Abstract
Introduction
Current radiofrequency ablation (RFA) techniques require invasive needle placement and are limited by accuracy of targeting. The purpose of this study was to test a novel non-invasive radiowave machine that uses RF energy to thermally destroy tissue. Gold nanoparticles were designed and produced to facilitate tissue heating by the radiowaves.
Methods
A solid state radiowave machine consisting of a power generator and transmitting/receiving couplers which transmit radiowaves at 13.56 MHz was used. Gold nanoparticles were produced by citrate reduction and exposed to the RF field either in solutions testing or after incubation with HepG2 cells. A rat hepatoma model using JM-1 cells and Fisher rats was employed using direct injection of nanoparticles into the tumor to focus the radiowaves for select heating. Temperatures were measured using a fiber-optic thermometer for real-time data.
Results
Solutions containing gold nanoparticles heated in a time- and power-dependent manner. HepG2 liver cancer cells cultured in the presence of gold nanoparticles achieved adequate heating to cause cell death upon exposure to the RF field with no cytotoxicity attributable to the gold nanoparticles themselves. In vivo rat exposures at 35W using gold nanoparticles for tissue injection resulted in significant temperature increases and thermal injury at subcutaneous injection sites as compared to vehicle (water) injected controls.
Discussion
These data show that non-invasive radiowave thermal ablation of cancer cells is feasible when facilitated by gold nanoparticles. Future studies will focus on tumor selective targeting of nanoparticles for in vivo tumor destruction.
Keywords: Radiofrequency ablation, nanoparticles, liver
INTRODUCTION
Despite a myriad of efforts aimed at improving detection and treatment, cancer continues to be the second leading cause of death among Americans. Due to the high incidence of metastatic disease, adjuvant therapies are an important component in caring for patients with cancer. The liver is a frequent site of metastatic disease from many solid tumors. In fact, metastatic disease in the liver is more common than primary liver cancer in the Unites States. Worldwide, however, primary hepatocellular cancer (HCC) remains the fifth most common malignancy, and accounts for over 500,000 deaths annually. While current therapeutic options for liver cancer (primary or metastatic) include hepatic resection, liver transplantation, chemotherapy (systemic or liver directed), or ablative techniques, significant limitations exist. It is estimated that only 10-20% of patients with liver tumors are surgical candidates (1,2). In the remainder of cases, other modalities of treatment must be entertained with RFA having gained significant popularity in the last decade.
RF current, which has a frequency between 10kHz to 900MHz, has been applied to medical contexts for nearly a century in the form of electrocautery. RFA results in thermal injury that occurs as a consequence of friction that is generated by the agitation of ions as they attempt to follow the alternating current as it flows within tissue (3). RFA was limited to use in neurosurgical and cardiac procedures until the early 1990s when two groups proposed it as an effective method for destroying unresectable, malignant liver tumors (4,5). Subsequent animal and human trials suggested that RFA is safe and effective in the treatment of liver tumors (6-9). Limitations of current RFA technology include the requirement for invasive needle placement, accuracy of image-guidance, tumor size limits, operator dependence, and collateral damage to non-tumorous liver parenchyma and adjacent structures. In addition, long-term follow-up studies have established that a learning curve exists (10) as well as the fact that local tumor recurrence rates are higher than first reported, even in experienced hands (11,12). These studies reinforce that surgical resection remains the “gold-standard” for the treatment of liver tumors. However, the fact that the majority of liver cancer patients are inoperable at the time of diagnosis coupled with the limitations of conventional, invasive RFA procedures is what provides the impetus for developing a noninvasive manner in which RF current can be used for tumor ablation.
The use of nanoparticle technology in oncologic applications has been the focus of recent study (13). Specifically, gold nanoparticles have been used to image tumor vasculature (14) and can serve as potential diagnostic markers for cancer (15). Furthermore, they have shown promise in targeting and enhancing the uptake of chemotherapeutic agents to cancer cells (16,17). One study has demonstrated the variable bioavailability of nanoparticles to mouse tumor models based on their physical properties (18). These studies highlight the potential use of gold nanoparticles for specific targeting of cancer cells. Herein, we describe our initial experience using a novel, noninvasive radiowave machine coupled with gold nanoparticle enhancer solutions to thermally ablate tissue and cancer cells in both in vitro and in vivo systems.
