Abstract
Dirhodium caprolactamate is the most efficient catalyst for the oxidation of Δ5-steroids to 7-keto- Δ5-steroids by 70% tert-butyl hydroperoxide in water (T-HYDRO®). Isolated product yields range from 38% to 87%.
Few substrates have commanded as much attention for allylic oxidation as have Δ5-steroids. Intense interest has been emerging concerning the biological effects of the 7-keto steroid oxidation products that are indicated in their increasingly evident physiological effects.1,2 Selenium dioxide is inappropriate as an oxidant because this reagent favors oxidation at the 4-position,3 and stoichiometric chromium(VI) oxidations4 are disfavored because of the relative severity of reaction conditions and environmental concerns.1a Recently, tert-butyl hydroperoxide has become the oxidant of choice for steroidal allylic oxidations, but the defining ingredient in these oxidations is the promoter or catalyst, and the optimal methodology is unclear. Since 2000 alone, allylic oxidation methodologies for steroids using tert-butyl hydroperoxide (TBHP) have been reported to be catalyzed or promoted by sodium chlorite, 5 chromium (VI),6 manganese(III) acetate,7 bismuth(III) salts,8 copper iodide,9 and cobalt. acetate 10 Chromium mediated oxidations have received the most attention,11 but ruthenium(III) chloride catalysis in large-scale applications was found to be more advantageous.12 We reported in 2004 a new catalytic system for allylic oxidations using TBHP that was extraordinarily effective for simple cyclic systems, providing enone product with complete regioselectivity and in high yield with the use of catalyst loadings of between 0.1 and 1.0 mol % dirhodium caprolactamate, Rh2(cap)4.13 The tert-butyl hydroperoxide oxidant was the anhydrous reagent in decane that has been favored by several investigators in order to avoid hydrolysis of the catalyst. We can now report that dirhodium caprolactamate is exceptionally effective for the oxidation of Δ5-steroids, and even those that possess oxidatively sensitive groups, using the much less expensive 70% TBHP in water (T-HYDRO®).
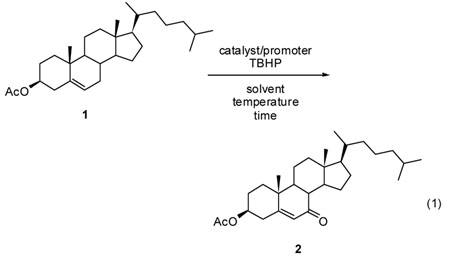
Previous reports for oxidations by TBHP cover a wide range of conditions, so direct comparison is difficult. However, cholesteryl acetate (1) is common to virtually all studies, and comparative results for its conversion to the 7-keto-Δ5 product (2, eq. 1) are described in Table 1, along with that for the Rh2(cap)4-catalyzed oxidation. As can be seen from the Table, the catalytic use of Rh2(cap)4 has distinct advantages over other existing methodologies: high product yield, use of T-HYDRO® instead of tert-butyl hydroperoxide in decane, catalyst loading down to 0.1 mol %, less T-HYDRO® than most methods, moderate temperatures and time. In contrast to the other catalysts or promoters, the oxidized Rh2(cap)4 that is formed upon reaction with TBHP is soluble in both water and the organic solvent, thus minimizing operational concerns that are related to two-phase reaction systems.
Table 1.
Method comparison for oxidation of cholestryl acetate (1) by tert-butyl hydroperoxide.
