Ladies and gentlemen, it is a distinct honor for me to be invited by Dr. Vanek to give the 2008 Jonathon E. Rhoads Lecture. In preparation for this talk, this summer I read Dr. Rhoads’ biography by Dr. Rombeau and Muldoon to gain some insight into the character and personality of this intriguing leader in surgery and nutriton1. I had met him on a few occasions at A.S.P.E.N. meetings and but never really had an opportunity know him. In the book I was struck by a quote from his friend, Mr. Schumaker, describing Dr. Rhoads: “The amazing thing is that I recall him as exactly the same sort of person he is today and has been for decades: quiet, deliberate, dignified, courteous, and wise. He had the same tendency to hesitate a moment before responding- a moment during which one could almost see something going on in his mind, quickly analyzing, judging, reaching a decision and organizing thoughts so that the words which followed were well chosen, brief, and to the point, with a penetrating logic that commanded attention and brought respect. 1 “ Quite a compliment, indeed! I particularly appreciated this quote from Dr. Rhoads himself 1, ‘The successful, scientific investigator is a person of great dedication to a given task. He must be willing to stick at it through many difficulties and discouragements. He must often be willing to work very much alone and without the daily appreciation and thanks that do so much to sustain the practitioner during the long days and night hours that his work entails. He must be looking for the way around obstacles and he should be constantly on alert for pearls scattered along the wayside and needs to be possessed of unusual judgment about when to stop and pick up such pearls (serendipity) and when to press on towards more ultimate goals.’ I would like to address several of these points. First of all, there is the need to stay alert for serendipitous pearls and new insights to take advantage of them. The second is to recognize and abandon unfruitful directions while pressing on towards focused goals. As I tell my laboratory colleagues, the research path is analogous to a golf course; our goal is to get to the 18th hole and avoid wasting time in the roughs. One should always ponder a unexpected result rather than dismissing it. If it does not agree with preconceived notions, the data is not usually wrong, the hypothesis is! As I stated in my Presidential address, “The eyes do not see what the mind does not know.” 2
While the University of Pennsylvania may have been the alpha site for specialized nutrition support, my training at Ohio State convinces me that OSU was an important beta site. With two future presidents of A.S.P.E.N.- Jeff Fabri and Phil Schneider -and with the early leadership of Dr. Ruberg (who had been involved with Dr. Dudrick earlier at Penn and became the head of Plastic Surgery at Ohio State), this group introduced me to specialized nutrition support. Working with my close friend and colleague, Jay Mirtallo, another leader in A.S.P.E.N., this environment provided very fertile ground. It was the time I took during my residency at the University of California, San Francisco under the tutelage of Dr. George Sheldon, now retired chairman at the University of North Carolina Chapel Hill, that I found my area of interest: the importance of route of nutrition on susceptibility to infection. I inherited a project established by Dr. Scott Peterson and Dr. Sheldon in which animals were given septic peritonitis3. While 70% of well-nourished rats survived the insult, only 10% of malnourished rats survived (Figure 1). Protein depleted rats that were then refed had the same survival rate as well nourished rats. But the two groups that received parenteral nutrition - either with or without fat - had the worst results of all. When I assumed leadership of the project, we felt was that there was something wrong with the composition of the TPN solution. But there was an important confounding variable in that experiment: the parenteral animals received a liquid diet via the vein while well-nourished or repleted rats received a solid diet via the gut. We eliminated these confounding variables by letting one group drink the TPN and pair-fed the other group intravenously4. To our surprise, serendipity showed that nothing was wrong with the TPN solution; previously malnourished4 or well- nourished5 animals that drank it survived like well-nourished animals and those given it intravenously survived like malnourished animals. I had found one of Dr. Rhoads’ “pearls” and have pursued that topic my entire career. This work preceded a dramatic increase in the number of publications on enteral feeding in the subsequent years (Figure 2).
Figure 1.
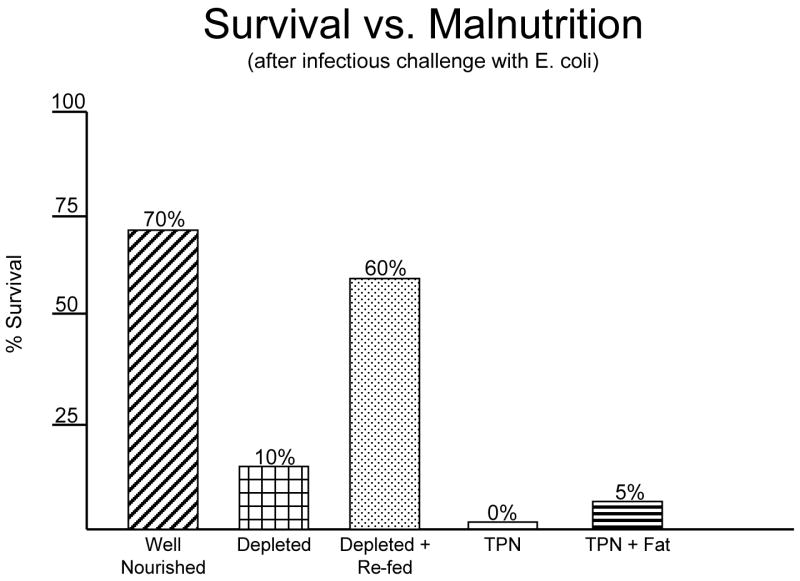
Survival of malnourished animals to an infectious challenge after nutrient manipulation. Protein depletion significantly reduced survival compared to well nourished animals. Refeeding improved the survival of protein depleted rats. Parenteral feeding either with or without fat failed to improve survival.
Figure 2.
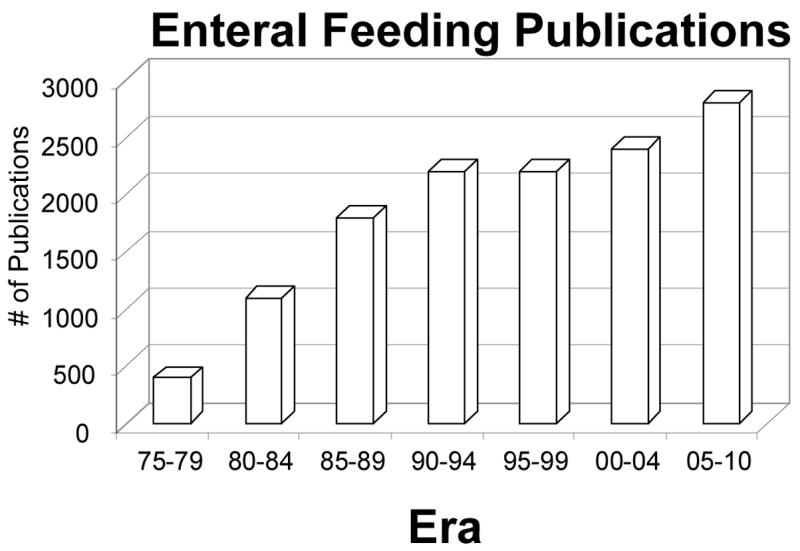
The number of enteral feeding publications in five year blocks from 1975 projected through 2010.
I remained on staff at O.S.U.for four years after finishing my residency and subsequently left for the University of Tennessee, Memphis to work at the Elvis Presley Trauma Center which cared for 4500 trauma patients annually. I had the great fortune of working with great people who really were important in the development of this project. Rex Brown, my PharmD colleague, Gayle Minard and I worked on a number of clinical projects. Critically important to our success in the laboratory was Jian Li MD, currently a very prominent business man in China. I was fortunate to work with Bobbi Langkamp-Henken PhD RD while she obtained her PhD. One of the superstars in my group was Kazuhiko Fukatsu MD PhD, a brilliant researcher who regularly contributes to A.S.P.E.N. and JPEN. He is a real leader in the field of nutritional support.
At Tennessee, Dr. Brown, myself and Dr. Minard worked on a project randomizing approximately 100 patients to be fed enterally or parenterally 6. In this trial we confirmed earlier work by Dr. Moore7 that delivery of nutrients via the gut significantly reduced the incidence of infection, particularly pneumonia, but we also showed that severity of injury was important. We used two severity scores to stratify patients: the Injury Severity Score (ISS) which quantifies the magnitude of total body injury- head, chest, abdomen, and 3 other systems and the Abdominal Trauma Index (ATI) which quantifies the severity of and risk of sepsis from intraabdominal injury. Our trial showed that if a patient’s ISS and ATI scores were low, there was no difference in infectious complications - they probably didn’t need to be fed. But if a patient had a high ISS, a high ATI, or both, their risk for infection increased by 6, 7 and 11 times with parenteral nutrition, respectively. Interestingly, the most important effect occurred in the resistance to pneumonia which dropped from 31% down to 11% with enteral feeding in the entire population and from 55% down to approximately 13% in the most severely injured subpopulation6.
