Abstract
Drug addiction is a state of altered brain reward and self-regulation mediated by both neurotransmitter and hormonal systems. Although an organism’s internal system attempts to maintain homeostasis when challenged by exogenous opiates and other drugs of abuse, it eventually fails, resulting in the transition from drug use to drug abuse. We propose that the attempted maintenance of hormonal homeostasis is achieved, in part, through alterations in levels of processing enzymes that control the ratio of active hormone to pro-hormone. Two pro-hormone convertases, PC1/3 and PC2 are believed to be responsible for the activation of many neurohormones and expression of these enzymes is dependent on the presence of a cyclic-AMP response element (CRE) in their promoters. Therefore, we studied the effects of short-term (24-h) and long-term (seven-day) morphine treatment on the expression of hypothalamic PC1/3 and PC2 and levels of phosphorylated cyclic-AMP-response element binding protein (P-CREB). While short-term morphine exposure down-regulated, long-term morphine exposure up-regulated P-CREB, PC1/3 and PC2 protein levels in the rat hypothalamus as determined by Western blot analysis. Quantitative immunofluorescence studies confirmed these regulatory actions of morphine in the paraventricular and dorsomedial nucleus of the hypothalamus. Specific radioimmunoassays demonstrated that the increase in PC1/3 and PC2 levels following long-term morphine led to increased TRH biosynthesis as evidence by increased TRH/5.4 kDa C-terminal proTRH-derived peptide ratios in the median eminence. Promoter activity experiments in rat sommatomammotrope GH3 cells containing the mu-opioid receptor demonstrated that the CRE(s) in the promoter of PC1/3 and PC2 is required for morphine-induced regulation of PC1/3 and PC2. Our data suggest that the regulation of the prohormone processing system by morphine may lead to alterations in the levels of multiple bioactive hormones and may be a compensatory mechanism whereby the organism tries to restore its homeostatic hormonal milieu. The down-regulation of PC1/3, PC2 and P-CREB by short-term morphine and up-regulation by long-term morphine treatment may be a signal mediating the switch from drug use to drug abuse.
Keywords: Post-translational processing, opiates, endorphin, TRH, prohormone convertase, hypothalamus, drug addiction
INTRODUCTION
As drug addiction is now viewed as a brain disease (Leshner, 1997), there has been an attempt to understand which neurochemical systems are altered by drug use. Abnormalities in neuroendocrine systems, such as corticotrophin-releasing hormone (CRH), pro-opiomelanocortin (POMC) and dynorphin have been reported in animals and humans exposed to drugs of abuse (Garcia de Yebenes and Pelletier, 1993, Spangler et al., 1996, Zhou et al., 1996, Rodriguez de Fonseca et al., 1997). For example, exogenous opiate administration down-regulates both brain POMC mRNA and plasma β-endorphin levels (Ho et al., 1980, Bronstein et al., 1990, Garcia de Yebenes and Pelletier, 1993). However, little is known if the changes in neurohormone(s) with drug intake are isolated or part of an altered hormonal environment and if these changes may account for some of the physiological effects of abused substances.
The precursors of opioid peptides, like most prohormones, are synthesized in an inactive form and are converted to the biologically active hormone by cleavage at paired basic residues. Two members of the family of prohormone convertases (PCs), PC1/3 and PC2, are located primarily in neuroendocrine tissues (Seidah et al., 1990, Hakes et al., 1991, Seidah et al., 1991, Day et al., 1992) and mediate the processing of most prohormones (Seidah and Chretien, 1994). PC2 null mice lack proglucagon processing leading to hypoglycemia and also have impaired proinsulin processing (Furuta et al., 1997). POMC, proenkephalin and prodynorphin processing is blunted (Berman et al., 2000, Laurent et al., 2004). PC1/3 null mice are small due to impaired proGHRH processing and are hyperglycemic due to impaired proinsulin processing (Zhu et al., 2002a, Zhu et al., 2002b). The findings that the knockout mice have processing profiles that are expected from the known biochemistry of PC1/3 and PC2 support the notion that these two enzymes are necessary and sufficient for the cleavage of most prohormones/pro-neuropeptides at paired basic residues. Indirect experiments demonstrated that expression of PC1/3 is dependent on activation of the CREB/cAMP system (Jansen et al., 1995, Lamas et al., 1997). Since these PCs have the potential to process a wide variety of prohormones, alterations in the activities of these enzymes in brain areas rich in neuropeptides would be expected to change the ratio of active hormone to inactive precursor for many hormonal systems.
Opiates and other drugs of abuse affect several intracellular messengers. The most well studied of these is CREB, a transcription factor responsive to cAMP, that along with the cAMP response element modulator (CREM) and activation transcription factor-1 (ATF-1), belong to the bZIP superfamily. CREB is activated by phosphorylation at Serine-133 by cAMP-dependent protein kinase A (PKA) to become phosphorylated CREB (P-CREB). Changes in the phosphorylation of CREB and other cAMP-dependent transcription factors regulate transcription of genes that are dependent on activation of their cyclic AMP-response elements (CREs) (Guitart et al., 1992, Lane-Ladd et al., 1997). P-CREB is found in most tissues examined, with high levels in the brain (Martin and Kandel, 1996) and the pituitary (Sassone-Corsi, 1995) and is thought to be an important mediator in substance abuse (Blendy and Maldonado, 1998, Nestler, 2001, Chao and Nestler, 2004). In many brain regions such as the locus coeruleus, acutely administered morphine and other mu opioid receptor agonists bind to Gi proteins-coupled mu opioid receptors, leading to inhibition of adenylate cyclase followed by a decrease in cAMP-dependent phosphorylation of CREB (Duman et al., 1988, Guitart et al., 1992). In contrast, in some brain regions, chronic opiate administration increases the level of cAMP by activating adenylate cyclase leading to an increase in the levels of P-CREB (Lane-Ladd et al., 1997, Nestler and Aghajanian, 1997). It has been proposed that CRE-mediated transcription serves as a functional marker for neuronal plasticity during opiate use (Shaw-Lutchman et al., 2002). The effect of exogenous opiates on the CREB system in regions involved in biosynthesis of opioids and other neuropeptides, such as the hypothalamus, has only briefly been examined (Shaw-Lutchman et al., 2002).
In humans, most drug users do not become drug-dependent. However other drug users become addicted to drugs of abuse. Koob and Le Moal (Koob and Le Moal, 1997) postulated that the organism tries to maintain homeostasis when challenged by exogenous drugs such as opiates. However, it eventually fails to adapt to the environmental challenges, thereby the organism faces a new, albeit pathological, setpoint in which tolerance to the drug occurs. If drug intake ceases, withdrawal symptoms occur, leading to negative affects followed by craving, drug seeking behaviors and compulsive drug use (Leshner, 1997). Chronic drug exposure resulting in “the addicted brain” is likely to lead to an altered of different brain genes compared to short-term drug exposure. The altered levels of these genes and proteins are likely to be multiple and the sum of changes in expression is likely to be the “switch” that moves the individual into a state of compulsive drug seeking and use (Leshner, 1997). We propose that this “switch to addiction” may be achieved, in part, by a series of gene products that are regulated by opiates via changes in intracellular transcription factors. One example of proteins affected differentially by short-term versus long-term opiate use may be the CRE-dependent processing enzymes that control the ratio of active hormones to prohormones. We tested the hypothesis that opioids alter prohormone convertase levels and hence change the amount of active hormones via changes in CREB phosphorylation. The hypothalamus was chosen as it is a major site of neuropeptide biosynthesis and has an important role in addiction processes (DiLeone et al., 2003, de Lecea et al., 2006). Morphine, which acts primarily through the mu opioid receptors (Matthes et al., 1996), was chosen to study the effects of short-term and long-term morphine exposure on the levels of P-CREB and the prohormone convertases, PC1/3 and PC2.
EXPERIMENTAL PROCEDURES
Animals and Treatments
All experimental protocols and animal procedures were approved by the Institutional Animal Care and Use Committee of The Charles Drew University of Medicine & Sciences-UCLA School of Medicine and performed in compliance with the NIH Guidelines for the Use of Animals in Research.
Adult male Sprague-Dawley rats (Harlan Teklad; Madison, WI) weighing 200-230 g, were housed in a room with controlled light, temperature and humidity, and unrestricted access to food and water. To assess the effects of morphine on PC1/3, PC2 and P-CREB levels, rats were divided into four groups for both short-term and long-term studies; we used a 24-h morphine pellet regimen to mimic short-term opiate exposure and a seven-day morphine pellet regimen to mimic long-term opiate exposure. After rats were anesthetized with isofluorane (Attane, Minrad INC, Bethlehem PA, USA), pellets containing placebo or morphine [75 mg morphine pellets, matching placebo pellets, National Institute of Drug Abuse (NIDA)] were implanted subcutaneously between their scapulas and remained in place for 24 h. Rats were then euthanized to determine the effect of short-term morphine exposure on the expression of PC1/3, PC2 and levels of P-CREB (Boundy et al., 1998). To determine the effect of long-term morphine exposure on these parameters, rats were implanted with one placebo or morphine pellet as described above on day 0. Rats were then implanted with three additional pellets (morphine 75 mg, or placebo pellets) on day 4 and then euthanized on day 7. Pellet implantation was staggered so that all rats were euthanized on the same day. Between 4 and 8 animals were used for each experiment. For both groups, pellet implantation and euthanasia occurred at the same time between 1300 and 1500.
