Abstract
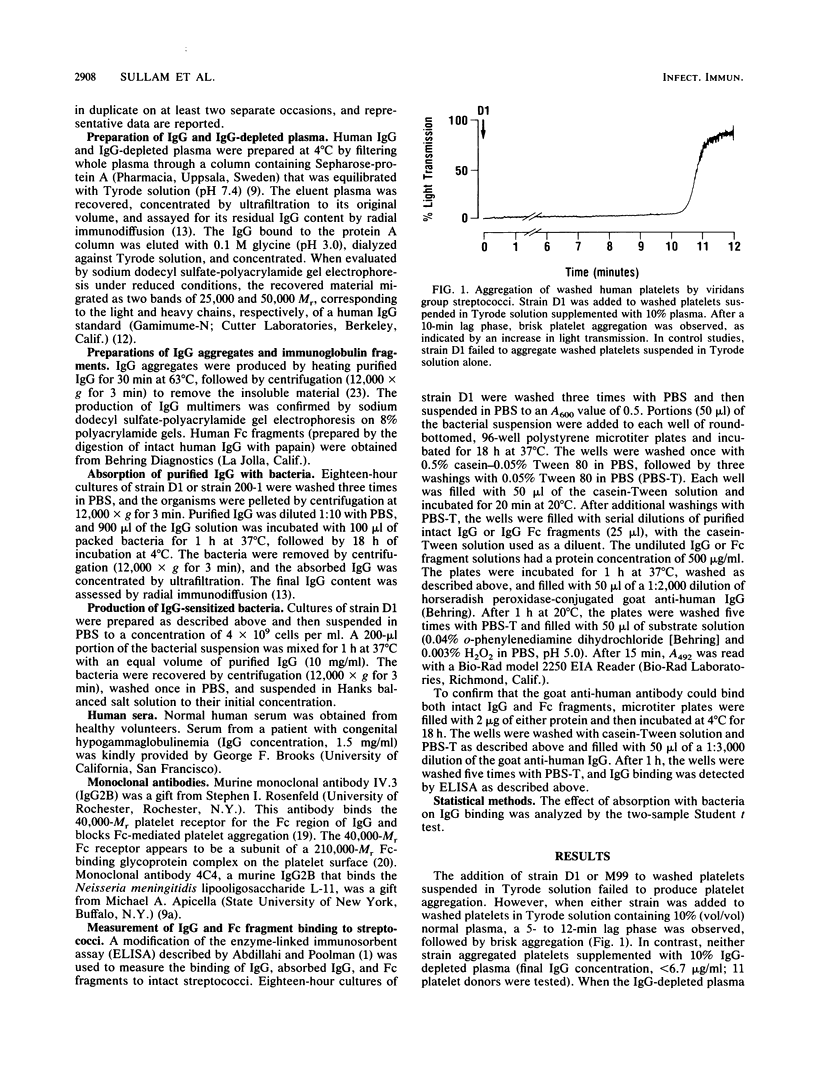
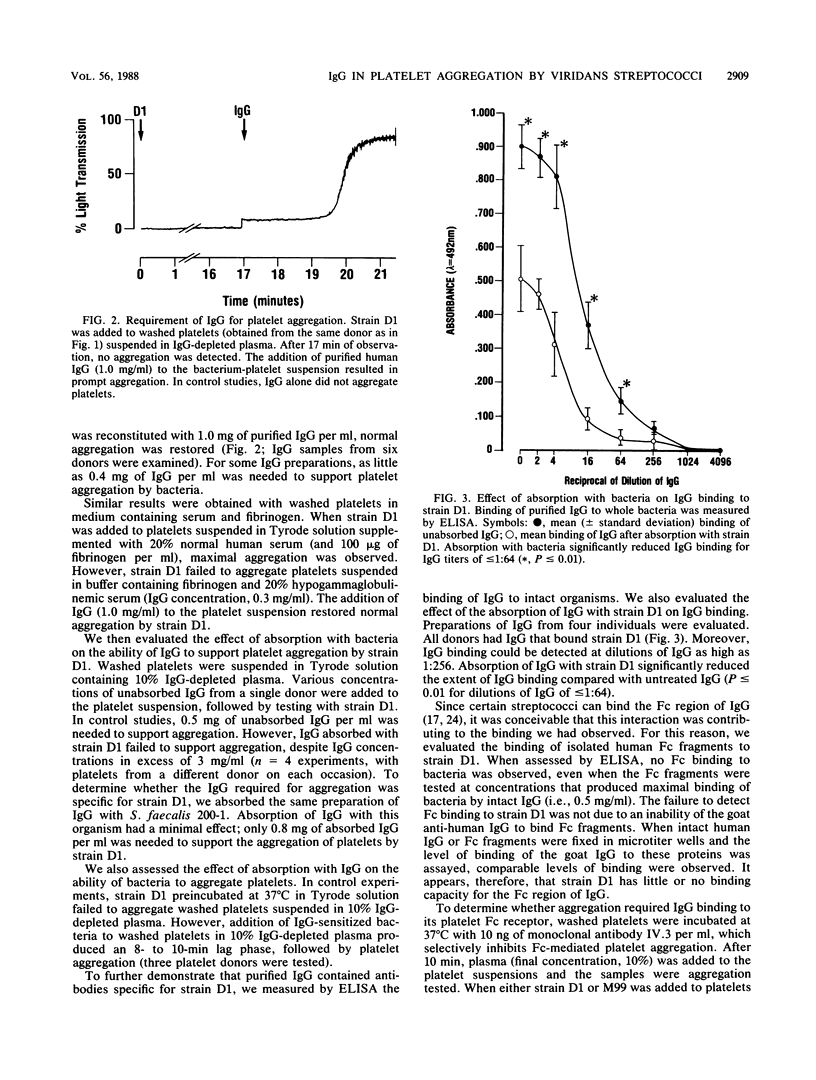
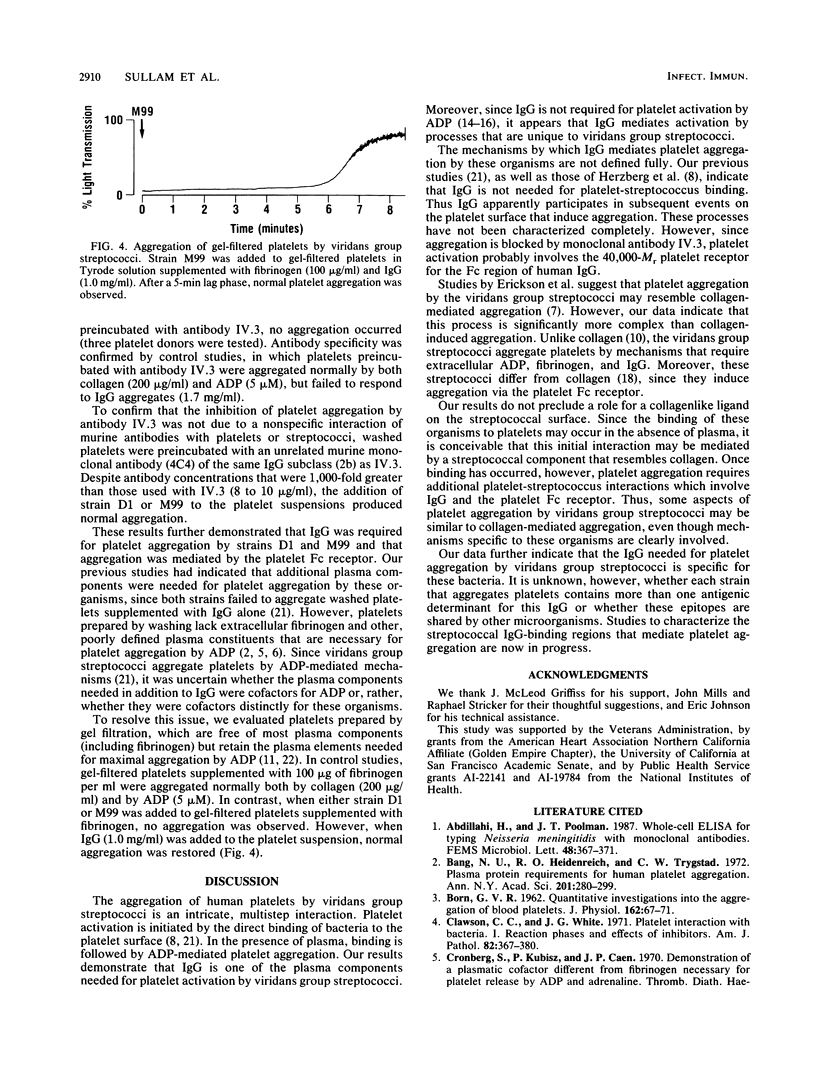
The aggregation of human platelets by the viridans group streptococci requires both direct platelet-bacterium binding and plasma components. Some of these extracellular constituents (e.g., fibrinogen) are cofactors for ADP, which mediates the terminal events in platelet activation by these organisms. In addition, other plasma components which are specific for viridans group streptococci are necessary. To better define these latter cofactors, we examined the role of immunoglobulin G (IgG) in platelet aggregation by two strains of viridans group streptococci. The addition of either strain to washed human platelets suspended in normal plasma resulted in a 5- to 12-min lag phase, followed by brisk and irreversible platelet aggregation. In contrast, neither strain aggregated platelets suspended in IgG-depleted plasma (IgG concentration, less than or equal to 6.7 micrograms/ml). The addition of IgG (1.0 mg/ml) to the platelet suspension restored normal aggregation. Absorption of the IgG with intact bacteria abolished its ability to support aggregation. Preincubation of washed platelets with a murine monoclonal antibody to the 40,000-Mr platelet Fc receptor blocked aggregation by both strains, but had no effect on aggregation by ADP (5 microM) or collagen (200 micrograms/ml). Neither strain aggregated gel-filtered platelets supplemented with fibrinogen (100 micrograms/ml), whereas ADP induced a maximal platelet response. When IgG (1.0 mg/ml) was added to the suspension of gel-filtered platelets, both strains produced normal aggregation. These results indicate that specific IgG is required for platelet aggregation by viridans group streptococci and that platelet activation is mediated through the 40,000-Mr Fc receptor on the platelet surface.