Abstract
We investigated the photodynamic effects of methylene blue (MB) on multi-species root canal biofilms comprising Actinomyces israelii, Fusobacterium nucleatum subspecies nucleatum, Porphyromonas gingivalis and Prevotella intermedia in experimentally infected root canals of extracted human teeth in vitro. The four test microorganisms were detected in root canals using DNA probes. Scanning electron microscopy (SEM) showed the presence of biofilms in root canals prior to therapy. Root canal systems were incubated with MB (25 µg/ml) for 10 minutes followed by exposure to red light at 665 nm with an energy fluence of 30 J/cm2. Light was delivered from a diode laser via a 250 µm diameter polymethyl methacrylate optical fiber that uniformly distributed light at 360°. Photodynamic therapy (PDT) achieved up to 80% reduction of colony-forming unit counts. We conclude that PDT can be an effective adjunct to standard endodontic antimicrobial treatment when the PDT parameters are optimized.
INTRODUCTION
The purpose and ultimate goal of endodontic treatment is to eliminate the bacterial infection in the root canal system and allow healing of apical periodontitis. Primary root canal therapy is a highly predictable procedure; however, inability to sufficiently disinfect the root canal system may lead to failure or persistent apical pathosis (1). While mechanical debridement combined with chemical irrigation removes the bulk of infecting microorganisms, residual bacteria are readily detectable in approximately one-half of teeth just prior to obturation (2). Certain operative problems such as inadequate instrumentation, a missed canal, or an inadequate restoration may lead to post-treatment endodontic disease (3). In addition, the anatomical complexity of the root canal system makes complete debridement of bacteria almost impossible even if conventional methods of chemo-mechanical debridement are performed to the highest technical standards (4). Since there is a marked decrease in the prognosis of endodontic retreatment, we sought adjuncts to standard endodontic antimicrobial procedures that may increase the effectiveness of orthograde endodontic treatment/retreatment (1).
The bacterial microflora of primary endodontic infection differs from that of post-treatment endodontic disease. Both cultural methods and polymerase chain reaction-based methods have demonstrated that primary endodontic infections are associated with polymicrobial and strictly anaerobic microorganisms (4, 5, 6). Endodontic treatment failures, however, are frequently associated with gram-positive aerobic and facultative microorganisms (6). The presence of Enterococcus faecalis in failed endodontic treatment is extensively covered in the literature (3, 7) and is rarely detected in primary infected and untreated cases. Yet, one can not discount the presence or significance of other microorganisms belonging to the genera Actinomyces, Propionibacterium, Porphyromonas and Prevotella which have been frequently detected in endodontic treatment failures (3, 7, 8).
Photodynamic therapy (PDT) was developed as a therapy for cancer and is based on the concept that a non-toxic photosensitizing agent, known as photosensitizer, can be preferentially localized in premalignant and malignant tissues and subsequently activated by light of the appropriate wavelength to generate singlet oxygen and free radicals that are cytotoxic to cells of the target tissue (9). Several studies have shown that oral bacteria are susceptible to PDT (10, 11). In recent years, PDT has been employed to target microorganisms in root canals in vitro (12–20) and in vivo (21–23). These studies suggested the potential of PDT as an adjunct to standard endodontic antimicrobial treatment. Methylene blue (MB), a well-established photosensitizer, has been used in PDT for targeting endodontic bacteria (12, 15, 18, 19) The hydrophilicity of MB (24), along with its low molecular weight and positive charge allows passage across the porin-protein channels in the outer membrane of gram-negative bacteria (25). Methylene blue predominantly interacts with the anionic macromolecule lipopolysaccharide resulting in the generation of MB dimers (25), which participate in the photosensitization process (25).
The present in vitro study evaluates the response of multi-species root canal biofilms of single-rooted extracted human teeth to PDT after sensitization with MB and exposure to red light at 665 nm. For the development of root canal biofilms, four bacterial species were used: the gram-positive rod Actinomyces israelii, and the gram-negative rods Fusobacterium nucleatum subspecies nucleatum, Porphyromonas gingivalis and Prevotella intermedia. All of these bacteria are key pathogens in infected root canals (26). A 250-µm diameter optical fiber made of polymethyl methacrylate was used for establishing substantially uniform illumination at 360°C within root canals.
MATERIALS AND METHODS
Photosensitizer
Methylene blue (Sigma, St Louis, MO) underwent dissolution in brain heart infusion (BHI) broth or phosphate buffered saline (PBS) broth and was filter-sterilized immediately prior to use. The final concentration used was 25 µg/ml (67 µM). The ultraviolet-visible absorption spectra of MB in BHI broth or PBS were recorded from 200 to 800 nm using quartz cuvettes with 1 cm path length on a diode-array spectrophotometer. The absorption spectra of MB in BHI broth or PBS were characterized by a long-wavelength maximum at 665 nm as shown previously (15).
Microorganisms
The following bacteria were used in this study: A. israelii (ATCC 12102), F. nucleatum subspecies nucleatum (ATCC 25586), P. gingivalis (ATCC 33277) and P. intermedia (ATCC 25611). Cultures of P. gingivalis were maintained by weekly subculture in plates comprised of Trypticase soy agar (Becton, Dickinson, and Co., Sparks, MD), BHI agar (Beckton, Dickinson, and Co.), yeast extract (Fisher Biotech, Fair Lawn, NJ), 5 µg/ml hemin, 0.3 µg/ml vitamin K, and 5% sheep blood. Trypticase soy agar with 5% sheep blood (Northeast Laboratories, Waterville, ME) was the culture media for the remaining bacterial species. Microorganisms were grown in an atmosphere of 80% N2, 10% H2, 10% CO2 at 35°C in an anaerobic chamber for 72 hr, harvested from plates, and suspended in BHI broth. Cells were dispersed by vortexing and repeated passage through Pasteur pipettes. Cell numbers were estimated by spectrophotometry at 600 nm in 1-ml cuvettes (0.1 optical density unit equals approximately 108 cells/ml).