MATERIALS AND METHODS
Noninvasive RF generator/Coupling System
A variable power (0-2 kilowatt) RF signal (13.56 megahertz) generator was built to specification and donated as a kind gift by Therm Med LLC, Erie, PA. This generator consists of a power source coupled to a transmitting and receiving head whose distance from one and other are adjustable as is the direction in which the field is generated. The electromagnetic field strength generated between the 2 heads was measured in a Farraday shielded room at low powers using an isotropic field monitor and probe (models FM2004 and FP2000, Amplifier Research Inc., Souderton, PA). A Hewlett-Packard Spectrum Analyzer (model 8566B; Agilent, Santa Clara, CA) was connected to the system to accurately measure transmitted power. Temperature measurement was achieved using a non-metallic fiber optic thermometer designed for use in high electromagnetic environments (model getTemp-4, getSpec, Dresden, Germany).
Solutions Testing
13 nanometer (nm) citrate coated gold nanoparticles (Au-NP) suspended in sterile water were a generous gift from David Waldeck, PhD (University of Pittsburgh, Department of Chemistry). Solution testing was performed on 0.5mL of either gold nanoparticles or water placed in test tubes and suspended in the field in a constant position while temperatures were measured continuously. Field strengths as well as the lengths of exposure were adjusted.
Cell Culture and in vitro exposures
HepG2 cells (ATCC, Monassas, VA) were cultured on 6 cm dishes in DMEM supplemented with 10% FBS along with 1% PS. Gold nanoparticles at a concentration of 4 nM (1mL) were added to the media of cells in culture 4 hours prior to exposure to the field. Gold nanoparticle-free media was used as control. The media on all cells was removed and replaced with fresh media with no nanoparticles after the 4 hour incubation to remove any residual nanoparticles that had not been incorporated into the cells. Cells were then exposed to the field at 35W while temperatures were continuously recorded. After exposure, cells were reincubated at 37°C, 5% CO2 for 4 more hours. At that time, supernatants were collected and frozen and cells were scraped into cold PBS and pelleted by centrifugation at 8000 rpm for 5 minutes.
Cell Viability Assay
Cell viability was assessed using an LDH assay kit (Roche Diagnostics, Indianapolis, IN) according to the manufacturer’s recommendations.
Western Blot Analysis
Whole cell lysis of HepG2 cells was performed by resuspending the cell pellets in an appropriate volume of 1x lysis buffer (CelLytic M, Sigma-Aldrich, St. Louis, MO) with 1mM PMSF for 30 minutes prior to high speed centrifugation. Protein concentrations were determined using the bicinchonic acid protein assay kit (Pierce, Rockford, IL). Supernatants from HepG2 cells incubated in the presence of gold nanoparticles alone or in combination with exposure to the RF field were collected, as were those from control plates, and mixed with equal volumes of 3X Laemelli sample buffer. Samples were loaded into a 12% nondenaturing, acrylamide gel and electrophoresed prior to being transferred to nitrocellulose membranes. After blocking in 5% milk overnight, membranes were probed using antibody for β-Actin (1:5000 in 1% milk overnight, Sigma Aldrich, St Louis, MO). Horse Radish Peroxidase tagged anti-rabbit secondary antibody (1:10,000 in 1% milk) was added for 1 hour after which membranes were developed with the Super Signal West Pico chemiluminescent kit (Pierce, Rockford, IL) and exposed to film.
In Vivo Subcutaneous Injections and Exposures
All animal experiments were carried out in accordance with guidelines established by the University of Pittsburgh’s Institutional Animal Care and Usage Committee. Male Buffalo rats weighing between 250-300g (Charles River, Wilmington, MA) were put to sleep in a CO2 chamber prior to being given an intraperitoneal injection of pentobarbital sodium (Ovation Pharmaceuticals, Deerfield, IL). Once adequately anesthetized, a 0.5mL subcutaneous injection of either sterile water or gold nanoparticles at a concentration of 13 nM was given into the shoulder. A temperature probe was placed into the injection site through a small incision followed by exposure to the RF field at a power of 35W. Temperatures were measured every minute for up to seven minutes. 48 hours were allowed to pass prior to harvesting the tissue at the site of injection/exposure.
