SUMMARY
Behavioral problems in young children can take on a variety of forms, which are linked to distinct antecedents and co-occurring markers. Internalizing difficulties in young children, for example, have been linked to individual differences in infant temperament and cortisol levels. In addition, there is growing evidence that these biobehavioral mechanisms are also shaped by gender. Four-year-old children participated in a study examining the relations between salivary cortisol and behavioral maladjustment as a function of gender and temperament. Both longitudinal (maternal report of infant temperament at 9 months) and concurrent (morning salivary cortisol at age 4) data were used to predict two forms of maladjustment: ‘Withdrawal’ (maternal report of internalizing behavior and laboratory observation of social reticence) and ‘Acting Out’ (maternal report of externalizing behavior and laboratory observation of solitary active play). High basal cortisol levels were strongly associated with Withdrawal in male participants. However, the relation was significant only in boys who exhibited high levels of negative temperament in infancy. There were no comparable findings with ‘Acting Out’ beyond a main effect of gender reflecting greater difficulty in boys. The data suggested that there are unique biobehavioral mechanisms shaping specific patterns of maladjustment in childhood.
Keywords: Cortisol, Temperament, Gender, Socioemotional Behavior, Early Childhood, Infancy
INTRODUCTION
The inability to adapt to evolving environmental demands can be linked to various forms of behavioral maladjustment ranging from the relatively benign (prolonged shyness when meeting new peers) to behaviors that mark a real impairment in functioning (as in the case of severe social anxiety). In addressing individual differences in adaptability, researchers have examined the forces that play a role in either hindering or facilitating the child’s ability to respond to his or her environment’s unique challenges. Throughout childhood, the environment and the demands it places on the individual are continuously evolving. As such, physiological systems involved in these transitions must be both pervasive and flexible in nature (Fox et al 2006).
One such mechanism, centered on the glucocorticoid cortisol, works to sustain circadian energy levels (Rosen & Schulkin 1998) and is sensitive to the ebb and flow of daily life. Although generally thought of as a stress hormone, cortisol coordinates adaptation to both environmental and internal conditions and plays a role in shaping both concurrent behavior and moderating subsequent patterns of development (see Shirtcliff et al 2005 for a discussion). In terms of the daily events in the life of a young child, cortisol levels can increase when entering a new classroom (Tout et al 1998) or social group (Granger et al 1994), return to normal levels at a regularly scheduled naptime (Watamura et al 2001), and then dip below baseline when actively engaged in a positive activity (Hertsgaard et al 1992, Legendre & Trudel 1996).
Cortisol levels reflect both individual differences in adjustment and the environmental factors that shape behavior. For example, Cicchetti and Rogosch(2001) found that maltreated children showed either depressed or elevated basal cortisol levels, depending on their behavioral symptomatology. O’Connor (O’Connor et al 2005) suggested that pre-natal anxiety can have long-lasting effects on cortisol secretion, potentially acting as a biological substrate for later psychopathology. Developmental work in normative samples has found that consistent individual differences in cortisol production are evident by nine months of age (Lewis & Ramsay 1995a, Lewis & Ramsay 1995b). Questions remain regarding the specificity of the link between basal cortisol levels and behavioral maladjustment. For example, while many studies emphasize the link between cortisol and internalizing difficulties (see Gunnar 2001 for a discussion), studies have linked increased cortisol levels in young children to both internalizing and externalizing difficulties (Essex et al 2002).
An individual differences approach may help explain the previously observed pattern of behavior. The current study examined factors strongly implicated in shaping profiles of behavioral maladjustment in early childhood, namely infant temperament, basal cortisol levels, and gender. In doing so, the study also examined the specificity or interactive nature of these relations by predicting both internalizing and externalizing behaviors.
Temperament refers to a constellation of individual traits that bias a child to interpret and react to the environment in a predictable manner and is linked to stable patterns of physiological, emotional, and cognitive reactivity (Fox et al 2001). Children with a ‘difficult’ or ‘negative’ temperament are viewed as less able to swiftly adjust to environmental demands and may be at greater risk for behavioral difficulties. Negative temperament or reactivity is marked by crying, fussing, and motoric agitation in infancy (e.g. Buss & Plomin 1984) and often leads to behavioral inhibition in response to novel objects, people, and events in early childhood (Kagan & Snidman 1991).
Behaviorally inhibited children appear to have higher baseline cortisol levels (Kagan et al 1988b) and behavioral inhibition, in conjunction with social wariness, can predict baseline cortisol levels in early childhood (see Schmidt et al 1997, but not Schmidt et al 1999). Elevated cortisol levels may also help explain the documented links between early negative temperament and later anxiety (Biederman et al 2001, Pérez-Edgar & Fox 2005). In the same vein, cortisol levels have been linked to activation in the central nucleus of the amygdala (Rosen & Schulkin 1998), a brain structure central to both behavioral inhibition (Kagan et al 1989, Pérez-Edgar et al 2007, Schwartz et al 2003) and anxiety (Thomas et al 2001a, Thomas et al 2001b).
The literature suggests that temperament measured in infancy or early childhood is a moderately stable trait with stability measures ranging from 0.3 to 0.6 (Degnan & Fox 2007). As expected, rates are higher when examining selected or at-risk samples rather than normative or randomly selected populations. In addition to issues of initial selection, a growing number of studies have found increased stability (as well as more extreme temperamental profiles) in boys (Fagan 1990, Fox et al 2001, Stevenson-Hinde & Glover 1996). This is in turn linked to increased behavior problems in pre-school (Stevenson-Hinde & Glover 1996), poor social skills in middle childhood (Rubin et al 1993), and lower self-esteem in adolescence (Morison & Masten 1991). In explaining this gender difference many have suggested that shyness or internalizing behaviors are less accepted in boys (Sadker & Sadker 1994), as seen in social interactions with parents (Crockenberg & Smith 1982, Radke-Yarrow et al 1988).
Supplementing this work are findings suggesting that gender differences in developmental trajectories are not due solely to differences in the environment. Biological and psychophysiological markers generally linked to shyness and internalizing behaviors also play different roles across gender. For example, negative reactivity in infancy predicts social wariness at age four only if the infant is male and shows right frontal EEG asymmetry (Henderson et al 2001). Interestingly, Buss et al. (2003) found that children with extreme right frontal asymmetry had higher levels of salivary cortisol than did peers with either extreme left frontal asymmetry or “average” levels of asymmetry.
