Abstract
IFIXα, a member of the interferon-inducible HIN-200 family, has been identified as a putative tumor suppressor. However, the molecular mechanisms underlying IFIXα-mediated tumor suppression are poorly understood. In the present study, we demonstrated that the metastasis suppressor maspin acts as the downstream target of IFIXα. IFIXα suppressed the invasion activity of MDA-MB-468 breast cancer cells, and its inhibitory effect was reversed by the knockdown of maspin. Both Maspin mRNA and protein were upregulated by IFIXα. Histone deacetylase (HDAC) inhibitors, but not DNA methyltransferase inhibitor upregulated maspin, and HDAC1 inhibited the transactivation of maspin promoter. Although the HDAC1 protein was downregulated in IFIXα-expressing cells, IFIXα did not affect HDAC1 mRNA levels. Conversely, a proteasome inhibitor restored the level of HDAC1 protein in IFIXα-expressing cells, and the polyubiqutination of HDAC1 was promoted by IFIXα, suggesting that HDAC1 is regulated by IFIXα through a ubiquitin-proteasome pathway. Together, these data provide novel insights into the tumor-suppressive function of IFIXα.
Keywords: IFIXα, maspin, HDAC1, breast cancer
INTRODUCTION
The HIN-200 family proteins are interferon (IFN)-inducible nuclear proteins that play critical roles in cellular responses to IFN, such as cell proliferation, differentiation, and apoptosis [1,2]. HIN-200 family has at least four members in humans and five in mice, and it shares the conserved 200X domain [1]. IFIXα is a member of the HIN-200 family that exhibits antitumor activity [3]. IFIXα expression is downregulated in patient samples of breast tumors and in metastatic breast cancer cell lines, and ectopic expression of IFIXα in breast cancer cells reduces cell growth and tumor formation in nude mice [3]. However, the molecular mechanisms underlying the anticancer activity of IFIXα is not well understood [3].
Maspin, a member of the serine protease inhibitor family, has been shown to be a potent metastasis suppressor [4–7]. The ectopic expression of maspin suppresses the invasive potential of breast cancer cells in vitro and the growth and metastasis of prostate and breast cancers in a mouse model [8,9]. Maspin is expressed ubiquitously in multiple tissues and normal epithelial cells but reduced in tumor cells [5,8]. However, since gross structural changes are not observed in the maspin gene of human cancer cells, the epigenetic change in the maspin gene caused by DNA methylation, histone-lysine methylation, and deacetylation appears to play a significant role in the tumor-cell-specific silencing of the maspin gene [10,11].
In this study, we show the functional link between IFIXα and maspin. We found that maspin is selectively upregulated in IFIXα-expressing cells and involved in anti-invasive activity of IFIXα. We also present evidence indicating that IFIXα downregulates histone deacetylase 1 (HDAC1), which is possibly involved in the silencing of the maspin gene in human breast cancer cells.
MATERIALS AND METHODS
Cell Culture
MDA-MB-468 and MCF7 control and IFIXα-expressing cells have been described previously [3]. All cells were maintained in DMEM/F12 supplemented with 10% fetal bovine serum and antibiotics. Transfection was performed as previously described [3,12].
Plasmid and siRNA
The IFIXα expression plasmid, siRNA against IFIXα [3,12], and maspin-promoter luciferase construct have been described previously [13]. The Maspin siRNA SMART pool was purchased from Dharmacon, Inc. (Lafayette, CO). The HDAC1 expression plasmid, pCMV6-XL5-HDAC1, was purchased from OriGene (Rockville, MD).
Immunoblot and Northern Blot
Cell lysates were prepared in RIPA buffer supplemented with protease and phosphatase inhibitors. Cell lysates were subjected to SDS–PAGE and transferred to a polyvinylidene fluoride (PVDF) membrane. The membranes were probed with the antibodies indicated in each figure. Anti-HDAC1 maspin monoclonal antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-HDAC1 polyclonal antibody was obtained from Cell Signaling (Beverly, MA). The use of anti-tubulin, Flag, IFIXα antibodies, and northern blotting has been described previously [3,12].