As a result of that study, the theme and focus of my research has been “why in the world is there this lower rate of infectious complications, particularly pneumonia, when nutrients are delivered via the gut?” A number of possible metabolic and immunologic reasons were postulated to explain the differences and our group pursued several. We studied potential differences in cathecholamine secretion8 due to a blunted metabolic response with enteral feeding after trauma but found no differences. We studied growth hormone9 or IGF-1 10 after trauma but only showed improved nitrogen retention with no obvious clinical effect on infections. We studied a diet supplemented with omega-3 fatty acids, glutamine, nucleic acids and branched chain amino acids compared to a standard diet11 recruiting only severely injured trauma patients with a high ISS and ATI. The results confirmed the observations of Moore et al 12 that the diet reduced the incidence of intraabdominal abscesses. Pneumonia was low in both groups presumably due to the enteral stimulation.
Could glucose control be the mechanism? Glucose control in critically ill patients with intensive insulin therapy became a hot topic which swept the ICU world in a very short time. We examined daily glucose levels in the patients recruited into the enteral-parenteral trial and found no significant differences in glucose levels between the enterally and parenterally fed patients or infected and non-infected patients until the fourth and fifth post injury day when developing infections and the attendant metabolic response rendered the parenteral group more hyperglycemic (Figure 3)13. Taking these results with the results of the Van den Berghe study14 which showed no improvement in mortality with intensive insulin therapy in the trauma population and Krinsley’s15 work (Table 1) showing a slight but insignificant increase in trauma mortality with strict glycemic control without any positive effect on respiratory infections, it is hard to believe that glucose explained the difference in our trial. Likewise, Speery et al16 showed that trauma patients with no glucose value > 200 mg/dL and average glucose values of 127 ± 2 mg/dL over the first 3 days with a maximum serum glucose of 169±25 mg/dL in the first 24 hours had the same rate of pneumonia as patients with a glucose value > 200 mg/dL and average glucose values of 135 ± 27 mg/dL over the first 3 days and a maximum level of 264±59 mg/dL in the first 24 hours (Table 1). We looked elsewhere for potential mechanisms to explain the higher pneumonia rate after trauma with parenteral nutrition.
Figure 3.
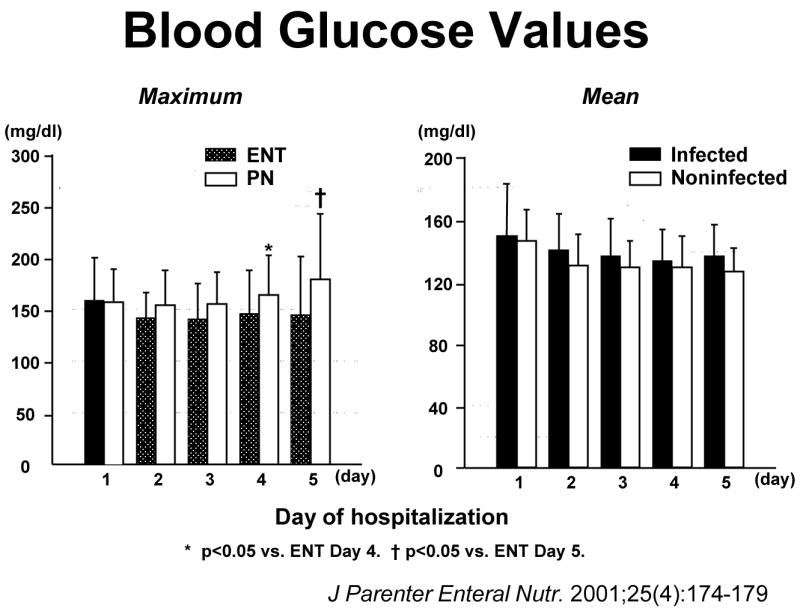
Blood glucose levels in trauma patients randomized to parenteral or enteral nutrition. The figure on the left shows maximal blood glucose levels after enteral or parenteral feeding during the first five days. There were no significant differences in maximal blood glucoses until the fourth or the fifth day when parenterally fed patients developed infectious complications. The right figure shows that there were no significant differences in mean blood glucose values over the first five days in patients who became infected compared to those that remain uninfected. (Reproduced with permission of Kudsk et al. J Parenter Enteral Nutr. 2001;25(4):174–179)
Table 1.
Glucose Control in Trauma
| Van den Berghe et al. NEJM2001;345:1359–67. | ||
|
| ||
| Insulin TX | CONVENTIONAL | |
| Trauma Mortality | 4/33 (12.1%) | 3/35 (8.6%) |
|
| ||
| Krinsley Mayo Proc 2004;79(8):992–1000 | ||
|
| ||
| Mortality | 19.5% | 17.8% |
| Respiratory Infections | 9/800 | 7/800 |
|
| ||
| Speery et al. J Trauma 2007;63:487–494 | ||
|
| ||
| n= 502 | n=348 | |
|
| ||
| Gluc 1st 24 hr | <200 | > 200 mg/dL |
| Max 3d | x= 169 ± 25 | 264 ± 59 p<.01 |
| 1st 3 d | x= 127 ± 21 | 135 ± 27 p<.01 |
| Pneumonia | 28.9% | 31.0% |
One avenue of investigation was especially disappointing to us. As you recall, the 1980’s propagated a concept of bacterial translocation and increased gut permeability as potential mechanisms for increased infections following injury. Dr. Langkamp- Henken and I ran a clinical study on permeability by infusing lactulose and mannitol through feeding jejunostomy tubes and measuring urinary excretion on days 1 and 7 after trauma to determine gut permiability17. Drs. Wilmore18 and Deitch19 had previously documented acute increases in the lactulose:mannitol ratio reflecting permeability increases in burned patients implicating it as an effect of subsequent stress and a potential cause of subsequent complications. We also documented acute increases in permeability on day 1 compared to day 7 after trauma17 but after analyzing our data and reexamining the data by Wilmore and Deitch, we were disappointed to find two patterns of response: a small group of trauma patients increased gut permeability which dropped to normal over time and a second group- the majority- who had no change. The permeablity change did not relate to subsequent infectious complications although the lactulose:mannitol ratio correlated with IL-6, a cytokine found in high systemic concentrations after injury20. In short, increased permeability failed to correlate with infections complications at all. I considered this disappointment and walked away from permeability and bacterial translocation as an explanation entirely. There was serendipitous finding in Dr. Langkamp-Henken’s laboratory results, however. While examining the response of enteral and TPN fed rats to gut ischemia/reperfusion (Figure 4), she noted that the gut of parenterally fed rats accumulated significant numbers of neutrophils reflected by increases in gut myeloperoxidase (MPO), an enzyme marker of neutrophils, compared to chow mice21. This “pearl” was pursued later by Dr. Fukatsu.
Figure 4.
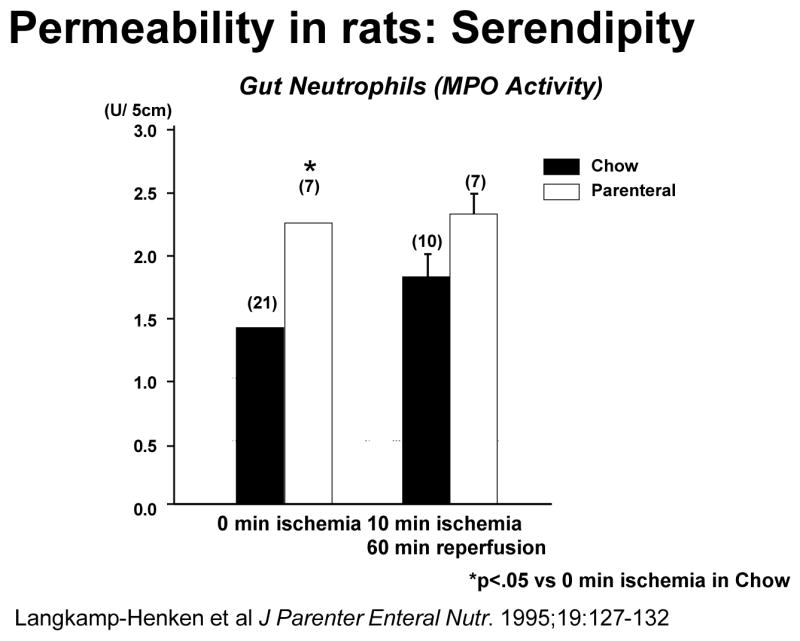
Animals randomized to parenteral feeding significantly increased gut neutrophil accumulation (as measured by myeloperoxidase (MPO) activity) prior to ischemia. (Reproduced with permission of Langkamp-Henken B et al. J Parenter Enteral Nutr. 1995;19:127–132)
While looking for the immunologic mechanism(s) that protect(s) the respiratory mucosa, I found that secretory immunoglobulin A (sIgA) provides the specific immune defense for the respiratory tract and discovered that these cells are sensitized to antigen in the Peyer’s Patches of the small intestine22. Figure 5 shows how the system works. Cell surface markers – L-selectin and α4β7 integrins- on naïve T&B cells recognize the specific molecule mucosal addressin cellular adhesion molecule- 1 (MAdCAM-1) on the high endothelial venule within the Peyer’s Patches and through their interaction migrate into the Peyer’s Patches23, 24. Eighty percent of the lymphocytes circulating in human blood and the blood of mice express L-selectin and α4β7 integrins, markers which distinguish them as destined for the mucosa-associated lymphoid tissue (MALT) 23,. The cells move into the Peyer’s patches and are sensitized to antigens taken from the gut lumen. They then migrate to the mesenteric lymph nodes, into the thoracic duct, and into the blood stream localizing back into the lamina propria of the gut as the gut-associated lymphoid tissue (GALT) and to extra-intestinal sites such as the mammary gland in lactating females, the genitourinary tract and the upper and lower respiratory tract25. In these sites IgA is produced under the influence of Th-2 type IgA-stimulating cytokines for transport across the mucosa and into the lumen as secretory IgA (sIgA) by a specific transport molecule, polymeric immunoglobulin receptor (pIgR) 26. Sixty percent of total body immunity is committed to this system. IgA is the most abundant antibody in the body and has a unique characteristic. It binds to bacteria antigens and prevents their attachment to the mucosal without killing them. If bacteria cannot attach, they cannot infect. We investigated experimentally whether that this system could explain the differences in vulnerability to pneumonia in the parenterally fed patients.