In Vivo Tumor Model
JM-1 hepatoma cells were cultured as described previously (19) and provided as a kind gift from George Michalopoulos, MD (University of Pittsburgh, Department of Pathology). After adequate induction of anesthesia, 1×106 cells suspended in 0.5mL of sterile PBS were injected subcutaneously into the shoulders of male Fisher rats (Charles River, Wilmington, MA) weighing between 300-400g. 14 days were allowed to pass before the subcutaneous tumor nodules were directly injected with either 0.5mL gold nanoparticles or an equal volume of sterile water prior to exposure in the RF field at 35W for 13 minutes. Temperatures at the injection site were measured every minute and 48 hours were allowed to pass prior to sacrificing the animals and harvesting the tumor nodules.
Histopathology
Formalin fixed, RF exposed subcutaneous injection site samples or tumor nodules were embedded in paraffin and cut to 6 μm thick sections. Tissues were stained with hematoxylin and eosin and slides were assessed by a liver pathologist (MN), who was blinded to treatment group, for evidence of RF induced tissue damage.
Statistical Analysis
Results are expressed as the mean ± SEM. Group comparisons were performed using Student’s t test or ANOVA. Differences were considered significant at P<0.05.
RESULTS
Gold nanoparticle containing solutions heat in time- and power-dependent manners when exposed to the RF field
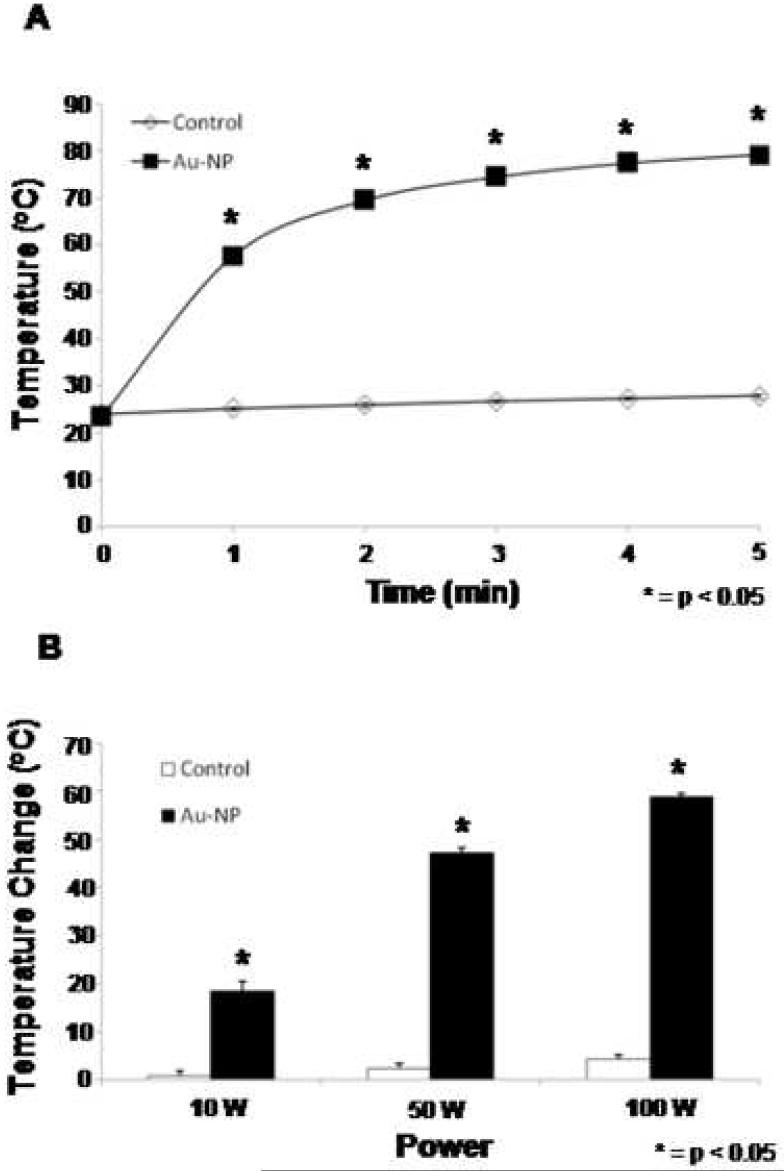
To begin testing whether gold nanoparticles were capable of serving as enhancers of heating in the externally generated RF field (Figure 1), solutions of citrate coated, colloidal gold nanoparticles were used. When exposed to the field at constant powers, nearly immediate heating was observed and temperatures continued to increase over a short time-course (Figure 2A). Previous studies have demonstrated that temperatures of approximately 50°C are required for thermally induced ablation. These temperatures were achieved within the first minute of our gold nanoparticle solutions tests. To determine the effect of RF field power on heating, solutions were exposed to the RF field at variable powers (10-100W) for 3 minutes. Heating was observed in a power-dependent manner in the nanoparticle containing solutions with no significant heating in the nanoparticle-free solutions (Figure 2B). This data demonstrates that gold nanoparticle-containing solutions can be heated selectively and to a degree (>50° C) that is sufficient to induce cell death upon exposure to the externally generated RF field.