Light Source
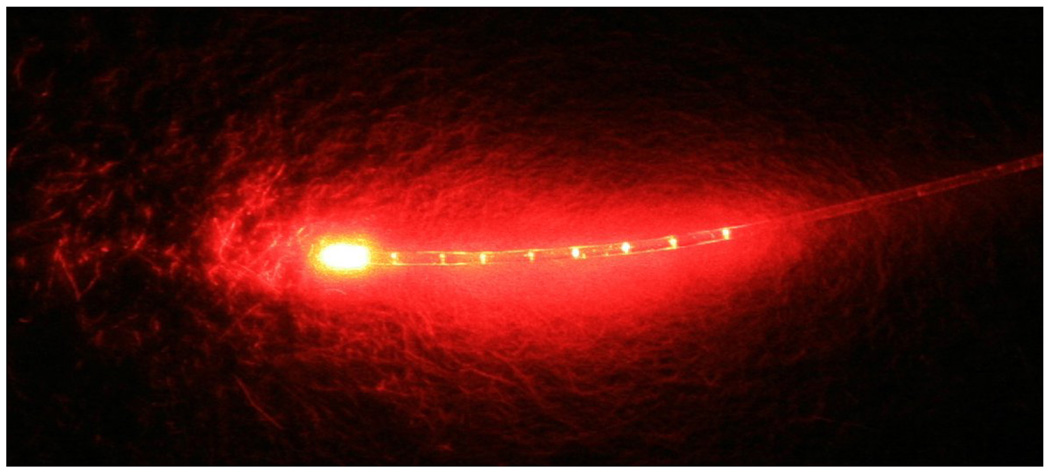
The irradiation source was a diode laser (BWTEK Inc., Newark, DE) with an output power of 1 Watt and a central wavelength of 665 nm. The system was coupled to a 250-µm diameter polymethyl methacrylate optical fiber that was mechanically notched over a one-centimeter length at approximately one-millimeter intervals (Schoelly Imaging Inc., Worcester, MA). The fiber was able to uniformly distribute light at 360° (Fig. 1).
Figure 1.
Optical fiber with a diameter of 250 µm that provided uniform illumination.
Tooth specimens
One hundred and twenty extracted single-rooted human teeth lacking radicular pathology or irregularity were stored in 0.5% NaOCl for 2–4 weeks. Specimens were decoronated to a standard 12 mm root segment length with a rotating diamond saw (#911H Brasseler USA, Savannah, GA) set at 20,000 rpm under water-coolent. Patency of apical foramina was established by inserting a size 15K-file (Dentsply Maillefer, Tulsa, OK). A file measurement was taken at the point where the size 15 K-file became visible at the apical foramen and 0.5 mm was subtracted to set the working length. The instrumentation sequence consisted of: Gates Glidden Burs (Dentsply Maillefer, Tulsa, OK) sizes 4 and 2 for the coronal 4 mm, ProTaper® S1 (Dentsply Tulsa Dental, Tulsa, OK), followed by .06 taper Profile® series 29® (Dentsply Tulsa Dental, Tulsa,OK) files sizes 4 (0.216 ISO equivalent) to 7 (0.465 ISO equivalent) in a crown-down manner, to achieve a master apical file size of 0.465 (ISO equivalent). An Aseptico Endo ITR™ (Dentsply Tulsa Dental, Tulsa, OK) electric motor was used with an 8:1 gear reduction mini-head contra-angle. Final apical patency was established with a size 25 K-file (Dentsply Maillefer, Tulsa, OK) in order to allow for an adequate apical aperture for flushing of microbial aggregates. RC Prep® (Premium Products, Plymouth Meeting, PA) was used as a lubricant and canals were irrigated with 6% sodium hypochlorite (NaOCl) throughout the instrumentation sequence. After canal preparation an aliquot of 1 ml of 17% ethylenediaminetetraacetic acid (EDTA) solution was left in situ for 3 min for smear layer removal, and was replaced by 1 ml of 6% NaOCl for 3 minutes. The tooth specimens were then placed in sterile microcentrifuge tubes containing 1ml of phosphate buffered saline (PBS). Teeth were then autoclaved at 121°C for 20 minutes. Following autoclave sterilization, PBS was aspirated from the microcentrifuge tubes under sterile conditions. The removal of the smear layer and the patency of dentinal tubules were previously demonstrated by scanning electron microscopy (SEM) (15).
Infection of root canals
One hundred and fourteen root specimens were transferred into sterile microcentrifuge tubes under sterile conditions. One milliliter of BHI broth containing 109 microorganisms (2.5 × 108 from each species) was injected into the prepared root canal system using a ProRinse® 30 gauge irrigation needle (Dentsply Tulsa Dental, Tulsa, OK). Cell numbers were estimated as described above in 1 ml cuvettes. After injection, each specimen was entirely submerged in BHI broth and the tubes were incubated anaerobically for 3 days. Following incubation, the medium was aseptically aspirated from the tubes. Three specimens were processed for SEM studies and 111 specimens were used for PDT studies.
Scanning Electron Microscopy
Three root specimens, prepared and infected as described above, were randomly selected for SEM analysis to demonstrate endodontic infection. Longitudinal grooves were cut with a diamond bur both on palatal/lingual and buccal surfaces of each root to facilitate vertical splitting with a chisel. Each sample was split into two halves with a stainless steel chisel. The sample half with the most visible part of the apex was fixed in 3.7% glutaraldehyde in 0.2 M sodium cacodylate buffered solution at 4°C for 24 hours. After dehydration in a graded ethanol concentration series, samples were air dried and mounted on an SEM stubs for gold sputtering and observation with a JEOL JSM 6400 scanning electron microscope (JEOL Corporation, Tokyo, Japan). SEM microphotographs were obtained at a standard magnification of 1500x at each third (coronal, middle and apical) and on the fracture surface.
