Abstract
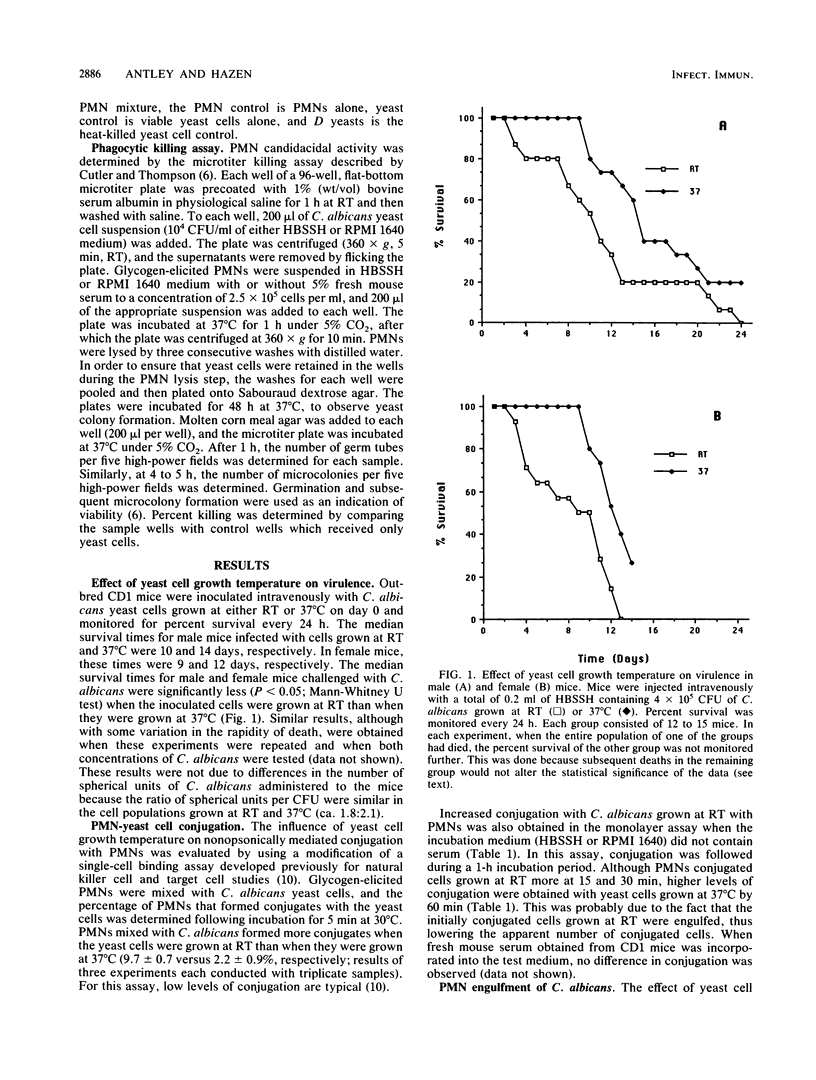
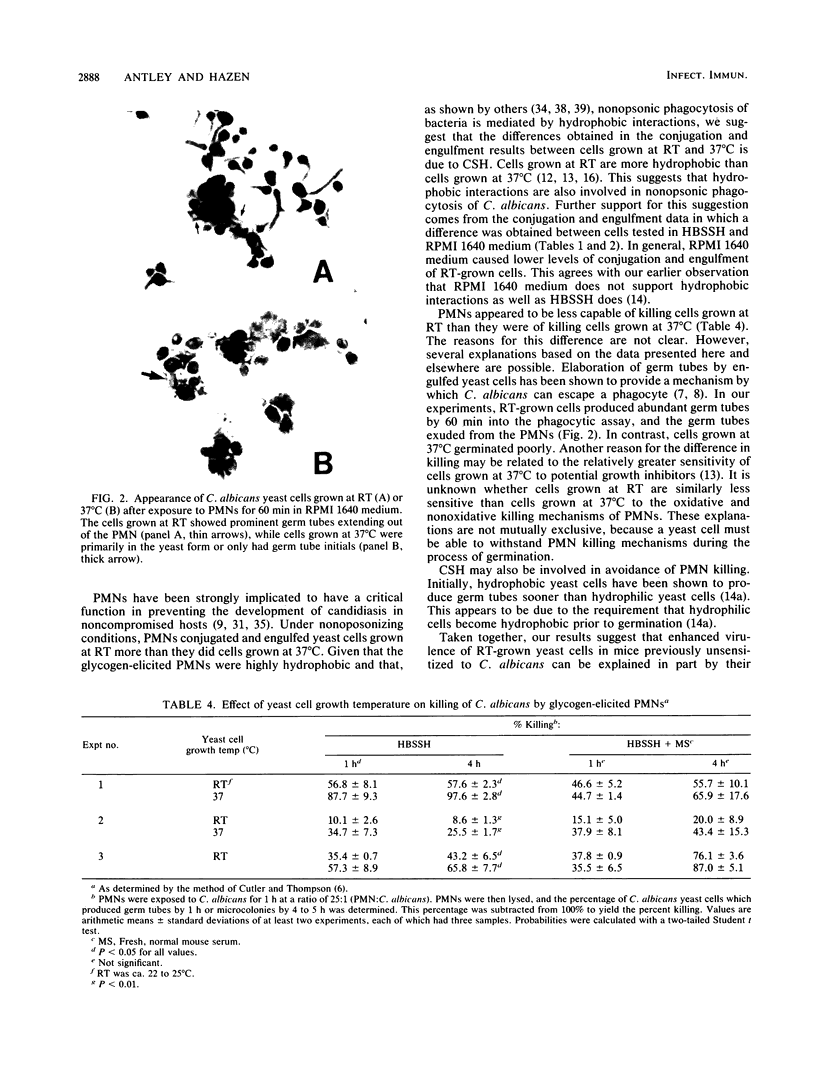
Previous studies have suggested that yeast cell growth temperature may influence the relative virulence of the opportunistic dimorphic fungus Candida albicans. To test this possibility, mice were challenged with C. albicans yeast cells which were grown at either room temperature or 37 degrees C, and their survival was monitored daily. Mice which received room temperature-grown cells died faster. The interaction of glycogen-elicited polymorphonucleated neutrophils (PMNs) with C. albicans yeast cells grown at the two temperatures was examined, because PMNs have been shown to have a critical role in preventing development of candidiasis in normal individuals. In the absence of serum (i.e., nonopsonic conditions), more PMNs conjugated and engulfed C. albicans cells grown at room temperature than those grown at 37 degrees C. However, PMNs were less able to kill cells grown at room temperature than cells grown at 37 degrees C. Cells grown at room temperature also produced abundant germ tubes after engulfment and were thus more likely to escape killing by phagocytes. These results suggest that cells grown at room temperature are more virulent because they are less likely to be killed by phagocytes and are more likely to disseminate. The possibility that expression of cell surface hydrophobicity is involved in these events is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Absolom D. R. Basic methods for the study of phagocytosis. Methods Enzymol. 1986;132:95–180. doi: 10.1016/s0076-6879(86)32005-6. [DOI] [PubMed] [Google Scholar]
- Barrett-Bee K., Hayes Y., Wilson R. G., Ryley J. F. A comparison of phospholipase activity, cellular adherence and pathogenicity of yeasts. J Gen Microbiol. 1985 May;131(5):1217–1221. doi: 10.1099/00221287-131-5-1217. [DOI] [PubMed] [Google Scholar]
- Borg M., Rüchel R. Expression of extracellular acid proteinase by proteolytic Candida spp. during experimental infection of oral mucosa. Infect Immun. 1988 Mar;56(3):626–631. doi: 10.1128/iai.56.3.626-631.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawner D. L., Cutler J. E. Cell surface and intracellular expression of two Candida albicans antigens during in vitro and in vivo growth. Microb Pathog. 1987 Apr;2(4):249–257. doi: 10.1016/0882-4010(87)90123-9. [DOI] [PubMed] [Google Scholar]
