Abstract
Background
Evidence suggests that cannabis users are at increased risk for cigarette smoking – if so, this may potentially be the single most alarming public health challenge posed by cannabis use. We examine whether cannabis use prior to age 17 is associated with an increased likelihood of DSM-IV nicotine dependence and the extent to which genetic and environmental factors contribute to this association.
Methods
A population-based cohort of 24–36 year old Australian male and female twins (N=6,257, 286 and 229 discordant pairs) was used. The cotwin-control method, with twin pairs discordant for early cannabis use, was used to examine whether after controlling for genetic and familial environmental background, there was evidence for an additional influence of early cannabis use on DSM-IV nicotine dependence. Bivariate genetic models were fitted to the full dataset to quantify the genetic correlation between early cannabis use and nicotine dependence.
Results
The early cannabis-using twin was about twice as likely to report nicotine dependence, when compared to their co-twin who had experimented with cigarettes but never used cannabis. Even when analyses were restricted to cannabis users, earlier age cannabis use onset conferred greater risk (1.7) for nicotine dependence than did later onset. This association was largely governed by common genetic liability to early cannabis use and nicotine dependence as demonstrated by genetic correlations of 0.41–0.52.
Conclusions
Early-onset cannabis users are at increased risk for nicotine dependence but this risk is largely attributable to common genetic vulnerability. There is no evidence for a causal relationship between cannabis use and nicotine dependence.
Keywords: cannabis, nicotine dependence, discordant twins, Mx, twin modeling, genetic
INTRODUCTION
It is well documented that cannabis use may contribute to an increased likelihood of experimenting with other illicit drugs [1–6]. However, researchers have recently begun to question whether cannabis use is also associated with increased risk for cigarette smoking. In two longitudinal samples of adolescents, those who initially abstained from cigarettes were more likely to report cigarette smoking at a subsequent assessment if they used cannabis in the interim [7,8]. There is also accumulating evidence that cannabis use may substantially impact subsequent important later stages of cigarette smoking, including nicotine dependence (ND) [7–9].
Two epidemiological studies have examined ‘reverse gateways’. Patton and colleagues [7], using a longitudinal sample of teens, reported that weekly cannabis use predicted an increase in tobacco initiation. The authors also reported that young adult smokers who used cannabis on a daily basis were at a nearly 3.6 increased odds of developing ND. In a sample of adolescents and young adults, Timberlake and colleagues [9] reported that 23–27 year-olds with a lifetime history of cannabis use (>10 times) were twice as likely to develop ND when compared to those young adults who reported no cannabis use at all.
While these studies demonstrate an association between cannabis use and cigarette smoking, they fail to address whether a common genetic predisposition to cannabis use and cigarette smoking is responsible for this association or whether this increase in risk is attributable to overlapping environmental influences. Empirical evidence demonstrates the importance of heritable influences on both cannabis use [10] and stages of cigarette smoking [11–16], but we are only aware of one genetically informative study of adult female twins [17] which showed that the association between cannabis use and cigarette smoking (experimentation and ND) was due to a correlation between the liability to use cannabis and experimentation with cigarettes, both of which are genetically influenced (h2cannabis= 0.40; h2nicotine= 0.67), but not due to any direct influence of cannabis use on ND or vice versa.
Several studies [3–6] show that those who initiate cannabis use earlier in life are at significantly greater risk for experimentation with other illicit drugs, illicit substance use disorders and for alcohol dependence. However, it remains to be seen whether individuals who initiate cannabis use at an early age are more likely to develop ND when matched for genetic background, and possibly for some environmental influences, with individuals who do not use cannabis at all and with individuals who use cannabis but only at a later age. The study of identical and fraternal twin pairs who are matched for familial environment and for genetic background allows us to test this important hypothesis.
In the current study, we use data gathered on young adult Australian twins to explore: (a) whether cannabis use prior to age 17 (early-onset) is associated with an increased risk for onset of DSM-IV ND and (b) the extent to which this association between early onset cannabis use and DSM-IV ND is explained by genetic and environmental commonality.
MATERIALS AND METHODS
Sample
A sample of 6,257 adult Australian male and female twins that included monozygotic (MZ) and dizygotic (DZ) pairs as well as twins from incomplete pairs, although these single-twins were not informative for the discordant twin pair analyses, were used. The full paired sample consisted of 494 MZ and 395 DZ same-sex male pairs and of 698 MZ and 513 DZ same-sex female pairs. Data on 661 DZ opposite sex pairs and 736 twins from pairs where a co-twin did not participate was also available. While opposite-sex pairs and single twins could not be used for the discordant twin pair analyses, data from these twins were utilized for twin analyses. Twins were aged 24–36 (mean 30 [2.5] years) years at the time of interviews conducted in 1996–2000 and these data alone were used for the analyses presented here. All twins were born between 1964 and 1971 and were initially recruited through the Australian school systems and via mass media appeals. Parents initially registered the adolescent twins in 1980–1982 and the twins themselves were interviewed via telephone in 1996–2000, after informed consent, as approved by the Institutional Review Boards of the Washington University in St. Louis, USA and the Queensland Institute of Medical Research, Australia, was obtained from all participants. Further details regarding sample ascertainment and collection are presented in related publications [3,18].
Measures
Diagnostic interviews were based on the SSAGA [19], which was updated for DSM-IV and adapted for telephone use in the Australian sample. ND measures were adapted for inclusion from the CIDI [20].