| catalyst/ additive (mol%) |
Oxidant | TBHP (equiv) |
Solvent | Time (h) |
temp. (°C) |
Yield 2, (%) |
|---|---|---|---|---|---|---|
| NaClO2 (116 %) |
T-HYDRO | 10 | MeCN | 80 | 60 | 66 ref. (5) |
| CrO3 (5 %)/NMI |
T-HYDRO | 7 | PhCF3 | 23 | 40 | 74 ref. (6) |
| Mn3(OAc)9 (10 %) |
t-BuOOH/ decane |
5 | EtOAc | 48 | 40 | 85 ref. (7) |
| BiCl3 (5 %) |
t-BuOOH/ decane |
10 | MeCN | 22 | 70 | 82 ref. (8) |
| CuI (3 %)/ Bu4NBr (6 %) |
T-HYDRO | 7 | benzene | 24 | 70 | 63 ref. (9) |
| Co(OAc)2 (2.2 %) |
t-BuOOH/ decane |
6 | benzene | 48 | 70 | 70 (ref. 10) |
| RuCl3 (0.7 %) |
T-HYDRO | 10 | Cyclohex ane |
24 | 20 | 75 (ref. 12) |
| Rh2(cap)4 (1.0 %) |
T-HYDRO | 5 | (CH2Cl)2 | 20 | 40 | 80 |
| Rh2(cap)4 (0.1 %) |
T-HYDRO | 8 | (CH2Cl)2 | 20 | 40 | 77 |
The Rh2(cap)4-catalyzed oxidation was performed on a mmole scale with Δ5-steroids using 0.1 to 1.0 mol % of Rh2(cap)4. 1,2-Dichloroethane was added to dissolve the steroid, the solution was heated to 40 °C, and then T-HYDRO® was added in one portion. The original blue color of Rh2(cap)4 turned deep-red upon mixing with tert-butyl hydroperoxide, consistent with formation of Rh(II)Rh(III) caprolactamate. The reaction solution was stirred for 20 h at 40 °C, after which the solution was concentrated under reduced pressure and subjected to purification by column chromatography (1-cm diameter, 15-cm long glass column) using hexane/ethyl acetate. Isolated yields were determined by mass of the purified product, and product identity was confirmed by NMR spectroscopy with comparison, whenever possible, to spectra of authentic samples.
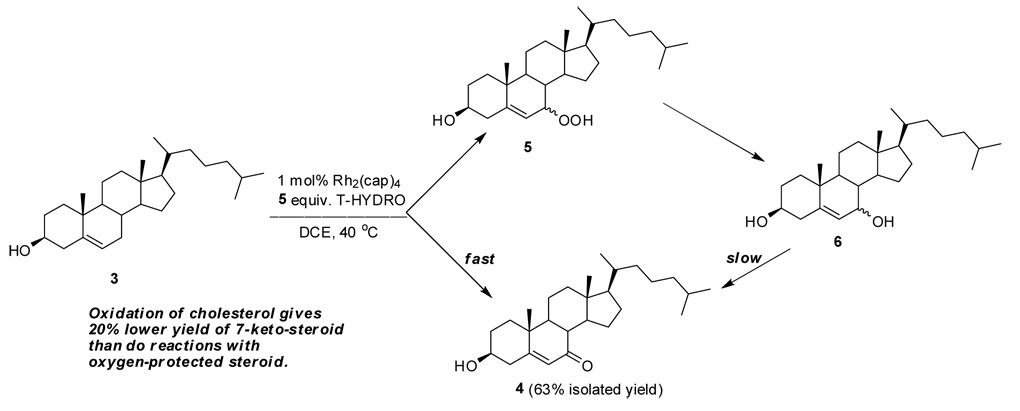
Oxidation of cholesterol (3) is more challenging than the oxidation of cholesteryl acetate, and we observe a comparative decrease in the isolated yield of 7-ketocholesterol (4) of 15–20 % with the use of tert-butyl hydroperoxide as the oxidant.14 Highly polar materials, believed to be acids from oxidative cleavage, are formed whose identity has been elusive. However, 7-ketocholesterol is easily isolated from the reaction mixture in pure form by standard chromatography (vide infra), and we have carried out the oxidation in gram-scale using 0.1 mol % Rh2(cap)4.