Figure 5.
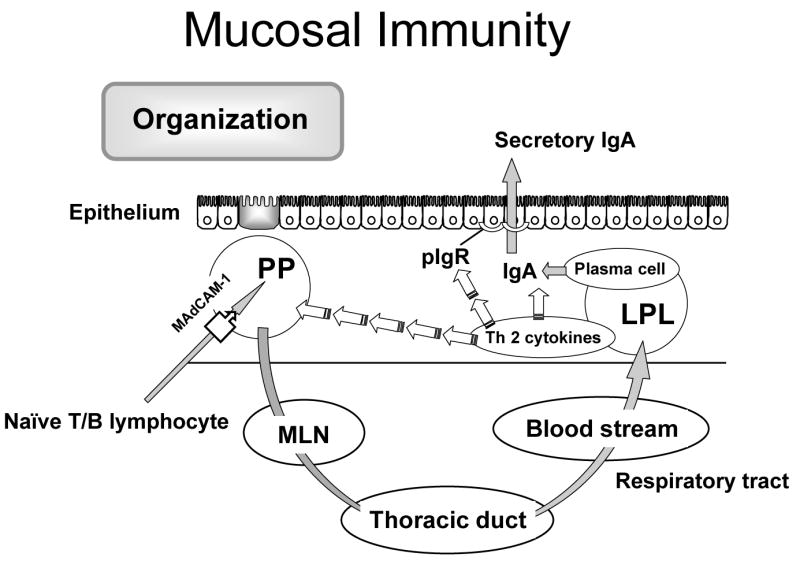
A scheme of the mucosa immune system describing the common mucosa immune hypothesis.
There are a couple of wrong ways to interpret this talk. A first impression might conclude that this work says TPN is bad; absolutely not! TPN has saved thousands of lives and reduced thousands of complications in patients who cannot or could not tolerate enteral feeding for prolonged periods of time. But experimentally TPN allows study of how enteral stimulation - or lack of enteral stimulation- affects intestinal processes such as immunology while preventing the development of malnutrition. Starved mice rapidly develop malnutrition and die within 3–4 days; parenteral nutrition prevents this. Secondly, one might conclude that this talk is just more babble about justifying enteral nutrition. Students and critics of this field certainly can point out discrepancies in results between individual studies, discrepancies between meta- analyses and so on in a the search for universal applicability of nutrition support 27. This talk is not meant to justify enteral nutrition. My point is this: if enteral feeding really reduces infections, particularly pneumonia, after trauma, there should be a definable reason, the reason should measurable and it should be testable in clinical populations. If specific mechanisms through which nutrition support works can be defined - and I suspect many exist- then small, focused clinical trials could allow testing of the effects of a specific nutritional manipulation on that specific mechanism in humans. If the new approach exerts a beneficial effect in the small clinical trial, larger scale, focused studies can be implemented to test the clinical effectiveness of the regimen using the smallest number of patients in the shortest time possible. So, whether one come from the field of Gastroenterology, Immunology, Physiology, Medicine, Surgery, Dietetics, Nursing or Pharmacy, current experimental work demonstrates that feeding or not feeding the gut influences: 1. 60% of the immune system (its function and histology), 2. Systemic inflammation, 3. The immune response to injury and 4. Other issues yet to be determined.
In our animal work, we use several different feeding models (Figure 6) including chow, a complex enteral diet (CED), intragastric parenteral nutrition (IG-TPN) or intravenous (IV-)TPN.28 IV-TPN and IG -TPN use the same formula to control for route of nutrient administration. The CED provides an isocaloric, isonitrogenous comparison to IG-TPN but provides insights into the role of fat and complex proteins and carbohydrates in GI responses. We assume that Chow provides the normal condition and introduces both complexity of diet and intermittency of feeding. One consistent finding in our work is that as the degree of stimulation drops from Chow to CED to IG-TPN to IV TPN, the markers of GI tract immunologic integrity decrease as well.
Figure 6.
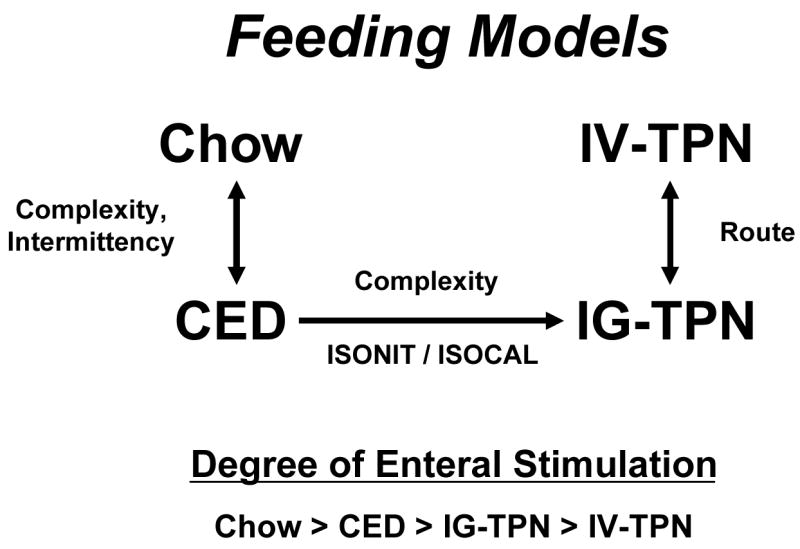
Animals groups used in studies of route and type of nutrition. Intravenous TPN (IV-TPN) and intragastric-TPN (IG-TPN) control for route of nutrition. IG-TPN and complex enteral diets (CED) are isonitrogenous and isocaloric and control for complexity of diets. Both CED and IG-TPN are continuously infused. Chow and CED diets allow comparisons of complexity of diet and intermittency of feeding with Chow representing normal conditions. In general, the beneficial effects of enteral feeding on gut associated lymphoid tissue decreases as the degree of enteral stimulation decreases.
