Abstract
During progression to type 1 diabetes (T1D), the pancreatic islets of humans and of the widely studied mouse model of T1D, the nonobese diabetic (NOD) mouse, are infiltrated by cells of the immune system. Here we report that infiltrated mouse islets (“translucent islets”) can be identified visually. We demonstrate the use of an efficient method for the isolation and culture of the islet-infiltrating cells of NOD mice, which results in a high percentage of CD8+ T cells after seven days, with minimal manipulation. We show that islet-infiltrating cells exit the islets soon after they are placed in culture and can be used in flow cytometric experiments several hours later. Importantly, we demonstrate that the cultured cells are antigen-responsive and that specificities present at the beginning of the culture are generally still present on day seven. In addition, some reactivities are undetected without culture, supporting the validity of the seven-day expansion period. This technique greatly facilitates investigations of the CD8+ T cell reactivities that play a pivotal role in the demise of pancreatic beta cells leading to the development of T1D.
Keywords: CD8+ T cell, islet infiltrate, NOD mice, type 1 diabetes
1. Introduction
Type 1 diabetes (T1D) is an autoimmune disease that results from the concerted attack of insulin-producing beta cells by cells of the immune system. Both in humans and in the widely used model of T1D, the nonobese diabetic (NOD) mouse, it is known that pathogenic T cell populations bearing T cell receptors specific for particular antigens infiltrate pancreatic islets and expand during progression to T1D (Roep, 2003). Therefore, islet-infiltrating cells are a critical target of investigation because they are actively involved in the destruction of the insulin-producing cells and because it is among these cells that the highest proportion of islet-reactive cells is found (Enee et al., 2008). Of particular interest are CD8+ T cells, which are essential for the development of the disease, as evidenced by the fact that when genetic manipulation and depleting antibody treatment have been used to render NOD mice deficient in CD8+ T cells, disease does not occur (Serreze et al., 1994; Wang et al., 1996; Pearson et al., 2003). In T1D patients, CD8+ T cells have been shown to be a prominent component of the immune infiltrate to pancreatic islets (Hanninen et al., 1992; Itoh et al., 1993; Santamaria et al., 1994; Somoza et al., 1994).
Unfortunately, islet-infiltrating cells are not available from T1D patients, from whom only peripheral blood and rarely pancreatic lymph nodes are available. However, recent work on CD8+ T cell reactivities in HLA-transgenic NOD mice has led to the identification of epitopes targeted in recent-onset T1D patients (Takaki et al., 2006; Blancou et al., 2007; Mallone et al., 2007; Jarchum et al., 2008; Martinuzzi et al., 2008), thus establishing these models as excellent tools for the discovery of relevant epitopes. In addition, studies of the peptide antigens targeted by islet-infiltrating cells in NOD mouse models have led to a much improved understanding of the underlying biology of the pathogenesis of T1D (e.g., Han et al., 2005b; Wong et al., 2006). Still, a large percentage of the antigens targeted by T cells found in the islet infiltrate of NOD mice (Lieberman et al., 2004), and possibly T1D patients, remains unidentified. Therefore, it is crucial to delineate methods for the study of islet-infiltrating cells, avoiding when possible their extensive manipulation in the laboratory.
Our group has successfully used such a method to study CD8+ T cells cultured from the islets of NOD mouse models which, as mentioned above, has led to a greater understanding of the pathogenesis of T1D as well as to novel antigen-specific therapies (Han et al., 2005a; Mukhopadhaya et al., 2008). However, widespread use of this powerful technique has not yet taken place, perhaps because islet isolation is done manually and is a time-consuming process. Nevertheless, we believe that its labor-intensive nature is outweighed, first, by the relevance of the T cell population under study, and second, by the fact that, as we describe here and may have been unrecognized until now, large numbers of functional CD8+ T cells can be obtained. It is also possible that common use of this technique has been hampered by a lack of information in the literature regarding the effect of culture on the islet-infiltrating T cell reactivities and the composition of the islet infiltrate. Our goal is to eliminate the potential aforementioned concerns and encourage its use with this careful description of the method.
While others have described the isolation of islet-infiltrating cells by pressing purified islets followed by filtering of the cells (Faveeuw et al., 1995) or by making single-cell islet preparations by passing them through needles (Lejon and Fathman, 1999), we propose the use of bile duct perfusion with collagenase followed by manual isolation of islets. We demonstrate here that heavily infiltrated islets can be microscopically identified and cultured separately if desired, yielding a higher number of lymphocytes, and that infiltrating cells exit the islet soon after they are placed in culture. Importantly, the quality of the cells obtained allows their use soon after isolation in flow cytometry assays that require adequate expression of cell surface markers. For specific experiments requiring large numbers of CD8+ T cells, which, as we demonstrate here, are a small percentage of islet-infiltrating cells, we have developed a method for the culture of islet-infiltrating cells that successfully allows the expansion of CD8+ T cells. In the present work, we describe careful analysis of the culture and report the changes in the composition of T cell subsets as well as T cell specificities over time. Importantly, the CD8+ T cell receptor specificities that were present in the original infiltrate are maintained over the culture period.
The study of the cells that lead to the demise of insulin-producing beta cells and finally insulin deficiency is critical as we make progress towards antigen-specific therapies. Some of these approaches, involving targeting of specific antigens for the establishment of peripheral tolerance (Mukhopadhaya et al., 2008) and the generation of antigen-specific regulatory T cells for modulation of disease progression in the pancreas (Tarbell et al., 2004), are emerging and may be the key to effective treatment of T1D.