Optimization of this oxidation was achieved by investigation of the change in % conversion and isolated yield as a function of temperature, amount of T-HYDRO®, solvent, and additive. Conversion is slow at room temperature, but complete within 20 h at 40 °C; at 60 °C oxidation is considerably faster, but the catalyst undergoes hydrolysis at a measurable rate. With 2.0 equiv of T-HYDRO® oxidation reached 83% conversion within 20 h and was at 95% conversion with 4.0 equiv of T-HYDRO® over the same time, the reason for which is competitive formation of both stereoisomers of 7-hydroperoxy- (5) and 7-hydroxy-cholesterol (6)15 that undergo slow oxidation to 7-ketocholesterol (Scheme 1). Dichloroethane was preferred over dichloromethane because of its higher boiling point. The use of toluene gave comparable results under comparable conditions; however, oxidation was slower in cyclohexane, and the use of either methanol or water without an organic co-solvent resulted, as expected from solubility considerations, in a much slower rate of oxidation. Inorganic bases that facilitated TBHP allylic oxidations which occurred under anhydrous conditions13 only inhibited oxidations that occurred with T-HYDRO®. When the same amount of TBHP in decane is used along with 50 mol % of potassium carbonate, instead of T-HYDRO®, % conversion only reaches 40 %. Thus, optimum conditions for allylic oxidations of cholesterol involve the use of 0.1 to 1.0 mol % of Rh2(cap)4, 4–5 molar equivalents of T-HYDRO® without base, a moderately polar organic solvent to dissolve the steroidal reactant, a temperature of 40 °C, and a reaction time of 20 h. Under these conditions 7-ketocholesterol was formed in 63% isolated yield with 1.0 mol % Rh2(cap)4 and in 58% isolated yield with 0.1 mol % Rh2(cap)4.16
Scheme 1.
To compare catalysts that are suitable for use with T-HYDRO ® under the same conditions that were optimized for cholesterol oxidation with Rh2(cap)4 catalysis, we substituted RuCl3(hydrate),12 CuI,9 and CuCl2 17 for Rh2(cap)4, all at 1.0 mol %. The copper catalysts were inadequate under these conditions, reaching only 61% conversion for CuI and 76% conversion with CuCl2 but with substantial amounts of partially oxidized 5 and 6 formed and remaining after 20 h; however, faster conversion rates occurred under anhydrous conditions (TBHP in decane) even though these catalysts were insoluble in the reaction medium. Ruthenium(III) chloride hydrate, on the other hand, catalyzed the clean conversion of cholesterol to 7-ketocholesterol, but the isolated yield of 7-ketocholesterol was only two-thirds of that obtained with the use of Rh2(cap)4; unlike oxidized Rh2(cap)4, oxidized RuCl3 is soluble in water but not in DCE. Reactions catalyzed by Rh2(cap)4 were also performed under conditions reported to be optimal for RuCl3 (0.7 mol % catalyst, 10 equiv T-HYDRO®, delivery by syringe pump, cyclohexane solvent, room temperature),12 and both catalysts gave the same outcome.
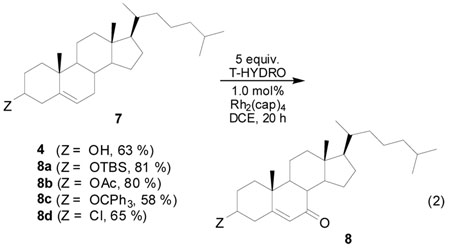
The usefulness of this oxidative methodology is linked to its functional group tolerance, selectivity, and product yield. Results from oxidations of cholesterol derivatives (7) by T-HYDRO® catalyzed by Rh2(cap)4 under conditions optimized for oxidation of cholesterol suggest high functional group compatibility (eq 2). Both acetyl and tert-butyldimethylsilyl cholesterol derivatives with their common hydroxyl protective groups undergo allylic oxidation to their respective 7-keto products in high yield; chloro- and trityl-derivatives give results comparable to cholesterol. Further optimization of conditions for specific substrates may be possible.