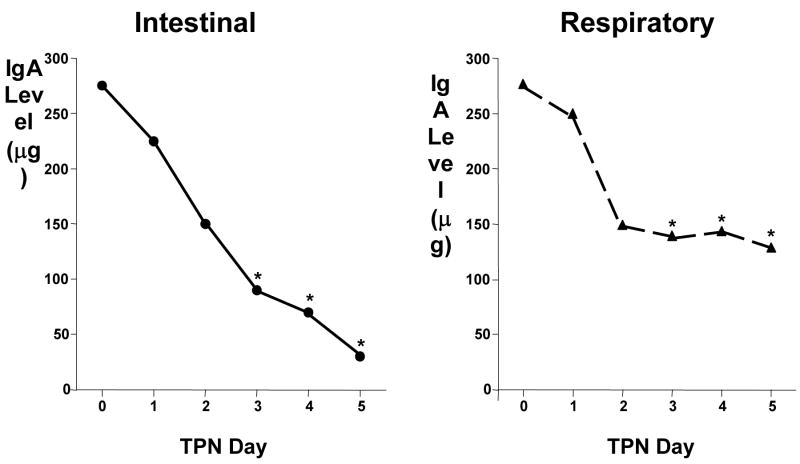
Looking at mucosal immunity, the overall effect of parenteral feeding without enteral stimulation is a reduction in sIgA. IgA levels drop over successive days of TPN in both the intestinal and respiratory tracts (Figure 7) 29. We demonstrated the significance of those decreases in two different experiments (Figure 8). If naïve mice are infected with a mouse-specific influenza virus, they become infected and shed the virus from the nasal passages for approximately 10 days30. Over those 10 days, new immune cells are created, IgA is produced, and the infection is cleared. Once immunized, a second dose of virus is cleared within a short period of time. We immunized mice with the virus, and inoculated immune mice with a second viral dose after they feeding them chow, a CED or IV-TPN. Immunity was maintained in all enterally stimulated mice but 50% of mice fed parenterally lost immunity and continued to shed virus31. In other words immune animals became non-immune when enteral stimulation was lacking. Memory was not lost since several days of chow refeeding returned immunity of IV-TPN mice to normal32. A second model may be more relevant to our patients, i.e. bacterial infections. If naïve animals are given increasing intra-tracheal doses of Pseudomonas (Ps) bacteria, mortality increases from 0 to 100% as the dose increases. Immunization of mice with bacterial antigens from that Ps decreases mortality from 90% to 10% in normal mice33. After generating Ps immunity, we randomized mice to either Chow, CED or IV-TPN for 5 days followed by a subsequent lethal Ps dose. Chow maintained the low mortality as did CED, but animals fed parenterally had the same mortality as non-immune animals34. We know that both the virus and Ps. defenses are IgA dependent after immunization so it is clear that this sIgA reduction in our animal models seems to be an important variable.
Figure 7.
IgA levels in intestinal and the respiratory tract washes drop over time if animals are fed parenteral nutrition (TPN) with no enteral stimulation (Adapted with permission of King et al Arch Surg. 1997;132:1303–1309. Copyright © 1999 American Medical Association. All Rights reserved).
Figure 8.
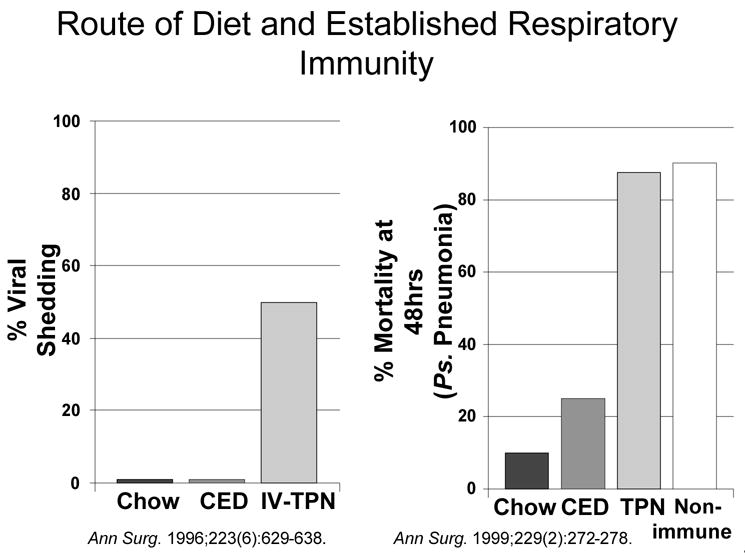
Left figure: In a model of continued viral infection and shedding, enterally fed mice receiving chow or a complex enteral diet (CED) maintain normal immunity while 50% of animals fed parenteral nutrition intravenously (IV-TPN) lost immunity. Right figure: Survival after intra-tracheal Pseudomonas (PS) is increased by previous immunization and reduces mortality from 90% in non-immune animals to 10% in immunized Chow fed mice. Feeding animals a complex enteral diet (CED) after immunization also improves survival but animals fed parenterally (TPN) lose all immunity. (Right figure reproduced with permission of King et al. Ann Surg. 1999;229(2):272–278.).
Let’s examine the MALT system in more detail. Entry of mucosal immunity cells into the Peyer’s Patches is dependent on two T & B cell integrins, α4β7 and L-selectin, expressed on their surfaces which interact with ICAM-1 and MAdCAM-1 expressed on high endothelial venules Peyer’s patches (Figure 9).35 We know these are important through blocking experiments performed in chow fed animals. Compared to lymphocytes recovered from Chow mice, blockade of either α4β7 or L-selectin reduce cell entry and PP lymphocyte numbers.23 Blockade of MAdCAM-1 but not ICAM-1 has the same effect.23,36 We have not investigated effects of lack of enteral stimulation on cell α4β7 or L-selectin expression yet but have focused on MAdCAM-1 and demonstrated that over 4, 8 12, 24, 48 and 72 hours of TPN, expression of MAdCAM-1 is significantly reduced with IV-TPN (Figure 10). 37 If animals fed IV-TPN for five days are given an enteral diet, MAdCAM-1 expression increases to normal in Peyer’s Patches within 12 hours.38 This is a very, very dynamic system which is extremely sensitive to the route of nutrition which directs cells into the Peyer’s Patches. As the enteral stimulus increases from none with IV-TPN to IG-TPN to CED to Chow, MAdCAM-1 expression reflects this stimulation by increasing as well which translates into a increase or decrease in Peyer’s Patch and lamina propria lymphocyte numbers which mirrors the MAdCAM-1 changes (Figure 11). Does this occur in humans? Dr. Fukatsu examined the number of GALT T cells in the lamina propria and intraepithelial space of patients undergoing elective resection of the bowel. 39 Patients fed enterally were compared to patients been fed parenterally prior to surgery. Using immunohistochemistry, they found that parenteral feeding significantly reduced the number of T cells within the lamina propria (Figure 12). Thus, the human data reflects changes seen in the animals work.
Figure 9.
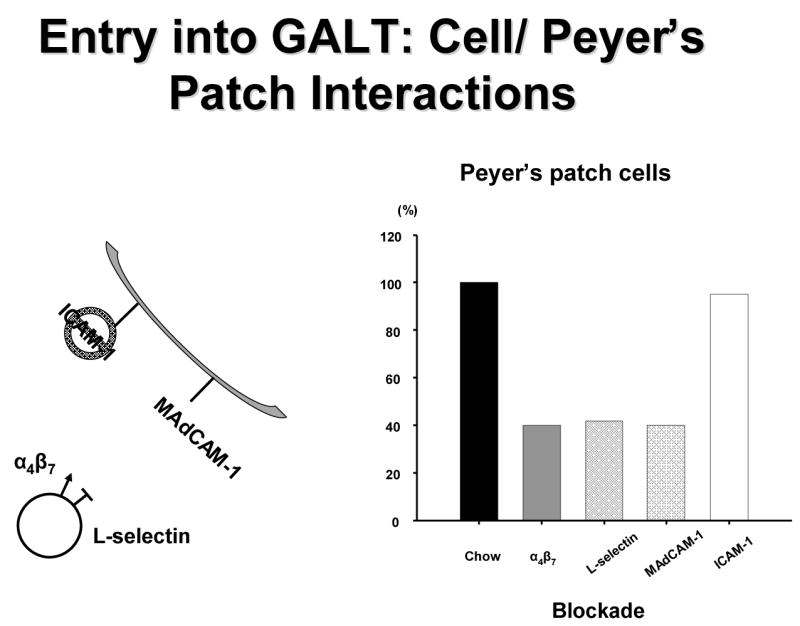
T and B cells enter into the gut associated lymphoid tissue (GALT) through interaction between α4β7 and L-selectin on the cell surfaces with intracellular adhesion molecule-1 (ICAM-1) and mucosal addressin cellular adhesion molecule-1 (MAdCAM-1) on the high endothelial venules on the Peyer’s patches. Blockade of α4β7 , L-selectin or MAdCAM-1 reduced cell entry into Peyer’s patches of chow fed mice whereas ICAM-1 blockade had no effect.
Figure 10.
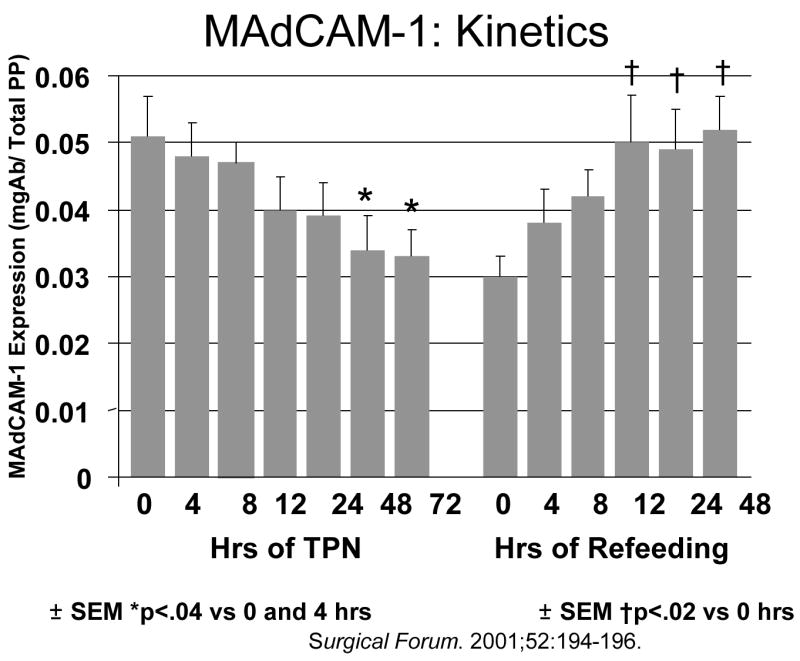
Mucosal addressin cellular adhesion molecule-1 (MAdCAM-1) expression in Peyer’s patches drops after feeding parenteral nutrition (TPN). If animals receive TPN for five days and are then given chow, MAdCAM-1 levels rapidly recover. (Reproduced with permission of Zarzaur et al Surgical Forum. 2001;52:194–196 and the American College of Surgeons).
