using the Kaplan-Meier method (43), and the 95% confidence interval (CI) for the median was based on the sign test. Exploratory differences in response rate and PFS were compared by Fisher's exact test and the Logrank test.
RESULTS
Patient characteristics
After a review of EGFR genotyped patients in our centers from 2004 to 2008, we identified 18 EGFR mutated patients that had received gefitinib and erlotinib. Clinical, demographic, pathologic and molecular characteristics of this cohort are displayed in Table 1. Sixty one percent of patients were women (11/18) and the majority never smokers (11/18). Ages varied between 43 to 80 years (Table 1). Almost all (16/18) patients had adenocarcinoma as the main histologic type of their tumor. Theses characteristics are similar to historic cohorts of EGFR mutated tumors (17). Exon 19 deletion-containing tumors were found in 13 patients (72%), L858R mutations in 4 patients (22%) and L861Q in 1 patient (Table 1).
Table 1.
Clinical, pathologic, demographic and molecular characteristics of the studied EGFR mutated patients
| Characteristic | no. of patients | % | |
|---|---|---|---|
| Age (years) | |||
| Median | 63 | ||
| Range | 43-80 | ||
| Sex | |||
| Female | 11 | 61% | |
| Male | 7 | 39% | |
| Smoking history | |||
| Never smoker | 11 | 61% | |
| Former smoker | 5 | 28% | |
| Smoker | 2 | 11% | |
| Histology | |||
| Adenocarcinoma | 16 | 89% | |
| NSCLC – NOS | 2 | 11% | |
| EGFR mutation | |||
| Exon 19 deletion* | 13 | 72% | |
| L858R | 4 | 22% | |
| L861Q | 1 | 6% | |
| Therapy prior to gefitinib | |||
| Platinum-based chemotherapy | 10 | 56% | |
| No prior therapy | 8 | 44% | |
| Therapy in between gefitinib and erlotinib | |||
| Experimental agent | 3 | 17% | |
| No therapy | 15 | 83% | |
no., number; NSCLC-NOS, non-small cell lung cancer-non otherwise specified;
specific EGFR sequences of the exon 19 deletions are detailed in Table 2.
Of the studied patients, 8 received gefitinib as their first anti-cancer therapy (44%) and 10 had received platinum-based chemotherapy previously (56%). Most patients (15/18, 83%) were not exposed to any other form of therapy between stopping gefitinib and prior to receiving erlotinib (Tables 1 and 2).
Table 2.
Clinical, pathologic, demographic, molecular characteristics, response to therapy, progression-free survival and overall survival in the studied patients
| Patient | Site | Age (yrs)/ Sex |
Histology | Smoking history |
Type of EGFR mutation |
Therapy prior to gefitinib |
Response - gefitinib 250 mg/d |
PFS - gefitinib (months) |
EGFR re- sequence |
MET amplification |
Therapy prior to erlotinib |
Response - erlotinib 150 mg/d |
PFS - erlotinib (months) |
Survival from gefitinib (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | BIDMC | 71/M | Adeno | former (40 py) |
delL747- S752 |
platinum- doublet |
CR | 24 | delL747- S752 + T790M |
ND | cetuximab, experimental Raf inhibitor |
PD | 1 | 30 |
| 2 | BIDMC | 74/F | Adeno | never smoker |
L858R | platinum- doublet |
PR | 40 | L858R + L747S |
No | experimental EGFR inhibitor |
PR | 6 | >62 (alive)# |
| 3 | BIDMC | 75/F | Adeno | never smoker |
delE746- A750 |
none | PR | 26 | ND | ND | none | PD | 2 | 35 |
| 4 | BIDMC | 77/F | Adeno | never smoker |
L858R | none | PR | 14 | ND | ND | none | PD | 3 | 27 |
| 5 | YCC | 45/F | Adeno | never smoker |
delL747- 751InsP |
platinum- doublet |
PR | 16.5 | ND | ND | none | SD | 3.7 | 39 |
| 6 | YCC | 56/F | Adeno | never smoker |
delL747- 751InsP |
platinum- doublet |
PR | 12 | delL747- 751InsP + T790M |
No | none | PD | 2 | 19 |
| 7 | YCC | 47/M | Adeno | smoker | delE746- T751InsA |
platinum- doublet |
PD | 1 | ND | ND | none | PD | 2 | 3 |
| 8 | YCC | 49/F | Adeno | never smoker |
delL747- 751InsP |
platinum- doublet |
PR | 3 | ND | ND | none | PD | 2 | 16 |
| 9 | YCC | 70/M | Adeno | smoker | delE746- A750 |
platinum- doublet |
PD | 2 | ND | ND | none | PD | 2 | 7 |
| 10 | DFCI | 43/M | NSCLC -NOS |
never smoker |
L858R | none | PR | 4 | ND | ND | none | PD | 2 | 9 |
| 11 | MGH | 64/M | NSCLC -NOS |
former (30 py) |
L858R | none | PR | 4 | ND | ND | none | PD | 2 | 21 |