Photodynamic treatment of biofilms
One hundred and eleven root specimens were prepared and infected as described above. Two sets of experiments utilizing 72 and 39 root specimens, respectively were performed. In the first set of experiments (72 root specimens), MB (25 µg/ml) was dissolved in BHI broth and the specimens were randomly assigned to the following four groups: 1) No light/No MB (control group, 19 specimens); 2) MB only (MB group, 18 specimens); 3) Light only (light group, 17 specimens); 4) Light and MB (PDT group, 18 specimens). Five separate experiments were carried out with 3–4 specimens per group in each experiment. In the second set of experiments (39 root specimens), MB (25 µg/ml) was dissolved in PBS and the specimens were randomly assigned to the following three groups: 1) No light/No MB (control group, 13 specimens); 2) MB only (MB group, 13 specimens); 3) Light and MB (PDT group, 13 specimens). Two separate experiments were carried out with 6 and 7 specimens per group in the first and second experiment, respectively. The rationale for using the above sets of experiments is based on previous studies, which showed reduction of the drug’s photodynamic effects in the presence of serum proteins (27). By dissolving the photosensitizer in PBS, the photodynamic effects of the drug are enhanced (28).
All individual specimens were placed in 1.5 ml microcentrifuge tubes under sterile conditions and then the canals of the MB only and PDT groups were filled to the level of the access cavity with MB solution (25 µg/ml) using a Pro Rinse® 30 gauge irrigation needle (Dentsply Tulsa Dental). After injection, the entire specimen was fully immersed in MB for 10 minutes. Specimens in the light and control groups were injected and fully immersed in sterile BHI broth or PBS. Following incubation, excess drug solution was aspirated and the root specimens were removed from the tubes. Light was then applied in the root canal system of the specimens in appropriate groups for 2.5 minutes followed by a break of 2.5 minutes and a second light exposure for 2.5 minutes using the optical fiber described above. The power density was calculated as previously described, to be 100 mW/cm2 (15). The total energy fluence dose was 30 J/cm2. The fiber optic was wiped with ethanol after the completion of each light exposure. Following all treatments, each specimen was aseptically mounted on a rubber dam, with the rubber dam frame attached to a rack. The contents of root canals were sampled by flushing the root canals with a coronal application of 1-ml of BHI broth with a Pro Rinse® 30 gauge irrigation needle (Dentsply Tulsa Dental). The bacterial suspension was collected in a 1.5 ml microcentrifuge tube positioned below the apical foramen and bacterial yielding was measured spectrophotometrically for each sample. After vortexing for 20 seconds, serial dilutions were prepared and 100 µl aliquots were inoculated onto blood agar and incubated anaerobically for 7 days. Survival fractions in each root specimen were calculated by counting the colonies on the plates and dividing by the number of colonies from controls (No light/No drug) that were kept at room temperature for periods equal to irradiation times.
Microbial analysis of control root canal biofilms
The microbial composition of root canal biofilms in control specimens was studied by cultural analysis and whole genomic probe assay (29).
Cultural analysis
Species were recognized by distinctive colony morphology for A. israelii (white, frequently irregular colonies), F. nucleatum (speckled colonies) or if pigmented (cream/greenish to brown/black) for P. gingivalis and P. intermedia. These latter species were differentiated by: for P. gingivalis lack of red fluorescence under long-wave UV light, and for P. intermedia; positive red fluorescence.
DNA probe analysis
Tris-EDTA buffer (1.5 ml) was added to the plates of control specimens and the bacterial colonies were harvested using glass rods. The cell suspensions were placed into individual Eppendorf tubes and sonicated for 10 sec to break up clumps. The optical density (OD) of each suspension was adjusted to a final OD of 1.0, which corresponded to approximately 109 cells. Ten µl of the suspension (107 cells) was removed and placed in another Eppendorf tube with 140 µl of TE buffer and 150 µl of 0.5M NaOH. The samples were lysed and the DNA was placed in lanes on a positively charged nylon membrane using a Minislot device (Immunetics, Cambridge, MA, USA). After fixation of the DNA to the membrane, the membrane was placed in Miniblotter 45 (Immunetics) with the lanes of DNA perpendicular to the lanes of the device. Digoxigenin-labeled whole genomic DNA probes against four species were hybridized in individual lanes of the Miniblotter. After hybridization, the membranes were washed at high stringency and the DNA probes were detected using antibody to digoxigenin conjugated with alkaline phosphatase for chemifluorescence detection. Signals were detected using AttoPhos substrate (Amersham Life Science, Arlington Heights, IL, USA) and were scanned using a Storm Fluorimager (Molecular Dynamics, Sunnyvale, CA, USA). Computer-generated images were analyzed to determine the fluorescence intensity associated with each sample and probe. Two lanes in each membrane contained DNA standards with 1 ng (105 bacteria) and 10 ng (106 bacteria) of each species. The sensitivity of the assay was adjusted to permit detection of 104 cells of a given species by adjusting the concentration of each DNA probe. The measured fluorescence intensities were converted to absolute counts by comparison with the standards on the same membrane. Failure to detect a signal was recorded as zero.
Confocal Scanning Laser Microscopy
Six root canal specimens, prepared as described above, were randomly selected for confocal scanning laser microscopy (CSLM). The root specimens were split with a diamond disk and both halves were placed in a tube and infected as described above. Three days later the root surface of one half of each root specimen was exposed to the reagents of the LIVE/DEAD BacLight Bacterial Viability Kit (Molecular Probes, Inc., OR) for 2 min to demonstrate the presence of biofilms. The root surface of the other half was exposed to MB (25 µg/ml) for 10 min followed by exposure to red light at 665 nm with a power density of 100 mW/cm2 and energy fluence of 30 J/cm2. For the irradiation the same diode laser was used as above coupled to a 1 mm optical fiber that delivered light into a lens, which formed a uniform circular spot, 2 cm in diameter. Following PDT, root surfaces were also exposed to the reagents of the LIVE/DEAD BacLight Bacterial Viability Kit as above to evaluate the effect of PDT. A Leica SP2 confocal scanning fluorescence microscope (Leica Inc., Malvern, PA) equipped with a 20x or 40x dry objective lens was employed to observe the fluorescence emission of SYTO 9 and propidium iodide. An argon laser (476 nm) was used as the excitation source of both reagents. Cross sections of biofilms were collected to assess the distribution of live and dead species within the biofilm matrices.
