Antimitotic Drugs Disrupt the Normal Function of the Mitotic Spindle and Cause Spindle Checkpoint – Mediated Mitotic Arrest
Microtubule inhibitors such as Vinka alkaloids (e.g., vinblastine and vincristine) and Taxans (e.g., taxol/paclitaxel and docetaxel) are potent therapeutic drugs for cancer treatment. The cellular consequences of taxol/paclitaxel treatments over a wide range of doses (1 nmol/L–100 µmol/L) have been reported. Overall, the main target of clinically relevant, low-to-moderate doses of taxol (5–200 nmol/L) is the mitotic spindle (1). In response to treatment with moderate doses of microtubule inhibitor, cells in culture and cells in tumor xenografts in nude mice exhibit mitotic arrest and/or apoptosis (2, 3). Other modes of cell death (e.g., lytic death) have been reported in mice treated with 30 mg/kg of i.v. docetaxel (4). However, the complexities of the cellular microenvironment in whole animals make the assessment of the mode of cell death in living animals somewhat more difficult. Recently, other mitosis-targeting drugs have been developed (e.g., inhibitors of mitotic kinesin motors such as KSP-IA, monastrol, and HR22C16; refs. 5–7). These potential therapeutic agents join the microtubule inhibitors in the general category of antimitotic drugs. Different types of antimitotic drugs are thought to impose different types of stress on kinetochores, which are considered to be a central origin for the generation of a signaling pathway called the mitotic spindle checkpoint or simply the spindle checkpoint. These different types of stress may activate different signaling cascades through the kinetochores, or may emphasize particular branches of checkpoint signaling [discussed in a reviewby Pinsky and Biggins (8)]. Some observations support this view. For example, microtubule-stabilizing drugs (e.g., taxol, epothilone B) lead A549 cells to aneuploidy and/or apoptosis (cells with sub-G1 DNA content) more efficiently than microtubule-destabilizing drugs (e.g., vinblastine, colchicine, nocodazole; ref. 9). Although aspects of signaling may differ among the major classes of antimitotic drugs, these drugs eventually lead cells to temporary or permanent mitotic arrest due to inhibition of the mitotic spindle or the proteins that regulate it.
Mitotic arrest induced by antimitotic drugs through the spindle checkpoint. The spindle checkpoint detects loss or impairment of functional connections between kinetochores and spindle microtubules during mitosis and disseminates signals that inhibit the anaphase-promoting complex/cyclosome. The anaphase-promoting complex/cyclosome is a ubiquitin ligase complex that catalyzes polyubiquitylation of a variety of targets including securin and mitotic cyclins during mitosis. Although the anaphase-promoting complex/cyclosome functions throughout the cell cycle with different activator subunits (Cdh1 and Cdc20), the major phenotype of the inhibition is mitotic delay/arrest. The inhibition of the anaphase-promoting complex/cyclosome causes high cyclin levels, sustained cyclin-dependent kinase 1 (cdk1) activity, and prolonged mitotic arrest. The mitotic arrest allows time for correction of chromosome connections to the spindle (10–12). Studies with cultured cells have suggested that the spindle checkpoint–mediated mitotic arrest may be a requirement for the subsequent cell death induced by the antimitotic drugs (5, 13).
The main scope of this reviewis to discuss the mitotic/postmitotic events and molecular signaling involved in treatments with antimitotic drugs, using information derived primarily from research with cultured cells. We will describe the events that occur during and after mitotic arrest, clarify the notion of cellular sensitivity to mitotic arrest, and discuss the correlation between spindle checkpoint function and antimitotic drug–mediated cell death. These are factors which are important in explaining the molecular basis for the efficacy of antimitotic drugs. Other cell biological effects induced by microtubule inhibitors during interphase prior to mitotic arrest may also be relevant to the induction of cell death. However, these interphase effects have been reviewed by others including Ganansia-Laymarie et al. (14) and Jordan and Wilson (11), and are not discussed here in detail. Mitotic catastrophe associated with DNA damage caused prior to M phase is also not discussed, although spindle checkpoint activation may play a role in the response to DNA damage as well (15–18).