| 12 | MGH | 64/F | Adeno | never smoker |
delE746- A750 |
none | PR | 7 | ND | ND | experimental Hsp90 inhibitor |
PD | 1 | >20 (alive)# |
| 13 | MGH | 69/F | Adeno | former (20 py) |
L861Q | none | SD | 4 | ND | ND | none | PD | 2 | 9 |
| 14 | MGH | 60/F | Adeno | former (5 py) |
delE746- A750 |
none | PR | 17 | ND | ND | none | SD | 5 | >49 (alive)# |
| 15 | MSKCC | 60/M | Adeno | never smoker |
delE746- A750 |
platinum- doublet |
PR | 16 | delE746- A750 + T790M* |
ND | none | PD | 4 | 32 |
| 16 | MSKCC | 52/F | Adeno | never smoker |
delE746- A750 |
platinum- doublet |
PR | 10 | delE746- A750 + T790M* |
ND | none | PD | 3 | >39 (alive)# |
| 17 | MSKCC | 62/F | Adeno | never smoker |
delE746- A750 |
platinum- doublet |
PR | 11 | delE746- A750 + T790M* |
ND | none | SD | 6 | >44 (alive)# |
| 18 | MSKCC | 80/M | Adeno | former (2 py) |
delE746- A750 |
none | SD | 11 | delE746- A750 + T790M* |
No* | none | PD | 2 | 32 |
BIDMC, Beth Israel Deaconess Medical Center; YCC, Yonsei Cancer Center; DFCI; Dana-Farber Cancer Institute; MGH, Massachussetss General Hospital; MSKCC, Memorial Sloan-Kettering Cancer Center; Adeno, adenocarcinoma; NSCLC-NOS, non-small cell lung cancer-not otherwise specified; EGFR, epidermal growth factor receptor; M, male; F, female; py, pack-years; Del 19, exon 19 deletion; ND, not done; CR, complete response; PR, partial response; PD, progressive disease; SD, stable disease; TTP, time to progression; yrs, years; Hsp90, heat shock protein 90;
EGFR re-sequence and/or MET amplification was obtained after exposure to gefitinib and erlotinib
latest survival data collected June 1st, 2008.
Initial response to gefitinib 250 mg/day
Fourteen out of the eighteen patients (78%) had radiographic responses to gefitinib (Table 2), a number that is compatible with retrospective and prospective data for EGFR mutated patients (11;19;44). 2 patients (11%) had stable disease (SD), and another 2 patients had de novo resistance to gefitinib with progressive disease (PD) as best response.
The median PFS was 11 months, with a 95% CI of 4 to 16 months (Figure 1). Five patients had responses that lasted more than 16 months (Table 2). All patients eventually displayed radiographic and clinical progression that required discontinuation of gefitinib. PFS was similar between patients that were chemotherapy-naïve or had received chemotherapy previously (Table 2).
Figure 1.
Kaplan-Meier curve for PFS of the EGFR mutated patients during gefitinib therapy
Response to erlotinib 150 mg/day
Patients were given erlotinib at an initial dose of 150 mg/day after their tumors had become gefitinib-resistant. The majority of patients had no additional systemic therapy between gefitinib and erlotinib (Table 1).
Fourteen out of the 18 patients (78%) had PD as the best response to erlotinib monotherapy, an additional 3 (16%) had brief periods of SD as best response, and only 1 patient (6%) had a radiographic partial response (PR) (Tables 2 and 3).
Table 3.
Response and PFS of EGFR mutated gefitinib-resistant patients on erlotinib monotherapy.
| PR | SD | PD | |
|---|---|---|---|
| Best radiographic response - no. pts (%) |
1 (6%) * | 3 (16%) | 14 (78%) |
| PFS - months (95% CI) |
2 (2-3) | ||
PR, partial response; SD, stable disease; PD, progressive disease; no, number; pts, patients; PFS, progression-free survival; CI, confidence interval;
The only patient with PR had the L858R-L747S EGFR mutation, which in vitro is sensitive to achievable serum levels of erlotinib 150 mg/day.
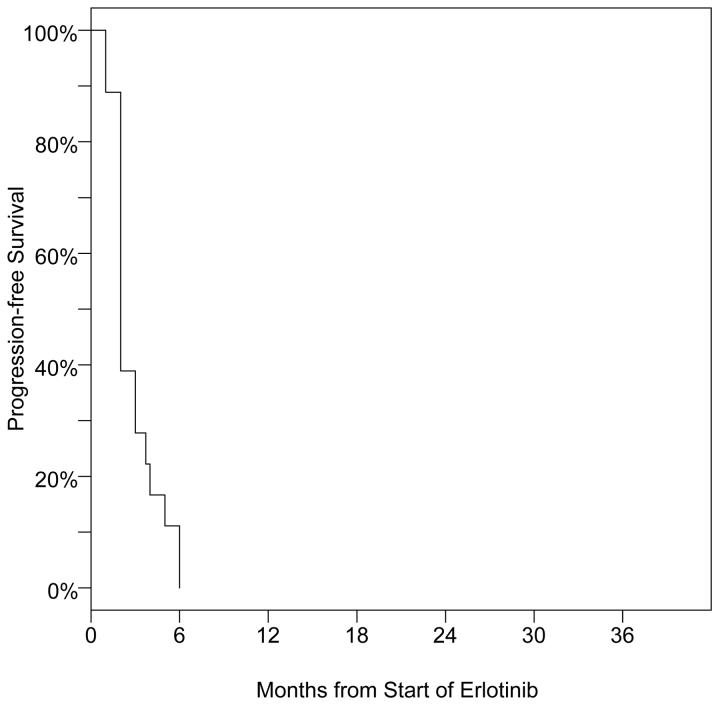
Median PFS was 2 months, with a 95% CI of 2 to 3 months (Figure 2). Only 2 patients (11%), one each with PR and SD, remained on erlotinib without progression for over 5 months and no patient had a PFS of over 6 months (Table 2). PFS was similar for patient that had or had not received chemotherapy as their fist line of systemic therapy (Table 2). 29% (4/14) of gefitinib responders had PR or SD after erlotinib compared to 0/4 of gefitinib non-responders. All 4 of the gefitinib non-responders progressed on erlotinib by 2 months, whereas half of the gefitinib responders had not progressed by 2 months.