2. Materials and methods
2.1 Animals
NOD, NOD.HHD, and NOD. β2m−/−.HHD mice (Takaki et al., 2006) have been previously described and are maintained by brother-sister mating at the Albert Einstein College of Medicine. In addition to expression of the class I MHC molecules Kd and Db, NOD.HHD mice express a monochain chimeric HLA-A*0201 molecule consisting of human β2-microglobulin covalently linked to the α1 and α2 domains of HLA-A*0201, followed by the α3, transmembrane, and cytoplasmic portions of H-2Db. NOD. β2m−/−.HHD mice are, in addition, deficient for β2 microglobulin, and therefore do not express Kd and Db. All animal experiments were approved by the Institutional Animal Care and Use Committee at Albert Einstein College of Medicine.
2.2 Peptides
Individual peptides, having a purity of ≥ 90%, were obtained from Mimotopes. Concentrated stocks (10 mM) were prepared in DMSO, and 10 µM working stocks were obtained by dilution in PBS.
2.3 Islet isolation
Islet isolation by collagenase perfusion of the common bile duct was modified from a previously described protocol (Leiter, 1997). A 30 gauge needle was attached to the end of a piece of siliconized tubing that was connected to a 10 cc syringe. The common bile duct was cannulated and the pancreas perfused with 1.5 ml of 0.625 mg/ml cold collagenase P (Roche, Indianapolis, IN) prepared in Hanks’ balanced salt solution (HBSS). (On rare occasions, newly prepared batches of collagenase will vary in potency from previous batches and the concentration may need to be adjusted by empirical testing). The inflated pancreas was removed, placed in a 50 ml conical tube with 0.5 ml collagenase P, and incubated for 14 min exactly at 37°C to digest exocrine tissue. This 14 min incubation was modified only for digestion of pancreas obtained from very young mice (4–5 weeks of age), when we incubated the tissue for 10 min at 37°C.
Digested tissue was dispersed by pipetting up and down several times with a 10 ml volume of 2% FCS/HBSS. This was followed by two washes with HBSS followed by a final spin at 39 × g for 1 min. Islets were then resuspended in HBSS containing DNase I (Worthington Biochemical, Lakewood, NJ) and handpicked into a dish containing 10 ml RPMI-10 (see below for recipe) using a micromanipulator (micrometer head, Mitutoyo U.S.A., Aurora, IL) with a homemade attachment of siliconized tubing and a siliconized fine glass tip under a dissecting microscope. Islets were handpicked again and counted, and placed for culture as described below. Islet preparations were kept on ice at all times except when under the microscope. Similar to others (Gotoh et al., 1985), we routinely obtained 120–250 islets from non-diabetic animals, while the yield in diabetic animals varied from 30–120 islets, depending on the stage of disease (however, islets from diabetic animals are usually very heavily infiltrated and a large number of lymphocytes can be obtained).
2.4 Propagation of islet-infiltrating T cells
Isolated islets were placed in culture immediately following two rounds of handpicking. Culture medium consisted of RPMI 1640 medium supplemented with 10% FBS (HyClone, Logan, UT), 1 mM sodium pyruvate, non-essential amino acids, 28 µM β-mercaptoethanol (designated RPMI-10), and 50 U/ml recombinant human IL-2 (PeproTech, Rocky Hill, NJ). About 50 islets/well were cultured in 24-well tissue culture plates at 37°C, 5% CO2 for 7 days. For experiments to assess the specificity of cells over the 7-day culture period, islets from several mice were pooled. When assayed, islets and islet-infiltrating cells were collected and passed through a 40 µm cell strainer. Islets were returned to culture with the infiltrating cells after a sample of infiltrating cells was removed.
2.5 IFN-γenzyme-linked immunospot (ELISPOT) assay
ELISPOT plates (MAHA S45 10; Millipore, Billerica, MA) were precoated with anti-mouse IFN-γ mAb (BD Biosciences, San Jose, CA) and blocked with 1% BSA (Fraction V; Sigma-Aldrich, St. Louis, MO). Mitomycin C-treated antigen-presenting cells, which were T2/Kd (generously provided by J. Yewdell, National Institute of Allergy and Infectious Diseases, Bethesda, MD) for studies with NOD.HHD mice or T2 cells (American Type Culture Collection) for NOD. β2m−/−.HHD mice, were added at 2 × 104 cells/well and pulsed with 1 µM peptide. Cultured islet-infiltrating T cells were added at 2 × 104 cells/well, and plates were incubated at 37°C for 40 h. IFN-γ secretion was detected with a second, biotinylated anti-mouse IFN-γ mAb (BD Biosciences) and spots were developed using streptavidin-alkaline phosphatase (Zymed Laboratories, Carlsbad, CA) and 5-bromo-4-chloro-3-indolyl-phosphate/nitroblue tetrazolium substrate (Sigma-Aldrich). Spots with a minimum size of 0.01 mm2 were counted using an automated ELISPOT reader system (Autoimmun Diagnostika, Strassberg, Germany). When expressed as spot-forming cells (SFC) / 2 × 104 cells, spot counts shown are background (PBS)-subtracted. The stimulation index was calculated as the ratio between SFC in response to a specific peptide divided by the SFC in the presence of PBS alone.
2.6 Detection of cell populations by FACS
Total B lymphocytes were detected by staining with polyclonal anti-mouse Ig+IgM and anti-mouse B220 (RA3-6B2, BD Biosciences). T cells were detected by staining with anti-mouse CD3 antibodies (mAb 145-2C11, BD Biosciences) and further characterized for CD4 expression using the mAb GK1.5 or for CD8 expression with the mAb 53-6.7 (BD Biosciences). NK cells were detected using the mAb DX5 against the pan-NK marker CD49b (BD Biosciences), and macrophages were detected with an anti-CD11b antibody (M1/70, BD Biosciences). Anti-mouse Foxp3 antibody (eBioscience, San Diego, CA) was used. Data acquisition was done in a FACS Calibur (BD Biosciences) and analyzed using FlowJo software.
