Abstract
Campylobacter pylori is the causative agent of gastritis and possibly of peptic and duodenal ulcers in adults. Histological observations show C. pylori attached to gastric epithelium as well as in the mucus layer of the stomach. We found that clinical isolates of C. pylori possess a cell-bound hemagglutinin detectable with human erythrocytes (all phenotypes tested) and those of a variety of animal species. The C. pylori hemagglutinin is antigenic, heat sensitive, and destroyed by pronase and papain but resistant to pepsin and trypsin. The hemagglutinin has fibrillar morphology; C. pylori-erythrocyte interaction displays very intimate contact, which is typical of fibrillae-mediated attachment. Fibrillae were removed from C. pylori by solubilization with N-octylglucose. After partial purification and removal of N-octylglucose by dialysis, the protein reaggregated, with the assembly of fibrillar structures. Hemagglutination inhibition was observed with the sialoproteins fetuin, alpha 2-macroglobulin, and glycophorin A but not with asialofetuin or asialoglycophorin A. The erythrocyte receptor was more sensitive to destruction by a neuraminidase specific for the N-acetylneuraminyl-alpha(2-3)-galactopyranosyl [NeuAc(2-3)Gal] sequence than one specific for NeuAc(2-6)Gal. Hemagglutination-inhibition assays with N-acetylneuraminyl-alpha(2-3)-lactose [NeuAc(2-3)-lactose] and NeuAc(2-6)-lactose confirmed that the C. pylori hemagglutinin preferentially binds to the NeuAc(2-3)Gal isomer of NeuAc-lactose. Based upon the above-described properties of the C. pylori fibrillar hemagglutinin, we conclude that this antigen should be designated as a putative colonization factor antigen.
Full text
PDF








Images in this article
Selected References
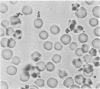
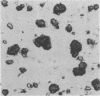
These references are in PubMed. This may not be the complete list of references from this article.
- Arai H., Munoz J. J. Fimbrial hemagglutinin in stationary and shake cultures of Bordetella pertussis. Infect Immun. 1979 Aug;25(2):764–767. doi: 10.1128/iai.25.2.764-767.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Blaser M. J. Gastric Campylobacter-like organisms, gastritis, and peptic ulcer disease. Gastroenterology. 1987 Aug;93(2):371–383. doi: 10.1016/0016-5085(87)91028-6. [DOI] [PubMed] [Google Scholar]
- Buchanan T. M. Antigen-specific serotyping of Neisseria gonorrhoeae. I. Use of an enzyme-linked immunosorbent assay to quantitate pilus antigens on gonococci. J Infect Dis. 1978 Sep;138(3):319–315. doi: 10.1093/infdis/138.3.319. [DOI] [PubMed] [Google Scholar]
- Buck G. E., Gourley W. K., Lee W. K., Subramanyam K., Latimer J. M., DiNuzzo A. R. Relation of Campylobacter pyloridis to gastritis and peptic ulcer. J Infect Dis. 1986 Apr;153(4):664–669. doi: 10.1093/infdis/153.4.664. [DOI] [PubMed] [Google Scholar]
- Drumm B., Sherman P., Cutz E., Karmali M. Association of Campylobacter pylori on the gastric mucosa with antral gastritis in children. N Engl J Med. 1987 Jun 18;316(25):1557–1561. doi: 10.1056/NEJM198706183162501. [DOI] [PubMed] [Google Scholar]
- Ehara M., Ishibashi M., Ichinose Y., Iwanaga M., Shimotori S., Naito T. Purification and partial characterization of fimbriae of Vibrio cholerae O1. Vaccine. 1987 Dec;5(4):283–288. doi: 10.1016/0264-410x(87)90153-8. [DOI] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Clegg S., Pauley J. A. Purification and characterization of the CFA/I antigen of enterotoxigenic Escherichia coli. Infect Immun. 1979 Aug;25(2):738–748. doi: 10.1128/iai.25.2.738-748.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G., Young L. S., Pitt J. Hemagglutination typing of Escherichia coli: definition of seven hemagglutination types. J Clin Microbiol. 1980 Aug;12(2):235–242. doi: 10.1128/jcm.12.2.235-242.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldhar J., Perry R., Golecki J. R., Hoschutzky H., Jann B., Jann K. Nonfimbrial, mannose-resistant adhesins from uropathogenic Escherichia coli O83:K1:H4 and O14:K?:H11. Infect Immun. 1987 Aug;55(8):1837–1842. doi: 10.1128/iai.55.8.1837-1842.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D. Y., Klein P. D. Campylobacter pyloridis gastritis: the past, the present, and speculations about the future. Am J Gastroenterol. 1987 Apr;82(4):283–286. [PubMed] [Google Scholar]