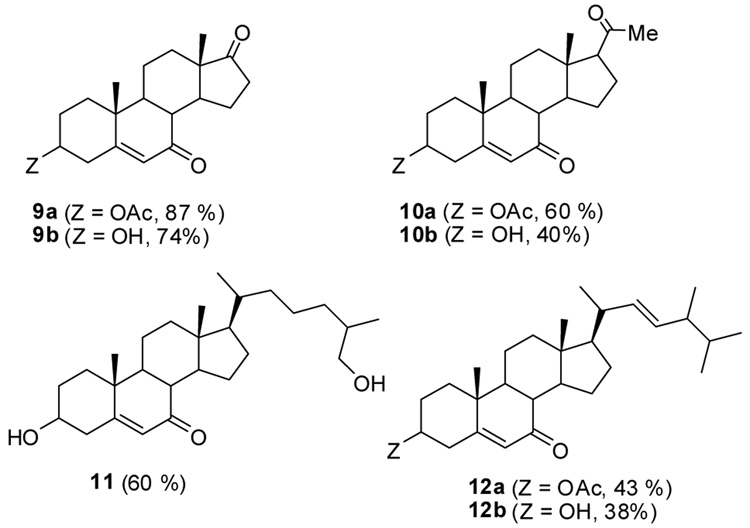
Oxidations of Δ5-steroids with functional groups at the 17-position or in the alkyl side chain at the 17-position under the same conditions gave variable 7-keto-Δ5- steroid product yields (9–12, Figure 1). In addition to results with dehydroandrosterone catalyzed by 1.0 mol % Rh2(cap)4, oxidation of this substrate with only 0.1 mol % Rh2(cap)4 formed 9b in 69 % yield. Although the presence of oxidizable functional groups in the alkyl side chain reduces the yield of 7-keto-Δ5-steroid product, only simple chromatography is required to obtain the allylic oxidation product. By-products from these oxidations were not identified but are of interest in efforts to further optimize product yields.
Figure 1.
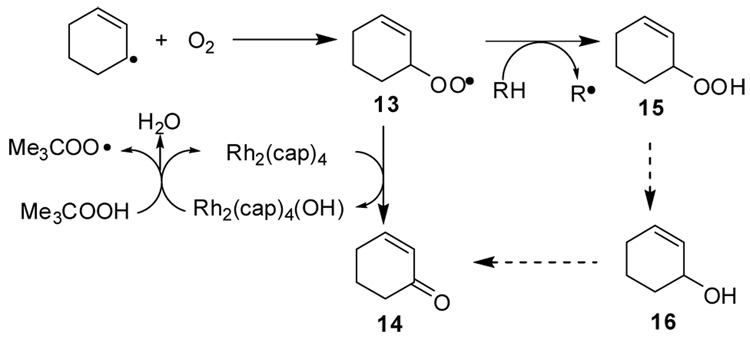
Allylic oxidations by TBHP occur through a free radical pathway for generation of the tert-butylperoxy radical that is common to metal catalyzed reactions of hydroperoxides.18 One-electron reduction of TBHP forms an oxidized metal hydroxide and the tert-butoxy radical that undergoes rapid hydrogen atom abstraction from tert-butyl hydroperoxide to produce the more stable tert-butylperoxy radical.19 The tert-butylperoxy radical imparts selectivity to hydrogen abstraction reactions;20 these processes are well documented and universally accepted.13,19–22 Trapping of the allyl radical by combination with dioxygen is well established.22
Selectivity in the conversion of the allylperoxy radical to ketone (13→14) in competition with hydrogen atom abstraction (13→15) is the core feature of this allylic oxidation process. Direct conversion of 13 to ketone could involve (a) a recently described23 hydrogen atom abstraction from the α-position to form an alkyl hydroperoxy radical, followed by oxidative transfer of hydroxyl to Rh2(cap)4, (b) occur through hydroperoxy radical capture by Rh2(cap)4, followed by O-O cleavage with hydrogen transfer (Scheme 2, cyclohexene model),24 or form a metal peroxide complex leading to, as yet, unprecedented intermediates. They are viable alternatives to bimolecular disproportionation of two peroxy radicals that would form alcohol and ketone together with dioxygen (the Russell mechanism).25 Efforts to determine the specific pathway or pathways for oxidation catalyzed by dirhodium compounds and methods to direct the reaction to a specific pathway are ongoing.
Scheme 2.
In summary, allylic oxidations of Δ5-steroids to 7-keto-Δ5-steroids by tert-butyl hydroperoxide is optimally conducted with T-HYDRO® in dichloroethane with dirhodium caprolactamate as the catalyst. This method is tolerant of a variety of functional groups and can be conducted at catalyst loadings as low as 0.1 mol %.