Figure 11.
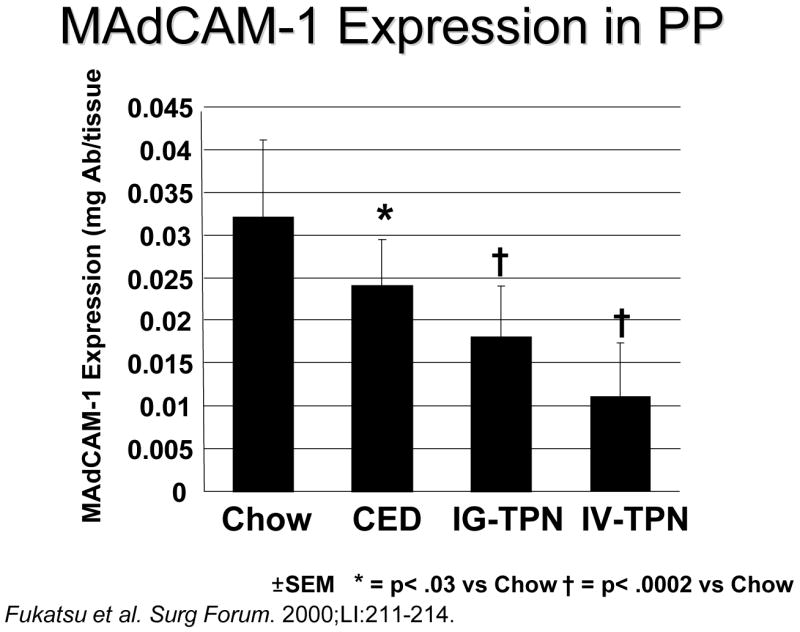
Mucosal addressin cellular adhesion molecule-1 (MAdCAM-1) expression in Peyer’s patches (PP) drops as the amount of enteral stimulation drops from Chow to a complex enteral diet (CED) to intragastric-parenteral nutrition (IG-TPN) to intravenous parenteral nutrition (IV-TPN). (Reproduced with permission of Fukatsu et al. Surgical Forum. 2000;51:211–214 and the American College of Surgeons)
Figure 12.
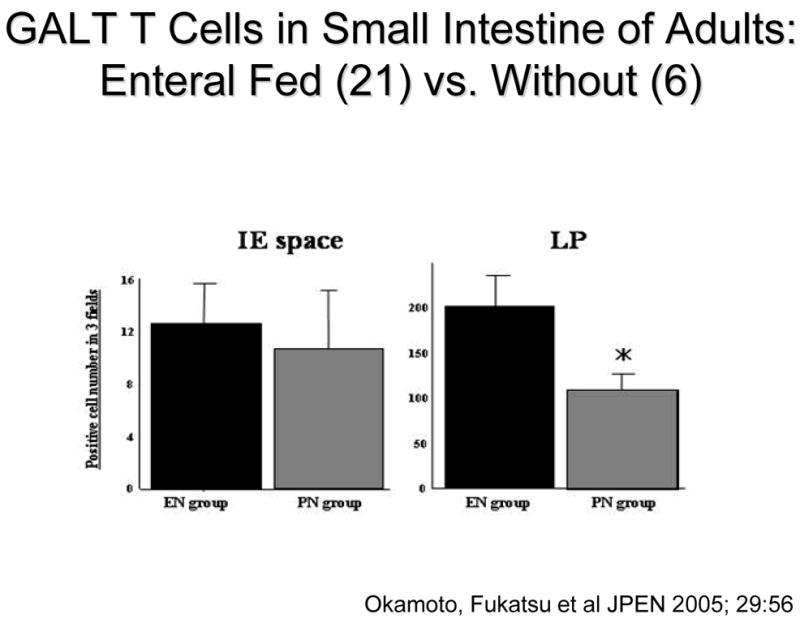
T cells within the gut associated lymphoid tissue (GALT) drop in the lamina propria (LP) of patients fed only parenteral nutrition (PN) preoperatively compared to individuals fed enterally (EN). This reached statistical significance in the Peyer’s patches (reproduced with the permission of Okamato et al, JPEN 2005; 29: 56.)
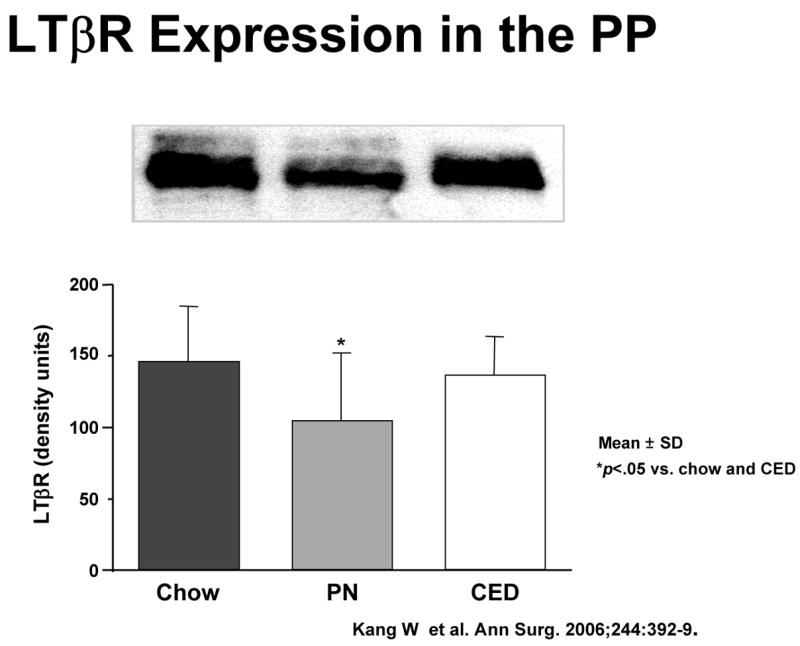
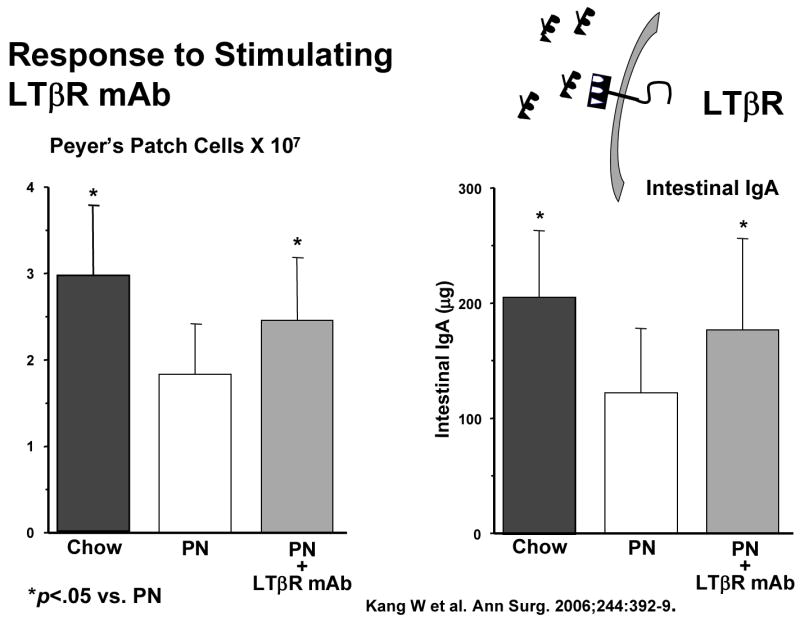
But MAdCAM-1 itself is the product of stimulation of another molecule, lymphotoxin beta receptor (LTβR). LTβR stimulation through interaction with activated T and B cells increases both the Th-2 cytokine IL-4, a co-stimulant of MAdCAM-1, as well as MAdCAM-1 through NFκB (Figure 13a).40 So what is the effect of lack of enteral feeding on this pathway? We’ve not yet investigated this effect upon in NFκB but IV-TPN is associated with a significantly depressed expression of LTβR compared to chow or CED (Figure 13b). 40 But association doesn’t establish cause and effect. To establish a direct link, we administered a LTβR- stimulating monoclonal antibody to parenterally fed mice i.e. used exogenous stimulation to drive the pathway rather than enteral feeding. 40 This stimulatory antibody given with parenteral nutrition significantly increased Peyer’s patch cells and intestinal IgA (Figure 13c). In addition, we blocked LTβR stimulation in chow fed mice using a ‘blocking’ fusion protein. 41 LTβR mRNA levels remained elevated due to enteral stimulation but interference with LTβR stimulation reduced MAdCAM-1 mRNA levels(Figure 13d). In summary, lack of enteral feeding reduces LTβR expression, and reduces MAdCAM-1. It also reduces IL-4, but IL-4 was studied in a different manner.