Invasion Assay
MDA-MB-468 control and IFIXα-expressing cells were resuspended in serum-free media and seeded into the upper Matrigel chamber of a transwell system. The bottom wells were filled with complete media containing 30 µg/mL of laminin. After incubating for 48 h, the cells that had invaded the Matrigel membrane were fixed with 4% formaldehyde and stained with 4′,6-diamidino-2-phenylindole (DAPI). The cells were then counted under a fluorescence microscope.
RESULTS AND DISCUSSION
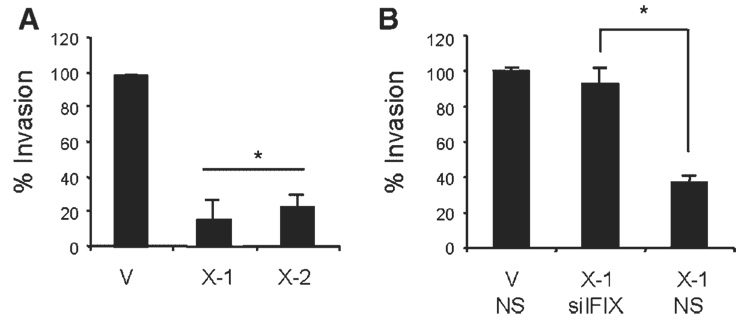
IFIXα expression is downregulated in patient samples of breast tumors and in metastatic breast cancer cell lines, suggesting that IFIXα may be involved in tumor metastasis [3]. Because invasion is a critical step to control tumor metastasis, we performed an in vitro invasion assay using MDA-MB-468 cells stably expressing IFIXα. As shown in Figure 1A, invasive activity in the two independent IFIXα stable clones (X-1 and X-2) was significantly suppressed. To confirm that this result was due to IFIXα, we knocked down IFIXα in the MDA-MB-468 cells and examined their invasive activity. As expected, the invasive activity in the IFIXα-expressing cells was restored to levels comparable to those in the control cells (Figure 1B). These results suggest that IFIXα is involved in metastasis by suppressing cellular invasive activity.
Figure 1.
IFIXα suppresses invasive activity in MDA-MB-468 cells. (A) MDA-MB-468 vector control (V) and IFIXα stable clones (X-1 and X-2) were subjected to an invasion assay as described in Materials and Methods Section. The number of invading control cells was set at 100%. Data are expressed as averages ± standard deviation (n = 3). *Significantly different from the control, P < 0.001. (B) MDA-MB-468 vector control (V) and IFIXα-expressing cells were transfected with IFIXα or nonspecific (NS) siRNA, and subjected to an invasion assay. The percent invasion relative to the control is indicated. Data are expressed as averages ± standard deviation (n = 3). *Significantly different, P < 0.001.
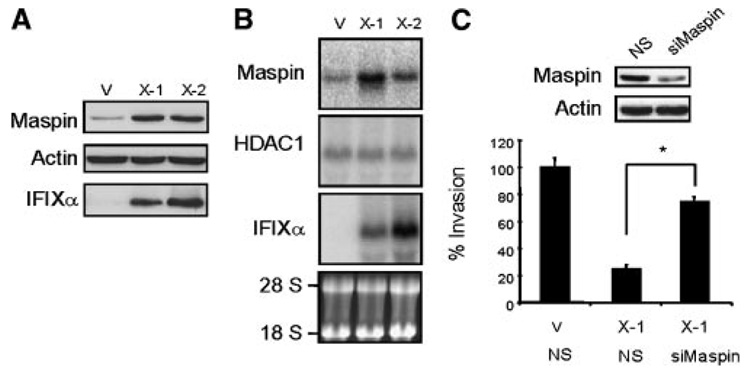
To further dissect the molecular mechanisms underlying the anti-invasive activity of IFIXα, we focused on the role of maspin in IFIXα-mediated anti-invasive activity. Then, we first determined the expression level of maspin in the IFIXα stable and control cells and found that maspin is upregulated in IFIXα-expressing cells (Figure 2A). Northern blot analysis likewise revealed that IFIXα increases maspin mRNA expression (Figure 2B).