The relations between cortisol and behavior may also differ as a function of gender (e.g., Jackson et al 2006), although most of this work has focused on adults, with relatively little work in children (Gunnar et al 1997, McBurnett et al 1991, van Goozen et al 1998). Four studies of note (Hatzinger et al 2007, Shirtcliff et al 2005, Smider et al 2002, Tout et al 1998) have directly examined gender differences in the relations between cortisol levels and behavioral adjustment in children, producing a complex pattern of findings.
Hatzinger and colleagues (2007) examined morning basal cortisol levels in a sample of kindergarten children. Their data indicate that increased levels of basal cortisol predicted increases in both internalizing and externalizing behaviors in boys, while decreased cortisol was linked to positive behavior in girls. In contrast, Smider and colleagues (2002) found no concurrent effects of afternoon cortisol levels in similarly aged children. Rather, they found predictive strength to age six with increased levels predicting increased withdrawal in girls and low levels in boys linked to more externalizing difficulty. The inverse relation among boys was also evident in a longitudinal study of children (ages 6 to 16 years) by Shirtcliff and colleagues (2005). However, another study (Tout et al 1998) found that decreased cortisol levels predicted internalizing difficulties in boys.
In contrast, studies with at-risk or clinical populations (Cicchetti & Rogosch 2001, Granger et al 1994, Granger et al 1998, Kagan et al 1988a, Kagan et al 1988b, Schmidt et al 1997) have generally found high levels of cortisol using both basal measures (e.g. Cicchetti & Rogosch 2001) and acute responses to novel stimuli or stressors (e.g. Granger et al 1998, Schmidt et al 1997). However, studies of at-risk populations often either do not address potential gender differences or have a single-sex sample (e.g. van Goozen et al 1998). Clearly, additional work will be needed to address the issue of potential gender differences in both normative and clinical populations.
As these data illustrate, questions remain concerning the specificity of the relations of interest. This is important as different forms of behavioral maladjustment are linked to differential outcomes into adolescence and adulthood. It is highly likely that different antecedent and underlying mechanisms may be at work with varying patterns of interrelation (Henderson et al 2004). To aid the validity and stability of the outcome points, the current study employed both laboratory observation and maternal reports of two distinct forms of behavioral functioning. In particular, we created a measure of ‘Withdrawal’, by bringing together maternal report of internalizing on the Child Behavior Checklist (Achenbach 1991) with laboratory observation of social reticence (Rubin 1989) in the presence of same-age, same-sex unfamiliar peers.
Reticent behavior is marked by a conflict between approach and avoidance motivations (Asendorpf 1990) in that the children do not engage in social interaction, although they persist in monitoring their peers. Reticence has been linked to behavioral inhibition, internalizing problems, and right frontal EEG asymmetry (Coplan 2000, Fox et al 2005, Henderson et al 2004).
In parallel, we created a measure labeled ‘Acting Out’ that employed externalizing scores on the CBCL with solitary active play in the same laboratory paradigm noted above. Solitary active behavior is marked by boisterous, repetitive behaviors and dramatizing. It is important to note that solitary active play is defined as behaviors carried out in the presence of peers, as opposed to with peers, which is more normative. Solitary active play, while infrequent, is negatively salient to peers (Rubin 1989) and has been linked to social immaturity, impulsivity, and externalizing behavior (Coplan & Rubin 1998, Coplan et al 2001b).
The current sample was selected for extreme negative reactivity at four months of age in preparation for a longitudinal study of behavioral inhibition and social withdrawal. Subsequently, the data in toddlerhood reflected this selection process finding high levels of withdrawal and very low levels of externalizing difficulties (Fox et al 1996). In addition, psychophysiological measures were also found to chiefly moderate developmental trajectories linked to withdrawal. As such, the current ‘Acting Out’ measure is designed to act as a counter weight to our central interest in the emergence of ‘Withdrawal’ behaviors. It is for this reason that we have also chosen to focus on negative affect at nine months, rather than the whole spectrum of temperament traits that may be assessed in infancy.
The present study examined the biological and behavioral factors that may shape developmental trajectories leading to behavioral maladjustment in young children. In doing so, the analyses brought together factors previously shown to influence developmental outcomes: Infant temperament, gender, and basal cortisol levels. To examine the specificity of these relations, two forms of maladjustment were used, ‘Withdrawal’ (internalizing and social reticence) and ‘Acting Out’ (externalizing and solitary active play).
Hypothesis one predicted that negative temperament in infancy would be associated with increased levels of Withdrawal at age four, particularly when coupled with concurrently high levels of basal cortisol. The second hypothesis predicted that this relation would be particularly strong in boys. Hypothesis three further limited the scope of the mechanism by predicting that the model would not hold for Acting Out behaviors.
METHOD
Participants
Children were drawn from two independent cohorts participating in longitudinal studies of temperament and affect regulation. Initially, four-month-old infants were screened for motoric and emotional reactivity in response to the presentation of novel visual and auditory stimuli (for details see Calkins et al 1996, Kagan & Snidman 1991). Infants from both cohorts were selected based on the amount of motor reactivity as well as positive and negative affect expressed during the presentation of the novel sights and sounds. Of the 433 infants screened, 153 were selected for inclusion in the longitudinal studies. The families were Caucasian and of middle-class background, living in the greater Washington, DC area. Approximately 68% of the mothers and 72% of the fathers were college educated. One-third of the children were first born. Previous analyses involving these children can be found in Fox et al. (2001, 1995). After initial selection, the children returned to the laboratory at multiple age points (Calkins et al 1996, Fox et al 2001).
This study relied on four measures collected at two time points: nine months and four years of age. As a result, the N’s varied across each of the measures (see below). In order to create a stable study population, the findings presented below are drawn solely from the 83 children (41 male) who had viable data on all measures.
Preliminary analyses found no significant differences between the children in this study and the remaining cohort with missing data (all p’s > 0.12). To ensure that the children included in this study reflect their relative position within the full cohort, any data manipulations (e.g., standardization, composite creation) were completed using the full cohort before removing the children with missing data.
Infant Temperament
At nine months of age, maternal reports of temperament were gathered using the Infant Behavior Questionnaire (IBQ; Rothbart 1981). The IBQ is an 87-item parent rating form in which parents are asked to rate the frequency of specific infant behaviors as they occurred in the previous week. Scaled scores are derived from the measure by taking the mean ratings on all items in the particular scale, omitting the items marked as ‘Does not apply’. The composite measure ‘Negative Affect’ was created by summing the children’s scores on two scales, ‘Distress to Limitations’ and ‘Distress to Novelty’. Data were available for 141 infants.