- Bridges C. G., Dasilva G. L., Yamamura M., Valdimarsson H. A radiometric assay for the combined measurement of phagocytosis and intracellular killing of Candida albicans. Clin Exp Immunol. 1980 Nov;42(2):226–233. [PMC free article] [PubMed] [Google Scholar]
- Cutler J. E., Thompson B. D. A simple and inexpensive method for assessing in vitro candidacidal activity of leukocytes. J Immunol Methods. 1984 Jan 20;66(1):27–33. doi: 10.1016/0022-1759(84)90244-8. [DOI] [PubMed] [Google Scholar]
- Diamond R. D., Krzesicki R., Jao W. Damage to pseudohyphal forms of Candida albicans by neutrophils in the absence of serum in vitro. J Clin Invest. 1978 Feb;61(2):349–359. doi: 10.1172/JCI108945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Oppenheim F., Nakagawa Y., Krzesicki R., Haudenschild C. C. Properties of a product of Candida albicans hyphae and pseudohyphae that inhibits contact between the fungi and human neutrophils in vitro. J Immunol. 1980 Dec;125(6):2797–2804. [PubMed] [Google Scholar]
- Grimm E. A., Bonavida B. Studies of the induction and expression of T cell mediated immunity. VI. Heterogeneity of lytic efficiency exhibited by isolated cytotoxic T lymphocytes prepared from highly enriched populations of effector-target conjugates. J Immunol. 1977 Sep;119(3):1041–1047. [PubMed] [Google Scholar]
- Hazen B. W., Hazen K. C. Dynamic expression of cell surface hydrophobicity during initial yeast cell growth and before germ tube formation of Candida albicans. Infect Immun. 1988 Sep;56(9):2521–2525. doi: 10.1128/iai.56.9.2521-2525.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazen B. W., Hazen K. C. Modification and application of a simple, surface hydrophobicity detection method to immune cells. J Immunol Methods. 1988 Mar 16;107(2):157–163. doi: 10.1016/0022-1759(88)90214-1. [DOI] [PubMed] [Google Scholar]
- Hazen K. C., Cutler J. E. Autoregulation of germ tube formation by Candida albicans. Infect Immun. 1979 Jun;24(3):661–666. doi: 10.1128/iai.24.3.661-666.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazen K. C., Hazen B. W. Temperature-modulated physiological characteristics of Candida albicans. Microbiol Immunol. 1987;31(6):497–508. doi: 10.1111/j.1348-0421.1987.tb03112.x. [DOI] [PubMed] [Google Scholar]