Statistical Analysis
In the first set of experiments (in BHI), data were obtained over a series of five experiments with n=3 or n=4 independent trials for each of the four treatments per experiment, a total of 72 observations. Colony forming units (CFU) were transformed to Log10 CFU values to reduce variance heterogeneity. Treatments were evaluated in a stratified (by experiment) analysis of variance with post hoc comparisons of treatment effects by the Least Significant Difference procedure. Stratification by experiment was used to control any systematic variation across experiments. In the second set of experiments (in PBS), data were obtained from two experiments with n=6 independent trials for each of three treatment groups in the first experiment and n=7 per group in the second, a total of 39 observations. As above, CFU were transformed to Log10 CFU values to reduce variance heterogeneity and treatments were evaluated in a stratified (by experiment) analysis of variance with post hoc comparisons by the Least Significant Difference procedure. In each set of experiments, survival fractions were computed from mean CFU values for each treatment relative to control within each experiment and then averaged across the set of experiments. The stratified analyses were carried out using the General Linear Models procedure in the Statistical Analysis System software (V9.1) from the SAS Institute.
RESULTS
Scanning Electron Microscopy
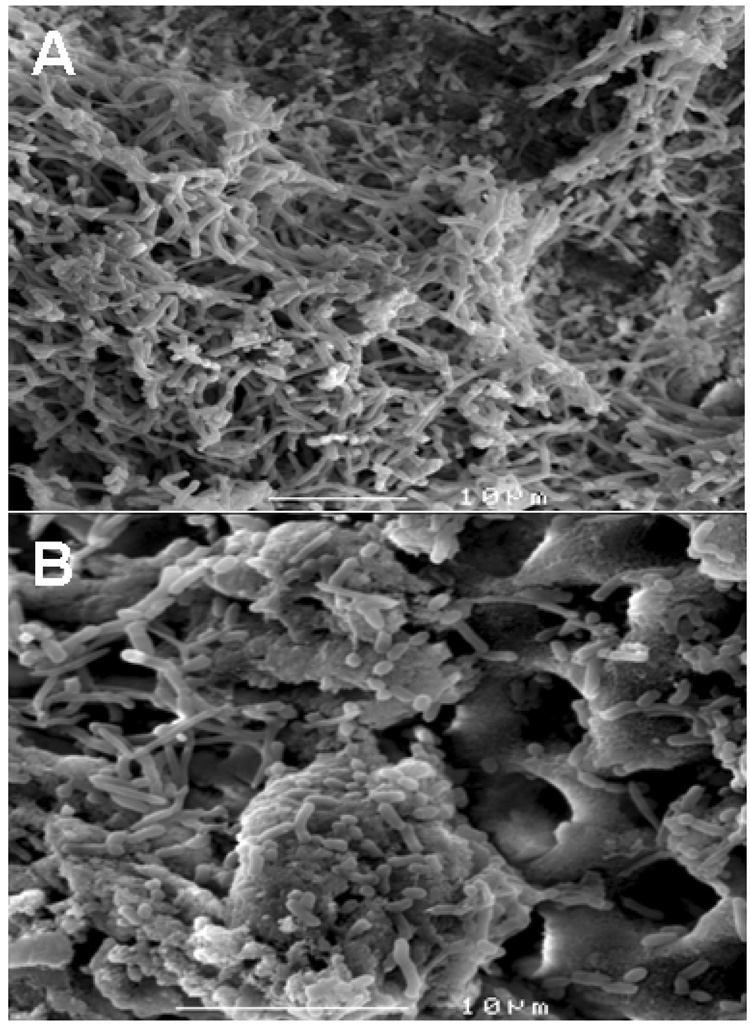
SEM demonstrated the development of multi-species biofilms in the root canal space (Fig. 2) three days after infection with the microorganisms.
Figure 2.
Multi-species biofilms on the root canal surface (A). Invasion of microorganisms in dentinal tubules (B).
Composition of control root canal biofilms
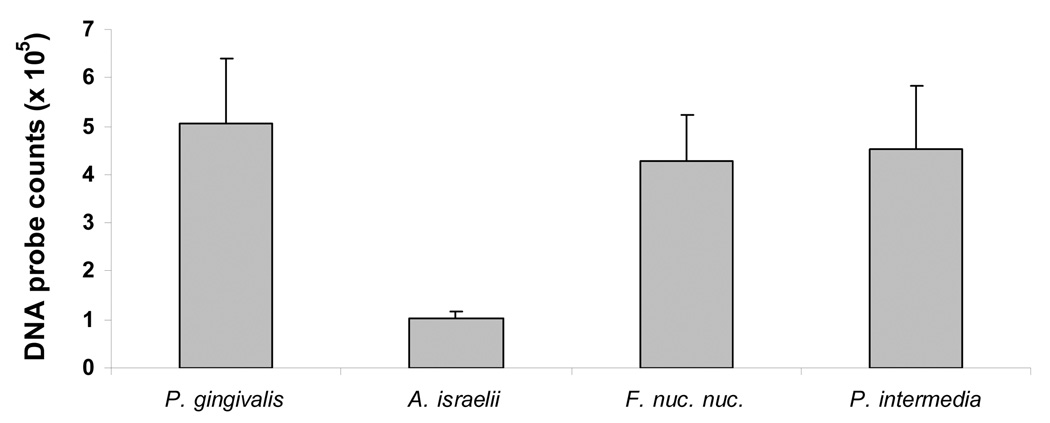
Cultural analysis demonstrated the presence of all species in control root canals (No light/No drug). DNA probe analysis of root canal biofilms from the control specimens also confirmed the presence of all four microorganisms (Fig. 3). The mean values of DNA probe counts for P. gingivalis, A. israelii, F. nucleatum subspecies nucleatum and P. intermedia were 5×105, 105, 4.3×105 and 4.5×105, respectively.
Figure 3.
Mean values of DNA probe counts for 4 species in root canal biofilms obtained from 5 experiments. Error bars denote the standard error of the mean.
