Abstract
Perilipin (PLIN) is the major protein surrounding lipid droplets in adipocytes and regulates adipocyte metabolism by modulating the interaction between lipases and triacylglycerol stores. Associations between PLIN gene polymorphisms and obesity risk have been described, but interactions with dietary macronutrients require further attention. We examined whether dietary macronutrients (e.g. carbohydrates and fats) modulated the associations of the common PLIN 11482G > A (rs894160) single nucleotide polymorphism with obesity. We studied a population-based sample of Caribbean-origin Hispanics (n = 920, aged 45-74 y) living in the Boston area. Obesity measures (waist and hip circumference, BMI) did not differ between GG subjects and carriers of the A allele (GA and AA). In multivariate linear regression models, we found a significant interaction between complex carbohydrate intake as a continuous variable and PLIN 11482 G > A genotype for waist circumference (P = 0.002). By dichotomizing complex carbohydrate intake, we found significantly different effects across PLIN 11482G > A genotypes. When complex carbohydrate intake was <144 g/d, waist circumference was larger in PLIN 11482G > A carriers (P = 0.024). Conversely, when complex carbohydrate intake was ≥144 g/d, waist and hip circumferences were less in PLIN 11482G > A carriers (P < 0.05). These interactions were not found for simple sugars or total carbohydrates. We identified a significant gene-diet interaction associated with obesity at the PLIN locus. In subjects with higher complex carbohydrate intake, the minor allele was protective against obesity, whereas in subjects with lower carbohydrate intake, the minor allele was associated with increased obesity. These interactions may be relevant to dietary management of obesity.
Introduction
Obesity is estimated to affect >320 million people globally and is a major risk factor for diabetes and cardiovascular disease (1-3). Obesity is a complex, multifactorial condition and its genetic and environmental contributors continue to be identified and refined; however, these factors often have been mainly evaluated independently of one another.
The role of macronutrient ratios and dietary composition in modulating obesity risk remains an open question (4-6). Although certain candidate genes have emerged to show consistent effects on obesity, the extent to which manipulation of specific macronutrient intakes may influence genetically based variability in obesity susceptibility remains largely unexplored. Increasing our understanding of the interactions between macronutrients and genetic variants may clarify some of the controversies about obesity and represents a step toward the development of targeted nutritional therapies for obesity management.
One established candidate gene for obesity is perilipin (PLIN),5 encoding an adipocyte-associated protein that influences obesity risk and insulin resistance through its regulation of adipocyte metabolism, lipolysis, and body fat accumulation (7-12). PLIN inhibits basal lipolysis and promotes triacylglycerol storage by limiting lipase access to triacylglycerol stores but also participates in catecholamine-stimulated lipolysis through an interaction with lipid droplet-associated hormone-sensitive lipase (13-15). Genetic variants in PLIN affect the PLIN protein content and lipolytic rates of adipocytes and also modify obesity risk in White and Asian populations (7-12).
Another candidate gene for obesity is PPARγ (PPARG), the gene encoding a nuclear transcription factor, which, like PLIN, plays a major role in adipocyte differentiation and function (16). Several (17-19) but not all (20) studies have reported associations between PPARG variants, specifically, the PPARG Pro12Ala polymorphism, and obesity. In addition, both PLIN and PPARG share a role in the regulation of lipolysis and adipogenesis and functional linkages between these proteins have been demonstrated. The PLIN promoter contains a PPAR-responsive element and PLIN mRNA is upregulated by synthetic PPAR agonists such as the insulin-sensitizing agent thiazolidinedione (21). These agents, although used therapeutically to treat diabetes, also cause weight gain (22).
Dietary factors interact with PLIN variants in modulating obesity and insulin resistance. Variation at the PLIN 11482G > A locus modulated weight loss in an energy-restricted, low-carbohydrate intervention (12). Furthermore, an interaction between macronutrients and obesity-related insulin resistance for the same PLIN variant was observed in a large multiethnic Asian population (11). In both studies, lower carbohydrate intake was associated with adverse metabolic consequences in those individuals carrying this minor PLIN allele. Dietary interactions have also been demonstrated for PPARG Pro12Ala, although dietary fats, rather than carbohydrates, have been the focus of previous studies (18).
Dietary intervention trials conducted over the past several decades have closely examined the role of carbohydrate and, particularly, specific types of carbohydrate such as complex carbohydrate in promoting weight management and improving glycemic control (6,23). Considerable evidence has accumulated in support of complex carbohydrate as a particularly beneficial macronutrient (24-27), while others have emphasized the negative role of carbohydrate, particularly in its refined form, through its effect on glycemic load (28-30). Overall consensus regarding the relative importance of specific macronutrients in the management of obesity does not exist. Collectively, results from dietary trials and previously reported PLIN-nutrient and PPARG-nutrient interactions suggest that genetic variation might be 1 source of variability in responses to dietary interventions. Therefore, in this study, we examined the interactions between PLIN single nucleotide polymorphisms (SNP), dietary composition, and obesity measures in a Caribbean-origin Hispanic population. Secondarily, based on widespread evidence for a role of PPARG Pro12Ala in obesity combined with evidence for possible functional relationships between PLIN and PPARG, we also examined these interactions for the PPARG SNP Pro12Ala.
Methods
Study design and subjects
Participants were recruited for a prospective 2-y cohort study of men and women of Puerto Rican origin aged 45-74 y living in the Boston, Massachusetts metropolitan area. Interviews to collect baseline demographic information, medical history, and dietary data were conducted between 2004 and 2007 by trained bilingual staff. The Institutional Review Board at Tufts University/New England Medical Center approved the protocol of the current study. Anthropometric data including height, weight, and waist and hip circumference were measured in duplicate consistent with the technique used by the NHANES. Blood was collected for biochemical analyses and genetic analysis and plasma was separated within 4 h in a refrigerated centrifuge and was stored at -70°C. Serum glucose was measured using an enzymatic, kinetic reaction with Olympus glucose reagents (OSCR6121). HDL cholesterol (HDL-C) was analyzed using EDTA plasma with the enzymatic endpoint reaction with Olympus HDL reagents (OSR6156) and triglycerides were analyzed using EDTA plasma with Olympus triglyceride reagents (OSR6033). Glucose, HDL-C, and triglyceride were measured using the Olympus AU400e (Olympus America).