- Hazen K. C., Plotkin B. J., Klimas D. M. Influence of growth conditions on cell surface hydrophobicity of Candida albicans and Candida glabrata. Infect Immun. 1986 Oct;54(1):269–271. doi: 10.1128/iai.54.1.269-271.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata K. Toxins produced by Candida albicans. Contrib Microbiol Immunol. 1977;4:77–85. [PubMed] [Google Scholar]
- Kennedy M. J., Volz P. A., Edwards C. A., Yancey R. J. Mechanisms of association of Candida albicans with intestinal mucosa. J Med Microbiol. 1987 Dec;24(4):333–341. doi: 10.1099/00222615-24-4-333. [DOI] [PubMed] [Google Scholar]
- Kimura L. H., Pearsall N. N. Relationship between germination of Candida albicans and increased adherence to human buccal epithelial cells. Infect Immun. 1980 May;28(2):464–468. doi: 10.1128/iai.28.2.464-468.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. C., King R. D. Characterization of Candida albicans adherence to human vaginal epithelial cells in vitro. Infect Immun. 1983 Sep;41(3):1024–1030. doi: 10.1128/iai.41.3.1024-1030.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Interaction of Candida albicans with human leukocytes and serum. J Bacteriol. 1969 Jun;98(3):996–1004. doi: 10.1128/jb.98.3.996-1004.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitz S. M., Diamond R. D. A rapid colorimetric assay of fungal viability with the tetrazolium salt MTT. J Infect Dis. 1985 Nov;152(5):938–945. doi: 10.1093/infdis/152.5.938. [DOI] [PubMed] [Google Scholar]
- Macura A. B. Hydrophobicity of Candida albicans related to their adherence to mucosal epithelial cells. Zentralbl Bakteriol Mikrobiol Hyg A. 1987 Oct;266(3-4):491–496. doi: 10.1016/s0176-6724(87)80231-6. [DOI] [PubMed] [Google Scholar]
- Morrison R. P., Cutler J. E. In vitro studies of the interaction of murine phagocytic cells with Candida albicans. J Reticuloendothel Soc. 1981 Jan;29(1):23–34. [PubMed] [Google Scholar]
- Odds F. C. Morphogenesis in Candida albicans. Crit Rev Microbiol. 1985;12(1):45–93. doi: 10.3109/10408418509104425. [DOI] [PubMed] [Google Scholar]