Early Cannabis use (EC)
Self-reports of having used cannabis at least once prior to age 17 – a definition previously shown by us to be highly associated with other illicit drug use [3];
Nicotine dependence (ND)
Defined using 7 DSM-IV criteria – clustering of three or more of these criteria in a single 12-month period led to a diagnosis of DSM-IV ND. Individuals who had smoked cigarettes at least once in their lifetime, but did not endorse smoking cigarettes 100 or more times [21] during their lifetime, were not queried about ND symptoms. These individuals were coded as unaffected for DSM-IV ND.
Covariates
To account for known correlates that may potentially mediate the association between EC and ND, we controlled for:
DSM-IV major depression: The association between depression and ND has been extensively studied (e.g. [22–24]) with evidence for common genetic influence. While the association between depression and cannabis use is controversial (see [25] for a review), genetic studies hint at some overlap [26,27] making this a potential mediating measure.
2 or more DSM-IV conduct problems: If the association between EC and ND represents a general proneness to delinquent behavior [28], including substance use, then controlling for a history of conduct problems is important.
Social anxiety (defined as experiencing fears, where doing something embarrassing/humiliating caused anxiety, or if experiencing fears caused problems with family, friends, work or other situations): Individuals with social anxiety tend to smoke cigarettes more frequently [29].
Exposure to childhood sexual abuse (self-reported rape or molestation or forced sexual contact with someone within or outside the family, prior to 17 years of age), which has been shown to be a potent risk factor for early onset of substance use and later dependence [30–35].
Lifetime use of other illicit psychoactive substances (cocaine, sedatives, stimulants, inhalants, solvents, opiates, hallucinogens or phencyclidine), which has been demonstrated to be an important outcome of EC [3–6], and with common genetic underpinnings.
Early drinking (drinking alcohol at least once a month for 6 months or longer when aged 16 years or younger) and early smoking (smoking at least once a week for 3 weeks or longer at age 15 or younger), as EC may simply be a marker for early onset of multiple substances [36].
Sample Characteristics
The full sample (N=6,257) had a mean age of 30 years and was 44.8% male –other characteristics are presented elsewhere [18]. Almost 89% of the full sample reported experimenting with cigarettes (3,028 male and 2,554 female twins) (see previous reports [15,37]). Of these ever-smokers, 56.7% reported smoking 100 or more cigarettes in their lifetime and 34.4% met criteria for DSM-IV ND (mean age of onset 21.2 [range 5–34 years]). In the full sample, 60.2% reported a lifetime history of cannabis use with 53.7% of these users (15.6% of the population) reporting initiation prior to 17 years of age (mean age of onset 15.2 [range 9–16 years]). In ever-smokers, the prevalence of EC was 19% and 12.7% in men and women respectively. Likewise, in individuals who reported experimentation with cigarettes and a lifetime history of cannabis use, the prevalence of ND was 42.8%.
Nearly 83% of all of our participants reported initiating cigarette smoking prior to 17 years of age. About 55% of the sample reported having smoked 100+ cigarettes prior to 17 years – these individuals are not informative in the discordant twin design and it is for this reason that we chose not to examine the association between EC and this measure. For ND, 8 (additionally, 6 did not report an age of onset) of the EC users reported onset of ND prior to EC and were deleted from those discordant twin analyses.
The mean age of twins constituting the discordant pairs was 30 years (SD 2.5) and 47.2% of the sample was male. 28.7% of the respondents had 10 or fewer years of education while 25.7% reported tertiary education (i.e. going to university). About 46% of the sample reported being married (or widowed) while 44% were never married and the remainder were separated or divorced at the time of interview. These estimates were highly comparable to the full sample.
Statistical Methods
Discordant twin design
To control for initiation of cigarette smoking, we selected only those twins who reported ever smoking cigarettes. Self-reported age of onset of cannabis use was used to select two subsamples of discordant twin pairs:
286 (129 MZ and 157 DZ) like-sex pairs, where one member reported EC while their co-twin reported cannabis use at a later age or did not report a lifetime history of cannabis use (i.e. including lifetime non-users);
229 (104 MZ and 125 DZ) like-sex pairs, where one member reported EC while their co-twin reported cannabis use after age 16 (i.e. excluding lifetime non-user co-twins);
The discordant twin method is a simple extension of matched pair conditional logistic regression where, along with matching for age and sex, there is also matching for other latent factors, such as genetic and familial environmental influences, which are shared by members of twin pairs. Kendler and colleagues [22] have previously presented the patterns of odds-ratios (O.R.) that we might expect, depending on whether the association between EC and ND is due to genetic or environmental factors or both:
If the association between EC and ND is exclusively due to genetic factors that influence both EC and ND, then the O.R. in MZ twins, who share all their genes (identical-by-descent), would not be significant. However, the O.R. would be elevated in DZ twins who are only matched for 50% of their genetic background;
If the association between EC and ND is exclusively due to shared environmental factors that influence both EC and ND, then we would expect no increase in the O.R. in either MZ or DZ discordant pairs, as both are assumed to equally share their familial environment. Presumably, when a combination of overlapping genetic and shared environmental influences is at play, we might expect some elevation in the DZ O.R.
If the association between EC and ND is neither due to genetic nor shared environmental influences, but instead is entirely due to unmeasured individual-specific environmental factors, or represents a truly causal relationship, then both MZ and DZ O.Rs. would be elevated.
Finally, if familial (genetic and/or shared environmental) and individual-specific environmental factors jointly contribute to the relationship between EC and ND, then again, both MZ and DZ O.R. would be elevated, however, the MZ O.R. would be smaller in magnitude than the DZ O.R.
It is important here to note that we are only considering pairs of twins discordant for EC and examining its association with ND in a conditional logistic regression framework (i.e. how likely is the EC member of a discordant pair to be ND and how likely is the non-EC member of a discordant pair likely to not be ND).