2.7 Statistical analysis
To analyze the statistical significance in the yield of cells from translucent and non-translucent islets, unpaired Student’s t test was used.
2.8 Islet visualization and imaging
A Nikon SMZ645 dissecting microscope (Nikon, Melville, NY) with an under-stage light source was used for handpicking islets. Islets shown in Fig. 1B (left panel), Fig. 1E, and Fig. 1F were imaged with a 4X phase contrast objective on a Nikon Diaphot inverted microscope with a heated chamber. Islets shown in Fig. 1B (right panel) were captured with a Zeiss SV11 stereo dissecting microscope (Zeiss, Thornwood, NY).
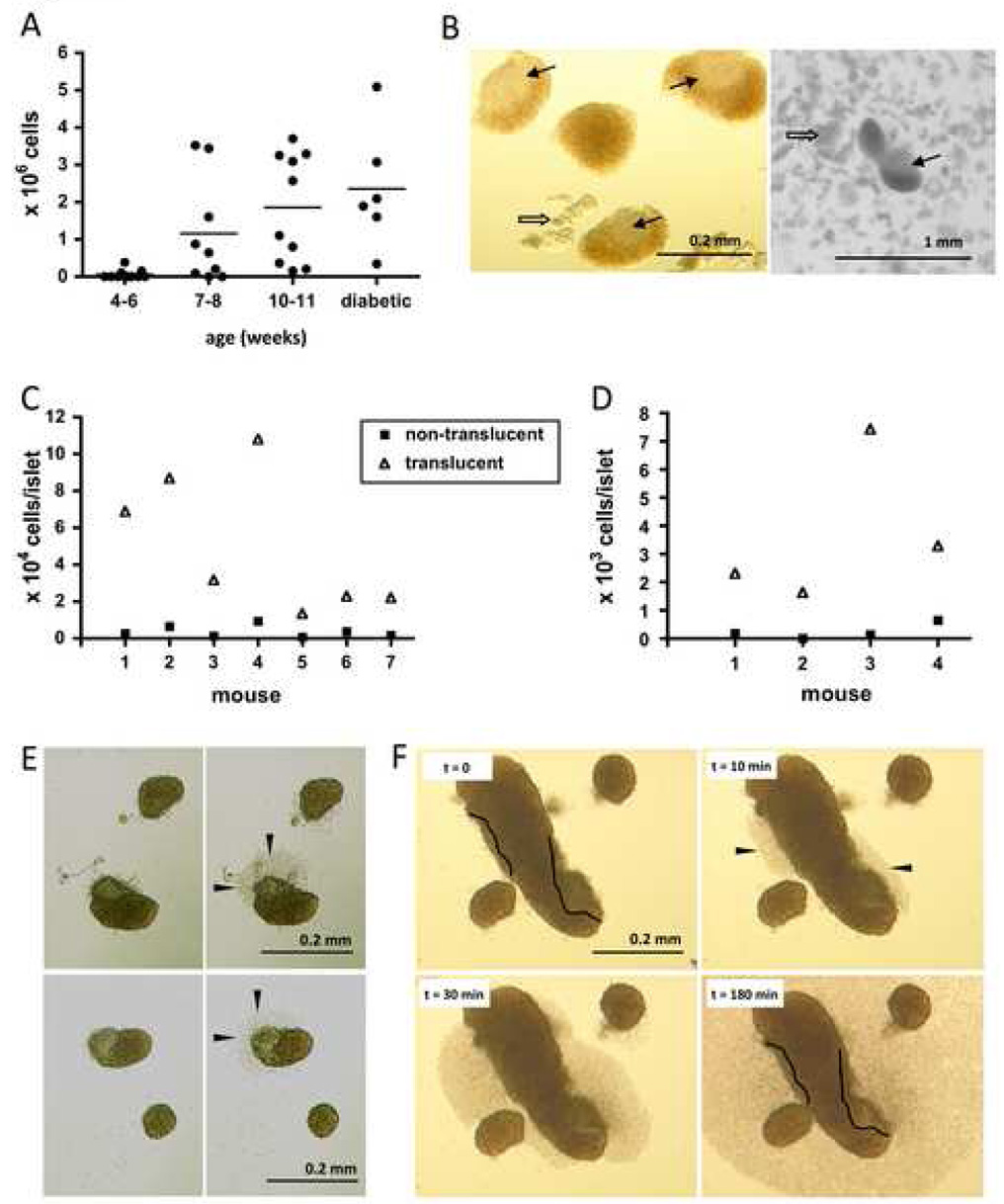
Figure 1.
Isolation of pancreatic islets from NOD mice and culture of islet-infiltrating cells. The total number of islet-infiltrating cells obtained from NOD mice of different ages after seven days in culture is shown in A, where each symbol represents one mouse. Islet infiltrates can be identified visually by grey color and translucency, as shown in the two images in B, obtained with different microscopes for more exhaustive visualization. Thin arrows indicate the translucent infiltrates and thick arrows point to digested exocrine tissue. The number of cells obtained after one week of culture from translucent and non-translucent islets from seven NOD mice (11 weeks of age) is shown in C. In D, the yield of infiltrating cells from translucent and non-translucent islets from four NOD mice (9 weeks of age) was assessed without culture (after only overnight incubation). In E, islets were imaged before and 30 minutes after (left and right panels, respectively) they were placed in culture. Infiltrating cells (arrow heads) can be seen exiting from the translucent regions of heavily infiltrated islets shown in E and F. Images in F show cells exiting the islets from infiltrated areas (delineated with a black line) over time.