Cellular “Sensitivity” Defined: Five Possible Outcomes of Antimitotic Drug – Mediated Mitotic Arrest
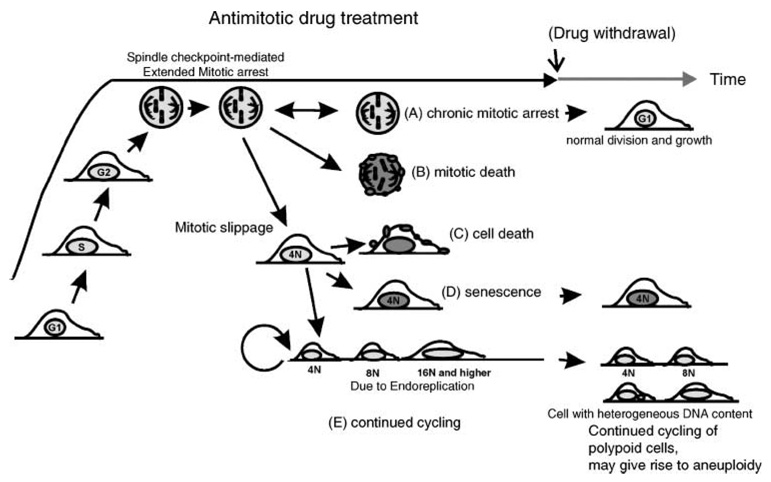
Cultured cells treated with antimitotic drugs transit through G1, S, and G2 cell cycle phases and, possibly after a G2 delay in some cases, eventually enter mitosis, where the cells arrest due to disruption of normal chromosome attachment to the spindle that results in activation of the spindle checkpoint. From the antimitotic drug–mediated, prolonged mitotic arrest, there are five observed outcomes (Fig. 1; ref. 19): the first is (A) chronic mitotic arrest. This is an outcome defined as one that is not committed to other outcomes at the time of observation. Thus, chronic mitotic arrest can eventually lead to other outcomes. Moreover, it is sometimes reversible (i.e., cells can undergo normal division) upon withdrawal of the antimitotic drug. The other outcomes include (B) mitotic death, in which cells directly initiate cell death during mitotic arrest, or (C–E) exit from mitosis to a polyploid condition (sometimes called mitotic slippage or adaptation). Mitotic slippage may or may not result in cell death. The polyploid cells can (C) initiate cell death, (D) remain in a metabolically active state without further cell division (a form of senescence), or (E) continue to divide with an abnormal genome content. Chromosomal instability in cycling polyploid cells may serve as a source for aneuploid cells (20). Such cells may undergo random and spontaneous chromosome loss leading to heterogenic cell populations, similar to the characteristic of many tumors.
Figure 1.
Five possible outcomes of antimitotic drug treatment at the cellular level, derived from cultured cell studies. Outcome A, chronic mitotic arrest (the double-headed arrow indicates that this outcome may give rise to other outcomes or it may lead to normal cell division after withdrawal of the antimitotic drug); outcome B, mitotic death; outcome C, mitotic slippage followed by cell death; outcome D, mitotic slippage followed by senescence; outcome E, continued cycling and endoreplication after mitotic slippage (after withdrawal of drug outcome, E cells may undergo abnormal division, generating heterogeneous populations of cells with varied chromosomal content). Modified from Weaver and Cleveland (19).
Cells showing “sensitivity” in response to antimitotic drugs exhibit cell death [outcomes (B) and (C)], senescence [outcome (D)], or remain alive and perhaps continue to cycle [a case of outcome (A) or (E)]. Understanding the determinant factors influencing the choice of outcome may aid us in designing therapies to bias the outcome in favor of death or senescence of the tumor cells, toward the outcomes (B), (C), or (D). It is likely that many factors affect treatment outcomes, and defining all the signaling pathways involved remains an important aspect of future studies in tumor cell biology. Later, we will describe how one such determining factor, p53 status, may influence the decision toward outcomes (D) and (E).
The Importance of Assays that Describe Sensitivity
How we assess the sensitivity of cells to antimitotic drugs depends strongly on what we measure (the type of assay) and when we measure (the time frame). We can roughly categorize these assays according to two types: short-term assays and long-term assays (Table 1). Short-term assays (usually done within 24–72 h) report the induction of apoptotic markers (e.g., poly-ADP-ribose polymerase fragment production, caspase activation, DNA damage, or a sub-G1 peak in fluorescence-activated cell sorting analysis) or cellular metabolic activity that detects lowered metabolism due to inhibition of cell growth and ongoing cell death [e.g., the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay]. These assays reliably report rapid onset of outcomes (B) and (C), but may miss outcome (D). Assays carried out over a longer time frame (over several days; e.g., cell growth assays such as colony formation or cell number counting) may be more informative in assessing the overall rate of cell death and/or growth retardation but are less frequently carried out. They may also be less informative regarding the mechanisms involved. Preferably to investigate the nature of the sensitivity of cells in culture to antimitotic drugs and to evaluate their implications for tumor therapy, it is desirable to compare data from several types of assays.
Table 1.