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bang N. U., Heidenreich R. O., Trygstad C. W. Plasma protein requirements for human platelet aggregation. Ann N Y Acad Sci. 1972 Oct 27;201:280–299. doi: 10.1111/j.1749-6632.1972.tb16305.x. [DOI] [PubMed] [Google Scholar]
- Clawson C. C., White J. G. Platelet interaction with bacteria. I. Reaction phases and effects of inhibitors. Am J Pathol. 1971 Nov;65(2):367–380. [PMC free article] [PubMed] [Google Scholar]
- Deykin D., Pritzker C. R., Scolnick E. M. Plasma co-factors in adenosine diphosphate-induced aggregation of human platelets. Nature. 1965 Oct 16;208(5007):296–298. doi: 10.1038/208296b0. [DOI] [PubMed] [Google Scholar]
- Erickson P. R., Herzberg M. C. A collagen-like immunodeterminant on the surface of Streptococcus sanguis induces platelet aggregation. J Immunol. 1987 May 15;138(10):3360–3366. [PubMed] [Google Scholar]
- Herzberg M. C., Brintzenhofe K. L., Clawson C. C. Aggregation of human platelets and adhesion of Streptococcus sanguis. Infect Immun. 1983 Mar;39(3):1457–1469. doi: 10.1128/iai.39.3.1457-1469.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelm H., Hjelm K., Sjöquist J. Protein A from Staphylococcus aureus. Its isolation by affinity chromatography and its use as an immunosorbent for isolation of immunoglobulins. FEBS Lett. 1972 Nov 15;28(1):73–76. doi: 10.1016/0014-5793(72)80680-x. [DOI] [PubMed] [Google Scholar]