3. Results and discussion
3.1 Infiltrated islets from the pancreas of NOD mice can be visually identified and isolated
Following bile duct cannulation, perfusion with collagenase and digestion, islets can be visualized under a dissection microscope and isolated using a micromanipulator, as described in the Materials and Methods. Using this method, islet-infiltrating cells can be obtained from NOD mice of different ages and cultured. Not surprisingly, larger numbers of islet-infiltrating cells (i.e., those cells obtained after passing the islet cultures through a 40 µm strainer) can be obtained from older mice as the disease progresses, with an average of 1.15 × 106 cells/pancreas at 7–8 weeks of age and 2.4 × 106 cells/pancreas at the onset of diabetes (Fig. 1A) after 7 days in culture. We expect that knowledge of these average yields will aid other researchers in experimental design.
While picking the islets of NOD mice, we noticed that there are visually identifiable differences among the islets. While some islets look intact, others can be distinguished by the presence of a translucent region of variable dimensions, and with a grey color rather than the typical brown color of the islet (Fig. 1B). Intrigued by this finding, and based on our extensive experience with islet isolation, as well as islet histology, we hypothesized that the translucent region that identified some islets (“translucent islets”) was the immune infiltrate known to result in destruction of the islets during development of T1D, and that non-translucent islets were either at a non-invasive stage of lymphocytic infiltration such as peri-insulitis, or completely intact. Therefore, we isolated translucent and non-translucent islets from several mice, cultured them separately, and determined the yield of infiltrating cells 7 days later. As seen in Fig. 1C, while non-translucent islets yielded 3.70 × 103 ± 3.14 × 103 cells/islet, translucent islets yielded 5.05 × 104 ± 3.70 × 104 cells/islet (p = 0.006). We then investigated whether this difference in cell number was also reflected without culturing the infiltrating cells. As shown in Fig. 1D, this difference was also detected after only overnight incubation, with an average of 0.24 × 103 ± 0.28 cells/islet from non-translucent islets and 3.67 × 103 ± 2.60 cells/islet from translucent islets (p = 0.04). Further, the ratio of infiltrating cells obtained from translucent/non-translucent islets is 15 and 14 before and after culture, respectively, suggesting cells from translucent and non-translucent islets expand similarly over the culture period.
We then sought to confirm that the translucent regions we were identifying as infiltrate were indeed the source of the cells. For this purpose, we imaged translucent and non-translucent islets before and after they were placed in culture at 37 °C and found infiltrating cells could be seen exiting the islet from translucent regions (Fig. 1E and F). Using time-lapse microscopy, we found that infiltrating cells can be seen exiting the islets as early as 10 minutes after these have been placed in culture (Fig. 1F). This novel finding is of interest particularly for investigations on the inflammatory composition of individual islets, where single infiltrated islets may need to be isolated, as well as for the generation of T cell clones derived from islet-infiltrating cells. In addition, it may aid investigations of the physical properties of infiltrated islets for the design of diagnostic and monitoring tools.
3.2 Cellular composition of the islet infiltrate in NOD mice during progression to T1D
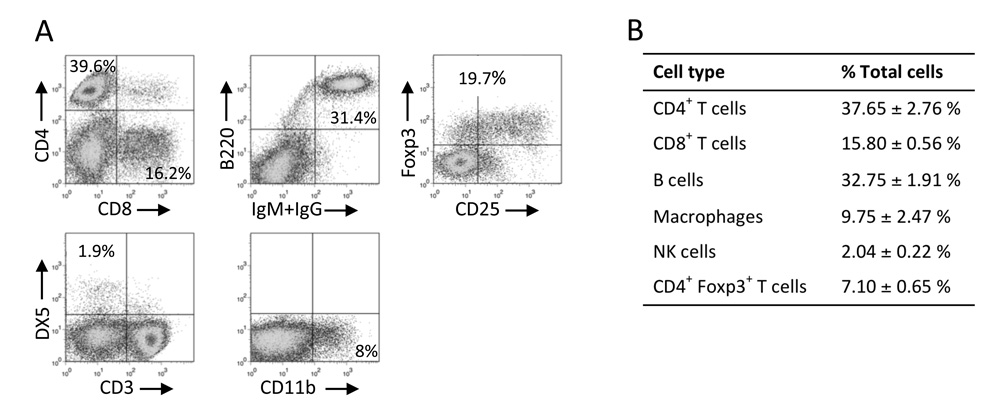
Upon demonstrating that infiltrating cells begin to exit the islet very soon after they are put in culture (Fig. 1E and F), we predicted that an overnight incubation would be enough time to allow for infiltrating cells to leave the islet but allow very limited proliferation of the infiltrating cells, and decided to use this approach to study the cellular composition of the islet infiltrate. We did not use trypsin, cell dissociation buffer, or mechanical methods to free the infiltrate, as these may damage cell surface proteins and seemed unnecessary when we found that only a few hours are needed for lymphocytes to exit the islet on their own. We isolated islets from non-diabetic NOD mice (9–11 weeks of age), allowed infiltrating cells to exit the islets overnight, and determined the number of cells belonging to several immunological compartments in the infiltrate by flow cytometry. As shown in Fig. 2A and B, the largest cell subset present in the infiltrate of NOD mice is CD4+ T cells, followed by B cells. However, a considerable percentage of the cells belong to the CD8+ T cell and macrophage compartments (15.80% and 9.75%, respectively), and NK cells were also represented (2%). Approximately 20% of CD4+ T cells in these mice were Foxp3+. These findings agree with previous reports of histological studies on T and B cells in the islet infiltrate (Signore et al., 1989; Kikutani and Makino, 1992), demonstrating that cell surface marker expression is maintained after cells are obtained by the methods proposed here.