Morphine-induced antinociceptive tolerance in rats exposed to short-term and long-term morphine
Rats were implanted with either a placebo or morphine pellet for 24 h or seven days and then tested for morphine-induced antinociception in the hot plate test. Rats were tested for baseline nociceptive latency, as described previously (Woolf and MacDonald, 1944), using a hot plate analgesia meter (Columbus Instruments, Columbus OH, USA). Briefly, rats were placed into an acrylic box with a metal floor preheated to 52.5°C and the latency for hindpaw shake or lick was recorded. Rats were then injected with morphine [10 mg/kg, intraperitoneal (i.p.)] and tested for changes in the nociceptive responses 30 min later. Each group contained 4 animals. A cut-off time of 60 sec was imposed as the maximum possible effect in order to prevent tissue damage. All animals were tested once per session in a random sequence. To test for opiate-mediated withdrawal, in a separate experiment, rats were implanted with placebo or morphine pellets as described above. Pellets were then removed 7-day post pellet implantation. Weight loss (over 30 min) and withdrawal signs (over 4 h) were monitored. Withdrawal signs were counted over each hour following pellet removal and % of animals showing each sign of withdrawal was depicted.
Protein Analysis by Immunoblotting
Morphine-treated and placebo-treated animals (n=4/group) were sacrificed by decapitation. The hypothalami were then carefully removed and homogenized immediately in ice-cold RIPA buffer (50 nmol/L Tris-HCl, 150 mmol/L NaCl, 1% sodium deoxycholate, 0.1% SDS, 100 mmol/L sodium orthovanadate, 1% Triton X-100, and EDTA-free protease inhibitors). Homogenates were then centrifuged at 4°C at 12,000g for 10 min and supernatants were collected. Protein concentration of the supernatants was measured with the Bradford assay (Bio-Rad protein assay kit, Hercules, CA, USA). Fifty micrograms of total protein were loaded and separated on 10% SDS polyacrylamide gels and then electrophoretically transferred to Hybond-ECL nitrocellulose membranes (Millipore corporation Bedford, MA, USA) in buffer containing 0.02% SDS and 20% methanol. Membranes were blocked with blocking solution (5% non-fat dry milk in PBS-T), washed three times (5 min each), and proteins detected using either polyclonal anti-phospho-CREB antibody (antibody raised against a synthetic phosphopeptide corresponding to residues 123-136 of rat cyclic AMP response element binding protein, Upstate, Lake Placid, NY, USA) at a dilution of 1:1000, or anti-PC1/3 or anti-PC2 antibodies at a dilution of 1:2000. The anti-phospho-CREB antibody cross-reacts with phosphorylated forms of CREB, CREM and ATF-1, but not with non-phosphorylated forms. Anti-PC1/3 and anti-PC2 antibodies were raised against either a PC1/3-glutathione fusion protein or a PC2-glutathione fusion protein (Hill et al., 1995) and generously provided by Nigel Birch, Ph.D. (University of Auckland, NZ). The PC1/3 antibody detects the 87, 74 and 66 kDa forms of PC1/3 while the PC2 antibody primarily detects the 64 kDa form of PC2 (Hill et al., 1995). Blots were then washed five times (5 min each) with PBS-T and incubated with anti-rabbit conjugated with horseradish peroxidase secondary antibody (Amersham Biosciences, Piscataway, NJ, USA) at a dilution of 1:2000 for 2 h. Bands were visualized using the ECL system (Amersham Biosciences). Band intensities were measured by optical densitometry of the auto-radiographs relative to the amount of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) following subtraction of background providing an objective measurement of signal intensity.
Tissue Preparation for immuno-imaging analysis
Four groups of animals (n= 4/group) were anesthetized with an intraperitoneal injection of ketamine (40 mg/kg, Sigma Chemical Co., St. Louis, MO, USA) and xylazine (5 mg/kg, Phoenix Scientific Inc, St. Joseph, MO, USA) and then perfused with phosphate-buffered saline (PBS), followed by 4% paraformaldehyde in PBS. Brains were carefully removed and post-fixed in 4% paraformaldehyde overnight at 4°C, then immersed in 25% sucrose until the tissues sunk and were then frozen on dry ice. Eight-μM-thick coronal cryostat sections of the brain region of interest [paraventricular nucleus (PVN) and dorsomedial nucleus (DMN) of the hypothalamus and the amygdala] were mounted onto slides (Superfrost ® Plus, Fisher-Scientific, Pittsburg, PA, USA) and stored at −80°C until processing. For better resolution, antigen retrieval was performed by heating the slides in a microwave oven with Antigen Unmasking Solution (Vector Inc, Burlingame, CA, USA) for three times (one min each) and then cooled for 20 min. Quenching of endogenous peroxidase activity was done by placing the slides in 0.3% H2O2 for 20 min, followed by rinsing with PBS.
Immunofluorescence
For immunofluorescence experiments, detection of P-CREB, PC1/3 and PC2 proteins was performed using the TSA™ Fluorescein System (Perkin Elmer LAS Inc, Boston, MA, USA) according to the manufacturer’s protocols. Polyclonal anti-phospho-CREB antibody and polyclonal anti-PC1/3 and anti-PC2 antibodies were obtained as described above. To avoid cross-reactions between the first and the second primary antiserum, both raised in the same species, primary and secondary antibodies were diluted in PBS containing 0.5% blocking reagent (TSA Fluorescein System kit) for PC1/3 and PC2 or normal donkey serum (NDS, Jackson ImmunoResearch Inc, West Grove, PA, USA) for P-CREB.
For the first primary antibody incubation, brain slices were incubated with TNB blocking buffer [0.1 M Tris pH 7.5; 0.15M NaCl; 0.5% blocking reagent (TSA kit)] for 40 min, and then incubated with PC1/3 or PC2 polyclonal antibodies (1:400) or P-CREB (1:1000) for 16 h at 4 °C. After rinsing with PBS, sections were incubated with Biotin-SP-conjugated AffiniPure Fab Fragment Donkey Anti-Rabbit IgG (H+L) (Jackson Immuno Research ) at a concentration of 1:400 for 60 min, followed by Streptavidin-HRP (SA-HRP, TSA kit) 1:100 for 30 min, and finally incubated in Fluorophore Tyramide Amplification Reagent (supplied in the TSA kit) at a concentration of 1:50 for 10 min. Unless indicated otherwise, slides were rinsed three times (five min each) in TNT buffer (0.1M Tris pH 7.5, 0.15M NaCl, 0.05% Tween®20) after each incubation. All incubations except for the primary antibody were carried out at room temperature in a humidified container and protected from light.
Microscopy and Image Analysis
Immunofluorescence images analyzed under microscopy were captured with SPOT RT (Diagnostic Instruments. Sterling Heights, MI) color digital camera attached to the microscope. Quantification of the levels of P-CREB, PC1/3 and PC2 was determined as integrated optical density (IOD) of staining using Image Pro Plus software (Media Cybernetics, Inc., Silver Spring, MD). In this software, when the images are properly calibrated for background intensity, IOD results are proportional to the mean optical density per area. This method determines the concentration of immunoreactive antigen. Two anatomically-matched adjacent sections from each animal were averaged and from each section, we analyzed approximately four images at 200X from each brain region. The entire section comprising the region of interest was analyzed and averaged to generate a single value from the first section that was averaged again with the value obtained in the same way from the second section. Thus, approximately 8 images per animal were analyzed and this value was averaged with the other 3 animals per group.