- Kinlough-Rathbone R. L., Mustard J. F., Packham M. A., Perry D. W., Reimers H. J., Cazenave J. P. Properties of washed human platelets. Thromb Haemost. 1977 Apr 30;37(2):291–308. [PubMed] [Google Scholar]
- Levy-Toledano S., Rendu F., Besson P., Caen J. Aggregation of human gel-filtered platelets. Requirement of apyrase and proteins. Rev Eur Etud Clin Biol. 1972 May;17(5):513–518. [PubMed] [Google Scholar]
- Lundblad J. L., Mitra G., Sternberg M. M., Schroeder D. D. Comparative studies of impurities in intravenous immunoglobulin preparations. Rev Infect Dis. 1986 Jul-Aug;8 (Suppl 4):S382–S390. doi: 10.1093/clinids/8.supplement_4.s382. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Mustard J. F., Packham M. A., Kinlough-Rathbone R. L., Perry D. W., Regoeczi E. Fibrinogen and ADP-induced platelet aggregation. Blood. 1978 Aug;52(2):453–466. [PubMed] [Google Scholar]
- Mustard J. F., Perry D. W., Ardlie N. G., Packham M. A. Preparation of suspensions of washed platelets from humans. Br J Haematol. 1972 Feb;22(2):193–204. doi: 10.1111/j.1365-2141.1972.tb08800.x. [DOI] [PubMed] [Google Scholar]
- Mustard J. F., Perry D. W., Kinlough-Rathbone R. L., Packham M. A. Factors responsible for ADP-induced release reaction of human platelets. Am J Physiol. 1975 Jun;228(6):1757–1765. doi: 10.1152/ajplegacy.1975.228.6.1757. [DOI] [PubMed] [Google Scholar]
- Myhre E. B., Kronvall G. Heterogeneity of nonimmune immunoglobulin Fc reactivity among gram-positive cocci: description of three major types of receptors for human immunoglobulin G. Infect Immun. 1977 Sep;17(3):475–482. doi: 10.1128/iai.17.3.475-482.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis H. K., Akkerman J. W., Houdijk W. P., Sixma J. J. Human blood platelets showing no response to collagen fail to express surface glycoprotein Ia. Nature. 1985 Dec 5;318(6045):470–472. doi: 10.1038/318470a0. [DOI] [PubMed] [Google Scholar]