Effects of partial inactivation of spindle checkpoint and overexpression of checkpoint proteins on sensitivity to treatment with antimitotic drugs
| Modulation on spindle checkpoint | Effect on spindle checkpoint function | Antimitotic drug tested | Drug sensitivity | Assay* | Assay length (h) | Reference |
|---|---|---|---|---|---|---|
| Cancer cell line variants |
Weakened | Nocodazole | Decreased | A, B | 48 | Masuda et al. (50) |
| Cancer cell lines | Weakened | Nocodazole | Increased | C, E | 48 | Lee et al. (13) |
| varying in BubR1 | Taxol | Increased | C, E | |||
| expression | ||||||
| Human T-cell leukemia | Weakened | Nocodazole | Decreased | F | 24–48 | Kasai et al. (52) |
| virus type I infection | Vincristine | Decreased | F | |||
| (Mad1 inactivation) | ||||||
| BubR1-siRNA | Weakened | Taxol | Decreased | D, F | 48 | |
| Mad2-siRNA | Weakened | Taxol | Decreased | D, F | 48 | Sudo et al. (53) |
| dnBub1 | Weakened | KSP-IA | Decreased | B, C, E | 48 | Tao et al. (5) |
| Taxol | Decreased (mildly) | B, C, E | ||||
| Mad2 haploinsufficiency | Weakened | KSP-IA | Decreased | B, C, E | ||
| Taxol | Decreased (mildly) | B, C, E | ||||
| Mad1-siRNA | Weakened | Nocodazole | Decreased | A, B | 40–48 | Kienitz et al. (54) |
| Taxol | Unchanged | A, B | ||||
| Monastrol | Unchanged | A, B | ||||
| Mad2 haploinsufficiency | Weakened | Nocodazole | Decreased | A, B | ||
| Taxol | Decreased | A, B | ||||
| Monastrol | Decreased | A, B | ||||
| Mad2 overexpression | Strengthened | Vincristine | Increased | A, C, G | A—72 | Wang et al. (55) |
| Taxol | Unchanged | A, C, G | C—96 | |||
| G—12 days | ||||||
| Mad2 overexpression in Mad2-siRNA |
Weakened then restored | Taxol | Increased | D | 48 | Sudo et al. (53) |
| Mad2 overexpression in BubR1-siRNA |
Weakened | Taxol | Unchanged | D | ||
| Overexpression of | ||||||
| BubR1 | Not determined | Nocodazole | Increased | B, C | 48–96 | Shin et al. (44) |
| Bub1 | Nocodazole | Increased (mildly) | B, C | |||
| Mad1 | Nocodazole | Unchanged | B, C | |||
| Mad2 | Nocodazole | Unchanged | B, C | |||
| Bub3 | Nocodazole | Unchanged | B, C |
“Short-term” assays: A, DNA fragmentation; B, caspase 3 activation, poly-ADP-ribose polymerase fragment generation; C, appearance of sub-G1 peak in fluorescence-activated cell sorting; D, trypan blue exclusion; E, colony formation assay after short drug exposure; F, cellular metabolism [e.g., 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]. “Long-term” assays: G, colony formation assay in continuous presence of drug.
Accessory Factors that Affect the Outcomes When Cells Are Treated with Antimitotic Drugs
Many factors not specifically involved in spindle checkpoint signaling affect the responses to antimitotic drugs. In earlier reports, requirements for p53 in antimitotic drug–mediated cell death seemed to vary, partially because no clear distinction was made regarding the mechanisms of cell death. For mitotic catastrophe induced by DNA damage, p53 is apparently not essential (15–18). The involvement of p53 in taxol-mediated cell death was implicated by a report that p53−/− knockout MEF cell lines were more susceptible to taxol treatment, although it was attributed to tumor necrosis factor-α activity (21). Further research in cultured cells suggests that p53 plays a role in postmitotic cells after mitotic slippage to inhibit continuing endoreplication and re-entry into mitosis (22–24). This function of p53 has been proposed to represent a postmitotic checkpoint (25). These results in cultured cells may reflect similar observations in mice. Mice with a deficient spindle checkpoint, e.g., Bub3-Rae1 double-haploinsufficient mice and BubR1 haploinsufficient mice, show early signs of aging, and fibroblasts from these mice accumulate senescence markers p16, p19, and p21. The mouse phenotype suggests that the p53-dependent cellular senescence pathway may be critical to induce senescence in cells with deficient spindle checkpoints (26, 27). Thus, normal p53 seems to bias cells toward senescence or death after aberrant mitotic exit [outcomes (C) and/or (D)]. In addition, functions of Raf1, p38MAPK, and p21-activated kinase have also been implicated in determining the fate of cells that exit mitosis abnormally (28, 29).
Spindle Checkpoint Function Determines the Effectiveness of Antimitotic Drugs in Yeast
Spindle checkpoint function provides cells with additional time to deal with problems during mitosis. Thus, the checkpoint helps to prevent mitotic errors and to maintain genome integrity. Spindle checkpoint genes were first isolated in yeast (MAD1-3, BUB1,3, MPS1; refs. 30–32). Apart from budding yeast MPS1 which has additional roles in spindle pole duplication (33), the spindle checkpoint proteins are not essential for overall survival in culture in both budding and fission yeast. Loss of any of these proteins causes yeast to suffer elevated chromosome segregation errors (30, 31, 34, 35). In the presence of microtubule inhibitors, the detrimental effect of mutations in spindle checkpoint genes in yeast is magnified and results in massive cell death. Mutants fail to arrest in mitosis, exit M phase without proper chromosome segregation, and show poor growth. Thus, the spindle checkpoint is a major determinant of the sensitivity of yeast to antimitotic drugs.
Spindle Checkpoint Genes Are Essential in Mammalian Cells
Mammalian orthologues of the core checkpoint proteins (e.g., Mad1, Mad2, BubR1/Mad3, Bub1, Bub3, and Mps1) were identified by sequence homology. Similar to their yeast counterparts, these proteins function by inhibition of anaphase-promoting complex/cyclosome (10). However, in mammalian cells, spindle checkpoint proteins seem to play a more essential role in normal cell division in the absence of spindle damage. Mad2−/−, BubR1−/−, and Bub3−/− knockout mice are embryonic lethal with failure of chromosome segregation (26, 36–39; for review, see ref. 40). Similarly, small interfering RNA (siRNA)–mediated inhibition of BubR1 or Mad2 expression results in the loss of spindle checkpoint function, massive chromosomal loss, and apoptotic death (41, 42).