Measurement of the 5.4 kDa proTRH-derived peptide and TRH in the median eminence
We wanted to determine if altered levels of PC1/3 and PC2 led to increased generation of higher levels of a biologically active hormone. TRH neurons in the PVN project to the median eminence (ME), where they are in close proximity to the capillaries of the hypophysial-portal system. Following placebo or morphine pellet treatment for 24 h or seven days, the rats were sacrificed by decapitation. The brains were dissected out and sliced into 1-mm sections with a brain matrix (Brain Tree Scientific, MA). Punches containing the arcuate nucleus and ME were taken with a stainless steel punch needle of 0.8 mm diameter (Brain Tree Scientific). ARC-ME punches (n=2 per rat) were taken from two consecutives sections between −2.6 and −3.8 mm relative to bregma according to the Paxinos and Watson “The Rat Brain in Stereotaxic Coordinates” (Paxinos and Watson, 1997) All RIAs and antibodies used in this study were developed in Nillni’s laboratory and fully described earlier (Nillni et al., 1995). The tracers were iodinated using the Chloramine T oxidation-reduction method followed by HPLC purification. The RIA assay to measured TRH was performed as standard in our laboratory using a specific TRH antiserum that recognizes only mature TRH peptide. For the detection of the 5.4 kDa C-terminal peptide, anti-preproTRH238-255 [pYE17, see Figure 1 of (Nillni and Sevarino, 1999)] antiserum was used (Nillni et al., 2002). All RIAs were performed using the same volume of material in duplicate. The sensitivity of the TRH and preproTRH238-255 assays were 2.0, 40.0, and 15.0 pg/tube, respectively. The intra- and inter-assay variability were 5-6% and 9-12%, respectively. The TRH antiserum does not cross-react with TRH-Gly, TRH-Gly-Lys or other extended forms of TRH (Nillni et al., 2002). The pYE17 antiserum does not cross-react with TRH (Nillni et al., 2002). The amount in pg of TRH and the 5.4 kDa C-terminal peptide was determined by RIA, the ratio of the two was calculated for each animal (n=3-4) and the ratios were averaged.
Fig. 1.
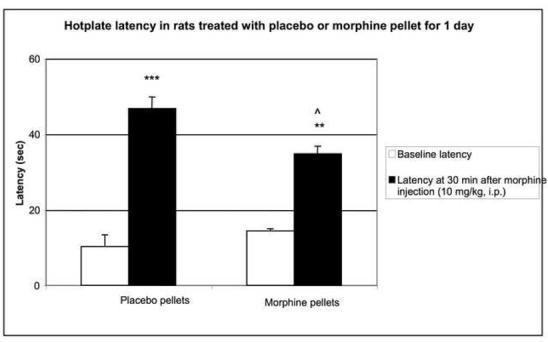
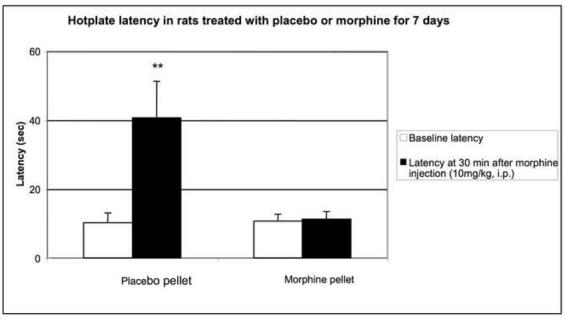
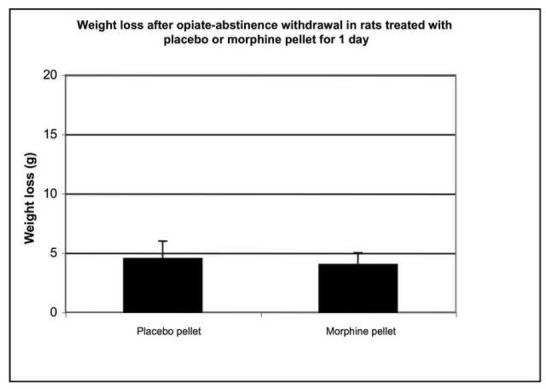
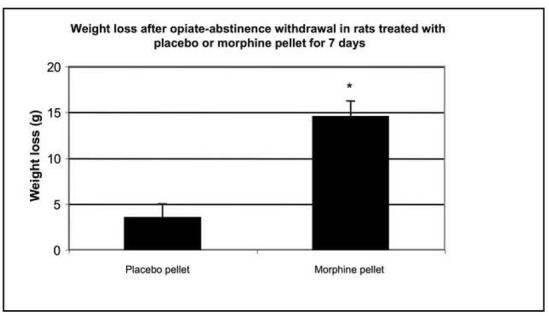
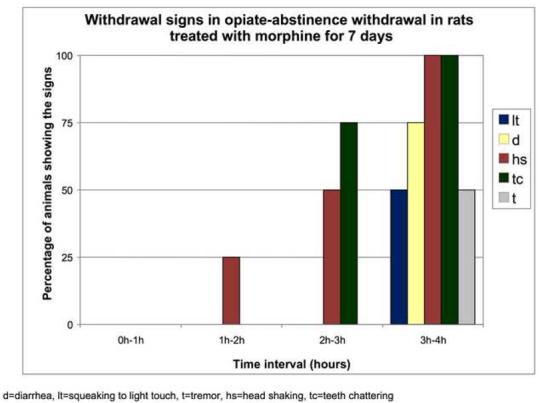
Morphine-induced antinociception and signs of abstinence withdrawal in rats treated with morphine or placebo for 24 hours or seven days. Rats were implanted with placebo or morphine pellets for 24 h or seven days (one pellet on day one and two pellets on day four. After 24-h (1A) or 7-day (1B) pellet implantation, rats were tested on a hot plate preheated to 52.5°C before and 30 min following an injection of morphine (10 mg/kg, ip). To test for signs of opiate-abstinence withdrawal, rats were implanted with a placebo or morphine pellet. Twenty-four hr or 7 days later, pellets were carefully removed and rats were monitored for weight loss (over 30 min) (1C, 1D) and withdrawal signs (over 4 h) (1E). Withdrawal signs were counted over each hour following pellet removal and % of animals showing that withdrawal sign is depicted. d, diarrhea; lt, squeaking to light touch; t, tremor; hs, head shaking; tc, teeth chattering. N=4 per group. **p<0.01 vs baseline, placebo pellet, ***p<0.001 vs baseline, placebo pellet. ^p<0.05 vs. post-morphine placebo pellet,
Cell Culture
The rat sommatomammotrope GH3 cell line was chosen since it expresses PC2 (Friedman et al., 1996) and would be expected to have transcription factors capable of binding to the PC1/3 and PC2 promoters. Because these cells do not endogenously express the mu-opioid receptor (Piros et al., 1995), we used GH3-μ cells [GH3 cells stably transfected with the full-length μ-opioid receptor cDNA (Piros et al., 1995)]. GH3-μ cells (generously provided by Dr. Tim Hales, Georgetown University) and wild-type GH3 cells (American Type Culture Collection, Rockville, MD, USA) were maintained at 37°C and 5% CO2 in Dulbecco’s Minimum Essential Medium (DMEM, Gibco BRL, Gaithersburg, MD, USA) with high glucose, 10% fetal bovine serum (FBS; Gibco BRL) supplemented with 1% antibiotic-antimycotic (Gibco BRL). GH3-μ cells were also grown in G418-Sulfate (Omega Scientific, Tarzana, CA, USA) (1 mg/mL).
Transfection of hPC1/3 promoter and hPC2 promoter into GH3-μ and GH3 cells
GH3-μ cells or GH3 cells in 6-well plates were allowed to adhere overnight and then placed in OPTI-MEM (Gibco BRL) medium and overlaid with DNA/cationic liposomes (Lipofectamine, Gibco BRL) mix. We used a human PC1/3-luciferase promoter construct containing the region of the human PC1/3 promoter from −971 bp to −1 bp relative to the translation initiation codon inserted in the pGL2-Basic vector which contains a luciferase reporter (Promega, Madison, WI, USA) (Jansen et al., 1995). To study the involvement of the CREs in the morphine regulation of PC1/3, the human PC1/3-promoter construct (−971 bp to −1 bp) with the CRE motifs of both CRE-1 and CRE-2 mutated by changing the central core AC dinucleotides to TG dinucleotides (PC1/3-minusCRE1/2) (Jansen et al., 1995). For the human PC2-luciferase promoter construct, we used the DNA from the human PC2 promoter (789 bp to −1 bp relative to the translation initiation codon) inserted in the pGL2-Basic vector (Jansen et al., 1997b). Cells were incubated with DNA (2 μg) for 6 h, then the medium was changed to DMEM serum-free medium for overnight incubation. Cells were then treated with 10 μM morphine (NIDA) or 10 μM morphine plus 10 μM naltrexone (NIDA) in 5% charcoal-stripped fetal bovine serum for 6 h. Cells were washed with PBS and then lysed in 25 mM tris-phosphate (pH 7.8), 10 mM MgCl2, 0.1% BSA, 15% glycerol, 1% Triton X-100 and 1 mM EDTA. After centrifugation, 180 μl of the cleared cell lysate was used for the luciferase assay. Luciferase activity was measured in a Berthold Lumat LB 9501 luminometer (Wallace, Inc., Gaithersburg, MD) in the presence of 0.8 mM ATP and 0.3 mM D-luciferin. Integrated light emission over 15 sec was measured. All transfections were done in duplicate and repeated at least three times.
Statistical analyses
The data were expressed as the mean ± SEM. Unpaired Student’s t-test was used to compare two groups and one-way analysis of variance with Newman-Keuls’s post-hoc t-tests to compare more than two groups. Significance level was set at p< 0.05.