Figure 2.
Cellular composition of the islet infiltrates of NOD mice. Islet-infiltrating cells from NOD mice (9–11 weeks of age) were obtained after overnight incubation and assayed by flow cytometry. Representative results of two or more experiments are shown in A and tabulated in B. In A, flow cytometry data for Foxp3 and CD25 expression was gated on CD4+ cells. About 98% of the cells in the islet infiltrate are accounted for in these experiments.
3.3 Culture conditions promote growth of CD8+ T cells
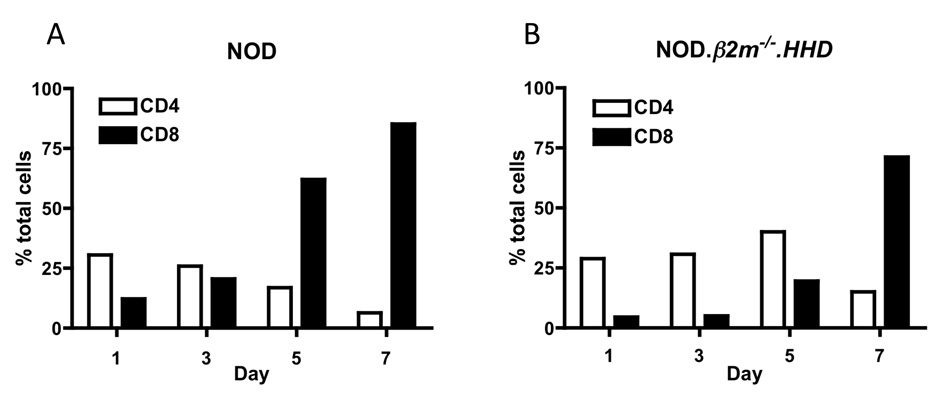
Because of the importance of CD8+ T cells in the pathogenesis of T1D, we were interested in developing culture conditions that promoted the growth and expansion of this particular cell type from the bulk of the islet infiltrate. We cultured islets in RPMI-10 medium supplemented with 50 U/ml IL-2 for 7 days and monitored the percentage of CD4+ and CD8+ T cells over the culture period. As shown in Fig. 3, after 7 days of culture in these conditions, an overwhelming percentage of cells are viable CD8+ T cells. Based on our findings, we have also used cells after 3–4 days in culture for the study of CD4+ T cells after enrichment of this population (data not shown). In addition, we performed similar experiments with islets isolated from NOD. β2m−/−.HHD mice, which have a lower percentage of CD8+ T cells in the spleen (Takaki et al., 2006) and in the infiltrate. Even when the starting population of CD8+ T cells is as low as 5% of total cells, 71% of cells are CD8+ T cells on day 7. Therefore, these culture conditions favor the growth and expansion of CD8+ T cells from islet infiltrates even when the starting percentage of CD8+ T cells is very low. We speculate that a factor contributing to the growth advantage of CD8+ T cells may involve direct antigen presentation by islet cells that express class I MHC molecules, whereas class II MHC-expressing cells in the culture may be lower in number. The large quantity of CD8+ T cells that can be obtained allows the study of these cells using methods that require a number of CD8+ T cells that exceeds that which can be isolated freshly from infiltrated islets, such as the use of peptide libraries to identify novel epitopes targeted (Takaki et al., 2006; Jarchum et al., 2007), tetramer analysis of the specificities present in the infiltrate (Lieberman et al., 2004), and identification of novel antigens when T cell clones or T cell receptor transgenic mice are not available, among others.
Figure 3.
Culture conditions favor the expansion of CD8+ T cells over time. Islet-infiltrating cells were assayed by flow cytometry over the seven-day culture period to assess changes in CD4+ and CD8+ T cell subsets. Percent cells from NOD (A) and NOD.β2m−/−.HHD (B) islet infiltrates over seven-day culture period. Results are representative of two or more independent experiments.
3.4 Cultured CD8+ T cells are antigen-responsive and their specificities were investigated over the culture period
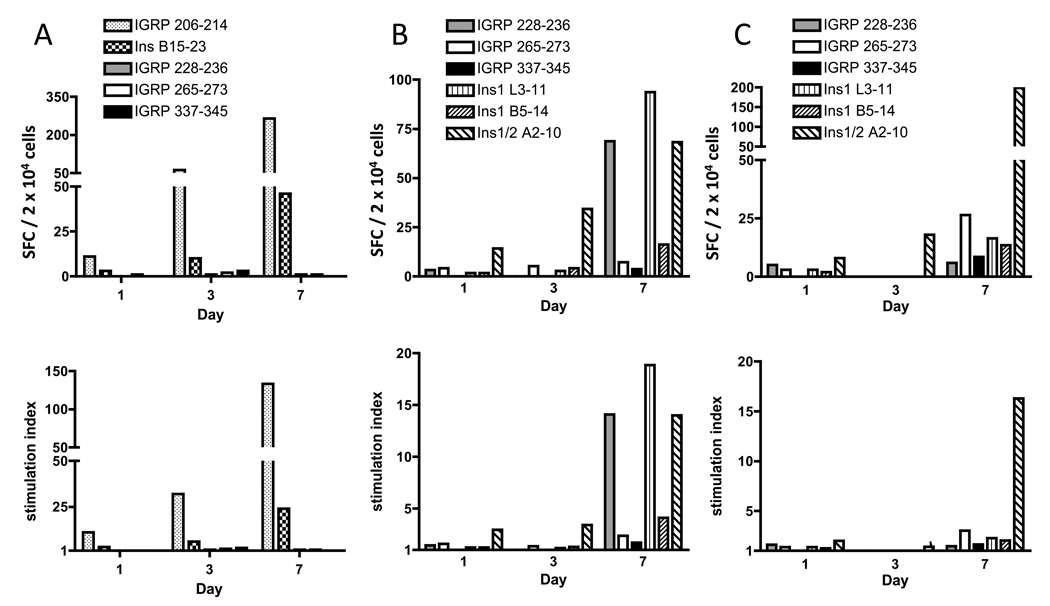
Once we had obtained large numbers of CD8+ T cells, we wanted to determine whether the specificities present at the beginning of the culture period were still represented after 7 days in culture, i.e., whether cultured cells could still be used to study specificities relevant to the development of T1D. Because the T cell specificities present in an individual islet can vary from one islet to another (Sarukhan et al., 1995), we pooled islets that were isolated from several mice and cultured them as described above. On days 1, 3, and 7, we collected a sample and continued to culture the rest of the cells with the islets. These samples were used in an IFN-γ ELISPOT assay to determine the number of cells specific for known epitopes and to monitor the specificities over the culture period. We initially used NOD.HHD mice for these experiments, reasoning that they would allow us to assess CD8+ T cell reactivity to several epitopes, including well-described NOD epitopes such as IGRP 206–214 and Ins B15-23, in addition to HLA-A2-restricted epitopes. Representative results of a total of two experiments using this approach are shown in Fig. 4A, and are expressed as SFC / 2 × 104 cells (top panel) and as stimulation index (bottom panel). Using both analytical methods, it can be observed that the rank order of T cells specific for IGRP 206–214 and Ins B15-23 is maintained throughout the culture period. However, because in these mice the CD8+ T cell response is strongly directed towards these two epitopes, studies with islet-infiltrating cells from NOD.HHD mice allowed us to study only a limited number of specificities. Therefore, we then investigated NOD. β2m−/−.HHD mice, in which we would be able