Figure 2.
Kaplan-Meier Curve for PFS of the gefitinib-resitant EGFR mutated patients during erlotinib therapy
EGFR re-sequencing after progression on EGFR TKI therapy and subsequent response to erlotinib
Seven of the 18 patients had their tumors sampled after progression on EGFR TKI therapy: 3 after gefitinib therapy, and the other 4 after gefitinib and erlotinib. Of these patients, 6 out of 7 (86%) had acquired the T790M EGFR mutation in association with their initial activating exon 19 deletions. Five out of the six (83%) T790M-carrying tumors displayed PD to erlotinib therapy (Table 2). One patient with exon 19 deletion (delE746-A750)-T790M had 6 months of SD on erlotinib, however since a biopsy was obtained after gefitinib and erlotinib we cannot excluded the possibility that T790M was acquired while on erlotinib therapy.
One patient had acquired the L747S secondary mutation in association with the activating L858R EGFR after exposure to gefitinib. The patient carrying L858R-L747S had a partial radiographic response to erlotinib 150 mg/day that lasted 6 months (Table 2).
MET amplification after progression on EGFR TKI therapy
Of the seven patients that had their tumors sampled after progression on EGFR TKI therapy, three had sufficient material for analysis of MET amplification. None of these 3 had amplification of the MET oncogene. Patients 2 and 18 were analyzed by quantitative PCR methods, and patient 6 by FISH (Table 2).
Overall survival from start of gefitinib
The median OS of all18 patients from start of gefitinib therapy to death was 30 months, 95% CI of 19 to 39 months. This is similar to OS reported for other series of EGFR mutated patients (17;19).
DISCUSSION
EGFR mutated cancers comprise a sub-set of NSCLC that are intrinsically sensitive to small molecule EGFR inhibitors (12;15;17). The current clinical experience with the use of gefitinib and erlotinib in EGFR mutated patients indicates that exon 19 deletion and L858R-bearing tumors commonly display radiographic responses to these drugs with disease control durations that sometimes reach a year or longer (17;19). Despite this unprecedented disease control rate, acquired resistance to EGFR TKIs invariably develops over the course of therapy and is becoming the main obstacle for management of this patient population (12).
The first mechanism of acquired resistance described was the acquisition of the T790M EGFR mutation (22;23). The methionine residue at position 790 generates a bulkier side chain that either affects binding of TKIs or enhances the affinity of the EGFR tyrosine kinase pocket to ATP, and this enhanced ATP affinity decreases the effective binding of gefitinib and erlotinib to the tyrosine kinase pocket of EGFR (22;45). There is a great deal of similarities among structures of tyrosine kinase receptors and some analogous acquired resistance mutations fall exactly in the same amino-acid residue. This is the case of the T315I, T670I, and T790M mutations in ABL1, KIT and EGFR, respectively, in CML, GIST and EGFR mutated NSCLC (46). Our groups have shown in multiple in vitro and in vivo models that T790M in cis to an activating mutation (either L858R or exon 19 deletions) negates the sensitivity to achievable doses of gefitinib or erlotinib (23;38). The in vitro concentrations of gefitinib/erlotinib that can inhibit T790M-EGFR and T790M-carrying cells exceed 5-10 μM (22;23;38;46). Very few other secondary EGFR mutations have been described (24;28). These have only been seen in patients receiving gefitinib who carried the L858R mutation. L858R-761Y (24) and L858R-L747S (28) in vitro shift the sensitivity curves for gefitinib and erlotinib when compared to L858R alone, however both mutations are hundred fold less “resistant” than L858R-T790M or exon 19 deletion-T790M. Most in vitro data would suggest that L858R-D761Y and L858R-L747S would be inhibited if the EGFR TKI dose reached 1-2 μM (24;28), which is achievable with 150 mg/day of erlotinib but not with 250 mg/day gefitinib. Gefitinib's clinical dose of 250 mg/day is far less than its maximum tolerated dose (MTD) of 1000 mg/day. The mean steady state serum concentration of gefitinib following 225 mg/day varied from 0.03-0.32 μg/mL in a phase I trial (36), with an average of 0.16 μg/mL or 0.358 μM. The mean concentration increases to 0.24 μg/mL at 300 mg/day, and to 1.1 μg/mL or 2.461 μM at 1000 mg/day of gefitinib (36). Erlotinib is used clinically at a dose of 150 mg/day (7), which is its MTD. The steady state trough concentrations at this dose ranged from 0.33 to 2.64 μg/mL in the phase I trial (37), with a median of 1.26 μg/mL or the equivalent to 2.930 μM.