RESULTS
Tolerance and withdrawal in rats implanted with morphine for 24-h or 7-day
To assess the degree of tolerance to and dependence on morphine in rats exposed to morphine for seven days as compared to 24-h, we performed the hot plate test and abstinence withdrawal studies. In the hot plate test, acute morphine (10 mg/kg) administration prolonged nociceptive latency in rats exposed to morphine- or placebo for 24 h (Fig. 1A). Although the response to morphine was significantly reduced in rats implanted with morphine for 24 hours compared to their placebo-implanted control group (p<0.05), it was still robust, showing some degree of tolerance to the antinociceptive effect of morphine. In contrast, rats treated for 7 days with morphine exhibited complete tolerance to the same dose of morphine (Fig. 1B). Opiate abstinence withdrawal, induced by morphine pellet removal, did not lead to weight loss in the 24-h morphine-pretreated rats (Fig. 1C), while substantial weight loss was observed in 7-day morphine-pretreated rats (Fig. 1D). Furthermore, withdrawal signs (diarrhea, squeaking to light touch, tremor, head shaking and teeth chattering) were seen in the rats following morphine pellet removal on day 7 (Fig. 1E). In contrast, no withdrawal signs were observed when morphine pellet was removed 24 h following implantation (data not shown). These results indicate that long-term morphine administration leads to a greater magnitude of tolerance and opiate dependency as compared to short-term morphine treatment. Thus, we used these two different forms (short- and long-term) of morphine treatment protocols to study the effect of short- and long-term morphine treatment on the level of PCs and P-CREB.
PC1/3, PC2 and P-CREB levels in morphine treated rats as determined by Western blot
We found a doublet of 43kDa and a 38 kDa bands corresponding to P-CREB and phosphorylated ATF-1, respectively, in the hypothalamus (Fig. 2A, B). We detected the 66 kDa form of PC1/3 and the 64 kDa form of PC2. We found a reduction in the levels of P-CREB (p< 0.05, Fig. 2A), PC1/3 (p< 0.05, Fig. 2C) and PC2 (p< 0.01, Fig. 2E) in the hypothalamus of rats implanted with morphine for 24 h, compared to placebo-treated rats. Seven-day morphine treatment induced a significant increase in the levels of hypothalamic P-CREB (Fig. 2B, p<0.01), PC1/3 (Fig. 2D, p<0.001) and PC2 (Fig. 2F, p<0.01) protein levels in comparison with animals implanted with placebo.
Fig. 2.
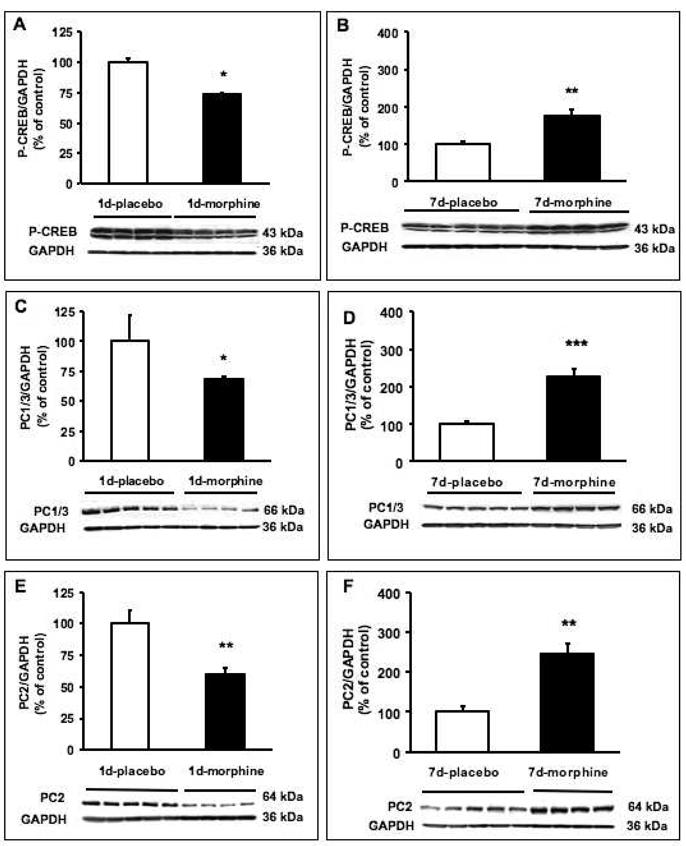
Effect of morphine on P-CREB, PC1/3 or PC2 protein levels in the hypothalamus in rats treated with morphine or placebo for 24 h or seven days. Rats were implanted with a placebo (open bars) or morphine (filled bars) pellet for 24 h (A, C, E) or seven days (B, D, F). Western blot measuring the level of P-CREB (A, B), PC1/3 (C, D) or PC2 (E, F) in hypothalamic extracts of the rats was performed. The same membranes were stripped and then immunoblotted with GAPDH antibody as a control. Densitometry of the bands expressing P-CREB, PC1/3 or PC2 was compared to GAPDH for quantitation. The data are expressed in arbitrary units as the mean +/- SEM. *p<0.05, **p<0.01, ***p<0.001.
P-CREB, PC1/3 and PC2 immunoreactivity in the PVN of morphine-treated rats
We performed single immunofluorescence against P-CREB, PC1/3 and PC2 protein and found that in the PVN of placebo-treated rats, the cells showed discrete staining for P-CREB (Fig. 3A, C), PC1/3 (Fig. 3E, G) and PC2 (Fig. 3I, K) protein. In the PVN of rats exposed to morphine for 24 h, there was a decrease in the levels of P-CREB (Fig. 3B, M; p<0.05) and PC1/3 (Fig. 3F, M; p<0.05) but not PC2 (Fig. 3J, O) positive neurons compared to their respective controls (Fig. 3A, E, I). This decrease was selective for P-CREB, as the decrease occurred in the parvocellular neurons located at the paraventricular hypothalamic medial area (PaMP) and the posterior region of the hypothalamic nucleus (PaPo) (Fig. 3A, B) but did not occur in other areas of the PVN for PC1/3 (Fig. 3E, F) or PC2 (Fig. 3I, J). CREB levels were not affected by short- or long-term morphine exposure in the PVN or other brain regions (data not shown).
Fig. 3.
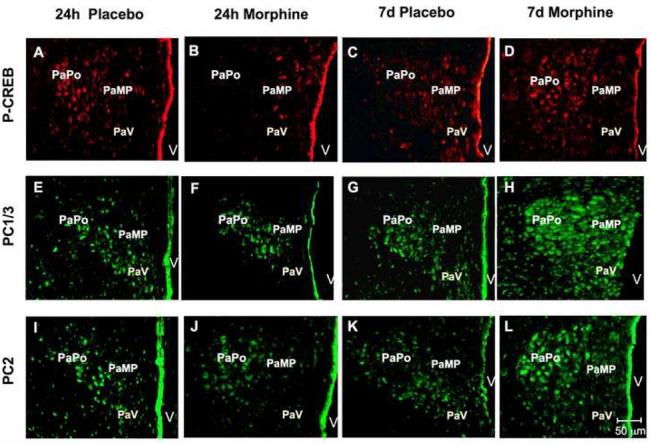
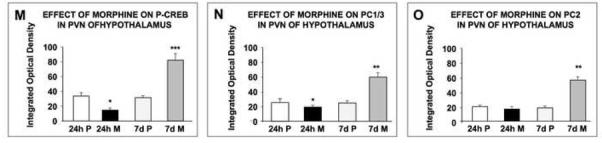
Changes in P-CREB, PC1/3 and PC2 levels in the paraventricular (PVN) nucleus of the hypothalamus of rats treated with morphine for 24 h or seven days as determined by immunofluorescence. Placebo or morphine pellets were implanted subcutaneously in the neck of rats for 24 h or seven days. Rats were then sacrificed, perfused with paraformaldehyde and their brains excised and fixed. Coronal sections of frozen slices (8-μM thick) of the paraventricular nucleus of the hypothalamus were cut, mounted, and subjected to immunofluorescence for P-CREB (A-D), PC1/3 (E-H) and PC2 (I-L). Figures are representatives of 4 rats treated/group. Quantification of P-CREB (M), PC1/3 (N) and PC2 (O) immunoreactivity in the PVN expressed as the Integrated Optical Density/area is also shown. Data is expressed as mean +/- SEM. n=4 rats per group. Magnification 200X. Bar = 50 μM, *p<0.05, **p<0.005. ***p<0.0005. P, placebo; M, morphine. PaV: paraventricular nucleus, ventral. PaMP: medial paraventricular nucleus, PaPo: paraventricular nucleus, posterior, V: third ventricle.