Genetic analysis
Genomic DNA was isolated from peripheral blood lymphocytes by standard methods as previously described (8,10). SNP PLIN 6209 T > C (rs2289487), PLIN 11482 G > A (rs894160), PLIN 13041 A > G (rs2304795), PLIN 14995 A > T (rs1052700), and PPARG Pro12Ala (rs1801282) were genotyped using the ABI Prism SNapShot multiplex system (Applied Biosystems).
Dietary assessment
Dietary intake was assessed using a FFQ that was designed for and tested in this population (31). Dietary data were linked to the Minnesota Nutrient Data system (1999, version 25) for nutrient analysis. Intakes of total fat, saturated fat, total carbohydrate, complex carbohydrate, and simple sugars were expressed as percentages of total energy intake and were included in analyses as both continuous and categorical variables. To construct categorical variables, intakes were classified into 2 groups according to the median intake of the population.
Statistical analyses
All continuous variables were examined for normal distribution. The relationship between PLIN and PPARG genotypes, dietary intakes, and anthropometric measures was evaluated using ANOVA techniques. The interactions between dietary macronutrient intakes (including total fat, total carbohydrate, complex carbohydrate, simple sugars) and polymorphisms were tested in a multivariate interaction model with control for potential confounders, including age, sex, alcohol (never, past, current), smoking (never, past, current), physical activity, diabetes medications, and dietary fiber. Total carbohydrate, complex carbohydrate, and simple sugar intakes were each adjusted for total energy intake using a residuals model. Macronutrient intake was regressed on total energy intake by computing residuals to which the predicted nutrient intake for the mean energy intake was added as a constant. The population medians for total carbohydrate, complex carbohydrate, and simple sugar intakes were used as cutoffs to dichotomize these variables. Additional adjustments for appropriate macronutrients were performed for each type of carbohydrate. Complex carbohydrate intake was also evaluated as a continuous variable by computing predicted values for each subject from the adjusted regression model and plotting those predicted values against complex carbohydrate intake depending on the PLIN genotype. Men and women were analyzed together to ensure adequate statistical power. SAS (version 9.1 for Windows) was used to analyze data. A P-value of 0.05 was considered significant.
Results
Demographic, biochemical, anthropometric, and genotypic data are presented in Table 1. Allele frequencies for the minor alleles of each SNP were 0.49 (PLIN 6209T>C), 0.27 (PLIN 11482G>A), 0.37 (PLIN 13041A > G), 0.22 (PLIN 14995A > T), and 0.06 (PPARG Pro12Ala). Genotype frequencies of the 5 genotyped SNP did not deviate from Hardy-Weinberg equilibrium expectations. No significant associations between obesity measures (waist and hip circumference, BMI) were observed for the 4 PLIN SNP or the PPARG SNP in this population. Interactions between dietary intakes and PLIN 11482G > A were examined by dichotomizing macronutrients according to the mean population intakes regressed on energy and evaluating their effects on obesity measures (Table 2). Homozygotes and heterozygotes of the variant alleles for PLIN 11482 G > A and PPARG Pro12Ala SNP were combined and compared with homozygous major allele subjects to increase statistical power. Multivariate adjustments for potential confounders included age, sex, smoking, alcohol, physical activity, diabetes medications, and dietary fiber. We did not find significant interactions between PLIN 6209T > C, PLIN 13041A > G, and PLIN 14995A > T polymorphisms and dietary intake for measures of obesity. Dietary interactions for PPARG Pro12ALA were examined for complex carbohydrate only. Results reported below pertain to PLIN 11482G > A and PPARG Pro12Ala.
TABLE 1.
Demographic, anthropometric, biochemical, dietary, and genotypic data in Caribbean-origin Hispanics1
| Men | Women | |
|---|---|---|
| n | 256 | 664 |
| Age, y | 57.6 ± 7.7 | 58.0 ± 7.2 |
| BMI, kg/m2 | 29.7 ± 5.3 | 33.1 ± 7.1 |
| Obese,2 % | 45.3 | 66.1 |
| Morbidly obese,2 % | 4.2 | 15.8 |
| Waist circumference, cm | 102 ± 14 | 102 ± 16 |
| Abdominal obesity,3 % | 46.5 | 84.8 |
| Plasma HDL-C, mmol/L | 1.04 ± 0.32 | 1.21 ± 0.32 |
| Plasma triglycerides, mmol/L | 2.06 ± 1.83 | 1.77 ± 1.15 |
| Serum glucose, mmol/L | 6.99 ± 3.11 | 6.72 ± 2.83 |
| Energy intake,4 kcal/d | 2370 ± 855 | 2023 ± 855 |
| Total fat, % of energy | 31.8 ± 5.3 | 30.7 ± 5.2 |
| Saturated fat, % of energy | 9.9 ± 2.4 | 9.5 ± 2.3 |
| Carbohydrate, % of energy | 50.2 ± 7.5 | 52.5 ± 7.6 |
| Complex carbohydrate, g/d | 176.3 ± 64.9 | 148.0 ± 63.2 |
| Complex carbohydrate, % of energy | 30.2 ± 5.9 | 29.9 ± 6.0 |
| Simple sugars, g/d | 119.2 ± 65.6 | 115.1 ± 68.9 |
| Simple sugar, % of energy | 20.0 ± 7.7 | 22.6 ± 9.0 |
| Fiber, g/d | 19.9 ± 7.9 | 17.7 ± 8.0 |
| Alcohol user, % | 50.6 | 33.2 |
| Current smoker, % | 31.8 | 20.0 |
| Diabetes,5 % | 42.2 | 41.2 |
| Diabetes medications, % | 32.8 | 34.3 |
| PLIN 11482 > A, n(%) | ||
| GG | 126 (50.0) | 354 (54.2) |
| GA | 106 (42.1) | 247 (37.9) |
| AA | 20 (7.9) | 51 (7.8) |
| PPARG Pro12Ala, n(%) | ||
| CC | 599 (88.1) | 232 (85.93) |
| CG | 78 (11.5) | 38 (14.07) |
| GG | 3 (0.44) | 0 (0) |
Values are means ± SD, %, or n(%).