Photodynamic treatment of root canals
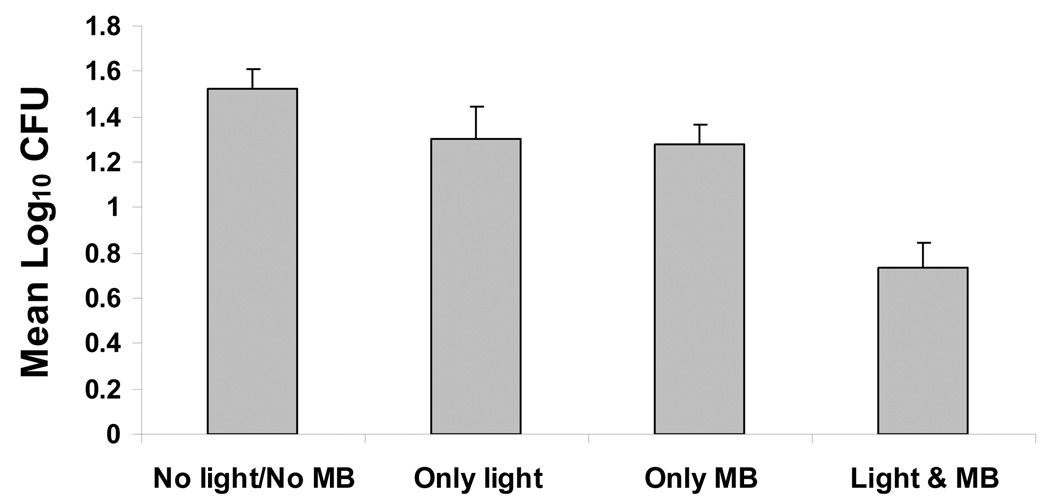
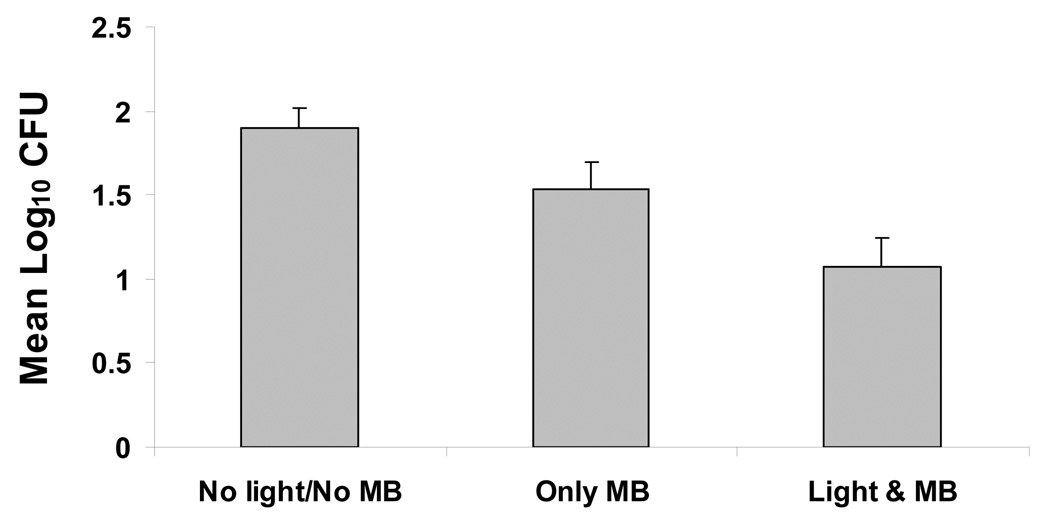
In the first set of five experiments (with MB dissolved in BHI broth), analysis of variance, stratified by experiment, indicated highly significant differences in mean Log10 CFU values among treatments (P<0.0001). The combination of light and MB gave the lowest mean counts, whereas the control gave the highest mean counts (Fig. 4A). Mean Log10 CFU levels for light alone and MB alone were similar (Fig. 4A). Pairwise comparisons among treatment means by the LSD procedure (at alpha=0.05) indicated that the combination of light and MB was significantly lower than control, light alone or MB alone. While both light and MB alone resulted in lower counts relative to control, neither was significant. Mean survival fractions showed little effect for either light or MB alone, but a reduction to about 27% survival relative to control for the combined treatment.
Figure 4.
Phototoxicity of multi-species root canal biofilms after incubation with 25 µg/ml MB dissolved in BHI broth (A) or in PBS (B) for 10 minutes followed by treatment with red light of 665 nm (30 J/cm2) and colony-forming assay. Each bar is the mean Log10 CFU levels (± Standard Error). The combination of light and MB was significantly lower than control, light alone or MB alone (A). The combination of light and MB was significantly lower than either control or MB alone (B).
In the second set of two experiments (with MB dissolved in PBS), analysis of variance, stratified by experiment, indicated highly significant differences in mean Log10 CFU counts between treatments (P=0.0018). Mean Log10 CFU counts were highest for controls, followed by MB alone and then the combination of light and MB (Fig 4B). Pairwise comparisons among treatment means by the LSD procedure (at alpha = 0.05) indicated that the combination of light and MB was significantly lower than either control or MB alone. MB alone was not quite significantly lower than control. Mean survival fractions showed a modest effect for MB alone (66%), but a reduction to about 20% of survival relative to control for light with MB.