In addition to secondary EGFR mutations, another mechanism of acquired resistance is an “oncogene switch” model. Our groups have recently shown that the acquired amplification of the MET oncogene occurs in approximately 20% of EGFR mutated patients with acquired resistance to gefitinib or erlotinib (26;27). MET couples with other ErbB members and activates down-stream signals that bypass the inhibited EGFR (27)(47). The in vitro resistance to erlotinib and gefitinib in this model was also in the range of 5-10 μM. Dual inhibition of EGFR and MET with tyrosine kinase inhibitors is able to overcome MET amplified EGFR TKI-resistant tumors (27). Of interest, in almost half of the patients with MET amplification, T790M was identified either in the same biopsy specimen or in biopsy specimens from other sites within the patient (26;27). This indicates that T790M will continue to be the most prevalent form of EGFR TKI resistance. Other oncogenes, such as the insulin-like growth factor I receptor (IGFIR) may also play a role in resistance to EGFR TKIs in non-EGFR mutated cells (48).
Despite a rapidly growing understanding of the molecular mechanisms of acquired resistance to EGFR inhibitors, there is no standard therapy for the expanding number of EGFR mutated tumors that become resistant to gefitinib. Since in an unselected population of platinum-refractory NSCLC patients gefitinib was not statistically better than placebo in controlling disease progression (49), the FDA restricted its use for patients previously benefiting from treatment or participating in clinical trials. Nonetheless, in the same phase III trial the never smoker and Asian group of patients had a clear clinical benefit (49). Gefitinib continues to be widely used in Eastern Asian countries and in EGFR genotyped patients (19;50). Erlotinib is approved for use in unselected patients after failure of platinum-based therapy (7), and it, like gefitinib, has excellent efficacy in EGFR mutated patients in retrospective and prospective series (12;17).
One question that remains unanswered is if gefitinib-resistant EGFR mutated patients could benefit from a switch to erlotinib. To address this, we retrospectively analyzed the clinical course of 18 EGFR mutated NSCLCs that were treated with gefitinib and, upon resistance, erlotinib. The patient characteristics, type of EGFR mutations (almost all had L858R or exon 19 deletions) and initial response to gefitinib 250 mg/day were consistent with previous experience in EGFR mutated patients (17;19). Our clinical observation was that the majority (over 83%) of the gefitinib-resistant patients given erlotinib 150 mg/day had radiographic progression within the first 2 to 4 months of exposure. This is consistent with our pre-clinical observations, since we expected gefitinib-resistant tumors to predominantly harbor T790M and/or MET amplification, which are cross-resistant to both EGFR TKIs as described above.
We had a second biopsy specimen in 7 of the 18 patients, and in 6 of them the T790M secondary mutation was identified together with the initial activating exon 19 deletion. None of the 3 patients analyzed had MET amplification (Table 2). Almost all of these gefitinib-resistant patients had rapid progression on erlotinib. Only 1 patient achieved a partial radiographic response upon switching to erlotinib (29). This patient had acquired the rare L747S mutation after exposure of the initial L858R-carrying tumor to gefitinib. As reported previously by our group, L858R-L747S is less sensitive to gefitinib and erlotinib than L858R in vitro (28). However, this compound mutation can be inhibited by increasing concentrations of gefitinib or erlotinib at a level that is clinically achievable for the later drug (29). We were not able to measure pharmacokinetic parameters of either gefitinib or erlotinib during the course of therapy in this patient, however the observed skin-related side effects (rash and pruritus) while on erlotinib 150 mg/day exceed in grade the effects while the patient was on gefitinib 250 mg/day (29), likely indicating a higher biologically active dose of the former compound in this individual. However, even in this patient the duration of response was relatively short and radiographic progression was noted after 6 months. Further biopsies were not available to test if the tumor had acquired additional mechanisms of resistance, such as T790M or MET amplification.
Two recent reports have described the clinical experience of using erlotinib following gefitinib failure in Asian patients. The first was a phase II trial of erlotinib 150 mg/day in patients with either primary or acquired resistance to gefitinib (41). In the initial report, none of the EGFR mutated patients had a radiographic response to erlotinib. All of the EGFR mutated patients from that study were included in our analysis and we report updated clinical data in their response to both gefitinib and erlotinib. The second study evaluated 14 unselected patients that had failed gefitinib, and 5 harbored EGFR mutations (51). Of the EGFR mutated patients, a clinical and radiographic response was described for 2 patients after exposure to erlotinib. However in 1 of these cases the patient progressed on erlotinib within the first 2 months of therapy. In the 5 EGFR mutated patients the time to progression on erlotinib averaged 3 months while the initial time to progression on gefitinib exceed 8 months (51). No molecular data was available for these patients after progression on gefitinib. Anecdotal reports of the use of erlotinib after failure of gefitinib have been published by many investigators (52-57) and recently summarized by one of us (58). Combining all reports and the data presented here by us, it seems that most of the patients that harbored an EGFR mutation, when the genotype was available, did not benefit significantly from erlotinib after they had received and progressed on gefitinib. In almost all patients that harbored an acquired T790M mutation after gefitinib, rapid progression was noted on erlotinib.