Antimitotic Drug – Mediated Cell Death Is Associated with Activation of the Apoptosis Machinery in Mammalian Cells
The aforementioned difference between yeast and mammalian systems (nonessential versus essential in normal mitosis, respectively) may be attributed in part to the robust apoptosis mechanism in mammalian cells. It has been hypothesized that malfunction of the spindle checkpoint in mammalian cells may not be inherently lethal, but the accumulation of mitotic errors and chromosome instability may trigger apoptosis (43). Some evidence supports this hypothesis. Although Mad2 knockout mice are inviable under any circumstances, two cell lines deficient in both Mad2 and p53 were derived from mouse blastocysts (43). These lines, though viable, show deficient spindle checkpoints and extraordinarily high rates of chromosome instability, reminiscent of spindle checkpoint–deficient mutants in yeast. Although loss of p53 seems important for the survival of these lines, it is possible that other genetic changes were also selected during outgrowth of the blastocysts. In HeLa cells in which p53 is inactivated by expression of the papilloma virus E6 protein, suppression of the checkpoint proteins Mad2 or BubR1 by transfection of plasmids encoding short hairpin RNA leads to massive cell death of the entire culture within 6 days (41).
There is other evidence that indicates the involvement of apoptosis in antimitotic drug–mediated cell death. Cell death after antimitotic drug treatment is associated with several apoptotic markers including activation of caspase 3 (44), Fas-associated death domain protein and caspase 10 (45), Bax (5), and accumulation of p53 (24). Cell death after knockdown of certain kinetochore components (i.e., hSpc24, hSpc25) that simultaneously causes loss of the spindle checkpoint function or direct knockdown of checkpoint molecules (i.e., BubR1, Mad2) induces apoptotic markers such as poly-ADP-ribose polymerase fragment generation, the appearance of Annexin V–positive cells (46), or caspase 3 activation (41). Consistently, pan-caspase inhibitors suppress cell death by mitotic kinesin inhibitors (5) and by a combinatorial challenge of hBUBR1 overexpression and nocodazole (44). Caspase 3 is reported to increase in amount at G2-M (47), and the increase may provide a favorable environment for the initiation of apoptosis. Thus, apoptosis-related proteins likely affect the outcomes for treatments with antimitotic drugs and act as a determinant of the efficacy of antimitotic drugs. However, the precise signaling pathways connecting checkpoint activation and apoptosis initiation remain unknown.
Apoptotic pathways may also cause feedback affecting the maintenance of the spindle checkpoint. The checkpoint proteins, Bub1 and BubR1, are caspase targets, and triggering caspases during mitotic arrest inactivates the spindle checkpoint and facilitates mitotic slippage (48, 49). Thus, at later stages, there may not be distinct boundaries between mitotic death and postmitotic cell death [Fig. 1, outcomes (B) and (C)].
Partial Abrogation of Spindle Checkpoint Function May Confer Resistance to Antimitotic Drugs in Mammalian Cells
The relationship between the function of the spindle checkpoint and cellular sensitivity to antimitotic drugs in mammalian cells is an issue of great general and clinical interest. However, because complete loss of the spindle checkpoint does not generally allow mammalian cells to survive, the correlation between checkpoint function and antimitotic drug sensitivity has been difficult to examine systematically. To investigate the effects of partial loss of spindle checkpoint function, researchers have used two systems: cancer cell lines with weakened spindle checkpoint function, or partial knockdown/impairment of spindle checkpoint proteins. For the latter, a variety of approaches have been used to compromise spindle checkpoint function experimentally, including viral infection, siRNA, dominant-negative protein expression, and haploinsufficiency.
Although gene mutations in known spindle checkpoint components are rare, some actively growing cancer cells have a weak spindle checkpoint, defined as a reduced ability to sustain mitotic arrest in the presence of an antimitotic drug. However, results from studies with several cancer cell lines have not yielded a clear consensus with regard to how spindle checkpoint status affects sensitivity to antimitotic drugs. Masuda et al. (50) showed that among 13 cancer cell lines tested, 4 cell lines had weakened spindle checkpoint function and were resistant to microtubule inhibitor–mediated induction of apoptosis. In these studies, drug sensitivity did not seem to be related to p53 status. In contrast, Lee et al. (13) reported elevated sensitivity to microtubule inhibitors among cancer cell lines with weakened spindle checkpoint function associated with the reduction of BubR1 protein expression. The sensitive cell lines were from the breast or ovary, organs whose cancers generally respond well to microtubule inhibitors. Although the cause of the discrepancy is unclear, one reconciliation of these apparently contradictory results may be that the inherent variability of cancer cells or the tissue specificity of the source plays an important role in cellular sensitivity to antimitotic drugs.