Supplementary Material
Procedures, product characterization, and full analysis of new compounds. This material is available free of charge via internet at http://pubs.acs.org.
Acknowledgment
The financial support of the National Institutes of Health (GM 46503) is gratefully acknowledged. We wish to thank Dr. Norman B. Javitt26 for his generous provision of 27-hydroxycholesterol.
References
- 1.Reviews: Parish EJ, Kizito SA, Qiu Z. Lipids. 2004:801. doi: 10.1007/s11745-004-1299-y. Arsenou ES, Fousteris MA, Koutsourea AI, Nikolaropoulos SS. Mini Revs. Med. Chem. 2003;3:557. doi: 10.2174/1389557033487953. Smith L. Lipids. 1996;31:453. doi: 10.1007/BF02522641. Peng S-K, Morin RJ. The Biological Effects of Cholesterol Oxides. Boca Raton, FL: CRC Press; 1992.
- 2.(a) Lee JW, Fuda H, Javitt NB, Strott CA, Rodriguez IR. Exp. Eye Res. 2006;83:465. doi: 10.1016/j.exer.2005.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Berthier A, Lemaire-Ewing S, Prunet C, Monier S, Athias A, Bessade G, Paisde Barros J-P, Laubriet A, Gambert P, Lizard G, Nacel D. Cell Death and Differ. 2004;11:897. doi: 10.1038/sj.cdd.4401434. [DOI] [PubMed] [Google Scholar]; (c) Deckert V, Duverneuil L, Poupon S, Monier S, de Guern N, Lizard G, Masson D, Lagrost L. Brit. J. Pharm. 2002;137:655. doi: 10.1038/sj.bjp.0704920. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Steffen Y, Wiswedel I, Peter D, Schewe T, Sies H. Free Radicals Biol. Med. 2006;41:1139. doi: 10.1016/j.freeradbiomed.2006.06.027. [DOI] [PubMed] [Google Scholar]; (e) Javitt NB. Curr. Opin. Lipidol. 2007;18:283. doi: 10.1097/MOL.0b013e328133851e. [DOI] [PubMed] [Google Scholar]
- 3.(a) Rabjohn N. Org. React. 1976;24:261. [Google Scholar]; (b) Crich D, Zou Y. Org. Lett. 2004;6:775. doi: 10.1021/ol036501h. [DOI] [PubMed] [Google Scholar]
- 4.(a) Hua Z, Carcache DA, Tian Y, Li Y-M, Danishefsky SJ. J. Org. Chem. 2005;70:9849. doi: 10.1021/jo051556d. [DOI] [PubMed] [Google Scholar]; (b) Bora U, Chaudhuri MK, Dey D, Kalita D, Kharmawphlang W, Mandal GC. Tetrahedron. 2001;57:2445. [Google Scholar]; (c) Kasal A. Tetrahedron. 2000;56:3559. [Google Scholar]; (d) Li D, Spencer TA. Steroids. 2000;65:529. doi: 10.1016/s0039-128x(00)00131-8. [DOI] [PubMed] [Google Scholar]; (e) Labaree D, Hoyte RM, Hochberg RB. Steroids. 1998;63:454. doi: 10.1016/s0039-128x(98)00069-5. [DOI] [PubMed] [Google Scholar]; (f) Parish EJ, Wei T-Y. Synth. Commun. 1987;17:1227. [Google Scholar]; (g) Dauben WG, Fullertyon DS. J. Org. Chem. 1971;36:3277. doi: 10.1021/jo00821a005. [DOI] [PubMed] [Google Scholar]
- 5.Silvestre SM, Salvador JAR. Tetrahedron. 2007;63:2439. [Google Scholar]
- 6.Fousteris MA, Koutsourea AI, Nikolaropoulos SS, Riahi A, Muzart J. J. Mol. Catal. A: Chem. 2006;250:70. [Google Scholar]
- 7.Shing TKM, Yeung YY, Su PL. Org. Lett. 2006;8:3149. doi: 10.1021/ol0612298. [DOI] [PubMed] [Google Scholar]