- Rosenfeld S. I., Looney R. J., Leddy J. P., Phipps D. C., Abraham G. N., Anderson C. L. Human platelet Fc receptor for immunoglobulin G. Identification as a 40,000-molecular-weight membrane protein shared by monocytes. J Clin Invest. 1985 Dec;76(6):2317–2322. doi: 10.1172/JCI112242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker R. B., Reyes P. T., Corash L., Shuman M. A. Evidence that a 210,000-molecular-weight glycoprotein (GP 210) serves as a platelet Fc receptor. J Clin Invest. 1987 Jun;79(6):1589–1594. doi: 10.1172/JCI112993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullam P. M., Valone F. H., Mills J. Mechanisms of platelet aggregation by viridans group streptococci. Infect Immun. 1987 Aug;55(8):1743–1750. doi: 10.1128/iai.55.8.1743-1750.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangen O., Berman H. J., Marfey P. Gel filtration. A new technique for separation of blood platelets from plasma. Thromb Diath Haemorrh. 1971 Jun 30;25(2):268–278. [PubMed] [Google Scholar]
- Valone F. H. Synergistic platelet activation by aggregates of IgG and the phospholipid platelet-activating factor 1-O-alkyl-2-acetyl-SN-glycero-3-phosphorylcholine. J Clin Immunol. 1986 Jan;6(1):57–64. doi: 10.1007/BF00915365. [DOI] [PubMed] [Google Scholar]
- von Mering G. O., Boyle M. D. Comparison of type III Fc receptors associated with group C and group G streptococci. Mol Immunol. 1986 Aug;23(8):811–821. doi: 10.1016/0161-5890(86)90066-0. [DOI] [PubMed] [Google Scholar]