However, we cannot exclude the possibility that continued EGFR inhibition, either with the original EGFR TKI or with a different anilinoquinazoline, benefits EGFR mutant patients. The re-administration of gefitinib or erlotinib in previously responsive patients that show radiographic progression has been reported to improve symptoms and the clinical course of patients (59;60), suggesting a role for continued TKI use to control the non-TKI resistant clones of these “oncogene addicted” cancers. Indeed, in our cohort of patients, we noted that patients with acquired resistance to gefitinib had modestly longer PFS on erlotinib than the ones that had de novo resistance; indicating that perhaps in EGFR mutated patients with a prior response to TKIs, control of non-resistance clones is achievable and may improve clinical outcomes. Ongoing phase II randomized trials are attempting to confirm if maintaining some form of EGFR TKI therapy in addition to other lines of therapy is better than placebo in EGFR mutated patients with resistance to gefitinib or erlotinib.
Initial steps have begun to use pre-clinical data for rationale design of clinical trials of patients with acquired resistance to gefitinib or erlotinib. Our groups have shown that some irreversible and second generation EGFR inhibitors in vitro can partially overcome the T790M mutation (22;38;46;61). This knowledge has spawned phase II trials of the HKI-272 (ClinicalTrials.gov identifier: NCT00266877), BIBW-2992 (ClinicalTrials.gov identifier: NCT00656136) and XL-647 (ClinicalTrials.gov identifier: NCT00522145) compounds in this selected patient population. However, in recent in vitro cell line models and in vivo mouse models, HKI-272 used at doses achieved in the phase I clinical trial (62) actually induced the acquisition of EGFR-T790M (63) or was ineffective generating a radiographic response in L858R-T790M tumors (64). Thus, it is possible that at the achievable clinical concentrations of this, and other novel EGFR inhibitors, T790M will still not be inhibited. Continued development of alternative EGFR inhibitors that have a better profile against EGFR mutated tumors with T790M, such as PF00299804 (65), and development of MET inhibitors may one day help circumvent acquired resistance to EGFR-targeted therapy.
In summary our data indicates that in EGFR mutated patients with acquired resistance to gefitinib at 250 mg/day, a switch to erlotinib at 150 mg/day does not lead to radiographic responses in most patients; despite the higher biologically active dose of erlotinib (36;37). The PFS was also short in these erlotinib-treated patients with a median of 2 months. These findings were expected, since pre-clinical models indicated that the two most common mechanisms of acquired resistance to gefitinib, EGFR-T790M and MET amplification, are highly resistant to achievable clinical concentrations of erlotinib (22;23;26;27). As expected from our pre-clinical models, the only patient that achieved a radiographic response harbored the borderline resistant L858R-L747S mutation, which, similar to L858R-D761Y, can be overcome by increasing concentrations of either gefitinib or erlotinib at 150 mg/day (24;28;29).
Acknowledgments
Funding/Grant Support: This work was supported by the National Institution of Health (NIH) grants K99CA126026 (to S.K.) and Specialized Program of Research Excellence (SPORE) in Lung Cancer PA20-CA090578-01A1 (to D.G.T); a Young Investigator Award (YIA) by the American Society of Clinical Oncology (to D.B.C), an American Association for Cancer Research (AACR)-AstraZeneca-Cancer Research and Prevention Foundation Fellowship in Translational Lung Cancer Research (to D.B.C), a Clinical Investigator Training Program from Beth Israel Deaconess Medical Center - Harvard/MIT Health Sciences and Technology in collaboration with Pfizer Inc. and Merck & Co. (to D.B.C), and research funding by OSI-Pharmaceuticals or Genentech (to BH, PAJ and LVS).
Footnotes
Conflict of interest: The following authors received consulting fees or honoraria: DMJ (Roche < $10.000; Genentech <$10.000), GJR (Boehringer-Ingelheim <$10.000; Roche <$10.000); PAJ (Roche <$10.000; AstraZeneca <$10.000); LVS (Genentech <$10.000). Other remuneration: PAJ (Genzyme), WP (Molecular MD). No other conflict of interest is stated.