Morning Salivary Cortisol
During a laboratory visit for the larger longitudinal study, parents were instructed in how to collect salivary cortisol samples. They were then given a saliva collection kit and written instructions. At collection, the children were asked to chew for approximately 1 min on a dental cotton roll saturated with Kool-Aid© crystals. Schwartz (Schwartz et al 1998) has cautioned that citrus-based substances may interfere with some assays. However, Talge and colleagues (Talge et al 2005) recently completed a series of experiments showing that, when used consistently within a sample, the introduction of this oral stimulant does not affect the rank ordering of participants and does not compromise the quality of the salivary cortisol data. In our experience, these stimulants are important for maximizing compliance in children and have been previously used successfully in multiple laboratories (Schmidt et al 1997, Tout et al 1998).
The saliva absorbed by the cotton was squeezed into a cryogenic tube with a needleless syringe. The procedure was repeated, if necessary, until at least 500 µl were collected. Saliva samples were then frozen. Collection took place within 30 min of waking over the course of three consecutive days. Parents kept a written log of wake-up and sampling times. The three frozen saliva samples were then returned to the laboratory at a subsequent visit. Similar procedures have been previously used (Gunnar et al 1989, Schmidt et al 1997) with good results.
The saliva samples were assayed by the Clinical Neuroendocrinology Branch of the National Institute of Mental Health, Bethesda, MD. Samples were thawed, vortexed, and centrifuged for 30 min at 2,250 × g. Concentrations were determined using a solid phase radioimmunoassay (125I) (Coat-A-Count, Diagnostic Products Corporation, Los Angeles, CA), using 200 µl of saliva. Samples incubated for three hours at room temperature. Following aspiration, tubes were counted using an ICN Micromedic Systems, Apex Automatic Gamma Counter. Both samples and standards were determined in duplicate. Samples were performed simultaneously in order to eliminate interassay variability. The lower detection limit assay was 0.1 µg/dl.
A single composite measure of mean morning cortisol level (expressed in µg/dl) was computed by averaging across all useable morning samples. The average collection time (see Table 1) was 7:53 am and reliable data were available for 111 four-year-olds (51 males).
Table 1.
Cortisol sample collection times. Samples were collected within 30 minutes of rising for three consecutive mornings. Table A presents the data for the mean collection times for the three samples. Table B presents the elapsed time between the earliest and latest cortisol sample for each child.
| A. Mean collection times | ||||
|---|---|---|---|---|
| Mean | Minimum | Maximum | SD | |
| Overall | 7:53 am | 5:53 am | 10:10 am | 0:48 |
| Boys | 7:45 am | 5:53 am | 10:10 am | 0:54 |
| Girls | 8:04 am | 6:46 am | 9:15 am | 0:37 |
| B. Elapsed collection times | ||||
|---|---|---|---|---|
| Mean | Minimum | Maximum | SD | |
| Overall | 0:31 | 0:20 | 0:50 | 0:06 |
| Boys | 0:30 | 0:20 | 0:45 | 0:05 |
| Girls | 0:32 | 0:20 | 0:50 | 0:07 |
p<0.10
p<0.05
p<0.01
p<0.10
p<0.05
p<0.01
Two children (both male) were removed from analysis due to extreme scores (Z’s > 5). As such, the analyses reported below were completed with a sample of 81 children (39 male).
Initial analyses examined the range of collection times for the cortisol samples in relation to the measures of interest. There were no significant gender differences for mean collection time and mean time range, t’s <1.05, p’s > 0.30. In addition, there were no relations between collection time and mean time range and the other measures of interest, r’s < 0.18, p’s > 0.30. For the regression analyses presented below, collection data were included as predictors. However, there were no significant findings and will therefore not be presented here.
Maternal Reports of Behavioral Maladjustment
Maternal reports of the children’s behavioral adjustment were assessed using the Child Behavior Checklist (CBCL; Achenbach 1991) at age four. The CBCL is a 113-item checklist in which parents use a three-point scale to rate how descriptive a series of behavior problems are of their own child. Information concerning the reliability and validity of the CBCL can be found in Achenbach (1991). The CBCL yields eight narrow-band factors: social withdrawal, somatic problems, anxiety/depression, social problems, thought problems, attention problems, delinquency, and aggressive behavior. These factors can be further reduced to two broadband factors, internalizing and externalizing behavior problems. Data were available from 112 children.
All analyses relied on standardized T-scores for internalizing (mean=46.96, SD=8.44) and externalizing (mean=50.51, SD=8.35) problems. Initial analyses found that ten children met the threshold cutoffs (i.e., T’s greater than or equal to 60) for internalizing problems. Of these, nine were boys, χ2=7.50, p = 0.01. For externalizing problems, again 10 children met the threshold cutoff. However, here they were evenly divided between boys and girls, χ2=0.002, p = 0.97.
Laboratory Observations of Social Behavior
As part of the larger experimental battery, the children also participated in a group play session with three unfamiliar, same sex, same age peers. Each quartet consisted of one socially inhibited child, one non-inhibited child, and two average children. The children were assigned to quartets based on levels of behavioral inhibition noted at 24 months of age.
The four children were led into a playroom where several age-appropriate toys were accessible. The visit was split into several episodes, a complete description of which may be found in Fox et al. (1995). Behaviors were coded with Rubin’s (1989) Play Observation Scale (POS), scoring 10-second intervals for social participation and the cognitive quality of play. Three independent observers, with reliability greater than 0.8, coded the POS. For purposes of this study, data from two 15-minute free play sessions were used to calculate an index of reticent (the sum of onlooking and unoccupied behavior; Coplan et al 1994) and solitary active (solitary-functional and solitary dramatic play; Coplan et al 2001a) behavior. As expected, reticence behaviors (mean=0.163, SD=0.133) were more evident in this sample than solitary active behaviors (mean=0.066, SD=0.084). While boys and girls did not differ in their levels of reticence, t(79) = 0.586, p = 0.56, boys were more likely to display solitary active behavior, t(79) = 5.70, p < 0.001.
Composite Measures
In order to create a stable measure of adjustment at age 4, we created two composite scores bringing together maternal report and laboratory observations. Standardized laboratory reticence scores and standardized CBCL internalizing T scores were meaned to create a ‘Withdrawal’ score. In parallel, standardized laboratory scores of solitary active behavior and standardized CBCL externalizing T scores were meaned to create an ‘Acting Out’ measure.
RESULTS
Preliminary Data Analysis
Simple correlations were conducted to examine the relations between the four central measures of the study (Table 2). Withdrawal was significantly correlated with both negative affect, r(81) = 0.29, p = 0.01, and home cortisol, r(81) = 0.30, p = 0.01. The two measures were not significantly correlated with Acting Out, r’s < 0.07. In addition, there was a trend linking Withdrawal and Acting Out, r(81) = 0.19, p = 0.08.