to compare responses to six well-characterized HLA-A2-restricted epitopes, three from islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP) and three from insulin, which are presented and targeted during progression to T1D in these mice (Takaki et al., 2006; Jarchum et al., 2007). Two such experiments were performed and are shown in Fig. 4B and 4C. In the experiment shown in Fig. 4B, it can be observed that the predominant specificity detected on day 1, to Ins1/2 A2-10, was still highly represented on days 3 and 7. In addition, T cells that remained undetected early during culture, specific for other epitopes, were detectable after the seven-day expansion period. In the experiment shown in Fig. 4C, reactivity to Ins1/2 A2-10 is the most prominent specificity detected on days 1, 3, and 7 as seen both by SFC and stimulation index.
Figure 4.
CD8+ T cell specificities present in the islet infiltrate over the culture period. Islet-infiltrating cells from NOD.HHD mice of 14 weeks of age (A) were examined by IFN-γ ELISPOT for reactivity to two H-2Kd-restricted epitopes and three HLA-A2-restricted epitopes. Top panels show results expressed as SFC / 2 × 104 cells (background-subtracted) and bottom panels as stimulation index (the ratio of SFC in the presence of antigen divided by SFC in the absence of antigen). Islets were isolated from NOD.β2m−/−.HHD mice of 13–15 weeks of age (B) and 20–26 weeks of age (C) and examined as in A for reactivity to six known HLA-A2-restricted epitopes.
Even though cultures can be observed that retain the relative frequency of different specificities (e.g., Fig. 4A), in other cases, the relative frequency of these populations changes over culture (e.g., Fig. 4B). We believe this could be due to several reasons. We do not discard the possibility that experimental limitations in detecting these cells by IFN-γ ELISPOT over the earlier days of culture may contribute to these results. However, another, we believe more likely, explanation, is based on the observation that some specificities expand better than others over the culture period. As observed in Fig. 4A, even though IGRP 206–214 and Ins B15-23-reactive cells retain their rank order over the culture period, they do not expand to similar extents, as there is a larger expansion of the IGRP-reactive population by day 7 as compared to the Ins-reactive cells (24-fold and 15-fold expansion of IGRP and Ins-reactive cells, respectively, by SFC, and 12-fold and 8-fold expansion, respectively, by stimulation index). Greater expansion of particular specificities could be due to better presentation of some antigens over others and to differing affinities of the T cell receptors for peptide-MHC complex. Interestingly, because expansion of the cells occurs in vitro in the presence of the islets and with minimal manipulation, both of these possibilities would likely reflect the biology of CD8+ T cell expansion in vivo. In other words, during progressive infiltration of the islets, certain T cell specificities may expand better than others and contribute differently to beta cell killing over time. Another alternative or additional factor that may be contributing to a proliferative advantage of some specificities may be a higher state of activation of the cells at the initial stage of culture (e.g., higher CD25 expression), which would allow some cells to respond better to the culture conditions. Importantly, the observation that some reactivities cannot be detected without the 7 day expansion period confirms the importance and validity of this method, since those reactivities must have been present on day 1 although they may have been undetected. It is critical to note that we did not detect any major specificities on days 1 and 3 that were not still present on day 7.
As we report here, we conclude from our extensive experience that after 3–4 days of culture, islet-infiltrating cells can be enriched for CD4+ T cells and after seven days, islet-infiltrating cells are mostly CD8+ T cells and can therefore be used for a variety of experimental setups without additional enrichment. We do not recommend the use of islet-infiltrating cells cultured for 3–5 days in IFN-γ ELISPOT to assay CD8+ T cell responses without enrichment for this specific lymphocyte population. Although this can be achieved (Fig. 4), the background in the assay is often higher on days 3 and 5 when using total infiltrating cells without enrichment (data not shown), which may decrease accuracy of the spot count on these days. We speculate that the higher background may be due to the presence of other cell types in the culture, which may become non-specifically activated before undergoing apoptosis during the last few days of culture.