- 8.Salvador JAR, Silvestre SM. Tettrahedron Lett. 2005;46:2581. [Google Scholar]
- 9.Arsenou ES, Koutsourea AI, Fousteris MA, Nikolaropoulos SS. Steroids. 2003;68:407. doi: 10.1016/s0039-128x(03)00042-4. [DOI] [PubMed] [Google Scholar]
- 10.Salvador JAR, Clark JH. Chem. Commun. 2001:33. [Google Scholar]
- 11.(a) Baptistella LH, Sousa IMO, Gushikem Y, Aleixo AM. Tetrahedron Lett. 1999;40:2595. [Google Scholar]; (b) Muzart J. Synth. Commun. 1989;19:2061. [Google Scholar]
- 12.Miller RA, Li W, Humphrey GR. Tetrahedron Lett. 1996;37:3429. [Google Scholar]
- 13.Catino J, Forslund RE, Doyle MP. J. Am. Chem. Soc. 2004;126:13622. doi: 10.1021/ja045330o. [DOI] [PubMed] [Google Scholar]
- 14.Few publications report results for the oxidation of cholesterol; oxidation of the 3-OH-protected substrate is reported instead.1,5–12
- 15.(a) Akihisa T, Matsumoto T, Sakamaki H, Take M, Ichinohe Y. Bull. Chem. Soc. Jpn. 1986;59:680. [Google Scholar]; (b) Dang H-S, Davies AG, Davison IGE, Schiesser CH. J. Org. Chem. 1990;55:1432. [Google Scholar]; (c) Ponce MA, Ramirez JA, Galagovsky LR, Gros EG, Erra-Balsells R. J. Chem. Soc., Perkin Trans. 2000;2:2351. [Google Scholar]
- 16.Oxidations with 0.1 mol % Rh2(cap)4 were performed with 1.36 M TBHP (8 molar equiv) whereas those with 1.0 mol % Rh2(cap)4 were with 0.27 M TBHP (5 molar equiv).
- 17.Salvador JAR, Melo MLS, Neves ASC. Tetrahedron. Lett. 1997;38:119. [Google Scholar]
- 18.(a) Nagiev TM. Coherent Synchronized Oxidation Reactions by Hydrogen Peroxide. Amsterdam: Elsevier Science, Ltd; 2006. [Google Scholar]; (b) Bäckvall J, editor. Modern Oxidation Methods. New York, NY: Wiley-VCH; 2004. [Google Scholar]
- 19.(a) Snelgrove DW, Lusztyk J, Banks JT, Mulder P, Ingold KU. J. Am. Chem. Soc. 2001;123:469. [Google Scholar]; (b) MacFaul PA, Arends IWCE, Ingold KU, Wayner DDM. J. Chem. Soc. Perkin Trans. 1997;2:135. [Google Scholar]
- 20.Bravo A, Bjorsvik H-R, Fontana F, Liguori L, Minisci F. J. Org. Chem. 1997;62:3849. doi: 10.1021/jo970302s. [DOI] [PubMed] [Google Scholar]
- 21.Yu J-Q, Corey EJ. J. Am. Chem. Soc. 2003;125:3232. doi: 10.1021/ja0340735. [DOI] [PubMed] [Google Scholar]
- 22.Chavez FA, Mascharak PK. Acc. Chem. Res. 2000;33:539. doi: 10.1021/ar990089h. [DOI] [PubMed] [Google Scholar]
- 23.Bozzelli JW, Sheng C. J. Chem. Phys. A. 2002;106:1113. [Google Scholar]
- 24.Note that Scheme 3 also describes a catalytic interconversion between Rh25+ and Rh24+.
- 25.Miyamoto S, Martinez GR, Medeiros MHG, Di Mascio P. J. Am. Chem. Soc. 2003;125:6172. doi: 10.1021/ja029115o. [DOI] [PubMed] [Google Scholar]
- 26.Department of Medicine and Pediatrics. New York University; [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Procedures, product characterization, and full analysis of new compounds. This material is available free of charge via internet at http://pubs.acs.org.