Table 2.
Zero-order correlations between negative affect, home cortisol levels, and maladjustment composite scores. Negative affect was measured at nine months of age, while the other measures were each collected at four years of age. N's = 81.
| Negative Affect | Cortisol | Withdrawal | Acting Out | |
|---|---|---|---|---|
| Negative Affect | 1.00 | |||
| Cortisol | 0.116 | 1.00 | ||
| Withdrawal | 0.287** | 0.300** | 1.00 | |
| Acting Out | 0.067 | 0.074 | 0.194+ | 1.00 |
p<0.10
p<0.05
p<0.01
Initial analyses found no gender-linked differences in negative affect at 9 months, cortisol levels at age 4, and Withdrawal scores, t’s < 1.45, p’s > 0.15 (Table 3). In contrast, boys had significantly higher scores on the Acting Out measure, t(79) = 4.89, p < 0.001.
Table 3.
Cortisol levels, temperament measures, and maladjustment scores for boys and girls in the sample. Negative affect was measured at nine months of age, while the other measures were each collected at four years of age. Standard deviations are noted in parentheses.
| Overall | Boys | Girls | |
|---|---|---|---|
| Negative Affect | 6.29 (1.38) |
6.52 (1.28) |
6.08 (1.45) |
| Basal Cortisol | 0.41 (0.18) |
0.43 (0.17) |
0.39 (0.18) |
| Withdrawal | −0.05 (0.80) |
0.06 (0.94) |
−0.15 (0.63) |
| Acting Out | 0.00 (0.68) |
0.34** (0.66) |
−0.31** (0.54) |
p<0.10
p<0.05
p<0.01
Longitudinal Analyses
Two hierarchical multiple regression analyses were conducted to examine the full model. Regressions were run using data at age nine months (negative affect) and age four (cortisol), along with gender, to predict behavioral maladjustment (Withdrawal and Acting Out) at age 4.
For each analysis, the predictors were entered into the regression equation in the following order: (1) negative affect at nine months, (2) home cortisol levels, (3) gender, (4) negative affect X home cortisol levels, (5) negative affect X gender, (6) home cortisol X gender, (7) negative affect X home cortisol X gender. The dependent measures were (a) Withdrawal and (b) Acting Out. Predictive measures were mean centered before use in the regressions. The results of the hierarchical regression analyses are presented in Table 4.
Table 4.
Predicting Withdrawal and Acting Out scores at age four using measures of infant temperament (negative affect at 9 months), concurrent cortisol levels, and gender.
| Withdrawal | Acting Out | ||||||
|---|---|---|---|---|---|---|---|
| Predictor | β | ΔR2 | ΔF | Predictor | β | ΔR2 | ΔF |
| Negative Affect | 0.92** | 0.09 | 7.95** | Negative Affect | −0.35 | 0.00 | 0.33 |
| Cortisol | 1.47** | 0.05 | 4.25* | Cortisol | −0.01 | 0.00 | 0.28 |
| Gender | −0.02 | 0.00 | 0.02 | Gender | −0.49** | 0.23 | 23.34** |
| Negative Affect X Cortisol | 1.03* | 0.14 | 15.01** | Negative Affect X Cortisol | 0.17 | 0.00 | 0.01 |
| Negative Affect X Gender | −0.69* | 0.04 | 4.17* | Negative Affect X Gender | 0.35 | 0.01 | 0.91 |
| Cortisol X Gender | −0.82* | 0.03 | 3.59+ | Cortisol X Gender | 0.01 | 0.00 | 0.02 |
| Negative Affect X Cortisol X Gender | −0.47 | 0.01 | 1.62 | Negative Affect X Cortisol X Gender | −0.11 | 0.00 | 0.08 |
| F(7,73)=5.97, p<0.001 | F(7,73)=3.46, p<0.001 | ||||||
Note: Standardized β are presented.
p<0.10
p<0.05
p<0.01
Withdrawal
When predicting Withdrawal behavior at age four, the full model accounted for 36.1% of the total variance, F(7,73) = 5.97, p < 0.001. Negative affect significantly predicted Withdrawal, accounting for 9.0% of the variance, ΔF(1,73) = 7.95, p = 0.006. The main effect of home cortisol levels was also significant, accounting for 4.6% of the overall variance, ΔF(1,73) = 4.25, p = 0.04. These reflected the significant zero-order correlations noted above. The main effect of gender was not significant.
The interaction between negative affect and cortisol levels accounted for an additional 14.1% of the variance in Withdrawal, ΔF(1,73) = 15.01, p < 0.001. In order to interpret this interaction, the children were divided into two groups based on negative affect at nine months and the correlations between the concurrent cortisol levels and Withdrawal were then calculated separately for each group. For the children high in negative affect, there was a significant positive correlation between cortisol and Withdrawal, r(39) = 0.44, p < 0.01. In contrast, the children low in negative affect showed no relation, r(42) = 0.20, p = 0.22.
The interaction between negative affect and gender also accounted for 3.8% of the variance, ΔF(1,73) = 4.17, p = 0.05. To examine this finding, separate zero-order correlations between negative affect and Withdrawal were calculated for boys and girls. Boys showed a significant positive relation, r(39) = 0.46, p < 0.01, while girls showed no relation, r(42) = 0.15, p = 0.33.
There was an additional trend for an interaction between home cortisol levels and gender, accounting for 3.1% of the variance, ΔF(1,73) = 3.59, p = 0.06. As in the previous analyses, separate zero-order correlations found a significant relation between cortisol and Withdrawal for boys, r(39) = 0.55, p < 0.01, but not girls, r(42) = 0.03, p = 0.84.
Exploratory Analyses
Although the three-way interaction between negative affect, cortisol, and gender was not significant, ΔF(1,73) = 1.64, p = 0.21, our a priori hypotheses led us to further explore gender-linked differences in the relations of interest. Two separate regressions were completed for boys and girls using the following predictors: (1) negative affect at nine months, (2) home cortisol levels at age 4, and (3) negative affect X home cortisol levels (see Table 5).
Table 5.