In contrast, P-CREB (Fig. 3D, M; p<0.0005), PC1/3 (Fig. 3H, N; p<0.005) and PC2 (Fig. 3L, O; p<0.005) protein levels increased in PVN of rats treated with morphine for seven days. The increase in P-CREB, PC1/3 and PC2 was found to be most abundant in the ventral region of the paraventricular hypothalamic nucleus, posterior part (PaPo) and in the paraventricular hypothalamic nucleus, medial parvicellular part (PaMP) (Fig. 3). Preliminary experiments suggested a high degree of co-localization between PC1/3 and P-CREB as well as between PC2 and P-CREB (data not shown).
P-CREB, PC1/3 and PC2 immunoreactivity in the dorso-medial (DM) nucleus of morphine-treated rats
In the DM nucleus of rats treated with placebo pellets, the neurons showed discrete staining for P-CREB (Fig. 4A, C), PC1/3 (Fig. 4E, G) and PC2 (Fig. 4I, K) protein. Following exposure to morphine for 24 h, there was a decrease in the levels of P-CREB (Fig. 4B, M; p<0.05), PC1/3 (Fig. 4F, N; p<0.005) and PC2 (Fig. 4J, O; p<0.005) positive neurons in the DM nucleus compared to their respective controls (Fig. 4A, E, I). Rats treated with morphine for seven days showed a significant increase in the number of P-CREB (Fig. 4D, M; p<0.005), PC1/3 (Fig. 4H, N; p<0.005) and PC2 (Fig. 4L, O; p<0.005) positive neurons. The decrease with 24-h morphine and the increase with seven-day morphine treatment occurred throughout this brain region.
Fig. 4.
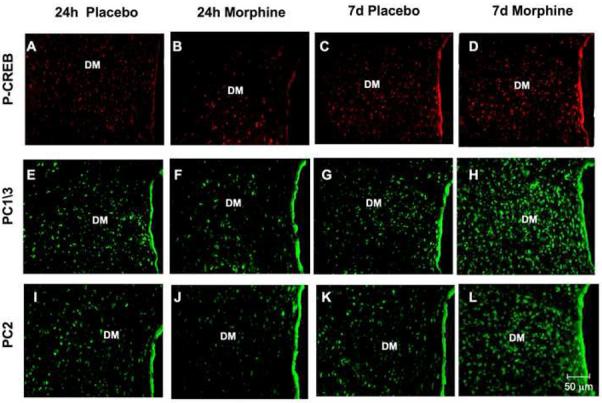
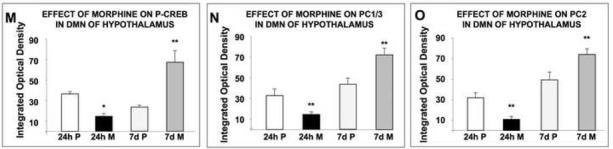
Changes in P-CREB, PC1/3 and PC2 levels in the dorsomedial (DM) nucleus of the hypothalamus of rats after short-term or long-term morphine treatment as determined by immunofluorescence. Rats were implanted with a placebo or morphine pellet for 24 h or seven day. Rats were then sacrificed, perfused with paraformaldehyde and their brains excised and fixed. Coronal sections of frozen slices (8-μm thick) of the dorsomedial nucleus of the hypothalamus were mounted and subjected to immunofluorescence for P-CREB (A-D), PC1/3 (E-H) and PC2 (I-L). Figures are representatives of four rats/group. Quantification of P-CREB (M), PC1/3 (N) and PC2 (O) immunoreactivity is expressed as the mean +/- SEM Integrated Optical Density/area. Magnification 200X. Bar = 50μm. *p<0.05, **p<0.005. P, placebo; M, morphine.
P-CREB, PC1/3 and PC2 immunoreactivity in the anterolateral nucleus of the amygdala (AHiAL) of morphine-treated rats
The amygdala is important for conditioned aspects of drug exposure (Chao and Nestler, 2004). We found that rats exposed to morphine for 24 h demonstrated a decrease in P-CREB levels (p<0.005; Fig. 5A, B, M), while no change was observed in the level of the PC1/3 (Fig. 5E, F, N) and PC2 (Fig. 5I, J, O) in these rats compared to their respective controls. In contrast, rats treated for seven days with morphine demonstrated a significant (p<0.005; Fig. 5M) increase in the levels of P-CREB (Fig. 5C, D), but no change in PC1/3 (Fig. 5G, H, N) and PC2 (Fig. 5K, L, O) expression.
Fig. 5.
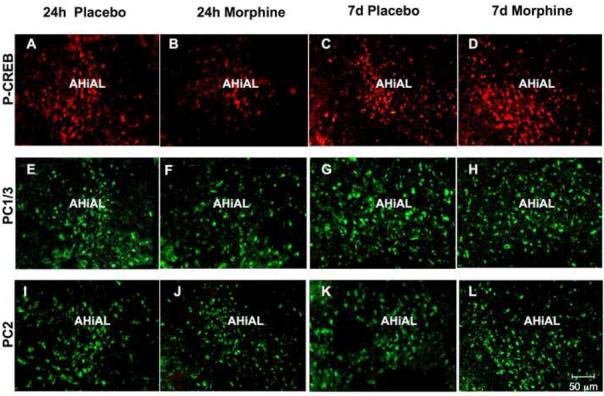
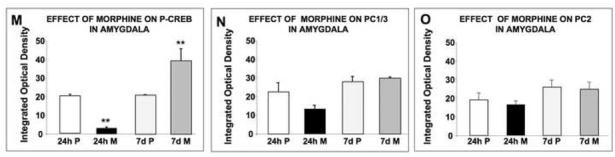
Changes in P-CREB, PC1/3 and PC2 levels in the anterolateral nucleus of the amygdala (AHiAL) of rats after short-term or long-term morphine treatment. Placebo or morphine pellets were implanted subcutaneously in the neck of rats for 24 h or seven days. Rats were then sacrificed, perfused with paraformaldehyde and their brains excised and fixed. Coronal sections of frozen slices (8-μM thick) were mounted and immunofluorescence for P-CREB (A-D), PC1/3 (E-H) and PC2 (I-L) was performed. Figures are representative of 4 rats/group analyzed. Quantification of P-CREB (M), PC1/3 (N) and PC2 (O) immunoreactivity is expressed as the mean +/- SEM Integrated Optical Density/area. n=4 rats per group. Magnification 200X. Bar = 50 μM. **p<0.005. P, placebo; M, morphine.
TRH/pro-TRH-derived peptide ratio in the ARC-ME of morphine-treated rats
To determine if the change in PC1/3 and PC2 levels alters the synthesis of a biologically active peptide, we measured the ratio between TRH and the 5.4 kDa C-terminal pro-TRH peptide after short-term and long-term morphine administration in the ARC-ME. The 5.4 kDa C-terminal pro-TRH-derived peptide is generated along with other intermediate peptides in equal molar amounts form the pre-pro-TRH molecule and can be used to normalize for the amount of prohormone present. As shown in Fig. 6, there was a significant effect of treatment (F3,10 = 32.1; p<0.0001) on the TRH/5.4 kDA peptide ratio. Post-hoc analysis revealed a significant increase (p<0.001) in the 7-day morphine treatment compared to 7-day placebo treatment. There was no effect of the 24-h morphine treatment compared to placebo.
Fig. 6.
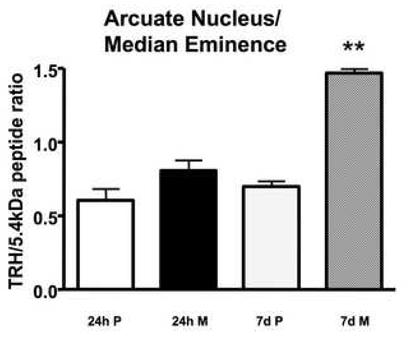
Measurement of the 5.4 kDa C-terminal proTRH-derived peptide and TRH in the arcuate nucleus/median eminence. Rats were implanted with placebo or morphine pellets for 24 h or seven days. The amount in pg of TRH and the 5.4 kDa C-terminal proTRH-derived peptide was determined by specific RIA, the ratio of the two was calculated for each animal (n=3-4) and the ratios were averaged. **p<0.001. P, placebo; M, morphine.
Regulation of human PC1/3 (hPC1/3) promoter and human PC2 (hPC2) promoter activity in GH3 cells by morphine
Our co-localization results suggest that 24-h exposure to morphine down-regulated PC1/3 and PC2 and seven-day morphine up-regulated PC1/3 and PC2 by phosphorylation of CREB. To provide causal evidence that the regulation of PC1/3 and PC2 by morphine involves the cAMP/CREB system, we transfected pituitary GH3 cells with promoters for human PC1/3 and PC2 and then examined the regulation of promoter activity by morphine. Human PC1/3 promoter activity was 73.1±6.1% in GH3-μ receptor cells receiving morphine compared to cells receiving media (p <0.05), and this reduction was reversed by naltrexone (Fig. 7A). Naltrexone alone stimulated PC1/3 promoter activity (p <0.05). In cells transfected with the PC1/3-minusCRE1/2 promoter construct, morphine did not reduce PC1/3-promoter activity (Fig. 7A) indicating that the morphine-induced inhibition of PC1/3 promoter activity occurs via these CREs. Human PC2 promoter activity was 31.5±1.1% in GH3-μ receptor cells receiving morphine compared to cells receiving media (Fig. 7B; p <0.0001), and this reduction was also reversed by naltrexone. In wild-type GH3 cells (without the mu-opioid receptor), neither human PC1/3 promoter nor PC2 promoter activity responded to morphine treatment (Fig. 7C). These results indicate that in this cell culture system, the regulation of PC1/3 promoter and PC2 promoter activity by morphine is mediated through the μ receptor.