4. Concluding remarks
In sum, the method proposed here is reliable and facilitates investigations of the islet infiltrate and of CD8+ T cells in particular, which are a critical population of effector cells participating in the destruction of beta cells and resulting in T1D. Studies of islet-infiltrating cells from NOD mice have led to important advancements in the understanding of T1D and to the development of tools that aid in the study of the disease in humans. As new immune therapies are developed for the treatment of T1D, it is crucial to establish accurate methods to monitor disease progression. Equally important, as prevention studies continue to be developed, highly accurate methods for prediction and early diagnosis of disease progression are needed. We speculate that in disease monitoring and diagnosis of T1D, as well as in the development of therapeutic interventions, antigen specificity will continue to take a prominent role, and the method described here has great potential to aid in this endeavor.
Acknowledgements
We thank the Albert Einstein College of Medicine’s Analytical Imaging Facility for technical assistance. This work was supported by National Institutes of Health grants DK64315 (T.P.D.), DK52956 (T.P.D.), DK77500 (T.P.D.), and DK20541 (Albert Einstein College of Medicine’s Diabetes Research and Training Center), and by grants to T.P.D. from the Juvenile Diabetes Research Foundation, the American Diabetes Association, and The Irma T. Hirschl/Monique Weill-Caulier Trust. The Flow Cytometry and Analytical Imaging Facilities at Albert Einstein College of Medicine are supported by National Institutes of Health Cancer Center Grant CA13330.
Abbreviations
- HBSS
Hanks’ balanced salt solution
- IGRP
islet-specific glucose-6-phosphatase catalytic subunit-related protein
- NOD
nonobese diabetic
- SFC
spot-forming cells
- T1D
type 1 diabetes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blancou P, Mallone R, Martinuzzi E, Severe S, Pogu S, Novelli G, Bruno G, Charbonnel B, Dolz M, Chaillous L, van Endert P, Bach JM. Immunization of HLA class I transgenic mice identifies autoantigenic epitopes eliciting dominant responses in type 1 diabetes patients. J. Immunol. 2007;178:7458. doi: 10.4049/jimmunol.178.11.7458. [DOI] [PubMed] [Google Scholar]
- Enee E, Martinuzzi E, Blancou P, Bach JM, Mallone R, van Endert P. Equivalent specificity of peripheral blood and islet-Infiltrating CD8+ T lymphocytes in spontaneously diabetic HLA-A2 transgenic NOD mice. J. Immunol. 2008;180:5430. doi: 10.4049/jimmunol.180.8.5430. [DOI] [PubMed] [Google Scholar]
- Faveeuw C, Gagnerault MC, Lepault F. Isolation of leukocytes infiltrating the islets of Langerhans of diabetes-prone mice for flow cytometric analysis. J. Immunol. Methods. 1995;187:163. doi: 10.1016/0022-1759(95)00180-i. [DOI] [PubMed] [Google Scholar]
- Gotoh M, Maki T, Kiyoizumi T, Satomi S, Monaco AP. An improved method for isolation of mouse pancreatic islets. Transplantation. 1985;40:437. doi: 10.1097/00007890-198510000-00018. [DOI] [PubMed] [Google Scholar]
- Han B, Serra P, Amrani A, Yamanouchi J, Maree AF, Edelstein-Keshet L, Santamaria P. Prevention of diabetes by manipulation of anti-IGRP autoimmunity: high efficiency of a low-affinity peptide. Nat. Med. 2005a;11:645. doi: 10.1038/nm1250. [DOI] [PubMed] [Google Scholar]
- Han B, Serra P, Yamanouchi J, Amrani A, Elliott JF, Dickie P, Dilorenzo TP, Santamaria P. Developmental control of CD8 T cell-avidity maturation in autoimmune diabetes. J. Clin. Invest. 2005b;115:1879. doi: 10.1172/JCI24219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanninen A, Jalkanen S, Salmi M, Toikkanen S, Nikolakaros G, Simell O. Macrophages, T cell receptor usage, and endothelial cell activation in the pancreas at the onset of insulin-dependent diabetes mellitus. J. Clin. Invest. 1992;90:1901. doi: 10.1172/JCI116067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N, Hanafusa T, Miyazaki A, Miyagawa J, Yamagata K, Yamamoto K, Waguri M, Imagawa A, Tamura S, Inada M. Mononuclear cell infiltration and its relation to the expression of major histocompatibility complex antigens and adhesion molecules in pancreas biopsy specimens from newly diagnosed insulin-dependent diabetes mellitus patients. J. Clin. Invest. 1993;92:2313. doi: 10.1172/JCI116835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarchum I, Baker JC, Yamada T, Takaki T, Marron MP, Serreze DV, DiLorenzo TP. In vivo cytotoxicity of insulin-specific CD8+ T-cells in HLA-A*0201 transgenic NOD mice. Diabetes. 2007;56:2551. doi: 10.2337/db07-0332. [DOI] [PubMed] [Google Scholar]
- Jarchum I, Nichol L, Trucco M, Santamaria P, DiLorenzo TP. Identification of novel IGRP epitopes targeted in type 1 diabetes patients. Clin Immunol. 2008;127:359. doi: 10.1016/j.clim.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikutani H, Makino S. The murine autoimmune diabetes model: NOD and related strains. Adv. Immunol. 1992;51:285. doi: 10.1016/s0065-2776(08)60490-3. [DOI] [PubMed] [Google Scholar]