Confocal Scanning Laser Microscopy
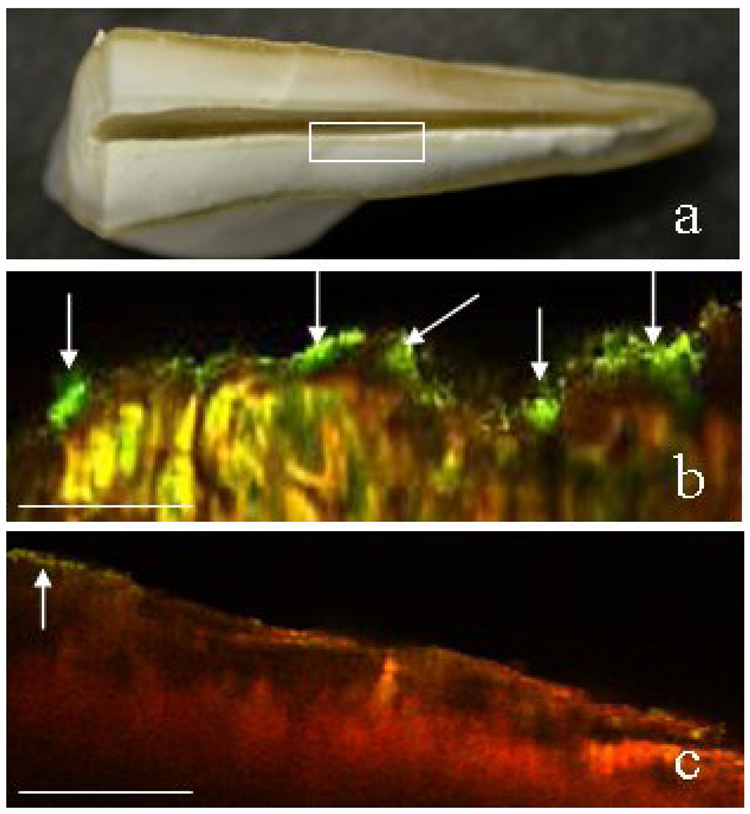
Images were obtained from the mid root area of canals (Fig. 5a, box). Fig. 5b is a X–Z confocal image that shows the presence of microbial biofilms (arrows) in the root canal system (green areas represent viable microbial masses that extend into the dentinal tubules) with a thickness of 20–25 µm. Another X–Z confocal image (Fig. 5c) demonstrates the destruction of biofilm species by PDT (red areas represent dead species). A few foci of residual live microorganisms (arrow) were also detected.
Figure 5.
Confocal scanning laser microscopy images (X–Z) obtained from the mid root area of canals (box) (a). Viable biofilms (green areas) were developed after infection with the mixture of 4 bacteria (b). The yellow areas represent reflected light (b). PDT induced destruction of root canal biofilms (red areas) (c). CSLM revealed the presence of foci of live bacteria following PDT (c, arrow). Scale bar = 100 µm.
DISCUSSION
The use of lasers is a new approach to eliminate microorganisms in root canal systems. The antibacterial effects of many of these lasers are a function of dose-dependent heat generation, which can char dentin, ankylose roots, melt cementum, and cause root resorption and periapical necrosis (30). These disadvantages may be overcome by the use of PDT, which has been recently employed to target microorganisms in the root canals (12–23). In the present study, we investigated the in vitro response of polymicrobial infection in root canals of single-rooted extracted human teeth to PDT after sensitization with MB and exposure to red light at 665 nm. Methylene blue, whose intravenous administration is FDA approved for methemoglobinemia, is a promising candidate for PDT of cancer. In addition, MB has been successfully used in PDT for targeting various gram-positive and gram-negative oral bacteria (31), and is also able to infiltrate dentinal tubules (32). In this study, we introduced a 250-µm diameter optical fiber made of polymethyl methacrylate that was mechanically notched over a one-centimeter length at approximately one-millimeter intervals. Notches provide a mechanical interruption to light propagation through the fiber, which was able to uniformly distribute light at 360° in the entire root canal system.
For the development of root canal biofilms, four bacterial species were used: the gram-positive rod A. israelii, and the gram-negative rods F. nucleatum subspecies nucleatum, P. gingivalis and P. intermedia. These species were selected for the following reasons: a) Although all of the above-mentioned bacteria are not frequently detected in endodontic failures (with the exception of A. israelii), they are key pathogens in infected root canals (26); b) Invasion of human dentinal tubules by P. intermedia in vitro has been reported (33), whereas P. gingivalis showed penetration of dentinal tubules of bovine roots (34). c) We have successfully developed biofilms of the four species in our laboratory (data not shown). In the above mixture of microorganisms we also tried to include E. faecalis without success. In the biofilm model employed in this study, DNA probe analysis demonstrated the presence of all four species. The proportion of A. israelii in these biofilms was 5 times lower compared with those of the other species indicating that A. israelii does not greatly benefit from the presence of the other three microorganisms. The presence of all four species was confirmed by cultural analysis. In addition, SEM and CSLM confirmed the development of root canal biofilms.
Our data demonstrated that sensitization of the four test microorganisms with 25 µg/ml of MB for 10 minutes followed by exposure to red light led to 73% and 80% reduction of CFU counts when MB was dissolved in BHI broth and PBS, respectively. Methylene blue alone reduced CFU by 25% (BHI) and 34% (PBS), whereas light alone did not have any effect on bacterial viability. The photodynamic effects of MB on root canal bacteria were probably affected by the presence of serum proteins in BHI broth (27). This has been previously demonstrated in our laboratory (28). The phototoxicity of a conjugate between poly-L-lysine and the photosensitizer chlorine6 on human dental plaque microorganisms derived from subgingival plaque samples was increased by 7% when the conjugate was dissolved in PBS (28), which is consistent with our current findings. In the present study, light was applied in the root canal system of the specimens for 2.5 minutes (15 J/cm2) followed by a 2.5 min break and a second light exposure for 2.5 min (15 J/cm2). Fractionating the exposure to light may have enhanced the efficacy of the PDT treatment. The PDT effects are abolished under anoxic conditions (35) and the dark interval of 2.5 minutes may have allowed time for oxygen to diffuse back into the anoxic root canal system.