Fig. 7.
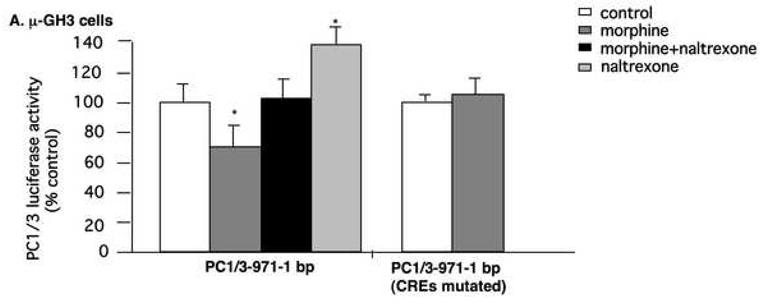
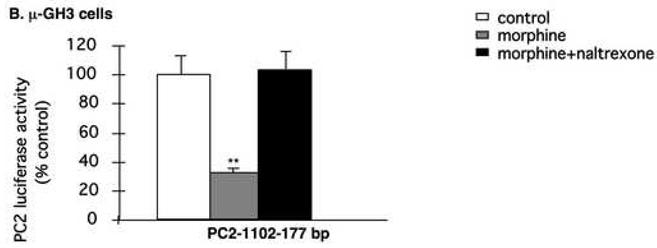
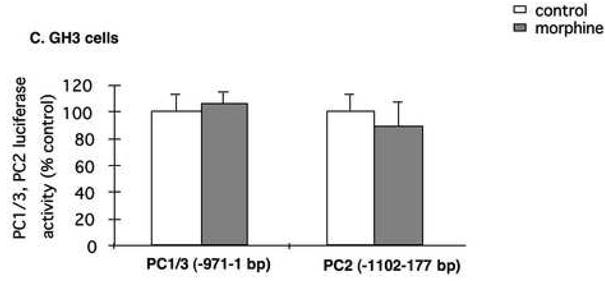
Regulation of hPC1/3 and hPC2 promoter activity by morphine. The human PC1/3-promoter-luciferase reporter construct, containing cDNA from −971 bp to −1 bp, relative to the translation initiation codon, and the PC1/3 construct containing cDNA from −971 bp to −1 bp with both CREs mutated were transiently transfected into GH3-μ receptor in 6-well plates (A). After six h, cells were incubated overnight in serum-free medium and subsequently treated with either 10 μM morphine, 10 μM naltrexone or 10 μM morphine plus 10 μM naltrexone for six h. Cells were then harvested and luciferase activity was measured. The results are expressed as mean ± SEM of luciferase activity compared to control cells of 3-4 experiments. Regulation of hPC2-promoter activity containing cDNA from −789 bp to −1 bp and hPC2-promoter containing cDNA from −789 bp to −1 bp promoter with its CREs mutated by morphine or morphine plus naltrexone in GH3-μ receptor cells (B). Regulation of hPC1/3 and hPC2 promoter activity by morphine in GH3 cells without expression of the mu-opioid receptor (C). *p<0.05, **p<0.0005.
DISCUSSION
Allostasis is a concept of physiology where an organism alters many parameters of its internal milieu to cope with external threats (McEwen, 1998). As these external disturbances continue, the organism must expend more energy to maintain internal stability at a new pathological setpoint. Eventually the organism can no longer compensate for this dysregulation, resulting in a disease state. It has been hypothesized that opiate intake is an example of an allostatic challenge causing dysregulation of the brain’s neurochemical system leading to loss of control of drug intake, followed by drug addiction (Koob and Le Moal, 1997). As the organism has a new, albeit pathological, setpoint, tolerance to the drug occurs, and if drug intake ceases, withdrawal symptoms occur. Thus, short-term drug use, chronic drug use, and withdrawal may be three distinct states, each with its own unique compensatory molecular processes. The molecular mechanisms of these changes may be mediated by alterations in intracellular cAMP levels. Short-term opiate exposure decreases intracellular cAMP levels in various brain regions, whereas chronic use increases cAMP levels and withdrawal causes a further increase in cAMP levels (Nestler and Aghajanian, 1997). This increased cAMP mediates parallel changes in the phosphorylation of CREB and other cAMP-dependent transcription factors (i.e. cAMP response element modulator; CREM) followed by changes in transcription of genes with promoters containing CREs (Guitart et al., 1992, Lane-Ladd et al., 1997). This pattern has been best studied in the locus coeruleus; however, Shaw-Lutchman et al. (Shaw-Lutchman et al., 2002) used CRE-LacZ transgenic mice expressing the reporter gene, Lac Z (encoding β-galactosidase) under the control of CRE-consensus sequences and found increased activity of the cAMP/CREB system following chronic morphine and naltrexone-precipitated withdrawal in several brain areas. Short-term morphine was not examined.
The CREB system plays a key role in drug dependence. CREBαΔ mutant mice showed reduced behavioral and physiological responses to opiate withdrawal in all nine withdrawal parameters examined (Maldonado et al., 1996), demonstrating that CREB is involved in opiate dependence. Naloxone-precipitated withdrawal induced similar changes in the immediate early genes (IEGs), c-fos and c-jun expression in CREBαΔ mutant mice and wild-type mice demonstrating that IEGs are not involved in CREB-mediated opiate withdrawal. The authors concluded that the effects of CREB on other target genes might lead to the altered behavioral response to opiate withdrawal. These other target genes are unknown; we speculate that some of them may be the important CRE-dependent processing enzymes, PC1/3 and PC2.
The proximal region of the PC1/3 promoter contains one consensus CRE (TGACGTCA, CRE-1) between −283 and −276 of the human PC1/3 promoter (relative to the translation start site) and one CRE-like motif (TGACGTGT, CRE-2) between −255 and −248 of the human PC1/3 promoter (Jansen et al., 1995). Both CREs are essential for cAMP-mediated activation of PC1/3 gene transcription (Jansen et al., 1995, 1997a) and are conserved between mice, rat and human. Moreover, site-specific mutagenesis of both elements completely blocked cAMP-mediated PC1/3 transcription by abolishing the binding of their respective trans-acting factors (Jansen et al., 1995, 1997a). The PC2 promoter contains a slightly different CRE-like sequence (TCACGTCA) at position −365-358 of the human PC2 promoter (Jansen et al., 1997b). Thus, the absolute dependence of PC1/3 (and most likely PC2) on activation of the P-CREB system led us to examine the effects of short-term morphine (which down-regulates P-CREB) and long-term morphine (which up-regulates P-CREB) on PC1/3 and PC2 levels. Our interest in how exogenous opiates affect neuropeptide biosynthesis led us to examine the regulation of P-CREB, PC1/3 and PC2 in the pro-neuropeptide-rich regions of the hypothalamus, as well as the amygdala, a brain region which is not rich in neuropeptides but chronic morphine was shown to up-regulate the cAMP system in this region (Terwilliger et al., 1991).
Our major findings were that short-term (24-h) morphine exposure down-regulated and long—term (seven-day) morphine treatment up-regulated P-CREB, PC1/3 and PC2 protein levels in the rat hypothalamus. In general, the increase in expression of these proteins was more pronounced after chronic morphine treatment than the decrease in their levels following short-term morphine treatment, and these results obtained by both Western blot analysis and immunofluorescence were in good agreement. Although the PC1/3 fusion antibody can detect the 87, 74 and 66 kDa forms of PC1/3 (Hill et al., 1995), the predominant form seen with this antibody in pituitary (Hill et al., 1995), AtT-20 cells (Li et al., 1999) and spleen (Nakashima et al., 2001) was the 66 kDa form, a finding confirmed in the hypothalamus in this paper. Similarly, we detected the 64 kDa form of PC2, a finding similar to previous reports (Hill et al., 1995, Nakashima et al., 2001). In the amygdala, we found that morphine regulated P-CREB, but not PC1/3 and PC2, a finding we explain as being due to the presence of different co-activators or co-repressors in this brain region, which limits the effect of changes in P-CREB on PC1/3 or PC2 levels.