Figure 13.
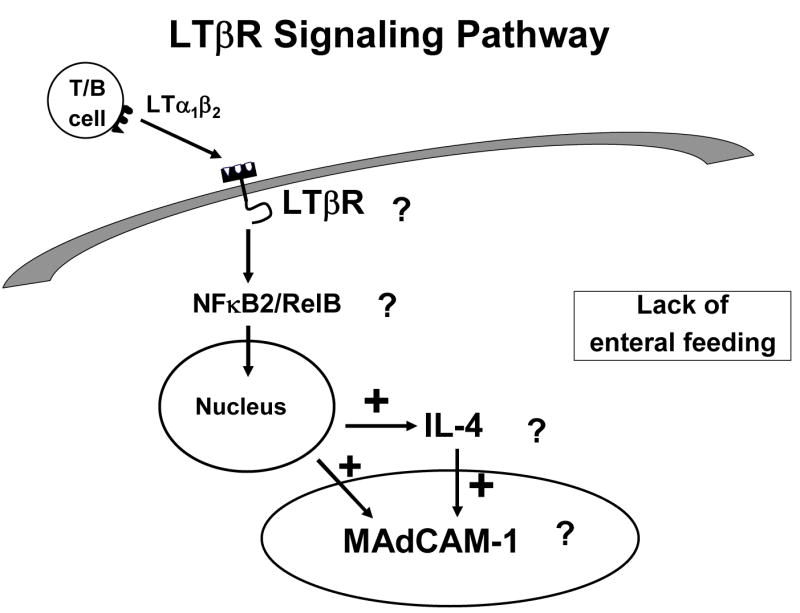
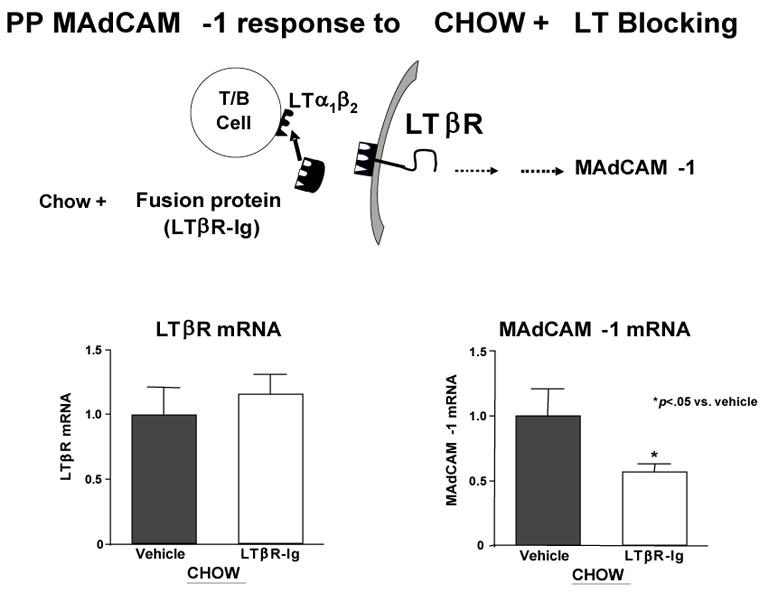
Panel A: The interaction of activated T and B lymphocytes with lymphotoxin beta receptor (LTβR) in the production of mucosal addressin cellular adhesion molecule-1 (MAdCAM-1) and IL-4. Stimulation of LTβR works through NFκB system to stimulate this pathway.
Panel B : LTβR expression on Peyer’s patches (PP) is significantly reduced in animals that receive only parenteral nutrition (PN) compared to feeding Chow or a complex enteral diet (CED) (Reproduced with permission of Kang et al Annals of Surgery 2006;244(3):392–399)
Panel C: Both Peyer’s patch cell numbers and intestinal IgA significantly increased after LTβR stimulation using a simulating monoclonal antibody (mAb) in PN mice compared to PN fed animals receiving a non-stimulating control antibody. (Reproduced with permission of Kang et al Annals of Surgery 2006;244(3):392–399)
Panel D: Chow feeding maintains LTβR mRNA levels while blockade of lymphotoxin α1β1 reduces MAdCAM-1 MRNA production by interfering with LTβR receptor stimulation. (Reproduced with permission of Kang et al JPEN 2007; 31:(5) 358–365)
Recognizing that lack of enteral feeding exerts deleterious effects upon MAdCAM-1, Peyer’s Patch and lamina propria lymphocyte numbers and sIgA, we examined the effects on Th-2 type IgA- stimulating cytokines together with cell phenotypes in the lamina propria. 42,43 Lack of enteral stimulation alters the ratio of CD4+:CD8+ cells from 2:1 level in chow fed mice to approximately 1:1 with IV-TPN. 28 Since T cells produce cytokines, we expected –and found- an effect on cytokines in the GALT. Normally, Th-1 type IgA-inhibiting cytokines such as IFNγ are counterbalanced by the Th-2 type IgA-stimulating cytokines, IL-4, IL-6, and IL-10. Lack of enteral feeding together with IV-TPN affects IL-4 and IL-10 levels and reduced their levels without affecting the IgA-inhibitory cytokine, IFNγ. As a result, as the degree of enteral stimulation decreases from chow to CED to IG TPN to IV TPN, IL-4 levels drop (Figure 14a) in association with reductions in intestinal IgA levels with loss of effective mucosal immunity (Figure 14b). 42,43
Figure 14.
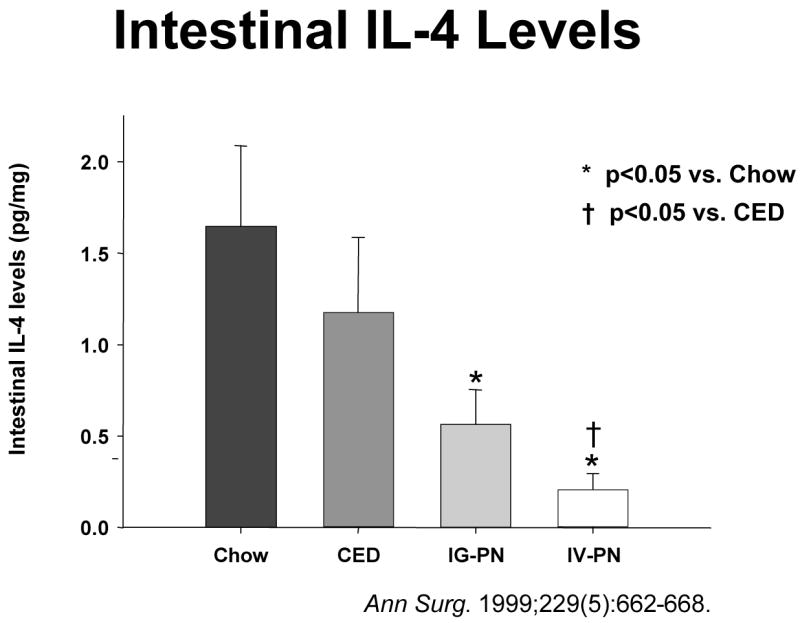
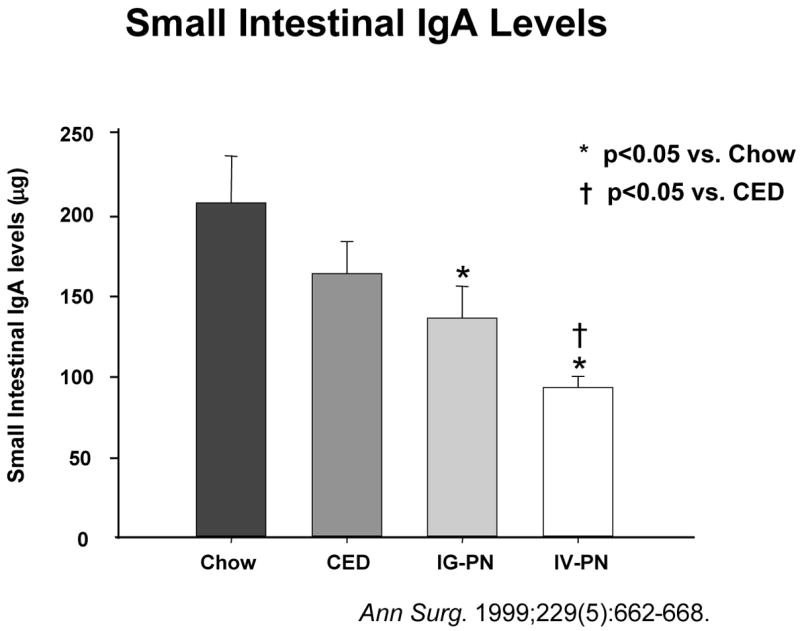
Intestinal interleukin-4 (IL-4) levels drop as the degree of enteral stimulation drops from chow to a complex enteral diet (CED) to intragastric parenteral nutrition (IG-PN) to intravenous PN (IV-PN). Simultaneously, small intestinal immunoglobulin-A (IgA) levels drop in intestinal washings.(Reproduced with the permission of Wu et al, Annals of Surgery 1999: 229 (5): 662–668)
But does enteral stimulation - or its absence- affect IgA transport? As shown in Figure 15, IgA is produced in a dimeric form which becomes a complex with the molecule, polymeric immunoglobulin receptor (pIgR) expressed on the basal surface of the epithelial cell. 44 This complex allows transportation of IgA across the cell into the lumen where part of the pIgR molecule remains within the cell, but part of the molecule (i.e. secretory component) stays attached to IgA converting it to sIgA. This addition distinguishes it from the forms of IgA found in the serum or within tissue. Dr. Sano studied this molecule and showed that lack of enteral stimulation reduces levels of the pIgR protein providing the evidence that enteral stimulation affects transport mechanisms (Figure 16). 44
Figure 15.