Conditional logistic regression models were fit in STATA. The within-pair association between EC and ND was examined in the first sample of twins where the unaffected co-twins included lifetime cannabis non-users, and then, the association was re-examined in the smaller sample where the co-twin population excluded lifetime non-users. All models were adjusted for covariates. The significance of interactions between gender and EC, and between zygosity and EC was tested. Analyses were re-done in MZ and DZ pairs discordant for EC use separately to tease apart the role of familial versus causal/non-causal unmeasured environmental influences.
Bivariate twin models
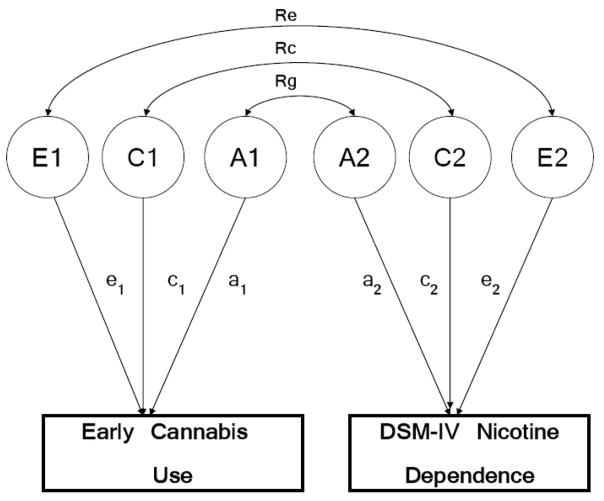
To test the robustness of our results to analytic strategy, we fit bivariate twin models utilizing data from the entire sample (i.e. not just pairs discordant for cannabis use), to examine the extent to which genetic (A), shared environmental (C) and non-shared environmental (E) factors were correlated across EC and ND. Figure 1 represents, using a path diagram, the within-twin (co-twin not shown) relationship tested in the bivariate model. In the figure, A1, C1 and E1, and A2, C2 and E2 are the genetic, shared and individual-specific influences on EC and ND respectively. Rg represents the extent to which A1 and A2, the genetic influences on EC and ND, are correlated. Rc and Re are shared and individual-specific environmental correlations respectively, between EC and ND. Note that, A1, the genetic influences on EC, are correlated 1.0 and 0.5 (across-twin within-trait) across members of a pair of MZ and DZ twins respectively, while Rg represents the within-twin across-trait correlation between EC and ND and may be suitably multiplied by 1.0 or 0.5 (MZ or DZ pair) to explain the across-twin across-trait correlation (i.e. the correlation between EC in twin 1 and ND in twin 2).
Figure 1.
The bivariate twin model, represented as a path diagram (circles=latent variables; A=additive genetic, C=shared environment, E=individual-specific environment; rectangles=observed variable; double-headed arrows=correlations Rg, Rc and Re). Shown here for one twin only.
Analyses were conducted on both definitions of EC i.e. where non-user co-twins were included as ‘0’ and where they were set to missing. For primary analyses, ND was set to missing in those who had never smoked even one cigarette – this is comparable to the discordant twin design. In secondary analyses, ND was set to missing in those who had never smoked cigarettes and in those who had smoked <100 cigarettes.
Models were fit to raw data using a full information maximum-likelihood (FIML) estimation procedure in the software package Mx [38]. A, C and E were allowed to vary, in addition to thresholds, in male and female twins. The correlation across twins was allowed to vary from 0.5 in DZ opposite-sex twin pairs as a test for qualitative sex differences. The fit of sub-models was tested using the difference in −2 loglikelihood fit of the models, which follows a chi-square distribution (Δχ2) for the given degrees of freedom (df).
RESULTS
In this entire sample of twins, EC users were 2.7 [95% C.I. 2.3–3.3] times more likely to report ND even after controlling for psychiatric covariates.
Discordant twin analyses
The unadjusted odds of the EC twin meeting criteria for ND were 1.9. This increased likelihood of ND remained even after adjusting for substance use and comorbid psychopathology – EC twins, when compared to their lifetime non-user or later-onset user co-twin, were 1.7 times more likely to meet criteria for ND (Table 1). Interactions between EC and gender or zygosity (being the member of a DZ versus MZ pair) were not significant.
Table 1.
Conditional odds-ratio between early cannabis use, and DSM-IV nicotine dependence in Australian same-sex twin pairs aged 24–36 years
| Unadjusted Odds-ratios
[95% C.I.] |
Adjusted Odds-ratios
[95% C.I.] |
Significant covariates | |
|---|---|---|---|
| Including those who never used cannabis
N=286 pairs |
1.9
[1.3–2.8] |
1.8
[1.1–2.7] |
Early smoking, major depression, childhood sexual abuse |
| Excluding those who never used cannabis
N=229 pairs |
1.7
[1.1–2.6] |
1.5
[0.9–2.7] |
Early smoking, major depression, childhood sexual abuse |
Note: Covariates tested include DSM-IV major depression, 2+ conduct problems, social anxiety, exposure to childhood sexual abuse, lifetime use of other illicit drugs, early drinking and early smoking
Even upon exclusion of co-twins who had never used cannabis, EC users were still at a 1.7 [95% C.I. 1.1–2.6] greater odds for ND. After adjusting for covariates, this odds-ratio was not statistically significant (O.R. 1.5, 95% C.I. 0.9–2.7, Table 1). Interactions with sex and zygosity were not significant.