- Poulain D., Hopwood V., Vernes A. Antigenic variability of Candida albicans. Crit Rev Microbiol. 1985;12(3):223–270. doi: 10.3109/10408418509104430. [DOI] [PubMed] [Google Scholar]
- Reinhart H., Muller G., Sobel J. D. Specificity and mechanism of in vitro adherence of Candida albicans. Ann Clin Lab Sci. 1985 Sep-Oct;15(5):406–413. [PubMed] [Google Scholar]
- Rogers T. J., Balish E. Immunity to Candida albicans. Microbiol Rev. 1980 Dec;44(4):660–682. doi: 10.1128/mr.44.4.660-682.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotrosen D., Edwards J. E., Jr, Gibson T. R., Moore J. C., Cohen A. H., Green I. Adherence of Candida to cultured vascular endothelial cells: mechanisms of attachment and endothelial cell penetration. J Infect Dis. 1985 Dec;152(6):1264–1274. doi: 10.1093/infdis/152.6.1264. [DOI] [PubMed] [Google Scholar]
- Saito H., Tomioka H., Watanabe T., Sato K. Mechanisms of phagocytosis of Mycobacterium leprae and other mycobacteria by human oligodendroglial cells. Infect Immun. 1986 Jan;51(1):163–167. doi: 10.1128/iai.51.1.163-167.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel J. D., Muller G., Buckley H. R. Critical role of germ tube formation in the pathogenesis of candidal vaginitis. Infect Immun. 1984 Jun;44(3):576–580. doi: 10.1128/iai.44.3.576-580.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel J. D., Myers P. G., Kaye D., Levison M. E. Adherence of Candida albicans to human vaginal and buccal epithelial cells. J Infect Dis. 1981 Jan;143(1):76–82. doi: 10.1093/infdis/143.1.76. [DOI] [PubMed] [Google Scholar]
- Speert D. P., Eftekhar F., Puterman M. L. Nonopsonic phagocytosis of strains of Pseudomonas aeruginosa from cystic fibrosis patients. Infect Immun. 1984 Mar;43(3):1006–1011. doi: 10.1128/iai.43.3.1006-1011.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speert D. P., Loh B. A., Cabral D. A., Salit I. E. Nonopsonic phagocytosis of nonmucoid Pseudomonas aeruginosa by human neutrophils and monocyte-derived macrophages is correlated with bacterial piliation and hydrophobicity. Infect Immun. 1986 Jul;53(1):207–212. doi: 10.1128/iai.53.1.207-212.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]