Obese, BMI ≥30 kg/m2. Morbidly obese, BMI ≥40 kg/m2.
Abdominal obesity, waist circumference ≥102 cm in men; waist circumference ≥88 cm in women.
1 kcal = 4.184 kJ.
Diabetes, fasting blood glucose ≥7 mmol/L or taking diabetes medications.
TABLE 2.
Interactions between PLIN 11482G > A and carbohydrate intake in association with obesity-related measures in Caribbean-origin Hispanics1
| GG | GA+AA | P-trend | P-interaction | |
|---|---|---|---|---|
| Complex CHO intake,3 g/d | ||||
| Waist, cm | ||||
| <1442 | 101 ± 1 | 105 ± 1 | 0.024 | 0.004 |
| ≥144 | 104 ± 1 | 101 ± 1 | 0.012 | |
| Hip, cm | ||||
| <144 | 107 ± 1 | 110 ± 1 | 0.060 | 0.009 |
| ≥144 | 109 ± 1 | 107 ± 1 | 0.016 | |
| BMI, kg/m2 | ||||
| <144 | 30.9 ± 0.5 | 32.0 ± 0.5 | 0.069 | 0.035 |
| ≥144 | 32.0 ± 0.4 | 31.2 ± 0.4 | 0.143 | |
| Total CHO intake,4 g/d | ||||
| Waist, cm | ||||
| <2652 | 103 ± 1 | 105 ± 1 | 0.093 | 0.065 |
| ≥265 | 102 ± 1 | 100 ± 1 | 0.242 | |
| Hip, cm | ||||
| <265 | 109 ± 1 | 110 ± 1 | 0.267 | 0.096 |
| ≥265 | 108 ± 1 | 106 ± 1 | 0.233 | |
| BMI, kg/m2 | ||||
| <265 | 31.7 ± 0.5 | 32.5 ± 0.5 | 0.210 | 0.265 |
| ≥265 | 31.2 ± 0.5 | 31.0 ± 0.5 | 0.744 | |
| Simple sugars intake,5 g/d | ||||
| Waist, cm | ||||
| <1112 | 104 ± 1 | 104 ± 1 | 0.894 | 0.821 |
| ≥111 | 101 ± 1 | 101 ± 1 | 0.762 | |
| Hip, cm | ||||
| <111 | 110 ± 1 | 109 ± 1 | 0.861 | 0.822 |
| ≥111 | 107 ± 1 | 107 ± 1 | 0.909 | |
| BMI, kg/m2 | ||||
| <111 | 32.0 ± 0.4 | 32.2 ± 0.4 | 0.811 | 0.764 |
| ≥111 | 31.0 ± 0.5 | 31.3 ± 0.6 | 0.591 | |
Data are means ± SE.
Dichotomized values for nutrients are adjusted for energy using the residuals method.
CHO = carbohydrate. Adjusted for age, sex, smoking, diabetes medication, physical activity, alcohol, saturated fat, sugar and dietary fiber.
Adjusted for age, sex, smoking, diabetes medication, physical activity, alcohol, saturated fat, and dietary fiber.
Adjusted for age, sex, smoking, diabetes medication, physical activity, alcohol, saturated fat, complex carbohydrate and dietary fiber. Simple sugars consist of sucrose, glucose, maltose, fructose, and lactose.
Complex carbohydrates were dichotomized based on median intake after energy adjustment using a residuals model into high (≥144 g/d) and low (<144 g/d) daily intakes and interaction terms between complex carbohydrate and genotype were obtained for waist (P = 0.004) and hip (P = 0.009) circumference and BMI (P = 0.035). For the PPARG SNP, no significant interaction terms between complex carbohydrate and genotype were obtained for obesity. Adjustment for saturated fat, simple sugars, and alcohol intakes were added to the model but did not alter significance. Analysis was also performed for PLIN 11482 G > A using a regression model that did not adjust for extraneous variability in total energy intake but was instead based on a dichotomized percentage of complex carbohydrate intake. Using this 2nd model, interaction terms for PLIN were obtained for waist (P = 0.014) and hip circumference (P = 0.048). Values in Table 2 and Figure 1 reflect the use of the residuals energy-adjusted model. For both high and low intakes of complex carbohydrate, significant differences in measures of obesity were observed for waist circumference between carriers of the PLIN variant allele (GA and AA) and noncarriers (GG). However, the direction of the difference depended on the level of complex carbohydrate intake. When daily intake of complex carbohydrate was low (<144 g/d), the presence of 1 or 2 variant alleles was associated with larger waist circumference (P = 0.024). Yet, for high daily intake of complex carbohydrate (≥144 g/d), carrier status was associated with lower waist (P = 0.012) and hip circumference (P = 0.016). Using the same statistical models, we did not find any significant interactions between PLIN 11482G > A polymorphism and simple sugar intake when it was dichotomized according to the energy-adjusted population median (111 g/d). Similarly, we did not find any significant interaction between this PLIN SNP and total carbohydrate intake when intake was dichotomized according to the median intake (265 g/d). We also examined interactions between the simple sugar:complex carbohydrate ratio, with both macronutrients dichotomized according to the median intake after adjustment for energy using the residuals model, and did not find significant interactions between the PLIN 11482 G > A and this ratio for obesity measures. Finally, we examined interactions between dietary fiber, PLIN 11482 G > A, and obesity, but did not observe any significant interactions for obesity (data not shown).
FIGURE 1.