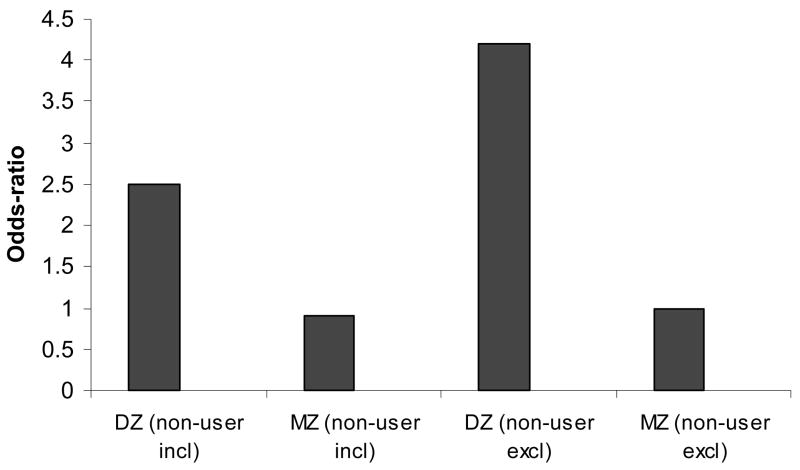
Even though interactions with zygosity were not significant in either case, this may have been a consequence of reduced power. When we examined the odds-ratios separately in discordant MZ pairs and DZ pairs, the association between EC (including or excluding cannabis non-user co-twins) and ND was significant only in DZ pairs (Figure 2). The pattern of MZ-DZ odds-ratios in Figure 2 is consistent with the conclusion that the association between EC and ND is due to those factors that are fully correlated across MZ pairs (thus, there is no excess risk) but only partially correlated across DZ pairs (thus, the excess risk).
Figure 2.
Pattern of MZ and DZ odds-ratios for the association between early onset cannabis use and DSM-IV nicotine dependence in adult Australian same-sex twins pairs discordant for early cannabis use.
Twin Modeling
Genetic (A) and non-shared environmental (E) factors influenced the liability to EC (a2=72% when non-users included; a2=66% when non-users excluded) and to ND (a2=48–58%) (Table 2). There was no evidence for the DZ opposite-sex correlation being different from the DZ same-sex twin correlation of 0.5 nor for varying magnitudes of A or E in men and women (Δχ2 = 10.03 for 10 df). Shared environmental factors (C) could be dropped without a significant deterioration of fit (Δχ2 ranged from 0 to 2.3 for 1 df). The total correlation between EC and ND was 0.34 when cannabis non-users were included and 0.23 when lifetime non-users were excluded. This correlation could be largely attributed to overlapping genetic (Rg=0.52) factors even when non-users of cannabis were excluded (Rg=0.41). Consistent with findings from the discordant twin models, there was no evidence for Re (Δχ2 = 2.4 for 1 df), or those individual-specific environmental factors that make one member of an MZ twin pair report EC and progress to ND but does not impact their co-twin. Fit statistics and the best-fitting model were largely unchanged when excluding non-users of cannabis from the definition of EC (Table 2).
Table 2.
Results from best-fitting bivariate model assessing the relationship between early-onset cannabis use and DSM-IV nicotine dependence in adult Australian same- and opposite-sex twins with a lifetime history of ever smoking cigarettes.
| h2 [95% CI] |
c2 [95% CI] |
e2 [95% CI] |
Rg | Rc | Re | Total Covariance | |
|---|---|---|---|---|---|---|---|
| Early cannabis use including non-users | |||||||
| Early Cannabis Use | 0.72
[0.64–0.80] |
- | 0.28
[0.20–0.36] |
- | - | ||
| Nicotine Dependence | 0.58
[0.50–0.66] |
- | 0.42
[0.34–0.50] |
0.52
[0.44–0.53] |
- | - | 0.34 |
| Early cannabis use excluding lifetime non-users | |||||||
| Early Cannabis Use | 0.66
[0.56–0.75] |
- | 0.34
[0.25–0.44] |
||||
| Nicotine Dependence | 0.48
[0.37–0.58] |
- | 0.52
[0.42–0.63] |
0.41
[0.29–0.53] |
- | - | 0.23 |
To account for the variance due to smoking 100+ cigarettes, ND was set to missing in those who had smoked less <100 cigarettes in their lifetime and models were tested. As expected, this substantially reduced the heritability of ND (a2=37%, 95% C.I. 25–49%, remainder due to E) as well as the heritability of EC (a2=52%, 95% C.I. 34–61%, remainder due to E). The genetic correlation between EC and ND was also reduced (Rg = 0.24–0.35), however, the genetic correlations were statistically significant and there was no evidence for shared or individual-specific environmental correlations.
DISCUSSION
In this study of adult male and female twins, onset of cannabis use prior to 17 years of age (EC) increased the odds of DSM-IV ND. However, our analyses also revealed that this excess risk for ND in EC users was largely due to common genetic influences on EC and ND.
Several investigators have now demonstrated the increased risk of problematic cigarette smoking in those with a history of cannabis use [8,9,39–41]. In prior work by us U.S. women, aged 18–29 years, who had used cannabis were 2.8 times more likely to transition from smoking 100+ cigarettes to ND [41]. There were, however, several limitations to these prior studies. First, none of these studies explored the possible role of EC, which is a potent predictor of other illicit drug use and psychopathology [3,27]. Second, none of them took into account the important etiological contributions of genetic influences and familial environment that may contribute, jointly, to risk for EC and also to progression to more involved stages of cigarette smoking, such as ND. If indeed, the association between EC and ND extends beyond a shared genetic (and/or environmental) vulnerability, then reducing early exposure to cannabis use may also assist with lower rates of ND. As demonstrated by our study, the risk for ND that is attributable to EC can be largely attributed to the effects of common genetic factors. These common genetic influences only partly overlap with the prior stage of a lifetime history of smoking 100+ cigarettes.