Methylene blue and red light at 665 nm were previously employed by our group for targeting E. faecalis in the root canals of extracted teeth (15, 19). The combined effect of MB and red light exhibited up to 97% reduction of bacterial viability. The results of both studies suggested the potential of PDT to be used as an adjunctive anti-microbial procedure after standard endodontic chemo-mechanical debridement, but also demonstrated the importance of further optimization of light dosimetry for bacterial photodestruction in root canals. Although the four microorganisms used in the present study show different susceptibilities to PDT (15), the bacterial killing induced by MB and light was significant, but not greater than 1 log10. The application of root canal PDT in vivo using tolonium chloride as the photosensitizer and light that was applied in the root canals via an emitter similar to that used in our study was recently reported (21, 22). It was suggested that PDT offered a means of destroying microorganisms remaining after using sodium hypochlorite alone (21) or citric acid and sodium hypochlorite as co-irrigants (22). Tolonium chloride and light eliminated bacterial monolayers (Streptococcus anginosus, E. faecalis, F. nucleatum) in infected root canals in vitro (20). When biofilms were present, the effect on bacterial eradication was reduced substantially as it was demonstrated by environmental scanning electron microscopy. In this study (20), the percentage of bacterial killing in biofilms following PDT was not reported. Recently, the photodynamic effects of a conjugate between polyethyleneimine and the photosensitizer chlorine e6 were investigated in association with endodontic treatment in vivo (23). PDT eliminated 82% of microorganisms that remained in root canals following standard endodontic treatment. A week later, 40% of the original bacterial load recolonized root canals and a second endodontic treatment followed by PDT was necessary to eliminate residual microorganisms. In this study (23), spiral movements of the optical fiber, from apical to cervical, were manually performed to deliver light in the root canal system. However, it is doubtful that this technique using a fiber without diffusers uniformly distributed light at 360°. In addition, the amount of light delivered within the root canal was not reported. Comparisons between our study and other studies that employed PDT for targeting root canal bacteria in vitro (14, 15, 18) are difficult, because these studies have used different photosensitizers, light parameters and light delivery techniques.
A logical conclusion of our present study is that increasing both the concentration of MB and the energy fluence of light may lead to greater than 1 log10 bacterial killing. Since only 20% of light energy delivered by our 250-µm fiber escapes from the root apex, there may be a safe therapeutic window for maximizing bacterial killing using even greater energy fluencies in a clinical setting. The use of naturally-infected teeth, which will contain a broader range of pathogens than our model system, would provide an excellent test of the potential of PDT in achieving root canal disinfection.
ACKNOWLEDGEMENTS
This work was supported by NIDCR grant RO1-DE-16922 and the Krakow Harvard-Forsyth Endodontic Research Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Sjögren U, Figdor D, Persson S, Sundqvist G. Influence of infection at the time of root filling on the outcome of endodontic treatment of teeth with apical periodontitis. Int Endod J. 1997;30:297–306. doi: 10.1046/j.1365-2591.1997.00092.x. [DOI] [PubMed] [Google Scholar]
- 2.Byström A, Sundqvist G. Bacteriologic evaluation of the effect of 0.5 percent sodium hypochlorite in endodontic therapy. Oral Surg Oral Med Oral Pathol. 1983;55:307–312. doi: 10.1016/0030-4220(83)90333-x. [DOI] [PubMed] [Google Scholar]
- 3.Sundqvist G, Figdor D, Persson S, Sjögren U. Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative re-treatment. Oral Surg Oral Med Oral Pathol. 1998;85:86–93. doi: 10.1016/s1079-2104(98)90404-8. [DOI] [PubMed] [Google Scholar]
- 4.Siqueira JF, Araújo MCP, Garcia PF, Fraga RC, Dantas CJS. Histological evaluation of the effectiveness of five instrumentation techniques for cleaning the apical third of root canals. J Endod. 1997;23:499–502. doi: 10.1016/S0099-2399(97)80309-3. [DOI] [PubMed] [Google Scholar]
- 5.Rolph HJ, Lennon A, Riggio MP, Saunders WP, MacKenzie D, Coldero L, Bagg J. Molecular identification of microorganisms from endodontic infections. J Clin Microbiol. 2001;39:3282–3289. doi: 10.1128/JCM.39.9.3282-3289.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siqueira JF., Jr Aetiology of root canal treatment failure: why well-treated teeth can fail. Int Endod J. 2001;34:1–10. doi: 10.1046/j.1365-2591.2001.00396.x. [DOI] [PubMed] [Google Scholar]
- 7.Hancock HH, 3rd, Sigurdsson A, Trope M, Moiseiwitsch J. Bacteria isolated after unsuccessful endodontic treatment in a North American population. Oral Surg Oral Med Oral Pathol. 2001;91:579–586. doi: 10.1067/moe.2001.113587. [DOI] [PubMed] [Google Scholar]
- 8.Pinheiro ET, Gomes BP, Ferraz CC, Sousa EL, Teixeira FB, Souza-Filho FJ. Microorganisms from canals of root filled teeth with periapical lesions. Int Endod J. 2003;36:1–11. doi: 10.1046/j.1365-2591.2003.00603.x. [DOI] [PubMed] [Google Scholar]
- 9.Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. Photodynamic therapy. J Natl Cancer Inst. 1998;90:889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson M. Photolysis of oral bacteria and its potential use in the treatment of caries and periodontal disease: a review. J App Bacteriol. 1993;75:299–306. doi: 10.1111/j.1365-2672.1993.tb02780.x. [DOI] [PubMed] [Google Scholar]
- 11.Soukos NS, Ximenez-Fyvie LA, Hamblin MR, Socransky SS, Hasan T. Targeted antimicrobial photochemotherapy. Antimicrob Agents Chemother. 1998;42:2595–2601. doi: 10.1128/aac.42.10.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silbert T, Bird PS, Milburn GJ, Walsh L. Disinfection of root canals by laser dye photosensitization. J Dent Res. 2000;79(Sp Issue):569. [Google Scholar]