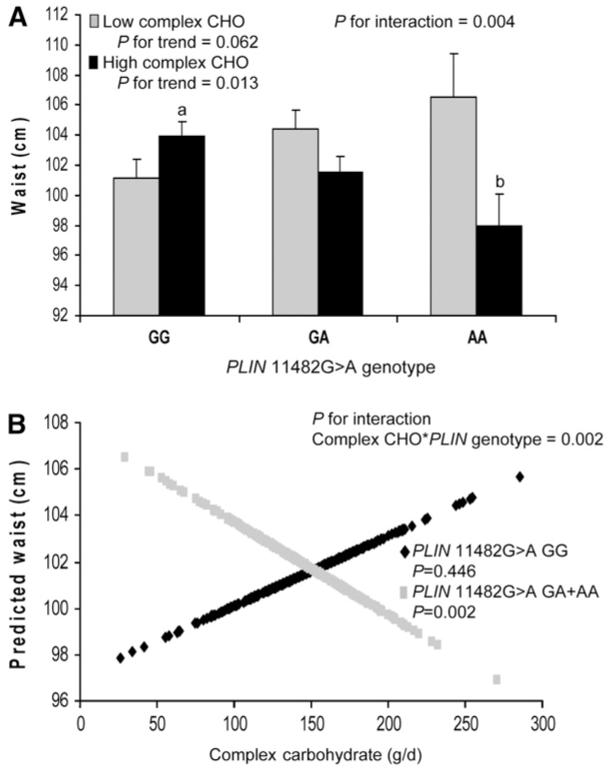
Waist circumference (A) by PLIN 11482G > A genotype and complex carbohydrate intake, adjusted for total energy intake using the residuals method. High complex carbohydrate ≥144 g/d. Low complex carbohydrate <144 g/d. Values are means + SE. Means were adjusted for age, sex, smoking, alcohol, physical activity, diabetes medications, saturated fat, dietary fiber, and simple sugars. P-values for trend were obtained through comparisons of means for genotype (GG, n = 480; GA, n = 353; AA, n = 71) according to complex carbohydrate intake. P for interaction was obtained for the interaction between genotype and complex carbohydrate intake. Means marked with different letters differ, P < 0.05. Predicted values of waist (B) by PLIN 11482G > A genotype (GG, n = 480; GA+AA, n = 424) plotted against complex carbohydrate intake (g/d, adjusted for total energy using residuals method) as a continuous variable. Predicted values for waist were calculated from the regression model after adjustment for age, sex, smoking, alcohol, physical activity, diabetes medications, saturated fat, and simple sugars. P-value for interaction indicates the significance of the interaction term for complex carbohydrate and PLIN genotype in the adjusted regression model. P-values for GG and GG+AA indicate the significance of the regression coefficients in the adjusted regression model.
We further examined interactions between complex carbohydrate intake and potential additive effects of the variant allele by evaluating waist circumference for each PLIN 11482G > A genotype (GG, GA, and AA) (Fig. 1A). In the context of high-complex carbohydrate intake, the presence of each additional variant allele was associated with an incremental decrease in waist circumference (P = 0.013) and mean waist circumference differed (P = 0.008) between GG and AA subjects. An interaction term was also obtained for dichotomized complex carbohydrate intake and genotype (P = 0.004). In contrast, when complex carbohydrate intake was low, the presence of each variant allele was associated with a marginal incremental increase in waist circumference (P = 0.062). Significant or almost significant interactions were observed for each PLIN genotype group (GG, GA, and AA) for waist circumference but not consistently for hip circumference or BMI (data not shown). When GA and AA were combined into a single group and compared against GG, then statistical power was sufficient to detect differences (Table 2).
We also examined interactions between complex carbohydrate intake as a continuous variable and PLIN 11482G > A on waist circumference. Predicted values for waist circumference are plotted against complex carbohydrate intake for carriers and noncarriers of the variant allele for PLIN 11482G > A (Fig. 1B). For carriers of the variant allele, as complex carbohydrate intake increased, predicted waist circumference decreased (P = 0.002). We also obtained an interaction term between genotype and complex carbohydrate for predicted waist circumference (P = 0.002).
Discussion
We have identified a significant interaction between PLIN 11482G > A polymorphism and dietary intake of complex carbohydrate in which the direction of the genetic effect on obesity is dependent upon intake of complex carbohydrate. When complex carbohydrate intake was ≥144 g/d (energy-adjusted population median intake), carriers of the variant allele exhibited smaller waist and hip circumferences compared with homozygotes for the major allele. In contrast, when complex carbohydrate was <144 g/d, variant allele carriers exhibited larger waist circumference. These associations between a PLIN polymorphism and obesity were not apparent when the population was considered in its entirety, independently of complex carbohydrate intake. Further, both the protective effect and the increased risk of obesity demonstrated additive properties; each additional variant allele was associated with an incremental decrease or increase in waist circumference depending on whether complex carbohydrate was high or low, respectively.
The interaction between PLIN 11482 G > A and diet for obesity was limited to complex carbohydrates and was not observed for dietary sugar or total carbohydrates. Whereas previous studies have established restricted energy intake as primary in the treatment of obesity, the role of dietary composition in obesity management continues to be debated. Proportions of carbohydrate, fats, and specific classes of these macronutrients have each been shown to modulate weight loss, but there is no overall consensus on the macronutrient composition that best sustains weight loss or agreement on whether macronutrients modify obesity independently of energy intake (4,6). At the same time, different proportions of carbohydrate, fats, and specific classes of these macronutrients have each been shown to modulate weight gain (5,26) Few studies have examined genetic factors as potential sources of variability in weight loss in response to dietary modifications (12,32). Our observations suggest that incorporating genetic information into dietary trials may help to reduce intra-individual variability in weight loss.
We did not observe an interaction between PLIN 11482G > A and obesity (waist, hip, or BMI), which was independent of complex carbohydrate intake. Of the 3 PLIN SNP with which obesity risk has been associated in previous studies, the 11482G > A SNP has been most extensively evaluated, both with and without the availability of dietary data. Whereas PLIN 11482G > A was shown to be protective against obesity in 2 White populations, carriers of the variant allele in a Mediterranean population were the most resistant to weight loss in an energy-restricted intervention trial (8,9,12). In contrast to the high complex carbohydrate intakes that were protective against obesity in PLIN 11482 G > A carriers in the current study, subjects in the weight loss trial consumed a diet that was designed to be lower in carbohydrate and relatively high in mono-unsaturated fats. In a separate study of PLIN 11482G > A, carbohydrate intake was shown to be protective against insulin resistance in 3 ethnic groups in a large Asian population (11). Although insulin resistance was not assessed in the current study, links between obesity and insulin resistance suggest that carbohydrate intake may be relevant to both processes (33-35).