This is the first study to document the effects of EC on ND using two complementary genetically informative methods. A feature of the current study is the consistency between the results of these two techniques: the discordant twin design and the bivariate twin model. While a twin model has previously been fitted to data on cannabis and cigarette smoking in adult female Virginia twins, it did not examine EC. These twin models are highly informative as they quantify the magnitude of genetic overlap between cannabis use and stages of cigarette smoking. As expected, these correlations were moderate to high suggesting that there may exist a cluster of genetic factors that influence an early onset of cannabis use and also influence progression to ND and persistent smoking. This common vulnerability to cannabis and tobacco use has been identified across numerous twin studies [17,42,43] and has been found to be influenced by genetic and shared environmental factors. It is plausible that the genetic influences on this non-specific component of risk may also extend to other aspects of disinhibited and externalizing behaviors [44–48] (for instance, GABRA2: gamma-aminobutyric acid receptor A, subunit 2). Initially identified for its role in alcohol dependence[49–52], recent work has implicated polymorphisms in the GABRA2 gene for their role in cannabis dependence [53], ND [54] and conduct disorder [55].
Genes common to cannabis and tobacco use alone, such as the cannabinoid receptor 1 (CNR1) gene may also contribute to the observed genetic correlation. As a target for endogenous cannabinoids [56], its role in mediating the effects of exogenous cannabinoids [57] is supported by animal [58,59] and human research with some studies reporting association between polymorphisms in CNR1 and cannabis-related behaviors [60–62] but not others [63]. CB1/CNR1 receptors have been implicated in ND as well. Nicotine increases levels of endogenous cannabinoids (e.g. anandamide) and influences reward sensitivity [64]. Rimonabant, a CB1 antagonist, is an emerging drug in tobacco cessation practice [65–70].
Some limitations to this study require discussion. First, our sample is an adult cohort of Caucasian men and women, and these findings may be sample specific. Second, retrospective recall may have affected reports of cannabis use and cigarette smoking. Third, we had less than 300 pairs of twins available for analysis so power may be a concern.
In conclusion, after accounting for genetic commonality, there remains no compelling evidence for causal processes linking EC to ND. This does not imply that reducing rates of EC will have no impact on cigarette smoking behaviors. Instead, it suggests that use of cannabis at an early age may serve as a marker for genetic vulnerability to a host of substance use behaviors. Providing specialized preventive techniques to such vulnerable adolescents will reduce their general likelihood of progression within their cigarette smoking trajectories and will also likely reduce their levels of general drug involvement.
Acknowledgments
This research is supported by DA023668 (A.A.), DA18660 & DA18267 (M.T.L.), DA019951 (M.L.P), DA12854 (PI Madden) and AA07728 (PI Heath), AA11998 (PI Heath) and AA13221 (PI Heath).
Reference List
- 1.Kandel D. Stages and Pathways of Drug Involvement. New York, NY: Cambridge University Press; 2002. [Google Scholar]
- 2.Hall W, Lynskey M. Is cannabis a gateway drug: Testing hypotheses about the relationship between cannabis use and use of other illicit drugs. Drug and Alcohol Review. 2005;24:39–48. doi: 10.1080/09595230500126698. [DOI] [PubMed] [Google Scholar]
- 3.Lynskey MT, Heath AC, Bucholz KK, Slutske W, Madden PA, Nelson EC, et al. Escalation of drug use in early-onset cannabis users vs co-twin controls. JAMA. 2003;289:427–433. doi: 10.1001/jama.289.4.427. [DOI] [PubMed] [Google Scholar]
- 4.Agrawal A, Neale MC, Prescott CA, Kendler KS. A twin study of early cannabis use and subsequent use and abuse/dependence of other illicit drugs. Psychol Med. 2004;34:1227–1237. doi: 10.1017/s0033291704002545. [DOI] [PubMed] [Google Scholar]
- 5.Lessem JM, Hopfer CJ, Haberstick BC, Timberlake D, Ehringer MA, Smolen A, et al. Relationship between Adolescent Marijuana Use and Young Adult Illicit Drug Use. Behav Genet. 2006;36:498–506. doi: 10.1007/s10519-006-9064-9. [DOI] [PubMed] [Google Scholar]
- 6.Lynskey M, Vink JM, Boomsma DI. Early onset cannabis use and progression to other drug use in a sample of Dutch twins. Behav Genet. 2006;36:195–200. doi: 10.1007/s10519-005-9023-x. [DOI] [PubMed] [Google Scholar]
- 7.Patton GC, Coffey C, Carlin JB, Sawyer SM, Wakefield M. Teen smokers reach their mid twenties. J Adolesc Health. 2006;39:214–220. doi: 10.1016/j.jadohealth.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 8.Patton GC, Coffey C, Carlin JB, Sawyer SM, Lynskey M. Reverse gateways? Frequent cannabis use as a predictor of tobacco initiation and nicotine dependence. Addiction. 2005;100:1518–1525. doi: 10.1111/j.1360-0443.2005.01220.x. [DOI] [PubMed] [Google Scholar]