Figure 1.
Equipment and set up of novel radiofrequency field generator. The RF field generator consists of a power source connected to transmitting and receiving heads. Solutions, cells in culture, or animals can be placed into the center of the field for exposure.
Figure 2.
Au-NP solutions are heated selectively and efficiently in RF field. A. Solutions of Au-NP (squares) or water (diamonds) were exposed to the RF field at 50W and temperatures were measured over time. Results reported are averages of three separate experiments. B. Solutions of Au-NP (black) or water (white) were exposed to the RF field at 10, 50, or 100W and temperatures were measured after 3 minutes. Results reported are the averages of three separate experiments.
HepG2 cells cultured in the presence of gold nanoparticles achieve adequate heating to cause cell death upon exposure to the RF field
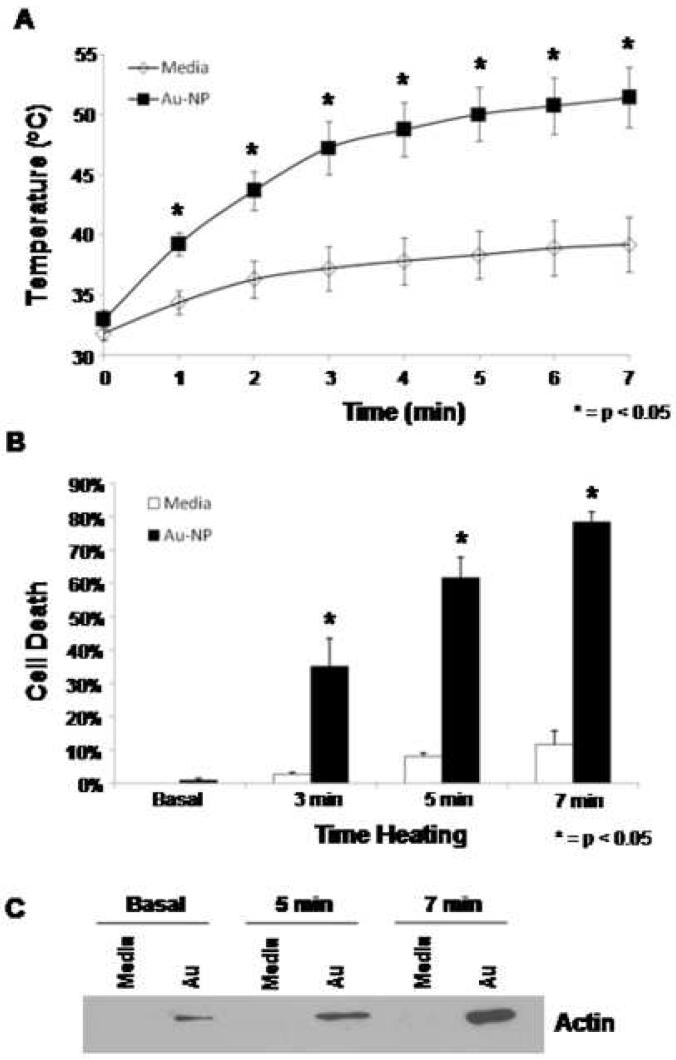
Given the results of the solutions testing described above, we set out to determine if a human liver cancer cell line when cultured in the presence of gold nanoparticles and subsequently exposed to the RF field could be heated enough to cause cell death. HepG2 cells cultured in the presence or absence of gold nanoparticles as described in the methods section were exposed to the field at 35W for up to 7 minutes. The presence of gold nanoparticles resulted in a time-dependent increase in heating (Figure 3A). Cell death as assessed by LDH assay correlated with temperature increases, with approximately 80% cell death achieved in the gold nanoparticle treatment group after 7 min of heating (Figure 3B). Additionally, these findings of cell death were confirmed by western blot analysis of the supernatants which demonstrated markedly increased β-actin release after 5 and 7 min of heating (Figure 3C) in the gold nanoparticle group. The presence of gold nanoparticles alone were not directly cytotoxic to our cell cultures, a finding that is consistent with previous reports (20). Further, primary rat hepatocytes incubated with gold nanoparticles at 37°C for up to 12 hours did not experience significant cell death compared to control cells (data not shown).