Mechanistic explanations for our observations of complex carbohydrate modulation of obesity for this PLIN SNP are unclear. One possibility is that complex carbohydrate modulates postprandial glucose and insulin responses, with subsequent effects on lipolysis and adipocyte energy homeostasis, both of which are regulated by PLIN. Evidence for a connection between PLIN and modulation of postprandial lipemia has been previously demonstrated (36). Early metabolic studies demonstrated increased insulin binding to adipocytes and increased insulin sensitivity in noninsulin-dependent diabetics consuming a high-starch/high-fiber/low-fat diet and more recent studies confirm that modestly increased carbohydrate intake improves insulin sensitivity in obese individuals with diabetes (37,38). Insulin exerts antilipolytic effects that are mediated through hormone-sensitive lipase and PLIN, and dietary interventions that alter insulin metabolism or reduce the hyperinsulinemia associated with type 2 diabetes might be expected to alter PLIN-regulated lipolysis (14,39). An enhanced lipolysis rate has been described for PLIN 11482G > A, but the implications of this altered function in response to dietary carbohydrate are unknown (7). Alternative hypotheses can be based on interactions between intake of specific forms of carbohydrate and altered lipid metabolism (40). De novo lipogenesis (DNL), in which lipids are synthesized from excess carbohydrates in the liver or adipose, is sensitive to the proportions of starch and sugar in the diet. A greater starch:sugar ratio is associated with reduced rates of DNL and higher sugar intake is associated with increased DNL (41-43). In the current study, intakes of complex carbohydrate in men and women were high and sugar intake in women was also high relative to average national intakes (NHANES 2003-04 shows complex carbohydrate and simple sugar intakes for 50- to 59-y-old men of 153 and 121 g/d, respectively, and 112 and 95 g/d, respectively, for 50- to 59-y-old women) in a population with a high prevalence of abnormal glucose and lipid metabolism. Although links between DNL and obesity are not clearly established, association of PLIN with adiposity is postulated to be related to its regulation of triacylglycerol storage and lipolysis where these regulatory mechanisms could be responsive to altered carbohydrate ratios (8-10,12). Ideally, exploration of the mechanisms underlying diet-associated modulation of PLIN and obesity would assess metabolic parameters including insulin sensitivity, plasma fatty acid concentrations, and adipocyte lipolysis rates.
Although mechanisms linking carbohydrate intake, triacylglycerol storage regulation, and obesity to PLIN are plausible, the issue of whether functionality can be ascribed to the PLIN 11482G > A SNP, as opposed to other PLIN SNP, is complicated by the SNP’s intronic location. The question of SNP functionality is not limited to the current study but is also implied in studies investigating a variety of obesity-related outcomes with which PLIN 11482G > A has been associated, including modulation of insulin resistance by dietary carbohydrate and fat (11), modulation of rosiglitazone-associated weight gain (22), altered weight loss-induced FFA levels (44), decreased adipocyte PLIN protein content, and increased lipolysis rates (7). Although the associations reported above are largely attributed to PLIN 11482G > A rather than other PLIN SNP, apparently conflicting associations for obesity and PLIN 11482G > A may appear to challenge these conclusions. For example, PLIN 11482G > A has been associated with increased obesity risk in Malays and Asian Indians (10) but reduced obesity risk in a Spanish population (9). Examination of linkage disequilibrium (LD) patterns in these groups revealed that in the Spanish, PLIN 11482G > A was strongly linked to PLIN 6209T > C and less strongly linked to PLIN 13041A > G and PLIN 14995A > T, whereas in the Asian populations, PLIN 11482G > A was in positive LD with PLIN 13041A > G and PLIN 14995A > T but in strong negative LD with PLIN 6209T > C. These examples illustrate the difficulties inherent in ascribing functional associations or interactions to particular SNP and as a result, we cannot eliminate the possibility that PLIN 11492G > A is a nonfunctional marker for another, functional locus.
For PPARG Pro12Ala, we did not observe associations with obesity independently of nutrients nor any interaction with complex carbohydrate. This SNP has been associated with obesity in 3 Chinese ethnic groups (Han, Kazaks, and Uygur) (19) and in Amerindians and Mexican Mestizos (17) but was not associated with obesity in a Spanish population (20). Allelic frequency is 1 potential source of variation, because frequencies of 0.12 (European Americans), 0.09 (Hispanics), and 0.04 (African Americans) have been reported (18). Associations of this SNP with obesity in Caribbean Hispanics, as opposed to Hispanic populations that may include non-Caribbeans, have not been previously described, nor have interactions with dietary carbohydrates.
Limitations of the current study include the high prevalence and severity of obesity in this population, particularly with respect to abdominal obesity. These population characteristics, along with a high prevalence of type 2 diabetes, may limit the extrapolation of our results to less obese, metabolically healthier populations. Further, as discussed earlier, variable LD patterns for PLIN SNP in different ethnic groups point out the need for additional caution in extrapolating results from this Caribbean Hispanic population to other ethnic groups. Associations between PLIN genotype and obesity in Hispanic populations, with or without dietary interactions, have not been previously described. In addition, although we recognize that complex carbohydrates include a wide range of glycemic index foods, which may have differing metabolic effects, we chose to examine complex carbohydrates in relation to PLIN based on previous studies suggesting interactions between total carbohydrate, insulin resistance (11), and resistance to weight loss (12) for carriers of the same PLIN variant. Interactions between total carbohydrate intake and obesity did not reach significance in the current study; however, sugar intake was high for women in this population and associations between sugar consumption and obesity have been described (45,46). Further, evidence for a relationship between glycemic index and obesity, like that of overall carbohydrate intake and obesity, is inconsistent, with some intervention trials supporting a benefit to low-glycemic diets (47), and others failing to demonstrate any benefit (6,48). Complicating the understanding of the role of glycemic index and obesity in the current population is a high intake of mixed foods (e.g. rice and beans, rice soups, chicken and rice). Combining lower glycemic foods such as beans or fat-containing foods with carbohydrate staples such as bread and rice consistently lowers the glycemic index of these high-glycemic index foods in other populations (49,50). Further evaluation of the relationships between glycemic index, food patterns, and obesity in the current population is warranted.