STATEMENT OF CLINICAL RELEVANCE:
EGFR mutated non-small cell lung cancers (NSCLCs) are sensitive to EGFR inhibitors in pre-clinical models. Clinical experience with the use of gefitinib/erlotinib in EGFR mutated patients indicates that many exon 19 deletion and L858R-bearing tumors display responses that sometimes reach a year, however acquired resistance to EGFR TKIs invariably develops. The secondary T790M mutation occurs in 50% and amplification of MET in 20% of TKI-resistant tumors. Few other secondary mutations (D761Y, L747S) have been described. Few therapies have been studied for the expanding number of EGFR mutated tumors that become resistant to gefitinib. Our data indicates that in EGFR mutated patients with resistance to gefitinib 250 mg/day, a switch to erlotinib 150 mg/day does not lead to radiographic responses in most patients despite the higher biologically active dose of erlotinib. Only a patient with the acquired L858R-L747S responded to erlotinib. Pre-clinical models indicated that the two most common mechanisms of acquired resistance to gefitinib, EGFR-T790M and MET amplification, are highly resistant to achievable clinical concentrations of erlotinib; while L858R-L747S is sensitive to erlotinib at 150 mg/day. The correlation of our findings with the molecular understanding of sensitivity and resistance of EGFR mutated systems underlines the need for genotype-based clinical studies to advance our understanding of treatment of this representative patient cohort.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Ardizzoni A, Boni L, Tiseo M, et al. Cisplatin- versus carboplatin-based chemotherapy in first-line treatment of advanced non-small-cell lung cancer: an individual patient data meta-analysis. J Natl Cancer Inst. 2007;99:847–57. doi: 10.1093/jnci/djk196. [DOI] [PubMed] [Google Scholar]
- 3.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–8. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 4.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 5.Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 6.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–97. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 7.Shepherd FA, Rodrigues PJ, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–32. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 8.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 9.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 10.Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101:13306–11. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97:339–46. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 12.Riely GJ, Politi KA, Miller VA, Pao W. Update on epidermal growth factor receptor mutations in non-small cell lung cancer. Clin Cancer Res. 2006;12:7232–41. doi: 10.1158/1078-0432.CCR-06-0658. [DOI] [PubMed] [Google Scholar]
- 13.Sequist LV, Joshi VA, Janne PA, et al. Response to treatment and survival of patients with non-small cell lung cancer undergoing somatic EGFR mutation testing. Oncologist. 2007;12:90–8. doi: 10.1634/theoncologist.12-1-90. [DOI] [PubMed] [Google Scholar]
- 14.Politi K, Zakowski MF, Fan PD, et al. Lung adenocarcinomas induced in mice by mutant EGF receptors found in human lung cancers respond to a tyrosine kinase inhibitor or to down-regulation of the receptors. Genes Dev. 2006;20:1496–510. doi: 10.1101/gad.1417406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ji H, Li D, Chen L, et al. The impact of human EGFR kinase domain mutations on lung tumorigenesis and in vivo sensitivity to EGFR-targeted therapies. Cancer Cell. 2006;9:485–95. doi: 10.1016/j.ccr.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 16.Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7:169–81. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 17.Sequist LV, Bell DW, Lynch TJ, Haber DA. Molecular predictors of response to epidermal growth factor receptor antagonists in non-small-cell lung cancer. J Clin Oncol. 2007;25:587–95. doi: 10.1200/JCO.2006.07.3585. [DOI] [PubMed] [Google Scholar]
- 18.Mitsudomi T, Kosaka T, Endoh H, et al. Mutations of the epidermal growth factor receptor gene predict prolonged survival after gefitinib treatment in patients with non-small-cell lung cancer with postoperative recurrence. J Clin Oncol. 2005;23:2513–20. doi: 10.1200/JCO.2005.00.992. [DOI] [PubMed] [Google Scholar]
- 19.Costa DB, Kobayashi S, Tenen DG, Huberman MS. Pooled analysis of the prospective trials of gefitinib monotherapy for EGFR-mutant non-small cell lung cancers. Lung Cancer. 2007;58:95–103. doi: 10.1016/j.lungcan.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamura K, Okamoto I, Kashii T, et al. Multicentre prospective phase II trial of gefitinib for advanced non-small cell lung cancer with epidermal growth factor receptor mutations: results of the West Japan Thoracic Oncology Group trial (WJTOG0403) Br J Cancer. 2008;98:907–14. doi: 10.1038/sj.bjc.6604249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sequist LV, Martins RG, Spigel D, et al. First-line gefitinib in patients with advanced non-small-cell lung cancer harboring somatic EGFR mutations. J Clin Oncol. 2008;26:2442–9. doi: 10.1200/JCO.2007.14.8494. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–92. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 23.Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balak MN, Gong Y, Riely GJ, et al. Novel D761Y and common secondary T790M mutations in epidermal growth factor receptor-mutant lung adenocarcinomas with acquired resistance to kinase inhibitors. Clin Cancer Res. 2006;12:6494–501. doi: 10.1158/1078-0432.CCR-06-1570. [DOI] [PubMed] [Google Scholar]