Figure 3.
In vitro cell culture demonstrates effective heating and cell death with Au-NP treated cells in the RF field. A. Temperature of HepG2 cells exposed to 35W in the RF field in the presence (squares) or absence (diamonds) of Au-NP over a timecourse. Results reported are the average of three separate experiments. B. Percent cell death as assessed by LDH assay of HepG2 cells exposed to 35W in the RF field for 3, 5 or 7 minutes in the presence (black) or absence (white) of Au-NP. Results reported are the average of three separate experiments. C. Western blot for β-actin on the supernatants of HepG2 cells exposed to 35W in the RF field in the presence or absence of Au-NP for 5 or 7 minutes.
In vivo subcutaneous injections of gold nanoparticles result in tissue destruction upon exposure to the RF field
Next, we aimed to establish that our in vitro results with gold nanoparticles could be duplicated in vivo. Male Buffalo rats were injected subcutaneously in the shoulder with gold nanoparticles and then exposed to the RF field at a power of 35W. These animals experienced significant increases in temperature at the soft tissue injection site compared to control (H2O-injected) animals (Figure 4A). Following RF exposure, tissue samples were obtained from injection sites for histopathologic assessment. Tissue taken from control animals demonstrated generally normal tissue architecture (Figure 4B), while tissue from gold nanoparticle injected animals revealed evidence of tissue destruction as evidenced by loss of normal tissue architecture, loss of membrane integrity, and infiltration of inflammatory cells (Figure 4C).
Figure 4.
Au-NP injection focuses the RF field for effective tissue ablation in vivo. A. Male Buffalo rats were injected subcutaneously in the shoulder with 0.5mL of either sterile water (white) or Au-NP (black) and were placed in the RF field at 35W for a duration of 7 minutes. Temperatures at the injection site were measured each minute. Results are reported as the change in temperature from baseline at the site of injection. B. Histologic assessment of subcutaneous injection site in sterile water injected animals reveals generally normal tissue architecture with no significant signs of inflammation. C. Tissue from Au-NP injected animals treated in the RF field demonstrates loss of tissue architecture and infiltration of inflammatory cells.
In vivo gold nanoparticle injections result in tumor ablation in a rat hepatoma model
In order to achieve proof of principle that gold nanoparticles could facilitate tumor ablation in the RF field, we utilized a rat hepatoma model. 1×106 JM-1 cells were injected subcutaneously into the shoulders of Fisher rats. 14 days were allowed to pass for tumor nodules to grow at which time the tumors were directly injected with either gold nanoparticles or sterile water (vehicle). Upon exposure to the RF field at 35W, greater temperature increases were achieved in the gold nanoparticle-injected animals as compared to controls (data not shown). Histopathologic assessment of tumor nodules harvested 48 hours after exposure demonstrated widespread tumor cell disaggregation coupled with nuclear hyperchromatism, cytoplasmic retraction, and areas of apoptosis and cell fragmentation (Figure 5). This pattern of necrosis was histologically reminiscent of thermal injury. A separate, patchy pattern of advanced confluent necrosis was also observed in both nanoparticle-injected and control water-injected tumors. Additional studies will be required to determine whether this latter change represents local in vivo effects of water inoculation or whether it is a feature of the growth characteristics of this tumor model. The widespread thermal pattern of tumor injury was not seen in control samples.
Figure 5.
Effect of RF field exposure on subcutaneous JM-1 hepatoma nodules. The photomicrograph compares RF exposure following injection of gold nanoparticles (A, B) or sterile water (C, D) into tumors. A. Low power photomicrograph shows relatively uniform cellular appearance of tumor with surrounding soft tissue present in upper portion of image (H&E, 100x). B. Closer view shows areas of tumor cell disaggregation, cell fragments, hyperchromatic nuclei and retracted cytoplasm. These features are compatible with thermal injury (H&E, 600x). C. Overview of control tumor shows area of confluent necrosis in the lower left-hand portion of the photograph with a sheet of viable tumor cells comprising the remainder of the image (H&E x100). D. Interface of viable tumor area (upper right) and necrotic region (lower left) (H&E x600).