Predicting Withdrawal scores at age four as a function of negative affect at nine months and cortisol levels at age 4. Analyses were conducted separately for boys and girls.
| Boys | Girls | ||||||
|---|---|---|---|---|---|---|---|
| Predictor | β | ΔR2 | ΔF | Predictor | β | ΔR2 | ΔF |
| Negative Affect | 0.33* | 0.18 | 8.57** | Negative Affect | 0.18 | 0.02 | 0.96 |
| Cortisol | 0.93** | 0.10 | 5.14* | Cortisol | 0.05 | 0.00 | 0.03 |
| Negative Affect X Cortisol | 0.81** | 0.23 | 16.92** | Negative Affect X Cortisol | 0.18 | 0.03 | 1.16 |
| F(3,35)=12.62, p<0.001 | F(3,38)=0.71, p=0.55 | ||||||
Note: Standardized β are presented.
p<0.10
p<0.05
p<0.01
When predicting Withdrawal in boys, the full model accounted for 51.3% of the variance, F(3,35) = 12.62, p < 0.001. Both the main effects of negative affect, ΔF(1,35) = 8.57, p < 0.01, 18.4% of variance, and home cortisol, ΔF(1,35) = 5.14, p = 0.03, 10.0% of variance, were significant. In addition, there was a significant interaction between negative affect and cortisol, ΔF(1,35) = 16.92, p < 0.001, 22.9% of variance.
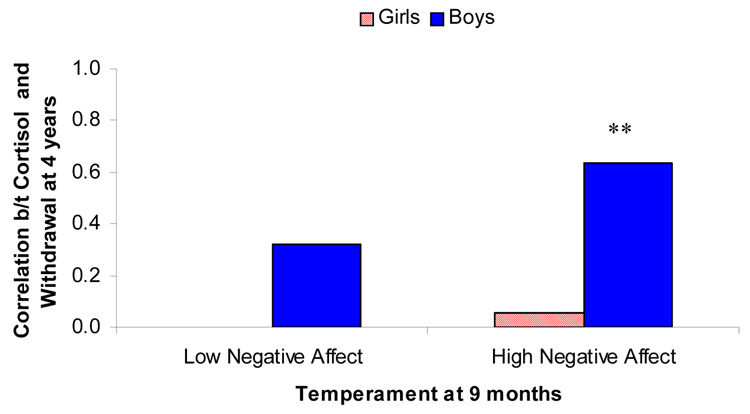
To examine this interaction, children were placed into two groups based on levels of negative affect in infancy (Figure 1). Simple correlations found that for boys high in early negative affect, home cortisol levels positively correlated with Withdrawal at age 4, r(24) = 0.63, p = 0.001. Boys with low levels of negative affect showed no relation, r(15) = 0.32, p = 0.24.
Figure 1.
Correlations between cortisol levels and Withdrawal as a function of infant temperament. The data are presented separately for boys and girls. (**p<0.01).
The equivalent analysis with girls found a non-significant model, F(3,38) = 0.71, p = 0.55, accounting for only 5.3% of the variance.
Acting Out
When predicting Acting Out, the full model accounted for 24.6% of the total variance, F(7,73) = 3.46, p < 0.001. This was driven entirely by the main effect of gender, ΔF(1,73) = 23.34, p < 0.001, which accounted for 22.9% of variance. This reflected the higher Acting Out scores in boys (0.34) than girls (−0.31), t(79) = 4.89, p < 0.001.
DISCUSSION
The current study assessed the relations between infant temperament, gender, and basal cortisol levels in shaping internalizing and externalizing forms of behavioral maladjustment in 4-year-old children. The data indicated that while gender-linked differences were evident for both outcome measures, the underlying mechanisms were quite distinct. As expected, infant temperament was linked to later Withdrawal. However, the relation was carried by children who also showed high levels of basal cortisol at age 4. Exploratory analyses also indicated that the mechanism was only evident in boys. In contrast, analyses using the Acting Out measure found only a main effect of gender, with boys showing more difficulties than girls.
The ability to effectively regulate cortisol levels to meet environmental and internal challenges is of great importance given that both chronic hypo- and hyper-reactivity have been linked to behavioral maladjustment (Gunnar & Donzella 2002), reflecting cortisol’s role as a central nervous system regulator, shaping basic mechanisms of arousal, attention, perception, and memory (Erickson et al 2003).
The current findings suggested that elevated cortisol may help sustain early-appearing biases from infancy into early childhood. This is in line with Gunnar’s (1999) contention that cortisol production is rooted in the child’s ability to cope with life stressors. A failure in the overall coping mechanism can, therefore, be displayed in a variety of ways, depending on individual, environmental, and experiential factors.
The accumulating data suggest that early temperamental biases, when coupled with a psychophysiological marker (cortisol in the current study, EEG asymmetry in Henderson et al 2001), will shape later development. Recent data indicating that differences in neural functioning can be detected decades after initial observations of temperamental inhibition (Guyer et al 2006, Pérez-Edgar et al 2007, Schwartz et al 2003) serve to underscore the stability and importance of these biobehavioral mechanisms.
The current study joins recent work (Hatzinger et al 2007, Shirtcliff et al 2005, Smider et al 2002, Tout et al 1998) examining potential gender-linked differences in the relations between cortisol levels and behavior. In the current sample, boys were at increased risk for both forms of behavioral maladjustment. This may in part reflect the high levels of co-morbidity often found in early childhood (e.g., Essex et al 2002). In addition, the children in this study were part of a selected sample at risk for behavioral inhibition (Fox et al 2001, Fox et al 1995). In these samples, boys often show increased temperamental stability and poorer outcomes. These data were therefore in line with other studies noting that the relation between cortisol and internalizing behaviors is most evident in selected or at-risk samples (e.g., Cicchetti & Rogosch 2001, Shirtcliff et al 2005). These data further illustrated the specificity of these findings in that the underlying mechanisms implicated in the overall patterns of behavior were quite distinct between Withdrawal and Acting Out.
The EEG asymmetry findings from Henderson et al (2001) suggest that the current data reflect a broad biobehavioral system at work. In that study, EEG asymmetry and negative affect at nine months predicted social withdrawal at age four only in boys. Building on these data, recent work (Martin McDermott, Pérez-Edgar, Henderson, Pine, & Fox, under review) finds an analogous pattern with the error-related negativity (ERN), a marker for error detection and performance monitoring, in adolescents who were behaviorally inhibited as young children. Here, a large ERN predicted clinical anxiety levels only in boys who were inhibited as children. Neither Henderson et al (2001), Martin McDermott et al (under review), nor the current study found a significant main effect of gender for any of the central psychophysiological measures (EEG Asymmetry, ERN, basal cortisol). This suggests that the findings were not an artifact of undifferentiated levels of reactivity in boys and girls, but rather reflected unique patterns of functioning linking biology to behavior.
The current study’s limitations should be noted when reviewing the findings. First, the current study was unable to address potential differences in the diurnal pattern of cortisol. While morning levels of cortisol were linked to differing patterns of maladjustment as a function of gender, we could not say if this measure is part of a larger pattern of hormonal secretion that may go beyond the relations noted here (Goodyer et al 1996).