- 25.Kosaka T, Yatabe Y, Endoh H, et al. Analysis of epidermal growth factor receptor gene mutation in patients with non-small cell lung cancer and acquired resistance to gefitinib. Clin Cancer Res. 2006;12:5764–9. doi: 10.1158/1078-0432.CCR-06-0714. [DOI] [PubMed] [Google Scholar]
- 26.Bean J, Brennan C, Shih JY, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci U S A. 2007;104:20932–7. doi: 10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–43. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 28.Costa DB, Halmos B, Kumar A, et al. BIM mediates EGFR tyrosine kinase inhibitor-induced apoptosis in lung cancers with oncogenic EGFR mutations. PLoS Med. 2007;4:1669–79. doi: 10.1371/journal.pmed.0040315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Costa DB, Schumer ST, Tenen DG, Kobayashi S. Differential responses to erlotinib in epidermal growth factor receptor (EGFR)-mutated lung cancers with acquired resistance to gefitinib carrying the L747S or T790M secondary mutations. J Clin Oncol. 2008;26:1182–4. doi: 10.1200/JCO.2007.14.9039. [DOI] [PubMed] [Google Scholar]
- 30.Rubin BP, Heinrich MC, Corless CL. Gastrointestinal stromal tumour. Lancet. 2007;369:1731–41. doi: 10.1016/S0140-6736(07)60780-6. [DOI] [PubMed] [Google Scholar]
- 31.Hehlmann R, Hochhaus A, Baccarani M. Chronic myeloid leukaemia. Lancet. 2007;370:342–50. doi: 10.1016/S0140-6736(07)61165-9. [DOI] [PubMed] [Google Scholar]
- 32.Kantarjian H, Pasquini R, Hamerschlak N, et al. Dasatinib or high-dose imatinib for chronic-phase chronic myeloid leukemia after failure of first-line imatinib: a randomized phase 2 trial. Blood. 2007;109:5143–50. doi: 10.1182/blood-2006-11-056028. [DOI] [PubMed] [Google Scholar]
- 33.Blanke CD, Demetri GD, von Mehren M, et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol. 2008;26:620–5. doi: 10.1200/JCO.2007.13.4403. [DOI] [PubMed] [Google Scholar]
- 34.Kantarjian HM, Giles F, Gattermann N, et al. Nilotinib (formerly AMN107), a highly selective BCR-ABL tyrosine kinase inhibitor, is effective in patients with Philadelphia chromosome-positive chronic myelogenous leukemia in chronic phase following imatinib resistance and intolerance. Blood. 2007;110:3540–6. doi: 10.1182/blood-2007-03-080689. [DOI] [PubMed] [Google Scholar]
- 35.Goodman VL, Rock EP, Dagher R, et al. Approval summary: sunitinib for the treatment of imatinib refractory or intolerant gastrointestinal stromal tumors and advanced renal cell carcinoma. Clin Cancer Res. 2007;13:1367–73. doi: 10.1158/1078-0432.CCR-06-2328. [DOI] [PubMed] [Google Scholar]
- 36.Baselga J, Rischin D, Ranson M, et al. Phase I safety, pharmacokinetic, and pharmacodynamic trial of ZD1839, a selective oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with five selected solid tumor types. J Clin Oncol. 2002;20:4292–302. doi: 10.1200/JCO.2002.03.100. [DOI] [PubMed] [Google Scholar]
- 37.Hidalgo M, Siu LL, Nemunaitis J, et al. Phase I and pharmacologic study of OSI-774, an epidermal growth factor receptor tyrosine kinase inhibitor, in patients with advanced solid malignancies. J Clin Oncol. 2001;19:3267–79. doi: 10.1200/JCO.2001.19.13.3267. [DOI] [PubMed] [Google Scholar]
- 38.Kobayashi S, Ji H, Yuza Y, et al. An alternative inhibitor overcomes resistance caused by a mutation of the epidermal growth factor receptor. Cancer Res. 2005;65:7096–101. doi: 10.1158/0008-5472.CAN-05-1346. [DOI] [PubMed] [Google Scholar]
- 39.Jackman DM, Yeap BY, Sequist LV, et al. Exon 19 deletion mutations of epidermal growth factor receptor are associated with prolonged survival in non-small cell lung cancer patients treated with gefitinib or erlotinib. Clin Cancer Res. 2006;12:3908–14. doi: 10.1158/1078-0432.CCR-06-0462. [DOI] [PubMed] [Google Scholar]
- 40.Riely GJ, Pao W, Pham D, et al. Clinical course of patients with non-small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with gefitinib or erlotinib. Clin Cancer Res. 2006;12:839–44. doi: 10.1158/1078-0432.CCR-05-1846. [DOI] [PubMed] [Google Scholar]
- 41.Cho BC, Im CK, Park MS, et al. Phase II study of erlotinib in advanced non-small-cell lung cancer after failure of gefitinib. J Clin Oncol. 2007;25:2528–33. doi: 10.1200/JCO.2006.10.4166. [DOI] [PubMed] [Google Scholar]
- 42.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 43.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 44.Kosaka T, Yatabe Y, Endoh H, et al. Mutations of the epidermal growth factor receptor gene in lung cancer: biological and clinical implications. Cancer Res. 2004;64:8919–23. doi: 10.1158/0008-5472.CAN-04-2818. [DOI] [PubMed] [Google Scholar]