In summary, we observed a modulation of the effects of PLIN 11482 G > A on several measures of obesity, which became apparent only when evaluated in light of complex carbohydrate intake. A single PLIN variant was associated with clinically important and opposite effects in a population exhibiting high rates of obesity and subsequent metabolic disturbance and these effects depended on intake of a specific macronutrient. Consideration of genotype in the evaluation of the effects of dietary factors may help to reconcile disparate results in dietary trials and may suggest more optimal dietary interventions that are based on individual genetic variants. Identifying population subsets that would benefit most from increased complex carbohydrate intake could aid in targeting nutritional advice specifically for these individuals.
Footnotes
Supported by the NIH, National Institute on Aging, grant number 5P01AG023394-02 and NIH/NHLBI grant number HL54776 and NIH/NIDDK DK075030 and contracts 53-K06-5-10 and 58-1950-9-001 from the USDA Agricultural Research Service.
- DNL
- de novo lipogenesis
- HDL-C
- HDL cholesterol
- LD
- linkage disequilibrium
- PPARG
- PPARγ
- PLIN
- perilipin
- SNP
- single nucleotide polymorphism
Literature Cited
- 1.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes and obesity-related health risk factors, 2001. JAMA. 2003;289:76–9. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 2.Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298:2028–37. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- 3.Yang W, Kelly T, He J. Genetic epidemiology of obesity. Epidemiol Rev. 2007;29:49–61. doi: 10.1093/epirev/mxm004. [DOI] [PubMed] [Google Scholar]
- 4.Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction. JAMA. 2005;293:43–53. doi: 10.1001/jama.293.1.43. [DOI] [PubMed] [Google Scholar]
- 5.Gardner CD, Kiazand A, Alhassan S, Kim S, Stafford RS, Balise RR, Kraemer HC, King AC. Comparison of the Atkins, Zone, Ornish and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A to Z weight loss study, a randomized trial. JAMA. 2007;297:969–77. doi: 10.1001/jama.297.9.969. [DOI] [PubMed] [Google Scholar]
- 6.Das SK, Gilhooly CH, Golden JK, Pittas AG, Fuss PJ, Cheatham RA, Tyler S, Tsay M, McCrory MA, et al. Long-term effects of 2 energy-restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: a 1-y randomized controlled trial. Am J Clin Nutr. 2007;85:1023–30. doi: 10.1093/ajcn/85.4.1023. [DOI] [PubMed] [Google Scholar]
- 7.Mottagui-Tabar S, Ryden M, Lofgren P, Faulds G, Hoffstedt J, Brookes AJ, Andersson I, Arner P. Evidence for an important role of perilipin in the regulation of human adipocyte lipolysis. Diabetologia. 2003;46:789–97. doi: 10.1007/s00125-003-1112-x. [DOI] [PubMed] [Google Scholar]
- 8.Qi L, Corella D, Sorli JV, Portoles O, Shen H, Coltell O, Godoy D, Greenberg AS, Ordovas JM. Genetic variation at the perilipin (PLIN) locus is associated with obesity-related phenotypes in White women. Clin Genet. 2004;66:299–310. doi: 10.1111/j.1399-0004.2004.00309.x. [DOI] [PubMed] [Google Scholar]
- 9.Qi L, Shen H, Larson I, Schaefer EJ, Greenberg AS, Tregouet DA, Corella D, Ordovas JM. Gender-specific association of a perilipin gene haplotype with obesity risk in a white population. Obes Res. 2004;12:1758–65. doi: 10.1038/oby.2004.218. [DOI] [PubMed] [Google Scholar]
- 10.Qi L, Tai ES, Tan CE, Shen H, Chew SK, Greenberg AS, Corella D, Ordovas JM. Intragenic linkage disequilibrium structure of the human perilipin gene (PLIN) and haplotype association with increased obesity risk in a multiethnic Asian population. J Mol Med. 2005;83:448–56. doi: 10.1007/s00109-004-0630-4. [DOI] [PubMed] [Google Scholar]
- 11.Corella D, Qi L, Tai ES, Deurenberg-Yap M, Tan CE, Chew SK, Ordovas JM. Perilipin gene variation determines higher susceptibility to insulin resistance in Asian women when consuming a high-saturated fat, low-carbohydrate diet. Diabetes Care. 2006;29:1313–9. doi: 10.2337/dc06-0045. [DOI] [PubMed] [Google Scholar]
- 12.Corella D, Qi L, Sorli JV, Godoy D, Portoles O, Coltell O, Greenberg AS, Ordovas JM. Obese subjects carrying the 11482G>A polymorphism at the perilipin locus are resistant to weight loss after dietary energy restriction. J Clin Endocrinol Metab. 2005;90:5121–6. doi: 10.1210/jc.2005-0576. [DOI] [PubMed] [Google Scholar]
- 13.Brasaemle DL, Rubin B, Harten IA, Gruia-Gray J, Kimmel AR, Londos C. Perilipin A increases triacylglycerol storage by decreasing the rate of triacylglycerol hydrolysis. J Biol Chem. 2000;275:38486–93. doi: 10.1074/jbc.M007322200. [DOI] [PubMed] [Google Scholar]
- 14.Miyoshi H, Souza SC, Zhang HH, Strissel KJ, Christoffolete MA, Kovsan J, Rudich A, Kraemer FB, Bianco AC, et al. Perilipin promotes hormone-sensitive lipase-mediated adipocyte lipolysis via phosphorylation-dependent and -independent mechanisms. J Biol Chem. 2006;281:15837–44. doi: 10.1074/jbc.M601097200. [DOI] [PubMed] [Google Scholar]
- 15.Aboulaich N, Vener AV, Stralfors P. Hormonal control of reversible translocation of perilipin B to the plasma membrane in primary human adipocytes. J Biol Chem. 2006;286:11446–9. doi: 10.1074/jbc.C500461200. [DOI] [PubMed] [Google Scholar]