Second, collection times for the cortisol samples varied among participants. Given the cyclical nature of cortisol secretions, there was a risk that this affected the findings presented here. Analyses, however, found no differences linked to cortisol levels, gender, or behavior problems. Future work would benefit from a more tightly regulated collection schedule.
Third, assessing cortisol in both early childhood and infancy may be of particular importance when considering long term adjustment given that stress early in life, such as maltreatment and abuse in the extreme, may alter the set point for the stress response well into adulthood (Heim et al 2001, King et al 2001). While there are some indications that individual patterns in cortisol levels may be evident as early as six months of age (Lewis & Ramsay 1995a, Lewis & Ramsay 1995b), concurrent cortisol measures in infancy would help extend the observed data.
The current study spanned a time period marked by important changes in the functioning and regulation of cortisol. Shirtcliff et al (2005) noted that most studies focus on children ages six to 12 (e.g. Smider et al 2002) due to the fact that major behavioral difficulties often first emerge at this time (Graber & Brooks-Gunn 1996). The functional and developmental significance of this system is closely tied to the context under which it is observed.
As such, rather than serving as an independent test of successful adaptation, cortisol may better serve our empirical and theoretical concerns when placed within a larger developmental profile that takes into account individual variations in biology, environment, and behavior. For example, previous studies have found that the cortisol wakening response and evening cortisol levels reflect independent characteristics of functioning (Netherton et al 2004, Rosmalen et al 2005), potentially leading to inconsistent findings across studies that employ different methodologies, populations, and measures. Work of this nature must take into account the (1) initiating stimulus, (2) the objective and subjective components of stimulus processing, and (3) the resulting response, which may incorporate both physiology and behavior. As such, it is difficult to a priori define and quantify the “load” or “burden” cortisol levels place on the individual (Levine & Ursin 1991, Steptoe 2000).
Indeed, the findings from the current study may be best understood in the context of a larger pattern of research showing a consistent link between underlying biological mechanisms (e.g., cortisol levels, frontal EEG asymmetry, limbic reactivity) and broad psychological and behavioral profiles (e.g., temperament, anxiety, externalizing difficulties) that is moderated by a number of factors that include an individual’s gender, environment, and genetic profile. In this sense, cortisol is one mechanism within a larger web of interconnected systems that may act independently or in tandem to shape developmental trajectories.
Acknowledgements
The authors would like to thank Kenneth H. Rubin and Amy Kennedy for the coding and analysis of the peer interaction data at age four. We would also like to thank Stacey Barton, Ariana Shahinfar, Genevieve Erb, Patricia Peters, Shari K. Young, Lisa Perry for their assistance in the longitudinal data collection. We would especially like to thank the parents of the children who participated and continue to participate in our studies. Funding for the study was provided by a grant from the John D. and Catherine T. MacArthur Foundation and NICHD grants (HD32666; HD17899) to Nathan A. Fox. Manuscript preparation was made possible by an NIMH grant (MH073569) to Koraly Pérez-Edgar.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Achenbach TM. Manual for Child Behavior Checklist/4-18 and 1991 Profile. Burlington, VT: University of Vermont, Department of Psychiatry; 1991. [Google Scholar]
- Asendorpf J. Beyond social withdrawal:Shyness, unsociability, and peer avoidance. Human Development. 1990;33:250–259. [Google Scholar]
- Biederman J, Hirshfeld-Becker DR, Rosenbaum JF, Herot C, Friedman D, et al. Further evidence of association between behavioral inhibition and social anxiety in children. American Journal of Psychiatry. 2001;158:1673–1679. doi: 10.1176/appi.ajp.158.10.1673. [DOI] [PubMed] [Google Scholar]
- Buss AH, Plomin R. Temperament:Early developing personality traits. Hillsdale, NJ: Erlbaum; 1984. [Google Scholar]
- Buss KA, Malmastadt Schumacher JR, Dolski I, Kalin NH, Goldsmith HH, Davidson RJ. Right frontal brain activity, cortisol, and withdrawal behavior in 6-month-old infants. Behavioral Neuroscience. 2003;117:11–20. doi: 10.1037//0735-7044.117.1.11. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Fox NA, Marshall TR. Behavioral and physiological antecedents of inhibited and uninhibited behavior. Child Development. 1996;67:523–540. [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. The impact of child maltreatment and psychopathology on neuroendocrine functioning. Development and Psychopathology. 2001;13:783–804. [PubMed] [Google Scholar]
- Coplan R. Assessing nonsocial play in early childhood. In: Gitlin-Weiner K, Sandgrund A, Schaefer C, editors. Play diagnosis and assessment. New York: Wiley; 2000. pp. 563–598. [Google Scholar]
- Coplan R, Gavinski-Molina M-H, Lagace-Seguin D, Wichmann C. When girls versus boys play alone: Nonsocial play and adjustment in kindergarten. Developmental Psychology. 2001a;37:464–474. doi: 10.1037//0012-1649.37.4.464. [DOI] [PubMed] [Google Scholar]
- Coplan R, Rubin KH. Exploring and assessing nonsocial play in preschool: The development and validation of the Preschool Play Behavior Scale. Social Development. 1998;7:72–91. [Google Scholar]
- Coplan R, Wichmann C, Lagace-Seguin D. Solitary-active play behavior: A marker variable for maladjustment in preschool? Journal of Research in Childhood Education. 2001b;15:164–172. [Google Scholar]
- Coplan RJ, Rubin KH, Fox NA, Calkins SD, Stewart SL. Being alone, playing alone, and acting alone: distinguishing among reticence and passive and active solitude in young children. Child Development. 1994;65:129–137. [PubMed] [Google Scholar]
- Crockenberg S, Smith P. Antecedents of mother-infant interaction and infant irritability in the first three months of life. Infant Behavior & Development. 1982;5:105–119. [Google Scholar]
- Degnan K, Fox NA. Behavioral inhibition and anxiety disorders: Multiple levels of a resilience process. Development and Psychopathology. 2007;19:729–746. doi: 10.1017/S0954579407000363. [DOI] [PubMed] [Google Scholar]