- Graham D. Y., Klein P. D., Evans D. J., Jr, Evans D. G., Alpert L. C., Opekun A. R., Boutton T. W. Campylobacter pylori detected noninvasively by the 13C-urea breath test. Lancet. 1987 May 23;1(8543):1174–1177. doi: 10.1016/s0140-6736(87)92145-3. [DOI] [PubMed] [Google Scholar]
- Hazell S. L., Lee A., Brady L., Hennessy W. Campylobacter pyloridis and gastritis: association with intercellular spaces and adaptation to an environment of mucus as important factors in colonization of the gastric epithelium. J Infect Dis. 1986 Apr;153(4):658–663. doi: 10.1093/infdis/153.4.658. [DOI] [PubMed] [Google Scholar]
- Hinson G., Knutton S., Lam-Po-Tang M. K., McNeish A. S., Williams P. H. Adherence to human colonocytes of an Escherichia coli strain isolated from severe infantile enteritis: molecular and ultrastructural studies of a fibrillar adhesin. Infect Immun. 1987 Feb;55(2):393–402. doi: 10.1128/iai.55.2.393-402.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T., Kasemsuksakul K., Oguchi T., Kohda M., Miwatani T. Production and partial characterization of pili on non-O1 Vibrio cholerae. J Infect Dis. 1988 Jan;157(1):217–218. doi: 10.1093/infdis/157.1.217. [DOI] [PubMed] [Google Scholar]
- Kaldor J., Tee W., Nicolacopolous C., Demirtzoglou K., Noonan D., Dwyer B. Immunoblot confirmation of immune response to Campylobacter pyloridis in patients with duodenal ulcers. Med J Aust. 1986 Aug 4;145(3-4):133–135. doi: 10.5694/j.1326-5377.1986.tb113771.x. [DOI] [PubMed] [Google Scholar]
- Kapperud G., Namork E., Skarpeid H. J. Temperature-inducible surface fibrillae associated with the virulence plasmid of Yersinia enterocolitica and Yersinia pseudotuberculosis. Infect Immun. 1985 Feb;47(2):561–566. doi: 10.1128/iai.47.2.561-566.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch H., Leying H., Büscher K. H., Kroll H. P., Opferkuch W. Isolation and separation of physicochemically distinct fimbrial types expressed on a single culture of Escherichia coli O7:K1:H6. Infect Immun. 1985 Feb;47(2):549–554. doi: 10.1128/iai.47.2.549-554.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutton S., Lloyd D. R., Candy D. C., McNeish A. S. Ultrastructural study of adhesion of enterotoxigenic Escherichia coli to erythrocytes and human intestinal epithelial cells. Infect Immun. 1984 May;44(2):519–527. doi: 10.1128/iai.44.2.519-527.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutton S., Lloyd D. R., McNeish A. S. Adhesion of enteropathogenic Escherichia coli to human intestinal enterocytes and cultured human intestinal mucosa. Infect Immun. 1987 Jan;55(1):69–77. doi: 10.1128/iai.55.1.69-77.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T. K., Väisänen-Rhen V., Rhen M., Pere A., Parkkinen J., Finne J. Escherichia coli fimbriae recognizing sialyl galactosides. J Bacteriol. 1984 Aug;159(2):762–766. doi: 10.1128/jb.159.2.762-766.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labigne-Roussel A. F., Lark D., Schoolnik G., Falkow S. Cloning and expression of an afimbrial adhesin (AFA-I) responsible for P blood group-independent, mannose-resistant hemagglutination from a pyelonephritic Escherichia coli strain. Infect Immun. 1984 Oct;46(1):251–259. doi: 10.1128/iai.46.1.251-259.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachica R. V., Zink D. L., Ferris W. R. Association of fibril structure formation with cell surface properties of Yersinia enterocolitica. Infect Immun. 1984 Oct;46(1):272–275. doi: 10.1128/iai.46.1.272-275.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marre R., Hacker J. Role of S- and common-type I-fimbriae of Escherichia coli in experimental upper and lower urinary tract infection. Microb Pathog. 1987 Mar;2(3):223–226. doi: 10.1016/0882-4010(87)90023-4. [DOI] [PubMed] [Google Scholar]
- Marshall B. J. Campylobacter pyloridis and gastritis. J Infect Dis. 1986 Apr;153(4):650–657. doi: 10.1093/infdis/153.4.650. [DOI] [PubMed] [Google Scholar]