- 16.Vidal-Puig A, Jimenez-Linan M, Lowell BB, Hamann A, Hu E, Spiegelman B, Flier JS, Moller DE. Regulation of PPAR gamma gene expression by nutrition and obesity in rodents. J Clin Invest. 1996;97:2553–61. doi: 10.1172/JCI118703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Canizales-Quinteros S, Aguilar-Salinas CA, Ortiz-Lopez MG, Rodriguez-Cruz M, Villarreal-Molina MT, Coral-Vazquez R, Huertas-Vazquez A, Hernandez-Caballero A, Lopez-Alarcon M, et al. Association of PPARG2 Pro12Ala variant with larger body mass index in Mestizo and Amerindian populations of Mexico. Hum Biol. 2007;79:111–9. doi: 10.1353/hub.2007.0022. [DOI] [PubMed] [Google Scholar]
- 18.Franks PW, Jablonski KA, Delahanty L, Hanson RL, Kahn SE, Altshuler D, Knowler WC, Florez JC, Diabetes Prevention Program Research Group The Pro12Ala variant at the peroxisome proliferator-activated receptor gamma gene and change in obesity-related traits in the Diabetes Prevention Program. Diabetologia. 2007;50:2451–60. doi: 10.1007/s00125-007-0826-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li LL, Ma XL, Ran JX, Sun XF, Xu LM, Ren J, Mao XM. Genetic polymorphism of peroxisome proliferator-activated receptor-gamma 2 Pro12Ala on ethnic susceptibility to diabetes in Uygur, Kazak and Han subjects. Clin Exp Pharmacol Physiol. 2008;35:187–91. doi: 10.1111/j.1440-1681.2007.04796.x. [DOI] [PubMed] [Google Scholar]
- 20.Rosado EL, Bressan J, Martins MF, Cecon PR, Martinez JA. Polymorphism in the PPARgamma2 and beta2-adrenergic genes and diet lipid effects on body composition, energy expenditure and eating behavior of obese women. Appetite. 2007;49:635–43. doi: 10.1016/j.appet.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Nagai S, Shimizu C, Umetsu M, Taniguchi S, Endo M, Miyoshi H, Yoshioka N, Kubo M, Koike T. Identification of a functional peroxisome proliferator-activated receptor responsive element within the murine perilipin gene. Endocrinology. 2004;145:2346–56. doi: 10.1210/en.2003-1180. [DOI] [PubMed] [Google Scholar]
- 22.Kang ES, Cha BS, Kim HJ, Kim HJ, Kim SH, Hur KY, Lee HJ, Shim WS, Ahn CW, et al. The 11482G >A polymorphism in the perilipin gene is associated with weight gain with rosiglitazone treatment in type 2 diabetes. Diabetes Care. 2006;29:1320–4. doi: 10.2337/dc05-2466. [DOI] [PubMed] [Google Scholar]
- 23.Frost GS, Brynes AE, Bovill-Taylor C, Dornhorst A. A prospective randomised trial to determine the efficacy of a low glycaemic index diet given in addition to healthy eating and weight loss advice in patients with coronary heart disease. Eur J Clin Nutr. 2004;58:121–7. doi: 10.1038/sj.ejcn.1601758. [DOI] [PubMed] [Google Scholar]
- 24.Anderson JW, Zeigler JA, Deakins DA, Floore TL, Dillon DW, Wood CL, Oeltgen PR, Whitley RJ. Metabolic effects of high-carbohydrate, high-fiber diets for insulin-dependent diabetic individuals. Am J Clin Nutr. 1991;54:936–43. doi: 10.1093/ajcn/54.5.936. [DOI] [PubMed] [Google Scholar]
- 25.Shah M, McGovern P, French S, Baxter J. Comparison of a low-fat, ad libitum complex-carbohydrate diet with a low-energy diet in moderately obese women. Am J Clin Nutr. 1994;59:980–4. doi: 10.1093/ajcn/59.5.980. [DOI] [PubMed] [Google Scholar]
- 26.Poppitt SD, Keogh GF, Prentice AM, Williams DE, Sonnemans HM, Valk EE, Robinson E, Wareham NJ. Long-term effects of ad libitum low-fat, high-carbohydrate diets on body weight and serum lipids in overweight subjects with metabolic syndrome. Am J Clin Nutr. 2002;75:11–20. doi: 10.1093/ajcn/75.1.11. [DOI] [PubMed] [Google Scholar]
- 27.Davis JN, Hodges VA, Gillham MB. Normal-weight adults consume more fiber and fruit than their age- and height-matched overweight/obese counterparts. J Am Diet Assoc. 2006;106:833–40. doi: 10.1016/j.jada.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 28.Pereira MA, Swain J, Goldfine AB, Rifai N, Ludwig DS. Effects of a low-glycemic load diet on resting energy expenditure and heart disease risk factors during weight loss. JAMA. 2004;292:2482–90. doi: 10.1001/jama.292.20.2482. [DOI] [PubMed] [Google Scholar]
- 29.Bellisle F, Dalix AM, De Assis MA, Kupek E, Gerwig U, Slama G, Oppert JM. Motivational effects of 12-week moderately restrictive diets with or without special attention to the glycaemic Index of foods. Br J Nutr. 2007;97:790–8. doi: 10.1017/S0007114507450309. [DOI] [PubMed] [Google Scholar]
- 30.Maki KC, Rains TM, Kaden VN, Raneri KR, Davidson MH. Effects of a reduced-glycemic-load diet on body weight, body composition, and cardiovascular disease risk markers in overweight and obese adults. Am J Clin Nutr. 2007;85:724–34. doi: 10.1093/ajcn/85.3.724. [DOI] [PubMed] [Google Scholar]
- 31.Tucker KL, Bianchi L, Maras J, Bermudez O. Adaptation of a food frequency questionnaire to assess diets of Puerto Rican and non-Hispanic adults. Am J Epidemiol. 1998;148:507–18. doi: 10.1093/oxfordjournals.aje.a009676. [DOI] [PubMed] [Google Scholar]