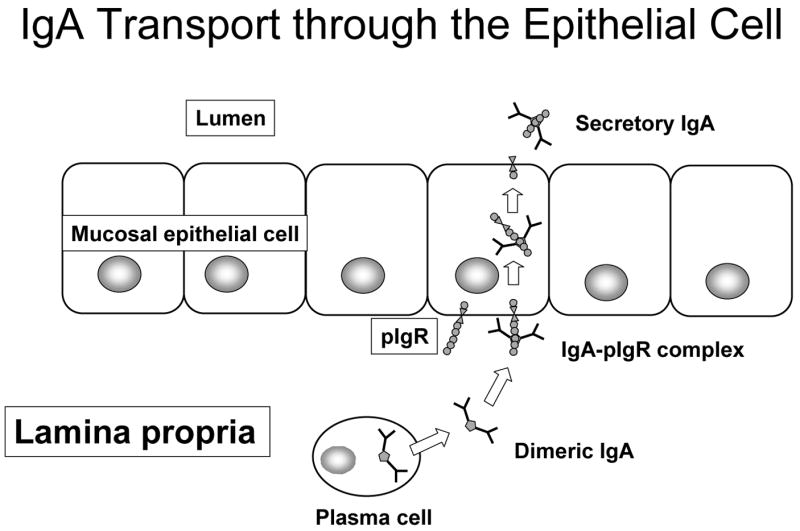
Immunoglobulin-A transport through the epithelial cell is dependant on polymeric immunoglobulin receptor (pIgR) expressed on the basal surface of the mucosal epithelial cells. After pIgR forms a complex with dimeric IgA, the complex is transported across cell and released into the lumen as secretory IgA (sIgA). A small component of the pIgR molecule remains attached to the IgA to distinguish dimeric IgA from secretory IgA.
Figure 16.
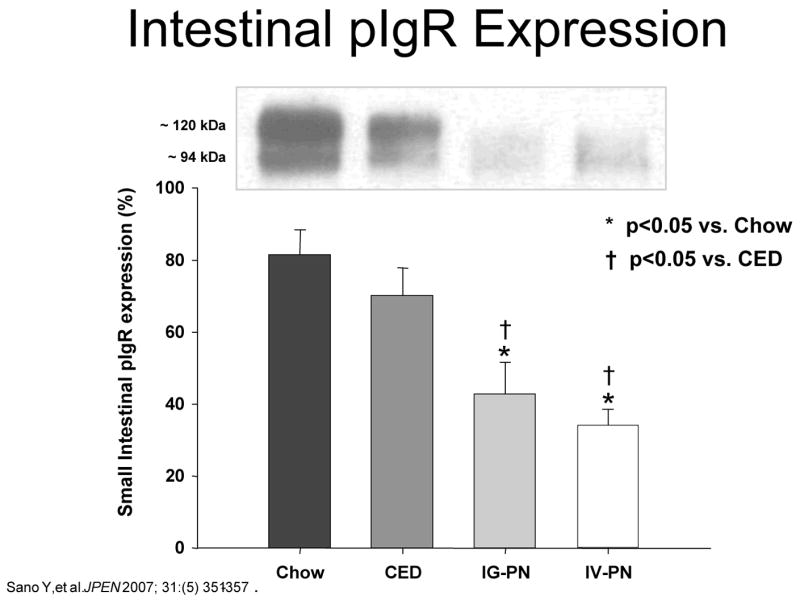
Intestinal polymeric immunoglobulin receptor (pIgR) expression drops as the degree of enteral stimulation decreases from Chow to a complex enteral diet (CED) to intragastric parenteral nutrition (IG-PN) to intravenous PN (IV-PN). (Reproduced with the permission of Sano et al JPEN 2007; 31:(5) 351–357)
In summary, the degree of enteral stimulation affects all of these mechanisms- lymphocyte entry and distribution, cytokine levels, IgA production and transport, and established anti-viral and anti-bacterial mucosal immunity. But does degree of enteral stimulation affect ability to respond to a new infectious complication? In that regard we studied the nasal associated lymphoid tissue (NALT) in rodents. In this system, naive T and B cells enter NALT, become sensitized to antigens, and migrate to the cervical lymph nodes and nasal passages where IgA is produced against the sensitizing antigen. By isolating cells from the nasal passages and plating then in a dish coated with the antigen of interest, we can stain for the cells producing antibody against that antigen and count the number of cells generating the antigen-specific immune response. Using the influenza virus described earlier, antigen specific cells proliferate over time with few appearing by 3 days but a peak at 10–13 days. 30 These immunoglobulin-producing cells include cells producing IgA, IgM and IgG. Clinically, the infection is eliminated during in this time as the immunoglobulins bind to the viral particles and stop the infection. Immunity and antigenic memory exist thereafter. We immunized naïve animal with the virus and then sacrificed groups fed chow, CED or IV-TPN at 6, 10 and 13 days. Mice fed enterally increased the populations of antigen-specific antibody-producing cells while parenterally fed mice developed an impaired response to the infectious challenge (Figure 17).30 Thus, lack of enteral nutrition impairs both established immunity against infections and the ability to generate an new immune response to a novel infectious challenge.
Figure 17.