The second approach is to specifically compare cells that are genetically identical except for specific targeting of checkpoint proteins. Jeang’s group first showed that the Tax oncoprotein encoded by human T-cell leukemia virus type I binds to and inhibits Mad1 (51). These researchers then showed that human T-cell leukemia virus type I infection of cultured cells results in mislocalization of Mad1 and Mad2 as well as diminished spindle checkpoint function. In this case, virus-infected cells were more resistant to nocodazole or vincristine compared with uninfected cells (52). Sudo et al. (53) transiently transfected MCF-7 cells with siRNA against BubR1 or Mad2, and showed that these cells were defective in spindle checkpoint function and were more resistant to taxol. Tao et al. (5) generated HeLa-based cell lines in which dominant-negative Bub1 could be expressed conditionally. Reduced checkpoint function correlated with reduced apoptosis in cells treated with taxol or the kinesin inhibitor KSP-IA. Bastians’ group generated HCT116-based cell lines in which Mad1 expression was reduced through siRNA expression (Mad1-kd) and cells with only one copy of functional Mad2 (Mad2+/−; ref. 54). Both cell lines showed higher resistance to nocodazole. Mad2+/− cells are resistant to taxol and monastrol as well. However, the Mad1-kd cell line was not resistant to taxol or monastrol.
Overexpression of spindle checkpoint proteins also seems to affect cell death induced by microtubule inhibitors. Mad2 overexpression in nasopharyngeal carcinoma cells sensitized these cells to vincristine, and to lesser extent, to taxol (55). Overexpression of BubR1 increased apoptosis in postmitotic polyploid cells in the presence of nocodazole (44).
Together, these results from the second approach generally support two conclusions: (a) antimitotic drug–mediated cell death may be affected by factors such as drug type and/or status of checkpoint proteins, and (b) a partial decrease of spindle checkpoint function in most, but not all, studies results in an increased resistance to antimitotic drugs in short-term apoptosis or growth assays. However, controversy persists and because so few studies have been done, it is difficult to determine what conclusions would be drawn from long-term assays of cancer cell viability.
In addition to the aforementioned experimental approaches, a species comparison is also informative. Cells derived from rodents seem to override the mitotic arrest induced with antimitotic drug treatment more readily than do cells from other mammalian species (including humans). These observations suggest that rodent cells have more “relaxed” spindle checkpoints (56). Consistent with the conclusions presented above, cells from rodents are generally more resistant to antimitotic drugs than are primate cells (e.g., ref. 57).
Does Inactivation of the Spindle Checkpoint Function through Down-Regulation of Cyclin-Dependent Kinase?
Spindle checkpoint activation causes mitotic arrest with sustained high cdk1-cyclin B activity. Inactivation of spindle checkpoint results in a drop in cdk1 activity and mitotic slippage. Does inactivation of the spindle checkpoint function solely to inactivate cdk1 with stimulation of the cell death mechanism? Alternatively, is postmitotic cell death triggered by two contradictory cues—active spindle checkpoint signaling juxtaposed with a decrease in cdk1 activity? Because spindle checkpoint function and the regulation of cdk1 activity are intertwined, they are difficult to affect independently. One approach would be to induce artificial mitotic slippage with cdk1 inhibitors. Tao et al. (5) showed that treatment of mitotic cells with the cdk inhibitor purvalanol A induced mitotic slippage and enhanced cell death (measured by the appearance of cells with sub-2N DNA content). They proposed that the activation of the spindle checkpoint and mitotic slippage are both required for subsequent activation of apoptotic machinery including Bax. As with all supposedly “specific” kinase inhibitors, caution regarding the interpretation of these results is required. However, the use of cdk inhibitors to force mitotic slippage and enhance the efficacy of antimitotic drugs deserves further investigation.
Conclusion
In this review, we have summarized recent information from studies in cultured cells on the correlation and possible linkage between spindle checkpoint–mediated mitotic arrest and antimitotic drug–mediated cell death. We developed definitions of the concept of sensitivity according to the possible outcomes of the mitotic arrest. Complete loss of the spindle checkpoint is, in most circumstances, incompatible with cell survival. However, partial loss of the spindle checkpoint may render cells somewhat more resistant to antimitotic drugs, at least in short-term assays. From a clinical standpoint, the ramifications are significant. By acquiring “weakened” spindle checkpoint function, cells may obtain two characteristics that increase their malignant potential: chromosome instability with concomitant changes in gene dosage as well as potentially increased resistance to antimitotic drugs. Weakened spindle checkpoint function is rather commonly observed among cancer cells, and thus the molecular linkages between this cancer-related change in cellular physiology and responses to treatment should be explored. At present, our understanding of the molecular interactions between mitotic arrest induced by antimitotic drugs and the induction of cell death remain rudimentary. Delineating the precise mechanisms of feedback among the signaling pathways involved will provide important insights into the rational development of therapies designed to leverage the differences between transformed and normal cells into novel effective strategies in anticancer treatment.
Acknowledgments
We thank our laboratory members for comments and support. Due to space limitations, we were unable to include all of the relevant work from colleagues.
Grant support: H.Y. Yamada is a recipient of a fellowship from the Breast Cancer Research Program of the U.S. Department of Defense (DAMD17-02-1-0532). G.J. Gorbsky is a recipient of a grant from the National Institute of General Medical Science (RO1-GM50412).