- Erickson K, Drevets W, Schulkin J. Glucocorticoid regulation of diverse cognitive functions in normal and pathological emotional states. Neuroscience and Biobehavioral Reviews. 2003;27:233–246. doi: 10.1016/s0149-7634(03)00033-2. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Klein MH, Cho E, Kalin NH. Maternal stress beginning in infancy may sensitize children to later stress exposure: Effects on cortisol and behavior. Society of Biological Psychiatry. 2002;52:776–784. doi: 10.1016/s0006-3223(02)01553-6. [DOI] [PubMed] [Google Scholar]
- Fagan J. The interaction between child sex and temperament in predicting behavior problems of preschool-age children in day care. Early Child Development and Care. 1990;59:1–9. [Google Scholar]
- Fox NA, Hane AA, Perez-Edgar K. Psychophysiological methods for the study of developmental psychopathology. In: Cicchetti D, Cohen DJ, editors. Developmental Psychopathology. Hoboken, NJ: John Wiley & Sons; 2006. pp. 381–426. [Google Scholar]
- Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: linking biology and behavior within a developmental framework. Annual Review Of Psychology. 2005;56:235–262. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: Psychophysiological and behavioral influences across the first four years of life. Child Development. 2001;72:1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Fox NA, Rubin KH, Calkins SD, Marshall TR, Coplan RJ, et al. Frontal activation asymmetry and social competence at four years of age. Child Development. 1995;66:1771–1784. [PubMed] [Google Scholar]
- Fox NA, Schmidt LA, Calkins SD, Rubin KH, Coplan RJ. The role of frontal activation in the regulation and dysregulation of social behavior during the preschool years. Development and Psychopathology. 1996;8:89–102. [Google Scholar]
- Goodyer I, Herbert J, Althman P, Pearson J, Secher S, Shiers H. Adrenal secretion during major depression in 8- to 16-year-olds, I: Altered diurnal rhythms in salivary cortisol and dehydroepiandrosterone (DHEA) at presentation. Psychological Medicine. 1996;26:245–256. doi: 10.1017/s0033291700034644. [DOI] [PubMed] [Google Scholar]
- Graber J, Brooks-Gunn J. Transitions and turning points: Navigating the passage from childhood through adolescence. Developmental Psychology. 1996;32:768–776. [Google Scholar]
- Granger D, Stansbury K, Henker B. Preschoolers’ behavioral and neuroendocrine responses to social challenge. Merrill-Palmer Quarterly. 1994;40:190–211. [Google Scholar]
- Granger DA, Serbin LA, Schwartzman AE, Lehoux P, Cooperman J, Ikeda S. Children's salivary cortisol, internalising behaviour problems, and family environment: Results from the Concordia longitudinal risk project. International Journal of Behavioral Development. 1998;22:707–728. [Google Scholar]
- Gunnar M. The role of glucocorticoids in anxiety disorders: A critical analysis. In: Vasey MW, Dadds MR, editors. The developmental psychopathology of anxiety. New York: Oxford University Press; 2001. pp. 143–159. [Google Scholar]
- Gunnar M, Mangelsdorf S, Larson M, Hertsgaard L. Attachment, temperament, and adrenocortical activity in infancy: A study of psychoendocrine regulation. Developmental Psychobiology. 1989;25:355–363. [Google Scholar]
- Gunnar M, Tout K, de Haan M, Pierce S, Stansbury K. Temperament, social competence, and adrenocortical activity in preschoolers. Developmental Psychobiology. 1997;31:65–85. doi: 10.1002/(sici)1098-2302(199707)31:1<65::aid-dev6>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Gunnar MR. Psychoendocrine studies of temperament and stress in early childhood: Expanding current models. In: Wachs JEBTD, editor. Temperament: Individual differences at the interface of biology and behavior. Washington D.C.: American Psychological Association; 1999. [Google Scholar]
- Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27:199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Nelson EE, Perez-Edgar K, Hardin MG, Roberson-Nay R, et al. Striatal function alteration in adolescents characterized by early childhood behavioral inhibition. Journal of Neuroscience. 2006;26:6399–6405. doi: 10.1523/JNEUROSCI.0666-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzinger M, Brand S, Perren S, von Wyl A, von Klitzing K, Holsboer-Traschler E. Hypothalamic-pituitary-adrenal (HPA) activity in kindergarten children: Importance of gender and associations with behavioral/emotional difficulties. Journal of Psychiatric Research. 2007;41:861–870. doi: 10.1016/j.jpsychires.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport D, Bonsall R, Miller A, Nemeroff C. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. American Journal of Psychiatry. 2001;158:575–581. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- Henderson HA, Fox NA, Rubin KH. Temperamental contributions to social behavior: The moderating roles of frontal EEG asymmetry and gender. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:68–74. doi: 10.1097/00004583-200101000-00018. [DOI] [PubMed] [Google Scholar]
- Henderson HA, Marshall PJ, Fox NA, Rubin KH. Psychophysiological and behavioral evidence for varying forms and functions of nonsocial behavior in preschoolers. Child Development. 2004;75:236–250. doi: 10.1111/j.1467-8624.2004.00667.x. [DOI] [PubMed] [Google Scholar]
- Hertsgaard L, Gunnar M, Larson M, Brodersen L, Lehman H. First time experiences in infancy: When they appear pleasant, do they activate adrenocortical stress response? Developmental Psychobiology. 1992;25:319–334. doi: 10.1002/dev.420250503. [DOI] [PubMed] [Google Scholar]
- Jackson E, Payne J, Nadel L, Jacobs W. Stress differentially modulates fear conditioning in healthy men and women. Biological Psychiatry. 2006;59:516–522. doi: 10.1016/j.biopsych.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Kagan J, Reznick J, Snidman N. Biological bases of childhood shyness. Science. 1988a;240:167–171. doi: 10.1126/science.3353713. [DOI] [PubMed] [Google Scholar]
- Kagan J, Reznick JS, Gibbons J. Inhibited and uninhibited types of children. Child Development. 1989;60:838–845. [PubMed] [Google Scholar]
- Kagan J, Reznick JS, Snidman N. The physiology and psychology of behavioral inhibition in children. Annual Progress in Child Psychiatry & Child Development. 1988b:102–127. [PubMed] [Google Scholar]
- Kagan J, Snidman N. Infant predictors of inhibited and uninhibited profiles. Psychological Science. 1991;2:40–44. [Google Scholar]
- King J, Mandansky D, King S, Fletcher K. Early sexual abuse and low cortisol. Psychiatry and Clinical Neurosciences. 2001;55:71–74. doi: 10.1046/j.1440-1819.2001.00787.x. [DOI] [PubMed] [Google Scholar]