Our findings of increased PC expression following long-term morphine administration agree with a clinical observation that post-mortem brains of heroin users had elevated PC2 expression in the striatum and nucleus accumbens shell, but not in the caudate nucleus and NAc core, compared to control brains (Drakenberg et al., 2006). In this paper, PC1/3 expression was not reported. Interestingly, opiate withdrawal, which also leads to increased P-CREB levels (Chao and Nestler, 2004) led to increased PC2 protein levels in the midbrain periaqueductal gray matter (PAG) and increased TRH levels in the lateral hypothalamus (Nillni et al., 2002). Neither TRH nor the 5.4 kDa C-terminal peptide was changed following opiate withdrawal in the median eminence implying a different mechanism of regulation or TRH biosynthesis in opiate withdrawal as compared to opiate addiction.
Most pro- and antinociceptive neuropeptides are synthesized as larger propeptides and cleaved to active neuropeptides by PC1/3 and PC2. These neuropeptides include enkephalins (ENKs), dynorphins (DYNs), β-endorphin, substance P, nociceptin/orphanin FQ, cholecystokinin, CRH, neurotensin/neuromedin N, somatostatin, oxytocin and galanin. It has been postulated that a delicate balance between pro- and antinociceptive peptides has an important role in addiction parameters (Koob and Le Moal, 1997). The differential regulation by short- and long-term morphine treatment may suggest that in short-term opiate exposure, the precursor to the opiate is responsive to opioids (end-product inhibition) and therefore there is decreased synthesis of endogenous opioid peptides and possibly other neuropeptide as well. On the other hand, we postulate that upon chronic morphine treatment, due to the development of tolerance and other compensatory changes, increased levels of the enzymes responsible for neuropeptide biosynthesis could lead to increased synthesis of opioids as well as anti-opioid peptides responsible for tolerance.
We found that in GH3-μ cells, short-term morphine treatment decreased PC1/3 and PC2 promoter activity, an effect blocked by naltrexone and absent in wild-type GH3 cells, lacking mu-opiate receptors. The effect of morphine was greater on the PC2 promoter than the PC1/3 promoter. This may be due to the fact that GH3 cells contain PC2 endogenously (Friedman et al., 1996) and presumably have cAMP-dependent transcription factors more capable of binding to the CRE on the PC2 promoter than the PC1/3 promoter. However, morphine also down-regulated PC1/3 promoter activity, demonstrating that GH3 cells contain also transcription factors capable of affecting the CRE on the PC1/3 promoter.
In conclusion, this study demonstrates that short-term morphine down-regulates and long-term morphine up-regulates hypothalamic P-CREB, PC1/3 and PC2 levels. This may be a potential pathway to uncover mechanisms of how opiate use leads to opiate addiction. Further studies are therefore needed to examine the physiological consequences of alterations of PC levels following opiate use.
Acknowledgments
This work was supported by NIH grants R01 DA14659 and K21 DA00276 and a Charles E. Culpeper Foundation Research Award to T.C.F., RCMI Program grant G21 RR03026-13, an NIH (R24DA017298) Minority Institution Drug Abuse Research Program (MIDARP) Program and a Center of Clinical Research Excellence grant (U54 RR14616-01) to The Charles Drew University of Medicine & Sciences. K.L. was supported by NIH R01 DA016682. Y.L. was supported by NIH K01 DK073272. E.A.N. was supported by NIH grants R01 DK58148 and R01 NS045231.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
At the 6th Gordon Research Conference on Proprotein Processing, Trafficking and Secretion (2004), the leading researchers agreed to use the terminology, PC1/3 to describe the identical PC1 and PC3 prohormone convertase.
REFERENCES
- Berman Y, Mzhavia N, Polonskaia A, Furuta M, Steiner DF, Pintar JE, Devi LA. Defective prodynorphin processing in mice lacking prohormone convertase PC2. J Neurochem. 2000;75:1763–1770. doi: 10.1046/j.1471-4159.2000.0751763.x. [DOI] [PubMed] [Google Scholar]
- Blendy JA, Maldonado R. Genetic analysis of drug addiction: the role of cAMP response element binding protein. J Mol Med. 1998;76:104–110. doi: 10.1007/s001090050197. [DOI] [PubMed] [Google Scholar]
- Boundy VA, Gold SJ, Messer CJ, Chen J, Son JH, Joh TH, Nestler EJ. Regulation of tyrosine hydroxylase promoter activity by chronic morphine in TH9.0-LacZ transgenic mice. J Neurosci. 1998;18:9989–9995. doi: 10.1523/JNEUROSCI.18-23-09989.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstein DM, Przewlocki R, Akil H. Effects of morphine treatment on proopiomelanocortin systems in rat brain. Brain Res. 1990;519:102–111. doi: 10.1016/0006-8993(90)90066-k. [DOI] [PubMed] [Google Scholar]
- Chao J, Nestler EJ. Molecular neurobiology of drug addiction. Annu Rev Med. 2004;55:113–132. doi: 10.1146/annurev.med.55.091902.103730. [DOI] [PubMed] [Google Scholar]
- Day R, Schafer MK-H, Watson SJ, Chretien M, Seidah NG. Distribution and regulation of the prohormone convertases PC1 and PC2 in the rat pituitary. Mol Endocrinol. 1992;6:485–497. doi: 10.1210/mend.6.3.1316544. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Jones BE, Boutrel B, Borgland SL, Nishino S, Bubser M, DiLeone R. Addiction and arousal: alternative roles of hypothalamic peptides. J Neurosci. 2006;26:10372–10375. doi: 10.1523/JNEUROSCI.3118-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLeone RJ, Georgescu D, Nestler EJ. Lateral hypothalamic neuropeptides in reward and drug addiction. Life Sci. 2003;73:759–768. doi: 10.1016/s0024-3205(03)00408-9. [DOI] [PubMed] [Google Scholar]
- Drakenberg K, Nikoshkov A, Horvath MC, Fagergren P, Gharibyan A, Saarelainen K, Rahman S, Nylander I, Bakalkin G, Rajs J, Keller E, Hurd YL. Mu opioid receptor A118G polymorphism in association with striatal opioid neuropeptide gene expression in heroin abusers. Proc Natl Acad Sci U S A. 2006;103:7883–7888. doi: 10.1073/pnas.0600871103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Tallman JF, Nestler EJ. Acute and chronic opiate-regulation of adenylate cyclase in brain: specific effects in locus coeruleus. J Pharmacol Exp Ther. 1988;246:1033–1039. [PubMed] [Google Scholar]
- Friedman TC, Cool DR, Jayasvasti V, Louie D, Loh YP. Processing of proopiomelanocortin in GH3 cells: inhibition by prohormone convertase 2 (PC2) antisense mRNA. Mol Cell Endocrinol. 1996;116:89–96. doi: 10.1016/0303-7207(95)03702-0. [DOI] [PubMed] [Google Scholar]
- Furuta M, Yano H, Zhou A, Rouille Y, Holst JJ, Carroll R, Ravazzola M, Orci L, Furuta H, Steiner DF. Defective prohormone processing and altered pancreatic islet morphology in mice lacking active SPC2. Proc Natl Acad Sci U S A. 1997;94:6646–6651. doi: 10.1073/pnas.94.13.6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia de Yebenes E, Pelletier G. Opioid regulation of proopiomelanocortin (POMC) gene expression in the rat brain as studied by in situ hybridization. Neuropeptides. 1993;25:91–94. doi: 10.1016/0143-4179(93)90087-q. [DOI] [PubMed] [Google Scholar]
- Guitart X, Thompson MA, Mirante CK, Greenberg ME, Nestler EJ. Regulation of cyclic AMP response element-binding protein (CREB) phosphorylation by acute and chronic morphine in the rat locus coeruleus. J Neurochem. 1992;58:1168–1171. doi: 10.1111/j.1471-4159.1992.tb09377.x. [DOI] [PubMed] [Google Scholar]
- Hakes DJ, Birch NP, Mezey E, Dixon JE. Isolation of two complementary deoxyribonucleic acid clones from a rat insulinoma cell line based on similarities to Kex2 and furin sequences and the specific localization of each transcript to endocrine and neuroendocrine tissues in rats. Endocrinology. 1991;129:3053–3063. doi: 10.1210/endo-129-6-3053. [DOI] [PubMed] [Google Scholar]
- Hill RM, Ledgerwood EC, Brennan SO, Loh YP, Christie DL, Birch NP. Characterization of the molecular forms and membrane association of Kex2/subtilisin-like serine proteases in neuroendocrine secretory vesicles. J Neurochem. 1995;65:2318–2326. doi: 10.1046/j.1471-4159.1995.65052318.x. [DOI] [PubMed] [Google Scholar]