References
- 1.Blagosklonny MV, Fojo T. Molecular effects of paclitaxel: myths and reality (a critical review) Int J Cancer. 1999;83:151–156. doi: 10.1002/(sici)1097-0215(19991008)83:2<151::aid-ijc1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 2.Yuan JH, Zhang RP, Zhang RG, et al. Growth-inhibiting effects of taxol on human liver cancer in vitro and in nude mice. World J Gastroenterol. 2000;6:210–215. doi: 10.3748/wjg.v6.i2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suzuki M, Nakamatsu K, Kanamori S, Masunaga S, Nishimura Y. Additive effects of radiation and docetaxel on murine SCCVII tumors in vivo: special reference to changes in the cell cycle. Radiat Res. 2003;159:799–804. doi: 10.1667/0033-7587(2003)159[0799:aeorad]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 4.Shimming R, Mason KA, Hunter N, Weil M, Kishi K, Milas L. Lack of correlation between mitotic arrest or apoptosis and antitumor effect of docetaxel. Cancer Chemother Pharmacol. 1999;43:165–172. doi: 10.1007/s002800050879. [DOI] [PubMed] [Google Scholar]
- 5.Tao W, South VJ, Zhang Y, et al. Induction of apoptosis by an inhibitor of the mitotic kinesin KSP requires both activation of the spindle assembly checkpoint and mitotic slippage. Cancer Cell. 2005;8:49–59. doi: 10.1016/j.ccr.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Mayer TU, Kapoor TM, Haggarty SJ, King RW, Schreiber SL, Mitchison TJ. Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science. 1999;286:971–974. doi: 10.1126/science.286.5441.971. [DOI] [PubMed] [Google Scholar]
- 7.Hotha S, Yarrow JC, Yang JG, et al. HR22C16: a potent small-molecule probe for the dynamics of cell division. Angew Chem Int Ed Engl. 2003;42:2379–2382. doi: 10.1002/anie.200351173. [DOI] [PubMed] [Google Scholar]
- 8.Pinsky BA, Biggins S. The spindle checkpoint: tension versus attachment. Trends Cell Biol. 2005;15:486–493. doi: 10.1016/j.tcb.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Chen JG, Horwitz SB. Differential mitotic responses to microtubule-stabilizing and -destabilizing drugs. Cancer Res. 2002;62:1935–1938. [PubMed] [Google Scholar]
- 10.Bhalla KN. Microtubule-targeted anticancer agents and apoptosis. Oncogene. 2003;22:9075–9086. doi: 10.1038/sj.onc.1207233. [DOI] [PubMed] [Google Scholar]
- 11.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 12.Kops GJ, Weaver BA, Cleveland DW. On the road to cancer: aneuploidy and the mitotic checkpoint. Nat Rev Cancer. 2005;5:773–785. doi: 10.1038/nrc1714. [DOI] [PubMed] [Google Scholar]
- 13.Lee EA, Keutmann MK, Dowling ML, Harris E, Chan G, Kao GD. Inactivation of the mitotic checkpoint as a determinant of the efficacy of microtubule-targeted drugs in killing human cancer cells. Mol Cancer Ther. 2004;3:661–669. [PubMed] [Google Scholar]
- 14.Ganansia-Leymarie V, Bischoff P, Bergerat JP, Holl V. Signal transduction pathways of taxanes-induced apoptosis. Curr Med Chem Anticanc Agents. 2003;3:291–306. doi: 10.2174/1568011033482422. [DOI] [PubMed] [Google Scholar]
- 15.Mikhailov A, Cole RW, Rieder CL. DNA damage during mitosis in human cells delays the metaphase/anaphase transition via the spindle-assembly checkpoint. Curr Biol. 2002;12:1797–1806. doi: 10.1016/s0960-9822(02)01226-5. [DOI] [PubMed] [Google Scholar]
- 16.Castedo M, Perfettini JL, Roumier T, et al. Mitotic catastrophe constitutes a special case of apoptosis whose suppression entails aneuploidy. Oncogene. 2004;23:4362–4370. doi: 10.1038/sj.onc.1207572. [DOI] [PubMed] [Google Scholar]
- 17.Nitta M, Kobayashi O, Honda S, et al. Spindle checkpoint function is required for mitotic catastrophe induced by DNA-damaging agents. Oncogene. 2004;23:6548–6558. doi: 10.1038/sj.onc.1207873. [DOI] [PubMed] [Google Scholar]
- 18.Vogel C, Kienitz A, Muller R, Bastians H. The mitotic spindle checkpoint is a critical determinant for topoisomerase-based chemotherapy. J Biol Chem. 2005;280:4025–4028. doi: 10.1074/jbc.C400545200. [DOI] [PubMed] [Google Scholar]
- 19.Weaver BA, Cleveland DW. Decoding the links between mitosis, cancer, and chemotherapy: The mitotic checkpoint, adaptation, and cell death. Cancer Cell. 2005;8:7–12. doi: 10.1016/j.ccr.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 20.Shi Q, King RW. Chromosome nondisjunction yields tetraploid rather than aneuploid cells in human cell lines. Nature. 2005;437:1038–1042. doi: 10.1038/nature03958. [DOI] [PubMed] [Google Scholar]