Figure 2.
IFIXα upregulates maspin mRNA and protein. (A) Maspin protein levels in MDA-MB-468 vector control (V) and IFIXα stable clones (X-1 and X-2) were determined using immunoblot analysis. (B) Total RNA was isolated from MDA-MB-468 vector control (V) and IFIXα stable clones (X-1 and X-2) and subjected to northern blotting using IFIXα, maspin, and HDAC1 cDNA as probes. The 18S and 28S rRNAs are shown as loading controls. (C) MDA-MB-468 control (V) and IFIXα-expressing cells (X-1) were transfected with maspin or nonspecific (NS) siRNA and subjected to an invasion assay. The percent invasion relative to the control is indicated. Data are expressed as averages ± standard deviation (n = 3). *Significantly different, P < 0.001. The immunoblots of maspin and actin are shown above the graph.
Subsequently, to validate the functional significance of maspin upregulation in IFIXα-expressing cells, we knocked down maspin by siRNA and examined the invasive activity. As shown Figure 2C, knockdown of maspin restored the IFIXα-expressing cells’ invasive activity to levels comparable to those in the control cells. Thus, maspin may be responsible for the anti-invasive activity of IFIXα.
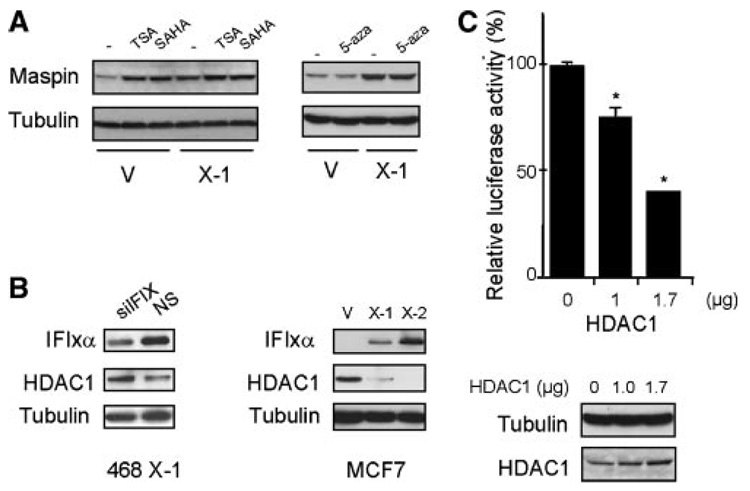
Next, we asked how maspin mRNA expression is controlled by IFIXα. It has been suggested that maspin expression is controlled by the epigenetic alteration of the maspin gene such as DNA methylation, histone-lysine methylation, and deacetylation in human cancer cells [10,11]. To determine if HDAC or DNA methylation is involved in maspin expression in MDA-MB-468 cells, we treated the cells with an HDAC inhibitor, either trichostatin A (TSA) or suberoylanilide hydroxamic acid (SAHA), or with the DNA methyltransferase inhibitor 5-aza-2′-deoxycytidine (5-aza) and then determined maspin expression using immunoblot analysis. As shown in Figure 3A (left panel), HDAC inhibitors restored the expression of the maspin protein in MDA-MB-468 control cells to levels comparable to those in the IFIXα-expressing cells, but the DNA methyltransferase inhibitor had no effect on the maspin protein level (Figure 3A, right panel). Moreover, we found that HDAC1 expression was lower in the IFIXα-expressing MDA-MB-468 cells (Figure 4A) than in the controls, and that knocking down IFIXα in these cells restored HDAC1 expression (Figure 3B, left panel). We also verified that HDAC1 was downregulated by IFIXα in MCF7 cells transfected with IFIXα (Figure 3B, right panel).
Figure 3.