- 9.Timberlake DS, Haberstick BC, Hopfer CJ, Bricker J, Sakai J, Lessem J, et al. Progression from marijuana use to daily smoking and nicotine dependence in a national sample of U.S. adolescents. Drug Alcohol Depend. 2007;88:272–281. doi: 10.1016/j.drugalcdep.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Agrawal A, Lynskey M. The Genetic Epidemiology of Cannabis Use, Abuse and Dependence: A Review. Addiction. 2006;101:801–812. doi: 10.1111/j.1360-0443.2006.01399.x. [DOI] [PubMed] [Google Scholar]
- 11.Madden PA, Heath AC, Pedersen NL, Kaprio J, Koskenvuo MJ, Martin NG. The genetics of smoking persistence in men and women: a multicultural study. Behav Genet. 1999;29:423–431. doi: 10.1023/a:1021674804714. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan PF, Kendler KS. The genetic epidemiology of smoking. Nicotine Tob Res. 1999;1 Suppl 2:S51–S57. doi: 10.1080/14622299050011811. [DOI] [PubMed] [Google Scholar]
- 13.Carmelli D, Swan GE, Robinette D, Fabsitz R. Genetic influence on smoking--a study of male twins. N Engl J Med. 1992;327:829–833. doi: 10.1056/NEJM199209173271201. [DOI] [PubMed] [Google Scholar]
- 14.Heath AC. Persist or quit? Testing for a genetic contribution to smoking persistence. Acta Genet Med Gemellol (Roma) 1990;39:447–458. doi: 10.1017/s0001566000003676. [DOI] [PubMed] [Google Scholar]
- 15.Lessov CN, Martin NG, Statham DJ, Todorov A, Slutske W, Bucholz KK, et al. Defining nicotine dependence for genetic research: evidence from Australian twins. Psychol Med. 2004;34:865–879. doi: 10.1017/s0033291703001582. [DOI] [PubMed] [Google Scholar]
- 16.Pergadia ML, Heath AC, Martin NG, Madden PA. Genetic analysis of DSM-IV nicotine withdrawal in adult twins. Psychol Med. 2006;36:963–972. doi: 10.1017/S0033291706007495. [DOI] [PubMed] [Google Scholar]
- 17.Neale MC, Harvey E, Maes HH, Sullivan PF, Kendler KS. Extensions to the modeling of initiation and progression: applications to substance use and abuse. Behav Genet. 2006;36:507–524. doi: 10.1007/s10519-006-9063-x. [DOI] [PubMed] [Google Scholar]
- 18.Heath AC, Howells W, Kirk KM, Madden PA, Bucholz KK, Nelson EC, et al. Predictors of non-response to a questionnaire survey of a volunteer twin panel: findings from the Australian 1989 twin cohort. Twin Res. 2001;4:73–80. doi: 10.1375/1369052012182. [DOI] [PubMed] [Google Scholar]
- 19.Bucholz KK, Cadoret RJ, Cloninger RC, Dinwiddie SH, Hesselbrock V, Nurnberger JI, et al. A New, Semi-Structured Psychiatric Interview For Use In Genetic Linkage Studies. J Stud Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- 20.Cottler LB, Robins LN, Grant BF, Blaine J, Towle SH, Wittchen HU, et al. The CIDI-core substance abuse and dependence questions: cross-cultural and nosological issues. The WHO/ADAMHA Field Trial. Br J Psychiatry. 1991;159:653–8. 653–658. doi: 10.1192/bjp.159.5.653. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention Cigarette smoking among adults -United States, 1991. Morbidity and Mortality Weekly. 1993;42:230–233. [PubMed] [Google Scholar]
- 22.Kendler KS, Neale MC, MacLean CJ, Heath AC, Eaves LJ, Kessler RC. Smoking and major depression. A causal analysis. Arch Gen Psychiatry. 1993;50:36–43. doi: 10.1001/archpsyc.1993.01820130038007. [DOI] [PubMed] [Google Scholar]
- 23.Johnson EO, Breslau N. Is the association of smoking and depression a recent phenomenon? Nicotine Tob Res. 2006;8:257–262. doi: 10.1080/14622200600576644. [DOI] [PubMed] [Google Scholar]
- 24.Johnson EO, Rhee SH, Chase GA, Breslau N. Comorbidity of depression with levels of smoking: an exploration of the shared familial risk hypothesis 1. Nicotine Tob Res. 2004;6:1029–1038. doi: 10.1080/14622200412331324901. [DOI] [PubMed] [Google Scholar]
- 25.Degenhardt L, Hall W, Lynskey M. Exploring the association between cannabis use and depression. Addiction. 2003;98:1493–1504. doi: 10.1046/j.1360-0443.2003.00437.x. [DOI] [PubMed] [Google Scholar]
- 26.Fu Q, Heath AC, Bucholz KK, Nelson E, Goldberg J, Lyons MJ, et al. Shared genetic risk of major depression, alcohol dependence, and marijuana dependence: contribution of antisocial personality disorder in men. Arch Gen Psychiatry. 2002;59:1125–1132. doi: 10.1001/archpsyc.59.12.1125. [DOI] [PubMed] [Google Scholar]
- 27.Lynskey MT, Glowinski AL, Todorov AA, Bucholz KK, Madden PA, Nelson EC, et al. Major depressive disorder, suicidal ideation, and suicide attempt in twins discordant for cannabis dependence and early-onset cannabis use. Arch Gen Psychiatry. 2004;61:1026–1032. doi: 10.1001/archpsyc.61.10.1026. [DOI] [PubMed] [Google Scholar]
- 28.Donovan JE, Jessor R. Structure of problem behavior in adolescence and young adulthood. J Consult Clin Psychol. 1985;53:890–904. doi: 10.1037//0022-006x.53.6.890. [DOI] [PubMed] [Google Scholar]