DISCUSSION
Our results demonstrate that gold nanoparticles coupled with a noninvasive radiofrequency generator are a potentially novel method for ablating tumors. Our in vitro cell culture tests demonstrate that colloidal gold nanoparticles are not directly cytotoxic but can, upon exposure to the RF field, be heated to a degree that results in cell death. We correlated our promising in vitro results with in vivo models in which gold nanoparticle injections resulted in ablation of either normal tissues or subcutaneous tumor nodules from a rat hepatoma model. The conceivable applications of this technology are broad and include the potential treatment of patients with nonoperative tumors as well as the possibility of treating multiple tumors in a single treatment session.
While the results of this study support further investigations into the use of gold nanoparticles in combination with the RF generator, there are a number of questions that remain to be answered as well as technical obstacles to be overcome in future studies. First and foremost, the aim of the project is to develop a noninvasive method of ablating tumors as an alternative to currently available RF probes. However, in our rat hepatoma model, gold nanoparticles were targeted to subcutaneous tumors via direct intra-tumoral injections. For this technology to be clinically applicable, nanoparticles will have to be administered noninvasively and subsequently targeted toward cancer cells. There are studies regarding the highly selective targeting of specific cells and tissues by nanoparticles through the modification of their physical properties (18). Recently, nanoparticle delivery systems (20nm-100nm) capable of escaping phagocytic clearance by the reticuloendothelial system have been investigated (21-23). Additionally, 33nm polyethylene glycol-coated gold nanoparticles have been incorporated with TNFα to enhance thermal induced tumor growth delay in a murine colon cancer model (24). Furthermore, 1.9nm gold nanoparticles delivered by IV injection have been used to enhance radiotherapy induced tumor ablation in a mammary cancer model (25). These reports suggest that research progress is rapidly being achieved for targeted nanoparticle delivery to cancer cells.
Glypican-3, a member of the glypican family of heparin sulfate proteoglycans, has been found to be overexpressed in hepatocellular cancer (26). Furthermore, epidermal growth factor receptor (EGFR), a transmembrane tyrosine kinase, is overexpressed in the fibrolamellar variant of HCC as well as other cancers for which RFA is commonly used including breast, gastric and colorectal (27). Anti-EGFR antibody conjugated gold nanoparticles have been described with applications including real time vital optical imaging as well as selective laser photo-thermal therapy. Therefore, future investigations will likely focus on developing antibody coated gold nanoparticles for targeted delivery. In addition to targeting gold nanoparticles to tumors, the radiofrequency generator has the potential to be modified in such a way that a more focused field is applied to specific sites of interest in a manner akin to cyberknife technology that is currently in clinical use for the delivery of targeted external beam radiation. Another limitation of the current study is that it does not address the diffusion of gold nanoparticles in vivo, or the size of the thermal ablation zone. However, the aim of the in vivo aspects of this study was simply to provide proof of concept that this novel technology can be used in conjunction with gold nanoparticles to cause thermally induced tissue injury. Certainly, future studies focusing on targeted delivery of gold nanoparticles will involve imaging to detect where systemically delivered nanoparticles accumulate as well as long-term follow-up to assess the ability of the non-invasive radiowave ablation to shrink or resolve established tumors.
Although at least 3-4 years away from clinical testing, it is important to mention that clinical monitoring that would be utilized with this non-invasive radiowave treatment. We envision that patients would be awake with IV sedation and would have cardiac and pulse oximetry monitoring during the procedure. Pain medications could be employed either prior to or during the procedure if the patient experiences pain. As for the strength of the current power generator, current radiofrequency ablation devices in clinical use are approved for up to 200 Watts. While the Kanzius power generator is capable of producing power up to 1000 W, our studies used 35-100 W for solution tests, and 35 W for cell culture and in vivo experiments, which is well below the current FDA limit of 200W.
We first described the use of this noninvasive radiowave technology for thermal ablation of cancer over a year ago (28). In that study, we used metal ion (copper, iron, and magnesium) injections to focus the radiowave heating, and have subsequently used gold nanoparticles as described in the current study. Recently, another group has reported using a similar radiowave machine in a rabbit hepatoma model utilizing single walled carbon nanotubes (29). However, like our study, the ablation model consisted of direct tumor injection of nanotubes, and thus selective, noninvasive cancer targeting remains elusive. Nonetheless, taken together these studies provide proof-of-principle that noninvasive radiowave ablation of cancer is technically feasible, particularly when facilitated by nanotechnologies. While this technology holds exciting potential for broad applications, significant further research is needed.