HDAC1 is negatively regulated by IFIXα. (A) MDA-MB-468 control (V) and IFIXα stable cells (X-1) were treated with 100 nM of TSA, 1 µM of suberoylanilide hydroxamic acid (SAHA), or 250 nM of 5-aza-2′-deoxycytidine (5-aza) for 14 h, and maspin and tubulin expression in both treated and untreated cells was determined by immunoblot analysis. (B) MDA-MB-468 IFIXα stable cells (X-1) were transiently transfected with siRNA against IFIXα (left panel). MCF7 cells were stably expressed with vector control (V) and IFIXα (X-1 and X-2). IFIXα, HDAC1, and tubulin expression were determined by immunoblot analysis. (C) H1299 cells were transiently co-transfected with 0.3 µg of Firefly luciferase plasmids containing maspin promoter, pRL-TK renilla luciferase plasmids, and the indicated amounts of HDAC1 expression plasmids. Twenty-four hours after transfection, the cells were harvested and subjected to a dual luciferase assay (Promega, Madison, WI). Data are expressed as averages ± standard deviation (n = 3). *Significantly different from the control, P < 0.001. The HDAC1 protein expression in H1299 cells are shown below the graph.
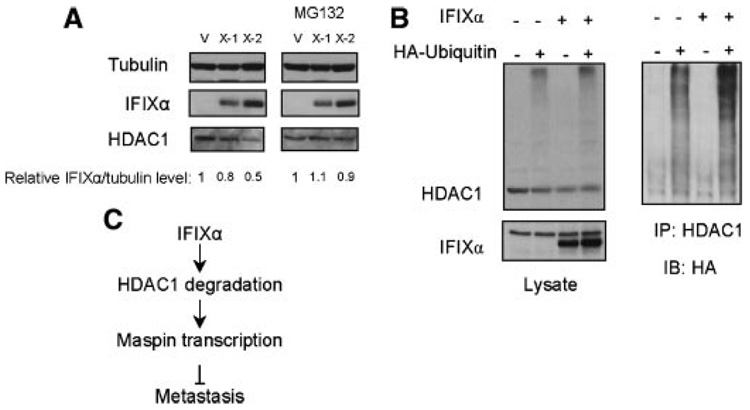
Figure 4.
IFIXα promotes HDAC1 polyubiquitination. (A) IFIXα-expressing cells were treated with 5 µM MG132 for 14 h, and both treated and untreated cells were subjected to immunoblot analysis with the indicated antibodies. Relative intensities of IFIXα/tubulin were shown. (B) Two hundred ninety-three cells were transiently transfected with the indicated expression plasmids. Twenty-four hours after transfection, the cells were treated with 5 µM MG132 for an additional 10 h and subjected to immunoprecipitation with an anti-HDAC1 monoclonal antibody and immunoblot analysis with the indicated antibodies. (C) A model of the IFIXα-mediated suppression of metastasis.
To confirm these results, we performed a luciferase assay using a maspin-promoter-luciferase plasmid. The results showed that HDAC1 overexpression suppressed the activity of the maspin promoter (Figure 3C). Therefore, our results suggest that IFIXα enhances maspin expression through the downregulation of HDAC1.
Although HDAC1 is downregulated by IFIXα, northern blot analysis revealed that the levels of HDAC1 mRNA are not affected by IFIXα (Figure 2B). Thus, to elucidate the potential mechanism by which IFIXα negatively regulates HDAC1, we examined HDAC1 protein expression in MDA-MB-468 cells treated with the proteasome inhibitor MG132. As shown in Figure 4A (right panel), the MG132 restored HDAC1 expression in the IFIXα-expressing cells, which suggests that IFIXα downregulates HDAC1 through a proteasome-mediated degradation pathway. Therefore, we next examined whether HDAC1 ubiquitination is promoted by IFIXα in vivo. First, HDAC1 and HA-ubiqutin were overexpressed in 293 cells with or without IFIXα. Then, HDAC1 was immunoprecipitated and subjected to an immunoblot analysis with an anti-HA antibody. The results showed that IFIXα enhanced HDAC1 polyubiquitination, suggesting that IFIXα controls the HDAC1 protein level by promoting its ubiquitination (Figure 4B).