- 21.Lanni JS, Lowe SW, Licitra EJ, Liu JO, Jacks T. p53-independent apoptosis induced by paclitaxel through an indirect mechanism. Proc Natl Acad Sci U S A. 1997;94:9679–9683. doi: 10.1073/pnas.94.18.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lanni JS, Jacks T. Characterization of the p53-dependent postmitotic checkpoint following spindle disruption. Mol Cell Biol. 1998;18:1055–1064. doi: 10.1128/mcb.18.2.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sablina AA, Agapova LS, Chumakov PM, Kopnin BP. p53 does not control the spindle assembly cell cycle checkpoint but mediates G1 arrest in response to disruption of microtubule system. Cell Biol Int. 1999;23:323–334. doi: 10.1006/cbir.1999.0362. [DOI] [PubMed] [Google Scholar]
- 24.Vogel C, Kienitz A, Hofmann I, Muller R, Bastians H. Crosstalk of the mitotic spindle assembly checkpoint with p53 to prevent polyploidy. Oncogene. 2004;23:6845–6853. doi: 10.1038/sj.onc.1207860. [DOI] [PubMed] [Google Scholar]
- 25.Margolis RL. Tetraploidy and tumor development. Cancer Cell. 2005;8:353–354. doi: 10.1016/j.ccr.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 26.Baker DJ, Jeganathan KB, Cameron JD, et al. BubR1 insufficiency causes early onset of aging-associated phenotypes and infertility in mice. Nat Genet. 2004;36:744–749. doi: 10.1038/ng1382. [DOI] [PubMed] [Google Scholar]
- 27.Baker DJ, Jeganathan KB, Malureanu L, Perez-Terzic C, Terzic A, van Deursen JM. Early aging-associated phenotypes in Bub3/Rae1 haploinsufficient mice. J Cell Biol. 2006;172:529–540. doi: 10.1083/jcb.200507081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bacus SS, Gudkov AV, Lowe M, et al. Taxol-induced apoptosis depends on MAP kinase pathways (ERK and p38) and is independent of p53. Oncogene. 2001;20:147–155. doi: 10.1038/sj.onc.1204062. [DOI] [PubMed] [Google Scholar]
- 29.Deacon K, Mistry P, Chernoff J, Blank JL, Patel R. p38 mitogen-activated protein kinase mediates cell death and p21-activated kinase mediates cell survival during chemotherapeutic drug-induced mitotic arrest. Mol Biol Cell. 2003;14:2071–2087. doi: 10.1091/mbc.E02-10-0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li R, Murray AW. Feedback control of mitosis in budding yeast. Cell. 1991;66:519–531. doi: 10.1016/0092-8674(81)90015-5. [DOI] [PubMed] [Google Scholar]
- 31.Hoyt MA, Totis L, Roberts BT. S. cerevisiae genes required for cell cycle arrest in response to loss of microtubule function. Cell. 1991;66:507–517. doi: 10.1016/0092-8674(81)90014-3. [DOI] [PubMed] [Google Scholar]
- 32.Weiss E, Winey M. The Saccharomyces cerevisiae spindle pole body duplication gene MPS1 is part of a mitotic checkpoint. J Cell Biol. 1996;132:111–123. doi: 10.1083/jcb.132.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fisk HA, Mattison CP, Winey M. A field guide to the Mps1 family of protein kinases. Cell Cycle. 2004;3:439–442. [PubMed] [Google Scholar]
- 34.He X, Patterson TE, Sazer S. The Schizosaccharomyces pombe spindle checkpoint protein mad2p blocks anaphase and genetically interacts with the anaphase-promoting complex. Proc Natl Acad Sci U S A. 1997;94:7965–7970. doi: 10.1073/pnas.94.15.7965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bernard P, Hardwick K, Javerzat JP. Fission yeast bub1 is a mitotic centromere protein essential for the spindle checkpoint and the preservation of correct ploidy through mitosis. J Cell Biol. 1998;143:1775–1787. doi: 10.1083/jcb.143.7.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dobles M, Liberal V, Scott ML, Benezra R, Sorger PK. Chromosome missegregation and apoptosis in mice lacking the mitotic checkpoint protein Mad2. Cell. 2000;101:635–645. doi: 10.1016/s0092-8674(00)80875-2. [DOI] [PubMed] [Google Scholar]
- 37.Michel LS, Liberal V, Chatterjee A, et al. MAD2 haplo-insufficiency causes premature anaphase and chromosome instability in mammalian cells. Nature. 2001;409:355–359. doi: 10.1038/35053094. [DOI] [PubMed] [Google Scholar]
- 38.Dai W, Wang Q, Liu T, et al. Slippage of mitotic arrest and enhanced tumor development in mice with BubR1 haploinsufficiency. Cancer Res. 2004;64:440–445. doi: 10.1158/0008-5472.can-03-3119. [DOI] [PubMed] [Google Scholar]