Acknowledgements
We thank Dr. George Michalopoulos for providing the JM-1 cells and Dr. David Waldeck for providing the gold nanoparticles. This work was supported in part by a grant from Labor, HHS, and Education Appropriations, as well as philanthropic contributions to the UPMC Liver Cancer Center Research Fund.
Abbreviations
- (gold)
Au nanoparticles
- (RFA)
Radiofrequency Ablation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tanabe KK, Curley SA, Dodd GD, Siperstein AE, Goldberg SN. Radiofrequency ablation: the experts weigh in. Cancer. 2004;100:641–50. doi: 10.1002/cncr.11919. [DOI] [PubMed] [Google Scholar]
- 2.Kuvshinoff B, Fong Y. Surgical therapy of liver metastases. Semin Oncol. 2007;34:177–85. doi: 10.1053/j.seminoncol.2007.03.003. Review. [DOI] [PubMed] [Google Scholar]
- 3.Goodman M, Geller DA. Radiofrequency ablation of hepatocellular carcinoma. In: Carr B, editor. Hepatocellular Cancer. Human Press; Totowa, NJ: 2005. pp. 171–183. [Google Scholar]
- 4.McGahan JP, Browning PD, Brock JM, Tesluk H. Hepatic ablation using radiofrequency electrocautery. Invest Radiol. 1990;25:267–270. doi: 10.1097/00004424-199003000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Rossi S, Fornari F, Pathies C, Buscarini L. Thermal lesions induced by 480 KHz localized current field in guinea pig and pig liver. Tumori. 1990;76:54–57. doi: 10.1177/030089169007600114. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg SN, Gazelle GS, Dawson SL, Rittman WJ, Mueller PR, Rosenthal DI. Tissue ablation with radiofrequency: effect of probe size, gauge, duration, and temperature on lesion volume. Acad Radiol. 1995;2(5):399–404. doi: 10.1016/s1076-6332(05)80342-3. [DOI] [PubMed] [Google Scholar]
- 7.Curley SA, Izzo F, Delrio P, Ellis LM, Granchi J, Vallone P, Fiore F, Pignata S, Daniele B, Cremona F. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg. 1999;230:1–8. doi: 10.1097/00000658-199907000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siperstein A, Garland A, Engle K, Rogers S, Berber E, String A, Foroutani A, Ryan T. Laparoscopic radiofrequency ablation of primary and metastatic liver tumors. Technical considerations. Surg Endosc. 2000;14:400–5. doi: 10.1007/s004640000067. [DOI] [PubMed] [Google Scholar]
- 9.Solbiati L, Livraghi T, Goldberg SN, Ierace T, Meloni F, Dellanoce M, et al. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology. 2001;221(1):159–166. doi: 10.1148/radiol.2211001624. [DOI] [PubMed] [Google Scholar]
- 10.Poon RT, Ng KK, Lam CM, Ai V, Yuen J, Fan ST, Wong J. Learning curve for radiofrequency ablation of liver tumors: prospective analysis of initial 100 patients in a tertiary institution. Ann Surg. 2004;239:441–9. doi: 10.1097/01.sla.0000118565.21298.0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdalla EK, Vauthey JN, Ellis LM, Ellis V, Pollock R, Broglio KR, Hess K, Curley SA. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818–25. doi: 10.1097/01.sla.0000128305.90650.71. discussion 825-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, Mickel L. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg. 2005;242:158–71. doi: 10.1097/01.sla.0000171032.99149.fe. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baptista P, Pereira E, Eaton P, Doria G, Miranda A, Gomes I, Quaresma P, Franco R. Gold nanoparticles for the development of clinical diagnosis methods. Anal Bioanal Chem. 2007 Dec 21; doi: 10.1007/s00216-007-1768-z. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 14.Cai QY, Kim SH, Choi KS, Kim SY, Byun SJ, Kim KW, Park SH, Juhng SK, Yoon KH. Colloidal gold nanoparticles as a blood-pool contrast agent for X-ray computed tomography in mice. Invest Radiol. 2007 Dec;42(12):797–806. doi: 10.1097/RLI.0b013e31811ecdcd. [DOI] [PubMed] [Google Scholar]