In summary, the present study provides a novel insight into the anticancer activity of IFIXα. We have previously demonstrated that IFIXα suppresses the growth of breast cancer in vitro, colony formation in soft agar, and tumorigenicity in nude mice [3]. In this study, we have shown that IFIXα suppresses the invasion of human breast cancer cells and identified maspin as a critical mediator of IFIXα’s anti-invasive activity. Maspin is a potent tumor suppressor that inhibits the invasion and metastasis of breast and prostate cancer cells [8]. Thus, in addition to the function of suppression of cancer cell growth, IFIXα may be involved in the inhibition of metastasis through upregulating maspin. We could not observe the correlation between expression levels of maspin and IFIXα in 468-IFIXα stalbe clones. Although this may be due to the diversity of each clone, one possibility is that IFIXα may activate some factor(s), which may in turn suppress the maspin induction via feed back mechanisms. Consistent with reports that epigenetic gene silencing, including DNA methylation and histone acetylation, regulates maspin expression [10,11], HDAC inhibitors effectively upregulated maspin expression in our study, but the DNA methyltransferase inhibitor did not (Figure 3A). The effects of HDAC inhibitors on the upregulation of maspin expression have been reported in prostate cancer cells [14]. Furthermore, the overexpression of HDAC1 suppressed maspin promoter activity (Figure 3C). We also showed that IFIXα downregulates the level of HDAC1 protein (Figure 3 and Figure 4). These results suggest that IFIXα prevents maspin expression though the downregulation of HDAC1 (Figure 4C). It has recently identified that HDAC is promising target of cancer treatments. HDAC deacetylates core histones and other transcription factors, resulting in the alteration of gene transcription profiles [15]. Mammalian HDAC has been classified four classes [16,17]. Class I HDAC including HDAC1 and 2 is ubiquitously expressed and appears to be critical for tumor cell proliferation [18]. HDAC inhibitors are highly active anticancer drugs that are in clinical trials for several cancer types [17,19]. Therefore, maspin may play a role in anticancer activity of HDAC inhibitors and IFIXα may exert its antimetastasis activity, at least in part, through the inhibition of HDAC1. However, we could not observed maspin induction by IFIXα in MCF7 cells although the expression level of HDAC1 is downregulated by IFIXα in these cells (Data not shown, Figure 3B). These results suggests that HDAC is involved in maspin silencing in some types of breast cancer whereas DNA methylation rather than histone acetylation may contribute to the maspin regulation in some breast cancer cells such as MCF7 cells.
In the present study, we showed that IFIXα promotes polyubiquitination and degradation of HDAC1, which in turn upregulates maspin (Figure 4). These findings do not coincide with the previous report that a proteasome inhibitor upregulates maspin expression in prostate cancer cells suggesting that maspin is also controlled by IFIXα-HDAC1-independent mechanisms [20]. Because IFIXα is not a ubiquitin ligase, the identification of the E3 ligase for HDAC1 will be required to further understand the molecular mechanism underlying the anti-invasive activity of IFIXα in future study. Moreover, it has recently been shown that maspin interacts with HDAC1 and inhibits its activity [14]. Thus, maspin may also amplify the effects of IFIXα through a feed forwards mechanisms.
ACKNOWLEDGMENTS
This work was supported by Susan G. Komen Breast Cancer Foundation grant BCTR76906 (to DHY and MCH) and National Institutes of Health Breast SPORE grant P50 CA116199 (to MCH).