- 13.Seal GJ, Ng YL, Spratt D, Bhatti M, Gulabivala K. An in vitro comparison of the bactericidal efficacy of lethal photosensitization or sodium hyphochlorite irrigation on Streptococcus intermedius biofilms in root canals. Int Endod J. 2002;35:268–274. doi: 10.1046/j.1365-2591.2002.00477.x. [DOI] [PubMed] [Google Scholar]
- 14.Garcez AS, Nunez SC, Lage-Marques JL, Jorge AO, Ribeiro MS. Efficiency of NaOCl and laser-assisted photosensitization on the reduction of Enterococcus faecalis in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:93–98. doi: 10.1016/j.tripleo.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Soukos NS, Chen PS, Morris JT, Ruggiero K, Abernethy AD, Som S, Foschi F, Doucette S, Bammann LL, Fontana CR, Doukas AG, Stashenko PP. Photodynamic therapy for endodontic disinfection. J Endod. 2006;32:979–984. doi: 10.1016/j.joen.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Williams JA, Pearson GJ, Colles MJ. Antibacterial action of photoactivated disinfection {PAD} used on endodontic bacteria in planktonic suspension and in artificial and human root canals. J Dent. 2006;34:363–371. doi: 10.1016/j.jdent.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Garcez AS, Ribeiro MS, Tegos GP, Nunez SC, Jorge AO, Hamblin MR. Antimicrobial photodynamic therapy combined with conventional endodontic treatment to eliminate root canal biofilm infection. Lasers Surg Med. 2007;39:59–66. doi: 10.1002/lsm.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.George S, Kishen A. Photophysical, photochemical, and photobiological characterization of Methylene blue formulations for light-activated root canal disinfection. J Biomed Opt. 2007;12:34029–34038. doi: 10.1117/1.2745982. [DOI] [PubMed] [Google Scholar]
- 19.Foschi F, Fontana CR, Ruggiero K, Riahi R, Vera A, Doukas AG, Pagonis TC, Kent R, Stashenko PP, Soukos NS. Photodynamic inactivation of Enterococcus faecalis in dental root canals in vitro. Lasers Surg Med. 2007;39:782–787. doi: 10.1002/lsm.20579. [DOI] [PubMed] [Google Scholar]
- 20.Bergmans L, Moisiadis P, Huybrechts B, Van Meerbeek B, Quirynen M, Lambrechts P. Effect of photo-activated disinfection on endodontic pathogens ex vivo. Int Endod J. 2008;41:227–239. doi: 10.1111/j.1365-2591.2007.01344.x. [DOI] [PubMed] [Google Scholar]
- 21.Bonsor SJ, Nichol R, Reid TM, Pearson GJ. An alternative regimen for root canal disinfection. Br Dent J. 2006;22:101–105. doi: 10.1038/sj.bdj.4813819. [DOI] [PubMed] [Google Scholar]
- 22.Bonsor SJ, Nichol R, Reid TM, Pearson GJ. Microbiological evaluation of photo-activated disinfection in endodontics (an in vivo study) Br Dent J. 2006;25:337–341. doi: 10.1038/sj.bdj.4813371. [DOI] [PubMed] [Google Scholar]
- 23.Garcez AS, Nunez SC, Hamblin MR, Ribeiro MS. Antimicrobial effects of photodynamic therapy on patients with necrotic pulps and periapical lesion. J Endod. 2008;34:138–142. doi: 10.1016/j.joen.2007.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wainwright M, Phoenix DA, Marland J, Wareing DRA, Bolton FJ. A study of photobactericidal activity in the phenothiazinium series. FEMS Immunol Med Microbiol. 1997;19:75–80. doi: 10.1111/j.1574-695X.1997.tb01074.x. [DOI] [PubMed] [Google Scholar]
- 25.Usacheva MN, Teichert MC, Biel MA. The interaction of lipopolysaccharides with phenothiazine dyes. Lasers Surg Med. 2003;33:311–319. doi: 10.1002/lsm.10226. [DOI] [PubMed] [Google Scholar]
- 26.Sundqvist G. Associations between microbial species in dental root canal infections. Oral Microbiol Immunol. 1992;7:257–262. doi: 10.1111/j.1399-302x.1992.tb00584.x. [DOI] [PubMed] [Google Scholar]
- 27.Bhatti M, MacRobert A, Meghji S, Henderson B, Wilson M. Effect of dosimetric and physiological factors on the lethal photosensitization of Porphyromonas gingivalis in vitro. Photochem Photobiol. 1997;65:1026–1031. doi: 10.1111/j.1751-1097.1997.tb07964.x. [DOI] [PubMed] [Google Scholar]
- 28.Soukos NS, Mulholland SE, Socransky SS, Doukas AG. Photodestruction of human dental plaque bacteria: enhancement of the photodynamic effect by photomechanical waves in an oral biofilm model. Lasers Surg Med. 2003;33:161–168. doi: 10.1002/lsm.10208. [DOI] [PubMed] [Google Scholar]
- 29.Socransky SS, Haffajee AD, Smith C, Martin L, Haffajee JA, Uzel NG, Goodson JM. Use of checkerboard DNA-DNA hybridization to study complex microbial ecosystems. Oral Microbiol Immunol. 2004;19:352–362. doi: 10.1111/j.1399-302x.2004.00168.x. [DOI] [PubMed] [Google Scholar]
- 30.Ramsköld LO, Fong CD, Stromberg T. Thermal effects and antibacterial properties of energy levels required to sterilize stained root canals with an Nd:YAG laser. J Endod. 1997;23:96–100. doi: 10.1016/S0099-2399(97)80253-1. [DOI] [PubMed] [Google Scholar]
- 31.Harris F, Chatfield LK, Phoenix DA. Phenothiazinium based photosensitisers - Photodynamic agents with a multiplicity of cellular targets and clinical applications. Curr Drug Targets. 2005;6:615–627. doi: 10.2174/1389450054545962. [DOI] [PubMed] [Google Scholar]
- 32.Absi EG, Addy M, Adams D. Dentine hypersensitivity. A study of the patency of dentinal tubules in sensitive and non-sensitive cervical dentine. J Clin Periodontol. 1987;14:280–284. doi: 10.1111/j.1600-051x.1987.tb01533.x. [DOI] [PubMed] [Google Scholar]
- 33.Berkiten M, Okar I, Berkiten R. In vitro study of the penetration of Streptococcus sanguis and Prevotella intermedia strains into human dentinal tubules. J Endod. 2000;26:236–239. doi: 10.1097/00004770-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Siqueira JF, Jr, De Uzeda M, Fonseca ME. A scanning electron microscopic evaluation of in vitro dentinal tubules penetration by selected anaerobic bacteria. J Endod. 1996;22:308–310. doi: 10.1016/S0099-2399(96)80265-2. [DOI] [PubMed] [Google Scholar]
- 35.Henderson BW. Probing the effects of photodynamic therapy through in vivo-in vitro methods. In: Kessel D, editor. Photodynamic therapy of neoplastic disease. Boca Raton (FL): CRC Press; 1990. pp. 169–188. [Google Scholar]