- Legendre A, Trudel M. Cortisol and behavioral responses of young children coping with a group of unfamiliar peers. Merrill-Palmer Quarterly. 1996;42:554–577. [Google Scholar]
- Levine S, Ursin H. What is stress? In: Brown M, Koob G, Rivier C, editors. Stress Neurobiology and Neuroendocrinology. New York: Marcel Dekker; 1991. pp. 3–21. [Google Scholar]
- Lewis M, Ramsay D. Developmental change in infants' responses to stress. Child Development. 1995a;66:657–670. doi: 10.1111/j.1467-8624.1995.tb00896.x. [DOI] [PubMed] [Google Scholar]
- Lewis M, Ramsay D. Stability and change in cortisol and behavioral response to stress during the first 18 months of life. Developmental Psychobiology. 1995b;28:419–428. doi: 10.1002/dev.420280804. [DOI] [PubMed] [Google Scholar]
- McBurnett K, Lahey B, Frick P, Risch C, Loeber R, et al. Anxiety, inhibition, and conduct disorder in children: II. Relation to salivary cortisol. Journal of the American Academy of Child and Adolescent Psychiatry. 1991;30:192–196. doi: 10.1097/00004583-199103000-00005. [DOI] [PubMed] [Google Scholar]
- Morison P, Masten AS. Peer reputation in middle childhood as a predictor of adaptation in adolescence: A seven-year follow-up. Child Development. 1991;62:991–1007. doi: 10.1111/j.1467-8624.1991.tb01585.x. [DOI] [PubMed] [Google Scholar]
- Netherton C, Goodyer I, Tamplin A. Salivary cortisol and dehydroepiandrosterone in relation to puberty and gender. Psychoneuroendocrinology. 2004;29 doi: 10.1016/s0306-4530(02)00150-6. [DOI] [PubMed] [Google Scholar]
- O’Connor T, Ben-Shlomo Y, Heron J, Golding J, Adams DVG. Prenatal anxiety predicts individual differences in cortisol in pre-adolescent children. Biological Psychiatry. 2005;58:211–217. doi: 10.1016/j.biopsych.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Pérez-Edgar K, Fox NA. Temperament and anxiety disorders. Child and Adolescent Psychiatric Clinics of North America. 2005;14:681–706. doi: 10.1016/j.chc.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Pérez-Edgar K, Roberson-Nay R, Hardin MG, Poeth K, Guyer AE, et al. Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. NeuroImage. 2007;35:1538–1546. doi: 10.1016/j.neuroimage.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke-Yarrow M, Richters J, Wilson W. Child development in a network of relationships. In: Hinde R, Stevenson-Hinde J, editors. Relationships within families: Mutual influences. Oxford, England: Clarendon Press; 1988. [Google Scholar]
- Rosen J, Schulkin J. From normal fear to pathological anxiety. Psychological Review. 1998;105:325–350. doi: 10.1037/0033-295x.105.2.325. [DOI] [PubMed] [Google Scholar]
- Rosmalen JGM, Oldehinkel AJ, Ormel J, de Winter AF, Buitelaar JK, Verhulst FC. Determinants of salivary cortisol levels in 10–12 year old children; A population-based study of individual differences. Psychoneuroendocrinology. 2005;30:483–495. doi: 10.1016/j.psyneuen.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Rothbart MK. Measurement of temperament in infancy. Child Development. 1981;52:569–578. [Google Scholar]
- Rubin K, Chen X, Hymel S. Socioemotional characteristics of withdrawn and aggressive children. Merrill-Palmer Quarterly. 1993;39:518–534. [Google Scholar]
- Rubin KH. The Play Observation Scale (POS) University of Waterloo; 1989. [Google Scholar]
- Sadker M, Sadker D. Failing at fairness: How America's schools cheat girls. New York: Scribner; 1994. [Google Scholar]
- Schmidt LA, Fox NA, Rubin KH, Sternberg EM, Gold PW, et al. Behavioral and neuroendocrine responses in shy children. Developmental Psychobiology. 1997;30:127–140. doi: 10.1002/(sici)1098-2302(199703)30:2<127::aid-dev4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Schmidt LA, Fox NA, Schulkin J, Gold PW. Behavioral and psychophysiological correlates of self-presentation in temperamentally shy children. Developmental Psychobiology. 1999;35:119–135. [PubMed] [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagan J, Rauch SL. Inhibited and uninhibited infants "grown up": Adult amygdalar response to novelty. Science. 2003;300:1952–1953. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- Schwartz D, Granger D, Susman E, Gunnar M, Laird B. Assessing salivary cortisol in studies of child development. Child Development. 1998;69:1503–1513. [PubMed] [Google Scholar]
- Shirtcliff E, Granger DA, Booth A, Johnson D. Low salivary cortisol levels and externalizing behavior problems: A latent state trait model in normally developing youth. Development and Psychopathology. 2005;17:167–184. doi: 10.1017/s0954579405050091. [DOI] [PubMed] [Google Scholar]
- Smider NA, Essex MJ, Kalin NH, Buss KA, Klein MH, et al. Salivary cortisol as a predictor of socioemotional adjustment during kindergarten: A prospective study. Child Development. 2002;73:75–92. doi: 10.1111/1467-8624.00393. [DOI] [PubMed] [Google Scholar]
- Steptoe A. Stress effects, overview. In: Fink G, editor. Encyclopedia of Stress. San Diego: 2000. pp. 510–511. [Google Scholar]
- Stevenson-Hinde J, Glover A. Shy girls and boys: A new look. Journal of Child Psychology and Psychiatry. 1996;37:181–187. doi: 10.1111/j.1469-7610.1996.tb01389.x. [DOI] [PubMed] [Google Scholar]
- Talge N, Donzella B, Kryzer E, Gierens A, Gunnar M. It's not that bad: Error introduced by oral stimulants in salivary cortisol research. Developmental Psychobiology. 2005;47:369–376. doi: 10.1002/dev.20097. [DOI] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, et al. Amygdala response to fearful faces in anxious and depressed children. Archives of General Psychiatry. 2001a;58:1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Whalen PJ, Eccard CH, Dahl RE, et al. Amygdala response to facial expressions in children and adults. Society of Biological Psychiatry. 2001b;49:309–316. doi: 10.1016/s0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- Tout K, de Haan M, Campbell EK, Gunnar MR. Social behavior correlates of cortisol activity in child care: Gender differences and time-of-day effects. Child Development. 1998;69:1247–1262. [PubMed] [Google Scholar]
- van Goozen S, Matthys W, Cohen-Kettenis P, Gispen-de Wiend C, Wiegant V, van Engeland H. Salivary cortisol and cardiovascular activity during stress in oppositional-defiant disorder boys and normal controls. Biological Psychiatry. 1998;43:531–539. doi: 10.1016/S0006-3223(97)00253-9. [DOI] [PubMed] [Google Scholar]
- Watamura S, Sebanc A, Gunnar M. Rising cortisol at childcare: Relations with nap, rest, and temperament. Developmental Psychobiology. 2001;40:33–42. doi: 10.1002/dev.10011. [DOI] [PubMed] [Google Scholar]