REFERENCES
- 1.Ludlow LE, Johnstone RW, Clarke CJ. The HIN-200 family: More than interferon-inducible genes? Exp Cell Res. 2005;308:1–17. doi: 10.1016/j.yexcr.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 2.Johnstone RW, Trapani JA. Transcription and growth regulatory functions of the HIN-200 family of proteins. Mol Cell Biol. 1999;19:5833–5838. doi: 10.1128/mcb.19.9.5833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ding Y, Wang L, Su LK, et al. Antitumor activity of IFIX, a novel interferon-inducible HIN-200 gene, in breast cancer. Oncogene. 2004;23:4556–4566. doi: 10.1038/sj.onc.1207592. [DOI] [PubMed] [Google Scholar]
- 4.Khalkhali-Ellis Z. Maspin: The new frontier. Clin Cancer Res. 2006;12:7279–7283. doi: 10.1158/1078-0432.CCR-06-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bailey CM, Khalkhali-Ellis Z, Seftor EA, Hendrix MJ. Biological functions of maspin. J Cell Physiol. 2006;209:617–624. doi: 10.1002/jcp.20782. [DOI] [PubMed] [Google Scholar]
- 6.Zhang M. Multiple functions of maspin in tumor progression and mouse development. Front Biosci. 2004;9:2218–2226. doi: 10.2741/1392. [DOI] [PubMed] [Google Scholar]
- 7.Lockett J, Yin S, Li X, Meng Y, Sheng S. Tumor suppressive maspin and epithelial homeostasis. J Cell Biochem. 2006;97:651–660. doi: 10.1002/jcb.20721. [DOI] [PubMed] [Google Scholar]
- 8.Zou Z, Anisowicz A, Hendrix MJ, et al. Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science. 1994;263:526–529. doi: 10.1126/science.8290962. [DOI] [PubMed] [Google Scholar]
- 9.Shi HY, Zhang W, Liang R, et al. Blocking tumor growth, invasion, and metastasis by maspin in a syngeneic breast cancer model. Cancer Res. 2001;61:6945–6951. [PubMed] [Google Scholar]
- 10.Maass N, Biallek M, Rosel F, et al. Hypermethylation and histone deacetylation lead to silencing of the maspin gene in human breast cancer. Biochem Biophys Res Commun. 2002;297:125–128. doi: 10.1016/s0006-291x(02)02136-8. [DOI] [PubMed] [Google Scholar]
- 11.Domann FE, Rice JC, Hendrix MJ, Futscher BW. Epigenetic silencing of maspin gene expression in human breast cancers. Int J Cancer. 2000;85:805–810. doi: 10.1002/(sici)1097-0215(20000315)85:6<805::aid-ijc12>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 12.Ding Y, Lee JF, Lu H, Lee MH, Yan DH. Interferon-inducible protein IFIXalpha1 functions as a negative regulator of H DM2. Mol Cell Biol. 2006;26:1979–1996. doi: 10.1128/MCB.26.5.1979-1996.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Z, Shi HY, Nawaz Z, Zhang M. Tamoxifen induces the expression of maspin through estrogen receptor-alpha. Cancer Lett. 2004;209:55–65. doi: 10.1016/j.canlet.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Yin S, Meng Y, Sakr W, Sheng S. Endogenous inhibition of histone deacetylase 1 by tumor-suppressive maspin. Cancer Res. 2006;66:9323–9329. doi: 10.1158/0008-5472.CAN-06-1578. [DOI] [PubMed] [Google Scholar]
- 15.Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK. Histone deacetylases and cancer: Causes and therapies. Nat Rev Cancer. 2001;1:194–202. doi: 10.1038/35106079. [DOI] [PubMed] [Google Scholar]
- 16.Xu WS, Parmigiani RB, Marks PA. Histone deacetylase inhibitors: Molecular mechanisms of action. Oncogene. 2007;26:5541–5552. doi: 10.1038/sj.onc.1210620. [DOI] [PubMed] [Google Scholar]
- 17.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5:769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 18.Senese S, Zaragoza K, Minardi S, et al. Role for histone deacetylase 1 in human tumor cell proliferation. Mol Cell Biol. 2007;27:4784–4795. doi: 10.1128/MCB.00494-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marks PA, Breslow R. Dimethyl sulfoxide to vorinostat: Development of this histone deacetylase inhibitor as an anticancer drug. Nat Biotechnol. 2007;25:84–90. doi: 10.1038/nbt1272. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Chen D, Yin S, et al. Maspin augments proteasome inhibitor-induced apoptosis in prostate cancer cells. J Cell Physiol. 2007;212:298–306. doi: 10.1002/jcp.21102. [DOI] [PubMed] [Google Scholar]