- Ho WKK, Wen HL, Ling N. Beta-endorphin like immunoreactivity in the plasma of heroin addicts and normal subjects. Neuropharmacology. 1980;19:117–120. doi: 10.1016/0028-3908(80)90175-6. [DOI] [PubMed] [Google Scholar]
- Jansen E, Ayoubi TA, Meulemans SM, Van de Ven WJ. Neuroendocrine-specific expression of the human prohormone convertase 1 gene. Hormonal regulation of transcription through distinct cAMP response elements. J Biol Chem. 1995;270:15391–15397. doi: 10.1074/jbc.270.25.15391. [DOI] [PubMed] [Google Scholar]
- Jansen E, Ayoubi TA, Meulemans SM, Van de Ven WJ. Cell type-specific protein-DNA interactions at the cAMP response elements of the prohormone convertase 1 promoter. Evidence for additional transactivators distinct from CREB/ATF family members. J Biol Chem. 1997a;272:2500–2508. doi: 10.1074/jbc.272.4.2500. [DOI] [PubMed] [Google Scholar]
- Jansen E, Ayoubi TA, Meulemans SM, Van De Ven WJ. Regulation of human prohormone convertase 2 promoter activity by the transcription factor EGR-1. Biochem J. 1997b;328:69–74. doi: 10.1042/bj3280069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Lamas M, Molina C, Foulkes NS, Jansen E, Sassone-Corsi P. Ectopic ICER expression in pituitary corticotroph AtT20 cells: effects on morphology, cell cycle, and hormonal production. Mol Endocrinol. 1997;11:1425–1434. doi: 10.1210/mend.11.10.9987. [DOI] [PubMed] [Google Scholar]
- Lane-Ladd SB, Pineda J, Boundy VA, Pfeuffer T, Krupinski J, Aghajanian GK, Nestler EJ. CREB (cAMP response element-binding protein) in the locus coeruleus: biochemical, physiological, and behavioral evidence for a role in opiate dependence. J Neurosci. 1997;17:7890–7901. doi: 10.1523/JNEUROSCI.17-20-07890.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent V, Jaubert-Miazza L, Desjardins R, Day R, Lindberg I. Biosynthesis of proopiomelanocortin-derived peptides in prohormone convertase 2 and 7B2 null mice. Endocrinology. 2004;145:519–528. doi: 10.1210/en.2003-0829. [DOI] [PubMed] [Google Scholar]
- Leshner AI. Addiction is a brain disease, and it matters. Science. 1997;278:45–47. doi: 10.1126/science.278.5335.45. [DOI] [PubMed] [Google Scholar]
- Li Q-L, Jansen E, Friedman TC. Regulation of prohormone convertase 1 (PC1) by gp130-related cytokines. Mol Cell Endocrinol. 1999;158:143–152. doi: 10.1016/s0303-7207(99)00168-9. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Blendy JA, Tzavara E, Gass P, Roques BP, Hanoune J, Schutz G. Reduction of morphine abstinence in mice with a mutation in the gene encoding CREB. Science. 1996;273:657–659. doi: 10.1126/science.273.5275.657. [DOI] [PubMed] [Google Scholar]
- Martin KC, Kandel ER. Cell adhesion molecules, CREB, and the formation of new synaptic connections. Neuron. 1996;17:567–570. doi: 10.1016/s0896-6273(00)80188-9. [DOI] [PubMed] [Google Scholar]
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dolle P, Tzavara E, Hanoune J, Roques BP, Kieffer BL. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- Nakashima M, Nie Y, Li QL, Friedman TC. Up-regulation of splenic prohormone convertases PC1 and PC2 in diabetic rats. Regul Pept. 2001;102:135–145. doi: 10.1016/s0167-0115(01)00311-1. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular neurobiology of addiction. Am J Addict. 2001;10:201–217. doi: 10.1080/105504901750532094. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Aghajanian GK. Molecular and cellular basis of addiction. Science. 1997;278:58–63. doi: 10.1126/science.278.5335.58. [DOI] [PubMed] [Google Scholar]
- Nillni EA, Friedman TC, Todd RB, Birch NP, Loh YP, Jackson IM. Pro-thyrotropin-releasing hormone processing by recombinant PC1. J Neurochem. 1995;65:2462–2472. doi: 10.1046/j.1471-4159.1995.65062462.x. [DOI] [PubMed] [Google Scholar]
- Nillni EA, Lee A, Legradi G, Lechan RM. Effect of precipitated morphine withdrawal on post-translational processing of prothyrotropin releasing hormone (proTRH) in the ventrolateral column of the midbrain periaqueductal gray. J Neurochem. 2002;80:874–884. doi: 10.1046/j.0022-3042.2002.00763.x. [DOI] [PubMed] [Google Scholar]
- Nillni EA, Sevarino KA. The biology of pro-thyrotropin-releasing hormone-derived peptides. Endocr Rev. 1999;20:599–648. doi: 10.1210/edrv.20.5.0379. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1997. [Google Scholar]
- Piros ET, Prather PL, Loh HH, Law PY, Evans CJ, Hales TG. Ca2+ channel and adenylyl cyclase modulation by cloned mu-opioid receptors in GH3 cells. Mol Pharmacol. 1995;47:1041–1049. [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Carrera M, Navarro M, Koob GF, Weiss F. Activation of corticotropin-releasing factor in the limbic system during cannabinoid withdrawal. Science. 1997;276:2050–2054. doi: 10.1126/science.276.5321.2050. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi P. Transcription factors responsive to cAMP. Ann Rev Cell Dev Biol. 1995;11:355–377. doi: 10.1146/annurev.cb.11.110195.002035. [DOI] [PubMed] [Google Scholar]
- Seidah NG, Chretien M. Pro-protein convertases of subtilisin/kexin family. Methods Enzymol. 1994;244:175–188. doi: 10.1016/0076-6879(94)44015-8. [DOI] [PubMed] [Google Scholar]
- Seidah NG, Gaspar L, Mion P, Marcinkiewicz M, Mbikay M, Chretien M. cDNA sequence of two distinct pituitary proteins homologous to Kex2 and furin gene products: tissue-specific mRNAs encoding candidates for pro-hormone processing proteinases. DNA Cell Biol. 1990;9:415–424. doi: 10.1089/dna.1990.9.415. [DOI] [PubMed] [Google Scholar]
- Seidah NG, Marcinkiewicz M, Benjannet S, Gaspar L, Beaubien G, Mattei MG, Lazure C, Mbikay M, Chretien M. Cloning and primary sequence of a mouse candidate prohormone convertase PC1 homologous to PC2, furin, and Kex2: distinct chromosomal localization and messenger RNA distribution in brain and pituitary compared to PC2. Mol Endocrinol. 1991;5:111–122. doi: 10.1210/mend-5-1-111. [DOI] [PubMed] [Google Scholar]
- Shaw-Lutchman TZ, Barrot M, Wallace T, Gilden L, Zachariou V, Impey S, Duman RS, Storm D, Nestler EJ. Regional and cellular mapping of cAMP response element-mediated transcription during naltrexone-precipitated morphine withdrawal. J Neurosci. 2002;22:3663–3672. doi: 10.1523/JNEUROSCI.22-09-03663.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler R, Zhou Y, Maggos CE, Zlobin A, Ho A, Kreek MJ. Dopamine antagonist and “binge’ cocaine effects on rat opioid and dopamine transporter mRNAs. Neuroreport. 1996;7:2196–2200. doi: 10.1097/00001756-199609020-00028. [DOI] [PubMed] [Google Scholar]
- Terwilliger RZ, Beitner-Johnson D, Sevarino KA, Crain SM, Nestler EJ. A general role for adaptations in G-proteins and the cyclic AMP system in mediating the chronic actions of morphine and cocaine on neuronal function. Brain Res. 1991;548:100–110. doi: 10.1016/0006-8993(91)91111-d. [DOI] [PubMed] [Google Scholar]
- Woolf G, MacDonald AD. The evaluation of analgesic action of pethidine hydrochloride (demerol) J Pharmacol Exp Ther. 1944;80:300–307. [Google Scholar]
- Zhou Y, Spangler R, LaForge KS, Maggos CE, Ho A, Kreek MJ. Corticotropin-releasing factor and type 1 corticotropin-releasing factor receptor messenger RNAs in rat brain and pituitary during “binge”-pattern cocaine administration and chronic withdrawal. J Pharmacol Exp Ther. 1996;279:351–358. [PubMed] [Google Scholar]
- Zhu X, Orci L, Carroll R, Norrbom C, Ravazzola M, Steiner DF. Severe block in processing of proinsulin to insulin accompanied by elevation of des-64,65 proinsulin intermediates in islets of mice lacking prohormone convertase 1/3. Proc Natl Acad Sci U S A. 2002a;99:10299–10304. doi: 10.1073/pnas.162352799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Zhou A, Dey A, Norrbom C, Carroll R, Zhang C, Laurent V, Lindberg I, Ugleholdt R, Holst JJ, Steiner DF. Disruption of PC1/3 expression in mice causes dwarfism and multiple neuroendocrine peptide processing defects. Proc Natl Acad Sci U S A. 2002b;99:10293–10298. doi: 10.1073/pnas.162352599. [DOI] [PMC free article] [PubMed] [Google Scholar]