- Leiter E. The NOD Mouse: A Model For Insulin-Dependent Diabetes Mellitus. In: Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Strober W, editors. Current Protocols in Immunology. Hoboken: John Wiley & Sons; 1997. p. 15.9.1. [DOI] [PubMed] [Google Scholar]
- Lejon K, Fathman CG. Isolation of self antigen-reactive cells from inflamed islets of nonobese diabetic mice using CD4high expression as a marker. J. Immunol. 1999;163:5708. [PubMed] [Google Scholar]
- Lieberman SM, Takaki T, Han B, Santamaria P, Serreze DV, DiLorenzo TP. Individual nonobese diabetic mice exhibit unique patterns of CD8+ T cell reactivity to three islet antigens, including the newly identified widely expressed dystrophia myotonica kinase. J. Immunol. 2004;173:6727. doi: 10.4049/jimmunol.173.11.6727. [DOI] [PubMed] [Google Scholar]
- Mallone R, Martinuzzi E, Blancou P, Novelli G, Afonso G, Dolz M, Bruno G, Chaillous L, Chatenoud L, Bach JM, van Endert P. CD8+ T-cell responses identify beta-cell autoimmunity in human type 1 diabetes. Diabetes. 2007;56:613. doi: 10.2337/db06-1419. [DOI] [PubMed] [Google Scholar]
- Martinuzzi E, Novelli G, Scotto M, Blancou P, Bach JM, Chaillous L, Bruno G, Chatenoud L, van Endert P, Mallone R. The frequency and immunodominance of islet-specific CD8+ T-cell responses change after type 1 diabetes diagnosis and treatment. Diabetes. 2008;57:1312. doi: 10.2337/db07-1594. [DOI] [PubMed] [Google Scholar]
- Mukhopadhaya A, Hanafusa T, Jarchum I, Chen YG, Iwai Y, Serreze DV, Steinman RM, Tarbell KV, DiLorenzo TP. Selective delivery of beta cell antigen to dendritic cells in vivo leads to deletion and tolerance of autoreactive CD8+ T cells in NOD mice. Proc. Natl. Acad. Sci. U S A. 2008;105:6374. doi: 10.1073/pnas.0802644105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson T, Markees TG, Serreze DV, Pierce MA, Marron MP, Wicker LS, Peterson LB, Shultz LD, Mordes JP, Rossini AA, Greiner DL. Genetic disassociation of autoimmunity and resistance to costimulation blockade-induced transplantation tolerance in nonobese diabetic mice. J. Immunol. 2003;171:185. doi: 10.4049/jimmunol.171.1.185. [DOI] [PubMed] [Google Scholar]
- Roep BO. The role of T-cells in the pathogenesis of type 1 diabetes: from cause to cure. Diabetologia. 2003;46:305. doi: 10.1007/s00125-003-1089-5. [DOI] [PubMed] [Google Scholar]
- Santamaria P, Lewis C, Jessurun J, Sutherland DE, Barbosa JJ. Skewed T-cell receptor usage and junctional heterogeneity among isletitis alpha beta and gamma delta T-cells in human IDDM [corrected] Diabetes. 1994;43:599. doi: 10.2337/diab.43.4.599. [DOI] [PubMed] [Google Scholar]
- Sarukhan A, Bedossa P, Garchon HJ, Bach JF, Carnaud C. Molecular analysis of TCR junctional variability in individual infiltrated islets of non-obese diabetic mice: evidence for the constitution of largely autonomous T cell foci within the same pancreas. Int. Immunol. 1995;7:139. doi: 10.1093/intimm/7.1.139. [DOI] [PubMed] [Google Scholar]
- Serreze DV, Leiter EH, Christianson GJ, Greiner D, Roopenian DC. Major histocompatibility complex class I-deficient NOD-β2mnull mice are diabetes and insulitis resistant. Diabetes. 1994;43:505. doi: 10.2337/diab.43.3.505. [DOI] [PubMed] [Google Scholar]
- Signore A, Pozzilli P, Gale EA, Andreani D, Beverley PC. The natural history of lymphocyte subsets infiltrating the pancreas of NOD mice. Diabetologia. 1989;32:282. doi: 10.1007/BF00265543. [DOI] [PubMed] [Google Scholar]
- Somoza N, Vargas F, Roura-Mir C, Vives-Pi M, Fernandez-Figueras MT, Ariza A, Gomis R, Bragado R, Marti M, Jaraquemada D. Pancreas in recent onset insulin-dependent diabetes mellitus. Changes in HLA, adhesion molecules and autoantigens, restricted T cell receptor V beta usage, and cytokine profile. J. Immunol. 1994;153:1360. [PubMed] [Google Scholar]
- Takaki T, Marron MP, Mathews CE, Guttmann ST, Bottino R, Trucco M, DiLorenzo TP, Serreze DV. HLA-A*0201-restricted T cells from "humanized" NOD mice recognize autoantigens of potential clinical relevance to type 1 diabetes. J. Immunol. 2006;176:3257. doi: 10.4049/jimmunol.176.5.3257. [DOI] [PubMed] [Google Scholar]
- Tarbell KV, Yamazaki S, Olson K, Toy P, Steinman RM. CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J. Exp. Med. 2004;199:1467. doi: 10.1084/jem.20040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Gonzalez A, Benoist C, Mathis D. The role of CD8+ T cells in the initiation of insulin-dependent diabetes mellitus. Eur. J. Immunol. 1996;26:1762. doi: 10.1002/eji.1830260815. [DOI] [PubMed] [Google Scholar]
- Wong CP, Li L, Frelinger JA, Tisch R. Early autoimmune destruction of islet grafts is associated with a restricted repertoire of IGRP-specific CD8+ T cells in diabetic nonobese diabetic mice. J. Immunol. 2006;176:1637. doi: 10.4049/jimmunol.176.3.1637. [DOI] [PubMed] [Google Scholar]