- 45.Yun CH, Mengwasser KE, Toms AV, et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci U S A. 2008;105:2070–5. doi: 10.1073/pnas.0709662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carter TA, Wodicka LM, Shah NP, et al. Inhibition of drug-resistant mutants of ABL, KIT, and EGF receptor kinases. Proc Natl Acad Sci U S A. 2005;102:11011–6. doi: 10.1073/pnas.0504952102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guo A, Villen J, Kornhauser J, et al. Signaling networks assembled by oncogenic EGFR and c-Met. Proc Natl Acad Sci U S A. 2008;105:692–7. doi: 10.1073/pnas.0707270105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guix M, Faber AC, Wang SE, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in cancer cells is mediated by loss of IGF-binding proteins. J Clin Invest. 2008;118:2609–19. doi: 10.1172/JCI34588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer) Lancet. 2005;366:1527–37. doi: 10.1016/S0140-6736(05)67625-8. [DOI] [PubMed] [Google Scholar]
- 50.Mitsudomi T, Yatabe Y. Mutations of the epidermal growth factor receptor gene and related genes as determinants of epidermal growth factor receptor tyrosine kinase inhibitors sensitivity in lung cancer. Cancer Sci. 2007;98:1817–24. doi: 10.1111/j.1349-7006.2007.00607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wong AS, Soong R, Seah SB, et al. Evidence for disease control with erlotinib after gefitinib failure in typical gefitinib-sensitive Asian patients with non-small cell lung cancer. J Thorac Oncol. 2008;3:400–4. doi: 10.1097/JTO.0b013e318168c801. [DOI] [PubMed] [Google Scholar]
- 52.Viswanathan A, Pillot G, Govindan R. Lack of response to erlotinib after progression on gefitinib in patients with advanced non-small cell lung cancer. Lung Cancer. 2005;50:417–8. doi: 10.1016/j.lungcan.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 53.Walther JC, Khorshid M, Gaya A, Plowman PN. Cross-over response to erlotinib of brain metastatic disease from bronchial adenocarcinoma after gefitinib failure, and an unusual rash. Clin Oncol (R Coll Radiol ) 2006;18:637–9. doi: 10.1016/j.clon.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 54.Garfield DH. Modern treatment of lung cancer: case 2. Response to erlotinib after failure of gefitinib in a patient with advanced non-small-cell lung carcinoma. J Clin Oncol. 2005;23:7738–40. doi: 10.1200/JCO.2005.02.4471. [DOI] [PubMed] [Google Scholar]
- 55.Gridelli C, Maione P, Galetta D, et al. Three cases of long-lasting tumor control with erlotinib after progression with gefitinib in advanced non-small cell lung cancer. J Thorac Oncol. 2007;2:758–61. doi: 10.1097/JTO.0b013e3180cc25b0. [DOI] [PubMed] [Google Scholar]
- 56.Wu SG, Shih JY, Yu CJ, Yang PC. Lung adenocarcinoma with good response to erlotinib after gefitinib treatment failure and acquired T790M mutation. J Thorac Oncol. 2008;3:451–2. doi: 10.1097/JTO.0b013e318169e32a. [DOI] [PubMed] [Google Scholar]
- 57.Chang JW, Chou CL, Huang SF, et al. Erlotinib response of EGFR-mutant gefitinib-resistant non-small-cell lung cancer. Lung Cancer. 2007;58:414–7. doi: 10.1016/j.lungcan.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 58.Cho BC, Kim JH. In Reply. J Clin.Oncol. 2008;26:1184–6. [Google Scholar]
- 59.Riely GJ, Kris MG, Zhao B, et al. Prospective assessment of discontinuation and reinitiation of erlotinib or gefitinib in patients with acquired resistance to erlotinib or gefitinib followed by the addition of everolimus. Clin Cancer Res. 2007;13:5150–55. doi: 10.1158/1078-0432.CCR-07-0560. [DOI] [PubMed] [Google Scholar]
- 60.Yokouchi H, Yamazaki K, Kinoshita I, et al. Clinical benefit of readministration of gefitinib for initial gefitinib-responders with non-small cell lung cancer. BMC Cancer. 2007;7:51. doi: 10.1186/1471-2407-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kwak EL, Sordella R, Bell DW, et al. Irreversible inhibitors of the EGF receptor may circumvent acquired resistance to gefitinib. Proc Natl Acad Sci U S A. 2005;102:7665–70. doi: 10.1073/pnas.0502860102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wong KK. HKI-272 in non small cell lung cancer. Clin Cancer Res. 2007;13:s4593–6. doi: 10.1158/1078-0432.CCR-07-0369. [DOI] [PubMed] [Google Scholar]
- 63.Godin-Heymann N, Ulkus L, Brannigan BW, et al. The T790M “gatekeeper” mutation in EGFR mediates resistance to low concentrations of an irreversible EGFR inhibitor. Mol Cancer Ther. 2008;7:874–9. doi: 10.1158/1535-7163.MCT-07-2387. [DOI] [PubMed] [Google Scholar]
- 64.Li D, Shimamura T, Ji H, et al. Bronchial and peripheral murine lung carcinomas induced by T790M-L858R mutant EGFR respond to HKI-272 and rapamycin combination therapy. Cancer Cell. 2007;12:81–93. doi: 10.1016/j.ccr.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 65.Engelman JA, Zejnullahu K, Gale CM, et al. PF00299804, an irreversible pan-ERBB inhibitor, is effective in lung cancer models with EGFR and ERBB2 mutations that are resistant to gefitinib. Cancer Res. 2007;67:11924–32. doi: 10.1158/0008-5472.CAN-07-1885. [DOI] [PubMed] [Google Scholar]