- 15.Qian X, Peng XH, Ansari DO, Yin-Goen Q, Chen GZ, Shin DM, Yang L, Young AN, Wang MD, Nie S. In vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags. Nat Biotechnol. 2008 Dec 23; doi: 10.1038/nbt1377. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 16.Podsiadlo P, Sinani VA, Bahng JH, Kam NW, Lee J, Kotov NA. Gold Nanoparticles Enhance the Anti-Leukemia Action of a 6-Mercaptopurine Chemotherapeutic Agent. Langmuir. 2007 Dec 5; doi: 10.1021/la702782k. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 17.Song M, Wang X, Li J, Zhang R, Chen B, Fu D. Effect of surface chemistry modification of functional gold nanoparticles on the drug accumulation of cancer cells. J Biomed Mater Res A. 2007 Dec 7; doi: 10.1002/jbm.a.31692. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 18.Balogh L, Nigavekar SS, Nair BM, Lesniak W, Zhang C, Sung LY, Kariapper MS, El-Jawahri A, Llanes M, Bolton B, Mamou F, Tan W, Hutson A, Minc L, Khan MK. Significant effect of size on the in vivo biodistribution of gold composite nanodevices in mouse tumor models. Nanomedicine. 2007 Dec;3(4):281–96. doi: 10.1016/j.nano.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Kar S, Wang M, Wilcox CS, Carr BI. Antitumor and anticarcinogenic actions of Cpd 5: a new class of protein phosphatase inhibitor. Carcinogenesis. 2003;24(3):411–416. doi: 10.1093/carcin/24.3.411. [DOI] [PubMed] [Google Scholar]
- 20.Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small. 2005;1(3):325–327. doi: 10.1002/smll.200400093. [DOI] [PubMed] [Google Scholar]
- 21.Mornet S, Vasseur S, Grasset F, Duguet E. Magnetic nanoparticle design for medical diagnosis and therapy. J Mater Chem. 2004;14:2161–2175. [Google Scholar]
- 22.Paciotti GF, Myer L, Weinreich D, Goia D, Pavel N, McLaughlin RE, et al. Colloidal gold: a novel nanoparticle vector for tumor directed drug delivery. Drug Deliv. 2004;11(3):169–183. doi: 10.1080/10717540490433895. [DOI] [PubMed] [Google Scholar]
- 23.Brigger I, Dubernet C, Couvreur P. Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliv Rev. 2002;54(5):631–651. doi: 10.1016/s0169-409x(02)00044-3. [DOI] [PubMed] [Google Scholar]
- 24.Visaria RK, Griffin RJ, Williams BW, Ebbini ES, Paciotti GF, Song CW, et al. Enhancement of tumor thermal therapy using gold nanoparticle-assisted tumor necrosis factor-alpha delivery. Mol Cancer Ther. 2006;5(4):1014–1020. doi: 10.1158/1535-7163.MCT-05-0381. [DOI] [PubMed] [Google Scholar]
- 25.Hainfeld JF, Slatkin DN, Smilowitz HM. The use of gold nanoparticles to enhance radiotherapy in mice. Phys Med Biol. 2004;49(18):N309–N315. doi: 10.1088/0031-9155/49/18/n03. [DOI] [PubMed] [Google Scholar]
- 26.Sung YK, Hwang SY, Park MK, Farooq M, Han IS, Bae HI, et al. Glypican-3 is overexpressed in human hepatocellular carcinoma. Cancer Sci. 2003;94(3):259–262. doi: 10.1111/j.1349-7006.2003.tb01430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buckley AF, Burgart LJ, Kakar S. Epidermal growth factor receptor expression and gene copy number in fibrolamellar hepatocellular carcinoma. Hum Pathol. 2006;37(4):410–414. doi: 10.1016/j.humpath.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 28.Klune JR, Jeyabalan G, Chory Es, Kanzius J, Geller DA. Pilot investigation of a new instrument for non-invasive radiowave ablation of cancer. J Surg Res. 2007 Feb.137:263. [Google Scholar]
- 29.Gannon CJ, Cherukuri P, Yakobson BI, Cognet L, Kanzius JS, Kittrell C, et al. Carbon nanotube-enhanced thermal destruction of cancer cells in a noninvasive radiofrequency field. Cancer. 2007;110(12):2654–2665. doi: 10.1002/cncr.23155. [DOI] [PubMed] [Google Scholar]