- 39.Kalitsis P, Earle E, Fowler KJ, Choo KH. Bub3 gene disruption in mice reveals essential mitotic spindle checkpoint function during early embryogenesis. Genes Dev. 2000;14:2277–2282. doi: 10.1101/gad.827500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baker DJ, Chen J, van Deursen JM. The mitotic checkpoint in cancer and aging: what have mice taught us? Curr Opin Cell Biol. 2005;17:583–589. doi: 10.1016/j.ceb.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 41.Kops GJ, Foltz DR, Cleveland DW. Lethality to human cancer cells through massive chromosome loss by inhibition of the mitotic checkpoint. Proc Natl Acad Sci U S A. 2004;101:8699–8704. doi: 10.1073/pnas.0401142101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Michel L, Diaz-Rodriguez E, Narayan G, Hernando E, Murty VV, Benezra R. Complete loss of the tumor suppressor MAD2 causes premature cyclin B degradation and mitotic failure in human somatic cells. Proc Natl Acad Sci U S A. 2004;101:4459–4464. doi: 10.1073/pnas.0306069101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burds AA, Lutum AS, Sorger PK. Generating chromosome instability through the simultaneous deletion of Mad2 and p53. Proc Natl Acad Sci U S A. 2005;102:11296–11301. doi: 10.1073/pnas.0505053102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shin HJ, Baek KH, Jeon AH, et al. Dual roles of human BubR1, a mitotic checkpoint kinase, in the monitoring of chromosomal instability. Cancer Cell. 2003;4:483–497. doi: 10.1016/s1535-6108(03)00302-7. [DOI] [PubMed] [Google Scholar]
- 45.Park SJ, Wu CH, Gordon JD, Zhong X, Emami A, Safa AR. Taxol induces caspase-10-dependent apoptosis. J Biol Chem. 2004;279:51057–51067. doi: 10.1074/jbc.M406543200. [DOI] [PubMed] [Google Scholar]
- 46.Bharadwaj R, Qi W, Yu H. Identification of two novel components of the human NDC80 kinetochore complex. J Biol Chem. 2004;279:13076–13085. doi: 10.1074/jbc.M310224200. [DOI] [PubMed] [Google Scholar]
- 47.Hsu SL, Yu CT, Yin SC, et al. Caspase 3, periodically expressed and activated at G2/M transition, is required for nocodazole-induced mitotic checkpoint. Apoptosis. 2006;11:765–771. doi: 10.1007/s10495-006-5880-x. [DOI] [PubMed] [Google Scholar]
- 48.Baek KH, Shin HJ, Jeong SJ, et al. Caspases-dependent cleavage of mitotic checkpoint proteins in response to microtubule inhibitor. Oncol Res. 2005;15:161–168. doi: 10.3727/096504005776367906. [DOI] [PubMed] [Google Scholar]
- 49.Kim M, Murphy K, Liu F, et al. Caspase-mediated specific cleavage of BubR1 is a determinant of mitotic progression. Mol Cell Biol. 2005;25:9232–9248. doi: 10.1128/MCB.25.21.9232-9248.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Masuda A, Maeno K, Nakagawa T, Saito H, Takahashi T. Association between mitotic spindle checkpoint impairment and susceptibility to the induction of apoptosis by anti-microtubule agents in human lung cancers. Am J Pathol. 2003;163:1109–1116. doi: 10.1016/S0002-9440(10)63470-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jin DY, Spencer F, Jeang KT. Human T cell leukemia virus type 1 oncoprotein Tax targets the human mitotic checkpoint protein MAD1. Cell. 1998;93:81–91. doi: 10.1016/s0092-8674(00)81148-4. [DOI] [PubMed] [Google Scholar]
- 52.Kasai T, Iwanaga Y, Iha H, Jeang KT. Prevalent loss of mitotic spindle checkpoint in adult T-cell leukemia confers resistance to microtubule inhibitors. J Biol Chem. 2002;277:5187–5193. doi: 10.1074/jbc.M110295200. [DOI] [PubMed] [Google Scholar]
- 53.Sudo T, Nitta M, Saya H, Ueno NT. Dependence of paclitaxel sensitivity on a functional spindle assembly checkpoint. Cancer Res. 2004;64:2502–2508. doi: 10.1158/0008-5472.can-03-2013. [DOI] [PubMed] [Google Scholar]
- 54.Kienitz A, Vogel C, Morales I, Muller R, Bastians H. Partial down-regulation of MAD1 causes spindle checkpoint inactivation and aneuploidy, but does not confer resistance towards taxol. Oncogene. 2005;24:4301–4310. doi: 10.1038/sj.onc.1208589. [DOI] [PubMed] [Google Scholar]
- 55.Wang X, Jin DY, Wong HL, Feng H, Wong YC, Tsao SW. MAD2-induced sensitization to vincristine is associated with mitotic arrest and Raf/Bcl-2 phosphorylation in nasopharyngeal carcinoma cells. Oncogene. 2003;22:109–116. doi: 10.1038/sj.onc.1206069. [DOI] [PubMed] [Google Scholar]
- 56.Kung AL, Sherwood SW, Schimke RT. Cell line-specific differences in the control of cell cycle progression in the absence of mitosis. Proc Natl Acad Sci U S A. 1990;87:9553–9557. doi: 10.1073/pnas.87.24.9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haller K, Kibler KV, Kasai T, et al. The N-terminus of rodent and human MAD1 confers species-specific stringency to spindle assembly checkpoint. Oncogene. 2006;25:2137–2147. doi: 10.1038/sj.onc.1209259. [DOI] [PubMed] [Google Scholar]