- Moch T., Hoschützky H., Hacker J., Kröncke K. D., Jann K. Isolation and characterization of the alpha-sialyl-beta-2,3-galactosyl-specific adhesin from fimbriated Escherichia coli. Proc Natl Acad Sci U S A. 1987 May;84(10):3462–3466. doi: 10.1073/pnas.84.10.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Mirelman D., Sharon N. Adherence of Escherichia coli to human mucosal cells mediated by mannose receptors. Nature. 1977 Feb 17;265(5595):623–625. doi: 10.1038/265623a0. [DOI] [PubMed] [Google Scholar]
- Orskov I., Birch-Andersen A., Duguid J. P., Stenderup J., Orskov F. An adhesive protein capsule of Escherichia coli. Infect Immun. 1985 Jan;47(1):191–200. doi: 10.1128/iai.47.1.191-200.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkkinen J., Rogers G. N., Korhonen T., Dahr W., Finne J. Identification of the O-linked sialyloligosaccharides of glycophorin A as the erythrocyte receptors for S-fimbriated Escherichia coli. Infect Immun. 1986 Oct;54(1):37–42. doi: 10.1128/iai.54.1.37-42.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punsalang A. P., Jr, Sawyer W. D. Role of pili in the virulence of Neisseria gonorrhoeae. Infect Immun. 1973 Aug;8(2):255–263. doi: 10.1128/iai.8.2.255-263.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauws E. A., Langenberg W., Houthoff H. J., Zanen H. C., Tytgat G. N. Campylobacter pyloridis-associated chronic active antral gastritis. A prospective study of its prevalence and the effects of antibacterial and antiulcer treatment. Gastroenterology. 1988 Jan;94(1):33–40. [PubMed] [Google Scholar]
- Rhen M., Klemm P., Korhonen T. K. Identification of two new hemagglutinins of Escherichia coli, N-acetyl-D-glucosamine-specific fimbriae and a blood group M-specific agglutinin, by cloning the corresponding genes in Escherichia coli K-12. J Bacteriol. 1986 Dec;168(3):1234–1242. doi: 10.1128/jb.168.3.1234-1242.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit H., Gaastra W., Kamerling J. P., Vliegenthart J. F., de Graaf F. K. Isolation and structural characterization of the equine erythrocyte receptor for enterotoxigenic Escherichia coli K99 fimbrial adhesin. Infect Immun. 1984 Nov;46(2):578–584. doi: 10.1128/iai.46.2.578-584.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulshen M. H., Rollo J. L. Pathogenesis of escherichia coli gastroenteritis in man--another mechanism. N Engl J Med. 1980 Jan 10;302(2):99–101. doi: 10.1056/NEJM198001103020207. [DOI] [PubMed] [Google Scholar]
- Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983 Jun 4;1(8336):1273–1275. [PubMed] [Google Scholar]
- Wadström T., Faris A., Freer J., Habte D., Hallberg D., Ljungh A. Hydrophobic surface properties of enterotoxigenic E. coli (ETEC) with different colonization factors (CFA/i, CFA/ii, K88 and K99) and attachment to intestinal epithelial cells. Scand J Infect Dis Suppl. 1980;Suppl 24:148–153. [PubMed] [Google Scholar]
- Watts T. H., Scraba D. G., Paranchych W. Formation of 9-nm filaments from pilin monomers obtained by octyl-glucoside dissociation of Pseudomonas aeruginosa pili. J Bacteriol. 1982 Sep;151(3):1508–1513. doi: 10.1128/jb.151.3.1508-1513.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. H., Knutton S., Brown M. G., Candy D. C., McNeish A. S. Characterization of nonfimbrial mannose-resistant protein hemagglutinins of two Escherichia coli strains isolated from infants with enteritis. Infect Immun. 1984 Jun;44(3):592–598. doi: 10.1128/iai.44.3.592-598.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. E., Straus D. C., Johanson W. G., Jr, Berry V. K., Bass J. A. Role of pili in adherence of Pseudomonas aeruginosa to mammalian buccal epithelial cells. Infect Immun. 1980 Sep;29(3):1146–1151. doi: 10.1128/iai.29.3.1146-1151.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wulffen H., Heesemann J., Bützow G. H., Löning T., Laufs R. Detection of Campylobacter pyloridis in patients with antrum gastritis and peptic ulcers by culture, complement fixation test, and immunoblot. J Clin Microbiol. 1986 Nov;24(5):716–720. doi: 10.1128/jcm.24.5.716-720.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]