- 29.Breslau N, Novak SP, Kessler RC. Psychiatric disorders and stages of smoking. Biol Psychiatry. 2004;55:69–76. doi: 10.1016/s0006-3223(03)00317-2. [DOI] [PubMed] [Google Scholar]
- 30.Dembo R, Dertke M, La VL, Borders S, Washburn M, Schmeidler J. Physical abuse, sexual victimization and illicit drug use: a structural analysis among high risk adolescents. J Adolesc. 1987;10:13–34. doi: 10.1016/s0140-1971(87)80030-1. [DOI] [PubMed] [Google Scholar]
- 31.Harrison PA, Fulkerson JA, Beebe TJ. Multiple substance use among adolescent physical and sexual abuse victims. Child Abuse Negl. 1997;21:529–539. doi: 10.1016/s0145-2134(97)00013-6. [DOI] [PubMed] [Google Scholar]
- 32.Neumann DA, Houskamp BM, Pollack VE, Briere J. The long term sequelae of childhood sexual abuse in women. Child Maltreatment. 1996;1:6–16. [Google Scholar]
- 33.Bushnell JA, Wells JE, Oakley-Browne MA. Long-term effects of intrafamilial sexual abuse in childhood. Acta Psychiatr Scand. 1992;85:136–142. doi: 10.1111/j.1600-0447.1992.tb01458.x. [DOI] [PubMed] [Google Scholar]
- 34.Dube SR, Anda RF, Whitfield CL, Brown DW, Felitti VJ, Dong M, et al. Long-term consequences of childhood sexual abuse by gender of victim. Am J Prev Med. 2005;28:430–438. doi: 10.1016/j.amepre.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 35.Nelson EC, Heath AC, Madden PA, Cooper ML, Dinwiddie SH, Bucholz KK, et al. Association between self-reported childhood sexual abuse and adverse psychosocial outcomes: results from a twin study. Arch Gen Psychiatry. 2002;59:139–145. doi: 10.1001/archpsyc.59.2.139. [DOI] [PubMed] [Google Scholar]
- 36.Collins LM, Graham JW, Long J, Hansen WB. Crossvalidation of latent class models of early substance use onset. Multivariate Behavioral Research. 1994;29:165–183. doi: 10.1207/s15327906mbr2902_3. [DOI] [PubMed] [Google Scholar]
- 37.Pergadia ML, Heath AC, Agrawal A, Bucholz KK, Martin NG, Madden PA. The Implications of Simultaneous Smoking Initiation for Inferences about the Genetics of Smoking Behavior from Twin Data. Behav Genet. 2006;1–10:1–10. doi: 10.1007/s10519-005-9042-7. [DOI] [PubMed] [Google Scholar]
- 38.Neale MC. Statistical Modeling with Mx. Dept of Psychiatry; Box # 980710, Richmond VA 23298: 2004. [Google Scholar]
- 39.Amos A, Wiltshire S, Bostock Y, Haw S, McNeill A. ‘You can’t go without a fag…you need it for your hash’--a qualitative exploration of smoking, cannabis and young people. Addiction. 2004;99:77–81. doi: 10.1111/j.1360-0443.2004.00531.x. [DOI] [PubMed] [Google Scholar]
- 40.Ream GL, Benoit E, Johnson BD, Dunlap E. Smoking tobacco along with marijuana increases symptoms of cannabis dependence. Drug Alcohol Depend. 2008;95:199–208. doi: 10.1016/j.drugalcdep.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Agrawal A, Madden P, Bucholz K, Heath A, Lynskey M. Transitions to Regular Smoking and to Nicotine Dependence in Women using Cannabis. Drug Alcohol Depend. 2008;95:107–114. doi: 10.1016/j.drugalcdep.2007.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han C, McGue MK, Iacono WG. Lifetime tobacco, alcohol and other substance use in adolescent Minnesota twins: univariate and multivariate behavioral genetic analyses. Addiction. 1999;94:981–993. doi: 10.1046/j.1360-0443.1999.9479814.x. [DOI] [PubMed] [Google Scholar]
- 43.Young SE, Rhee SH, Stallings MC, Corley RP, Hewitt JK. Genetic and environmental vulnerabilities underlying adolescent substance use and problem use: general or specific? Behav Genet. 2006;36:603–615. doi: 10.1007/s10519-006-9066-7. [DOI] [PubMed] [Google Scholar]
- 44.Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: modeling the externalizing spectrum. J Abnorm Psychol. 2002;111:411–424. [PubMed] [Google Scholar]
- 45.Elkins IJ, King SM, McGue M, Iacono WG. Personality traits and the development of nicotine, alcohol, and illicit drug disorders: prospective links from adolescence to young adulthood. J Abnorm Psychol. 2006;115:26–39. doi: 10.1037/0021-843X.115.1.26. [DOI] [PubMed] [Google Scholar]
- 46.Walden B, McGue M, Lacono WG, Burt SA, Elkins I. Identifying shared environmental contributions to early substance use: the respective roles of peers and parents. J Abnorm Psychol. 2004;113:440–450. doi: 10.1037/0021-843X.113.3.440. [DOI] [PubMed] [Google Scholar]
- 47.Iacono WG, Carlson SR, Taylor J, Elkins IJ, McGue M. Behavioral disinhibition and the development of substance-use disorders: findings from the Minnesota Twin Family Study. Dev Psychopathol. 1999;11:869–900. doi: 10.1017/s0954579499002369. [DOI] [PubMed] [Google Scholar]
- 48.Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- 49.Edenberg HJ, Dick DM, Xuei X, Tian H, Almasy L, Bauer LO, et al. Variations in GABRA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. Am J Hum Genet. 2004;74:705–714. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fehr C, Sander T, Tadic A, Lenzen KP, Anghelescu I, Klawe C, et al. Confirmation of association of the GABRA2 gene with alcohol dependence by subtype-specific analysis. Psychiatr Genet. 2006;16:9–17. doi: 10.1097/01.ypg.0000185027.89816.d9. [DOI] [PubMed] [Google Scholar]