- 32.Nieminen T, Matinheikki J, Nenonen A, Kukkonen-Harjula K, Lindi V, Hamelahti P, Laaksonen R, Fan YM, Kahonen M, et al. The relationship of sterol regulatory element-binding protein cleavage-activation protein and apolipoprotein E gene polymorphisms with metabolic changes during weight reduction. Metabolism. 2007;56:876–80. doi: 10.1016/j.metabol.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 33.Karter AJ, Mayer-Davis EJ, Selby JV, D’Agostino RB, Jr, Haffner SM, Sholinsky P, Bergman R, Saad MF, Hamman RF. Insulin sensitivity and abdominal obesity in African-American, Hispanic, and non-Hispanic white men and women. The Insulin Resistance and Atherosclerosis Study. Diabetes. 1996;45:1547–55. doi: 10.2337/diab.45.11.1547. [DOI] [PubMed] [Google Scholar]
- 34.Hanson RL, Imperatore G, Bennett PH, Knowler WC. Components of the “metabolic syndrome” and incidence of type 2 diabetes. Diabetes. 2002;51:3120–7. doi: 10.2337/diabetes.51.10.3120. [DOI] [PubMed] [Google Scholar]
- 35.Gastaldelli A, Cusi K, Pettiti M, Hardies J, Miyazaki Y, Berria R, Buzzigoli E, Sironi AM, Cersosimo E, et al. Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology. 2007;133:496–506. doi: 10.1053/j.gastro.2007.04.068. [DOI] [PubMed] [Google Scholar]
- 36.Perez-Martinez P, Yiannakouris N, Lopez-Miranda J, Arnett D, Tsai M, Galan E, Straka R, Delgado-Lista J, Province M, et al. Postprandial triglyceride metabolism is modified by the presence of genetic variation at the perilipin (PLIN) locus in two Caucasian populations. Am J Clin Nutr. 2008;87:744–52. doi: 10.1093/ajcn/87.3.744. [DOI] [PubMed] [Google Scholar]
- 37.Hjollund E, Pedersen O, Richelsen B, Beck-Nielson H, Sorensen NS. Increased insulin binding to adipocytes and monocytes and increased insulin sensitivity of glucose transport and metabolism in adipocytes from non-insulin-dependent diabetics after a low-fat/high-starch/high-fiber diet. Metabolism. 1983;32:1067–75. doi: 10.1016/0026-0495(83)90079-3. [DOI] [PubMed] [Google Scholar]
- 38.Sargrad KR, Homko C, Mozzoli M, Boden G. Effect of high protein vs high carbohydrate intake on insulin sensitivity, body weight, hemoglobin A1c, and blood pressure in patients with type 2 diabetes mellitus. J Am Diet Assoc. 2005;105:573–80. doi: 10.1016/j.jada.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 39.Egan JJ, Greenberg AS, Chang MK, Londos C. Control of endogenous phosphorylation of the major cAMP-dependent protein kinase substrate in adipocytes by insulin and beta-adrenergic stimulation. J Biol Chem. 1990;265:18769–75. [PubMed] [Google Scholar]
- 40.Chong MF, Fielding BA, Frayn KN. Metabolic interaction of dietary sugars and plasma lipids with a focus on mechanisms and de novo lipogenesis. Proc Nutr Soc. 2007;66:52–9. doi: 10.1017/S0029665107005290. [DOI] [PubMed] [Google Scholar]
- 41.Hudgins LC, Hellerstein MK, Seidman CE, Neese RA, Tremaroli JD, Hirsch J. Relationship between carbohydrate-induced hypertriglyceridemia and fatty acid synthesis in lean and obese subjects. J Lipid Res. 2000;41:595–604. [PubMed] [Google Scholar]
- 42.Parks EJ, Krauss RM, Christiansen MP, Neese RA, Hellerstein MK. Effects of a low-fat, high-carbohydrate diet on VLDL-triglyceride assembly, production, and clearance. J Clin Invest. 1999;104:1087–96. doi: 10.1172/JCI6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwarz JM, Linfoot P, Dare D, Aghajanian K. Hepatic de novo lipogenesis in normoinsulinemic and hyperinsulinemic subjects consuming high-fat, low-carbohydrate and low-fat, high-carbohydrate isoenergetic diets. Am J Clin Nutr. 2003;77:43–50. doi: 10.1093/ajcn/77.1.43. [DOI] [PubMed] [Google Scholar]
- 44.Jang Y, Kim OY, Lee JH, Koh SJ, Chae JS, Kim JY, Park S, Cho H, Lee JE, et al. Genetic variation at the perilipin locus is associated with changes in serum free fatty acids and abdominal fat following mild weight loss. Int J Obes (Lond) 2006;30:1601–8. doi: 10.1038/sj.ijo.0803312. [DOI] [PubMed] [Google Scholar]
- 45.Schulze MB, Matthias B, Manson JE, Ludwig DS, Colditz G, Stampfer MJ, Willett WC, Hu FB. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA. 2004;292:927–34. doi: 10.1001/jama.292.8.927. [DOI] [PubMed] [Google Scholar]
- 46.Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79:537–43. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- 47.LaHaye SA, Hollett PM, Vyselaar JR, Shalchi M, Lahey KA, Day AG. Comparison between a low glycemic load diet and a Canada Food Guide diet in cardiac rehabilitation patients in Ontario. Can J Cardiol. 2005;21:489–94. [PubMed] [Google Scholar]
- 48.Sichieri R, Moura AS, Genelhu V, Hu F, Willett WC. An 18-mo randomized trial of a low-glycemic-index diet and weight change in Brazilian women. Am J Clin Nutr. 2007;86:707–13. doi: 10.1093/ajcn/86.3.707. [DOI] [PubMed] [Google Scholar]
- 49.Sugiyama M, Tang AC, Wakaki Y, Koyama W. Glycemic index of single and mixed meal foods among common Japanese foods with white rice as a reference food. Eur J Clin Nutr. 2003;57:743–52. doi: 10.1038/sj.ejcn.1601606. [DOI] [PubMed] [Google Scholar]
- 50.Henry CJ, Lightowler HJ, Kendall FL, Storey M. The impact of the addition of toppings/fillings on the glycaemic response to commonly consumed carbohydrate foods. Eur J Clin Nutr. 2006;60:763–9. doi: 10.1038/sj.ejcn.1602380. [DOI] [PubMed] [Google Scholar]