- 51.Lappalainen J, Krupitsky E, Remizov M, Pchelina S, Taraskina A, Zvartau E, et al. Association between alcoholism and gamma-amino butyric acid alpha2 receptor subtype in a Russian population. Alcohol Clin Exp Res. 2005;29:493–498. doi: 10.1097/01.alc.0000158938.97464.90. [DOI] [PubMed] [Google Scholar]
- 52.Covault J, Gelernter J, Hesselbrock V, Nellissery M, Kranzler HR. Allelic and haplotypic association of GABRA2 with alcohol dependence. Am J Med Genet B Neuropsychiatr Genet. 2004;129:104–109. doi: 10.1002/ajmg.b.30091. [DOI] [PubMed] [Google Scholar]
- 53.Agrawal A, Edenberg HJ, Foroud T, Bierut LJ, Dunne G, Hinrichs AL, et al. Association of GABRA2 with Drug Dependence in the Collaborative Study of the Genetics of Alcoholism Sample. Behav Genet. 2006;36:640–650. doi: 10.1007/s10519-006-9069-4. [DOI] [PubMed] [Google Scholar]
- 54.Agrawal A, Pergadia ML, Saccone SF, Hinrichs AL, Lessov-Schlaggar CN, Saccone NL, et al. Gamma-Aminobutyric Acid Receptor Genes and Nicotine Dependence: Evidence for Association from a Case-Control Study. Addiction. 2008;103:1027–1038. doi: 10.1111/j.1360-0443.2008.02236.x. [DOI] [PubMed] [Google Scholar]
- 55.Dick DM, Bierut LJ, Hinrichs AL, et al. The role of GABRA2 in risk for conduct disorder and alcohol and drug dependence across different developmental stages. Behav Genet. 2006;36:577–590. doi: 10.1007/s10519-005-9041-8. [DOI] [PubMed] [Google Scholar]
- 56.Devane WA, Hanus L, Breuer A, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 57.Yamamoto T, Takada K. Role of cannabinoid receptor in the brain as it relates to drug reward. Jpn J Pharmacol. 2000;84:229–236. doi: 10.1254/jjp.84.229. [DOI] [PubMed] [Google Scholar]
- 58.Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci U S A. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lichtman AH, Martin BR. Cannabinoid tolerance and dependence. Handb Exp Pharmacol. 2005:691–717. doi: 10.1007/3-540-26573-2_24. [DOI] [PubMed] [Google Scholar]
- 60.Zhang PW, Ishiguro H, Ohtsuki T, Hess J, Carillo F, Walther D, et al. Human cannabinoid receptor 1: 5′ exons, candidate regulatory regions, polymorphisms, haplotypes and association with polysubstance abuse. Mol Psychiatry. 2004;9:916–931. doi: 10.1038/sj.mp.4001560. [DOI] [PubMed] [Google Scholar]
- 61.Hopfer CJ, Young SE, Purcell S, et al. Cannabis receptor haplotype associated with fewer cannabis dependence symptoms in adolescents. Am J Med Genet B Neuropsychiatr Genet. 2006;141:895–901. doi: 10.1002/ajmg.b.30378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zuo L, Kranzler HR, Luo X, Covault J, Gelernter J. CNR1 Variation Modulates Risk for Drug and Alcohol Dependence. Biol Psychiatry. 2007 doi: 10.1016/j.biopsych.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 63.Herman AI, Kranzler HR, Cubells JF, Gelernter J, Covault J. Association study of the CNR1 gene exon 3 alternative promoter region polymorphisms and substance dependence. Am J Med Genet B Neuropsychiatr Genet. 2006;141:499–503. doi: 10.1002/ajmg.b.30325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Siu EC, Tyndale RF. Non-nicotinic therapies for smoking cessation. Annu Rev Pharmacol Toxicol. 2007;47:541–64. 541–564. doi: 10.1146/annurev.pharmtox.47.120505.105354. [DOI] [PubMed] [Google Scholar]
- 65.Gelfand EV, Cannon CP. Rimonabant: a selective blocker of the cannabinoid CB1 receptors for the management of obesity, smoking cessation and cardiometabolic risk factors. Expert Opin Investig Drugs. 2006;15:307–315. doi: 10.1517/13543784.15.3.307. [DOI] [PubMed] [Google Scholar]
- 66.Le FB, Goldberg SR. Rimonabant, a CB1 antagonist, blocks nicotine-conditioned place preferences. Neuroreport. 2004;15:2139–2143. doi: 10.1097/00001756-200409150-00028. [DOI] [PubMed] [Google Scholar]
- 67.Castane A, Valjent E, Ledent C, Parmentier M, Maldonado R, Valverde O. Lack of CB1 cannabinoid receptors modifies nicotine behavioural responses, but not nicotine abstinence. Neuropharmacology. 2002;43:857–867. doi: 10.1016/s0028-3908(02)00118-1. [DOI] [PubMed] [Google Scholar]
- 68.Garwood CL, Potts LA. Emerging pharmacotherapies for smoking cessation. Am J Health Syst Pharm. 2007;64:1693–1698. doi: 10.2146/ajhp060427. [DOI] [PubMed] [Google Scholar]
- 69.Cahill K, Ussher M. Cannabinoid type 1 receptor antagonists (rimonabant) for smoking cessation. Cochrane Database Syst Rev. 2007:CD005353. doi: 10.1002/14651858.CD005353.pub2. [DOI] [PubMed] [Google Scholar]
- 70.Muccioli GG. Blocking the cannabinoid receptors: drug candidates and therapeutic promises. Chem Biodivers. 2007;4:1805–1827. doi: 10.1002/cbdv.200790153. [DOI] [PubMed] [Google Scholar]