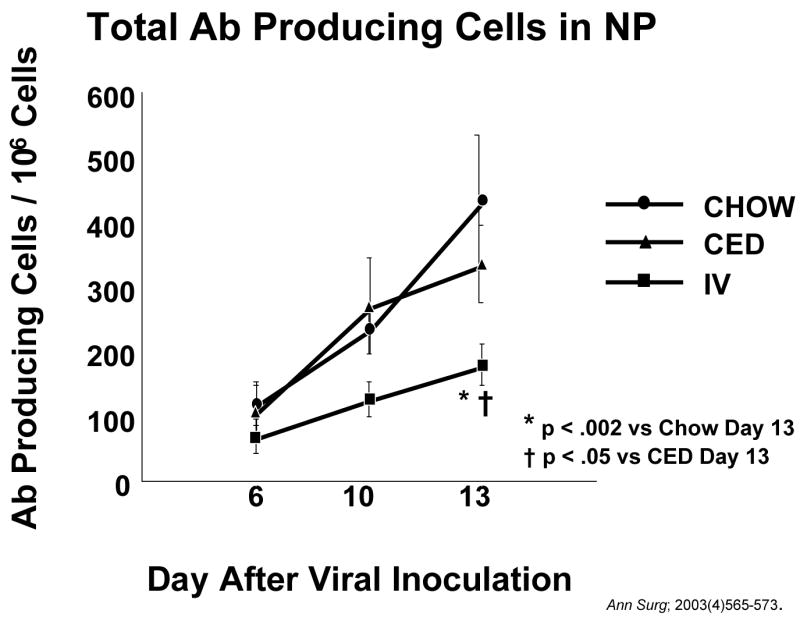
After inoculation with a new viral challenge, antibody (AB) producing cells increase in the nasal passages (NP) of animals fed chow or a complex enteral diet (CED). Accumulation of antibody producing cells is significantly depressed in animals fed parenteral nutrition (IV-TPN). (Reproduced with permission of Johnson et al Ann Surg; 2003(4)565–573)
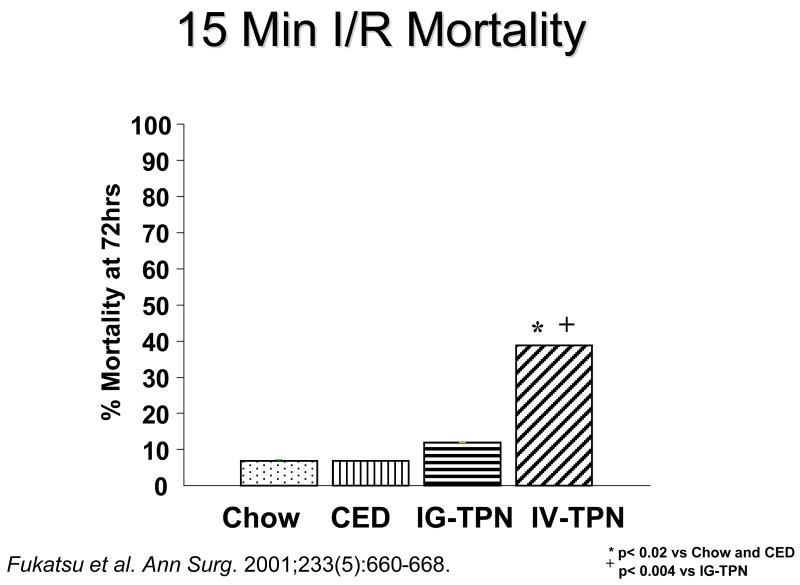
The alterations in cytokines affect the systemic mechanisms as well as IgA production, particularly inflammation. Figure 18a shows the role of cytokines on both the GALT and on the vascular endothelium. IL-4 and Il-10 increase IgA production for transport to the lumen while IFNγ exerts an inhibitory effect. Normally this balance is weighted in favor of IL-4 and Il-10. IL-4 and Il-10 also exert effects on the endothelium of the blood vessels within the GALT by suppressing ICAM-1 expression. 45 IFNγ, which suppresses IgA production, stimulates ICAM-1 expression. ICAM-1 plays an important role in attracting neutrophils to the endothelium leading to attachment, priming, and augmentation of their inflammatory response to subsequent injury. Since lack of enteral stimulation reduced IL-4 and IL-10, we examined effect of IV-TPN upon ICAM-1 and levels of neutrophils within the gut. Dr. Fukatsu deserves all credit for implementing this work which seemed reasonable given Dr. Langkamp-Henken’s serendipitous findings of increases MPO in the gut of starved rats. 21 Dr. Fukatsu showed that lack of enteral stimulation increased both ICAM-1 expression and neutrophil accumulation in the mouse intestine (Figure 18b). 45 This is important since this interaction primes the cells. Moore et al47 showed that cells primed by the gut during hemorrhagic shock were redistributed to the lungs during resuscitation. If animals were subjected to a second insult such as ischemia/reperfusion, these primed cells injured the lungs through the augmented inflammatory response. Dr. Fukatsu used IV-TPN to up-regulate the ICAM-1 and tested whether it also primed the cells. He showed that IV-TPN increased the expression of CD-18 on lung cells reflecting their activation while IG-TPN and chow feeding produced no such activation. 48 IV-TPN ‘priming’ resulted in increases in lung permeability and a significantly increased mortality (Figure 19). 49 Thus, lack of enteral feeding affect both mucosal immunity and systemic inflammation. This observation may explain the increases in multiple organ dysfunction noted after parenteral feeding.
Figure 18.
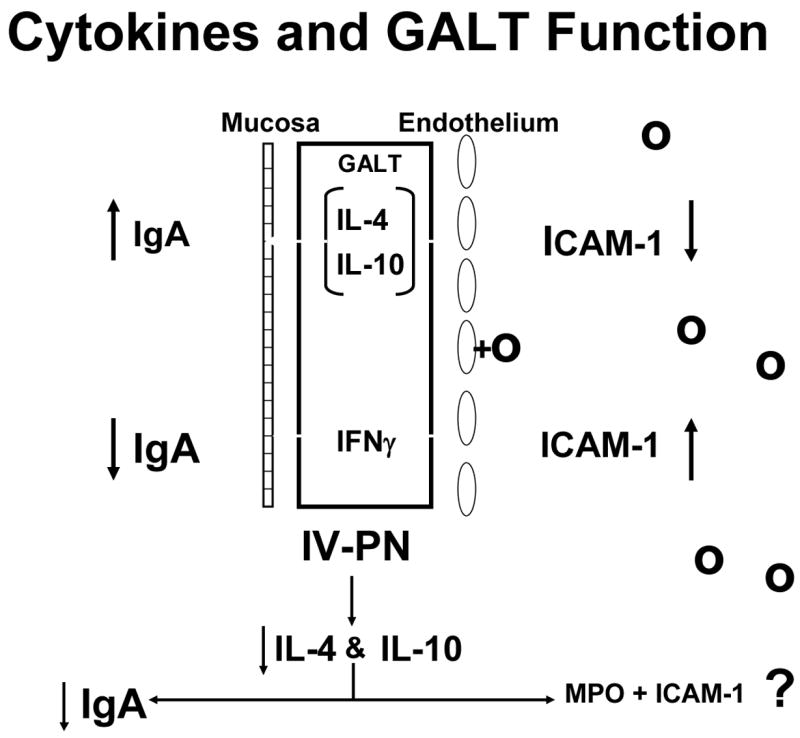
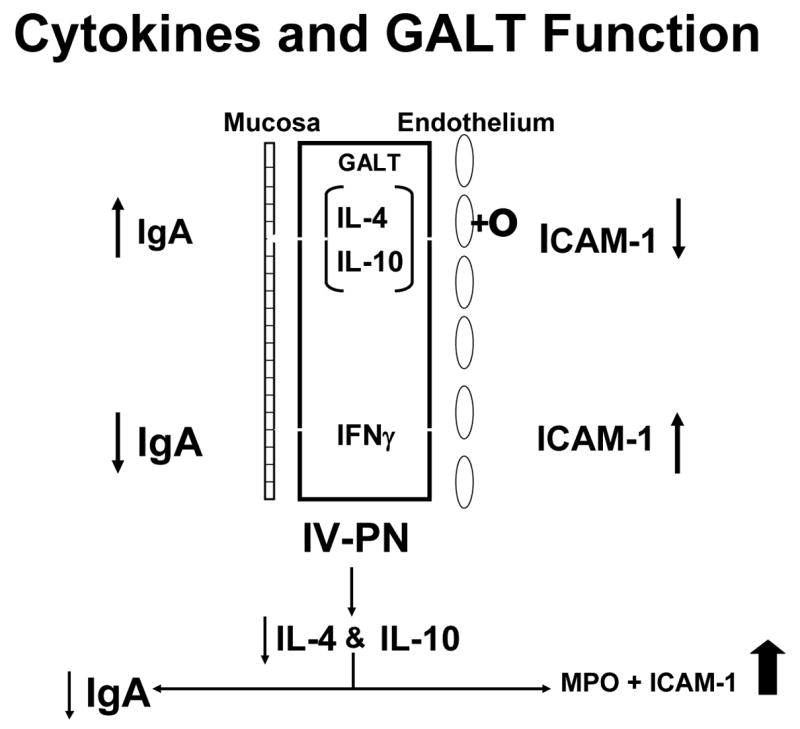
Figure 18a. The interaction between cytokines and gut associated lymphoid tissue (GALT) function is shown. Interleukin-4 (IL-4) and interleukin-10 (IL-10) stimulate IgA production with release of IgA into the lumen while inhibiting intercellular adhesion molecule-1 (ICAM-1) expression on endothelial cells of the vasculature. Interferon gamma (IFNγ) counteracts IL-4 to inhibit IgA production and augment ICAM-1expression.
Figure 18b: Intravenous parenteral nutrition (IV-PN) reduces IL-4 and IL-10 leading to an increase in ICAM-1 expression and lower IgA. (Figures 18 a and b reproduced with permission of Kudsk et al. Am J Surg. 2003;185(1)16–21 and The American Journal of Surgery)
Figure 19.
Fifteen minutes of gut ischemia/reperfusion (IR) increases the mortality of animals fed intravenous parenteral nutrition (IV-TPN) compared to animals fed chow, a complex enteral diet (CED) or intragastric PN (IG-TPN).
Many critically ill patients, however, cannot be fed enterally due to fistulas, lack of enteral access, profound ileus or other conditions which render them dependent on parenteral nutrition for support. Ideally, clinicians could add something to the parenteral solution that both feeds the patient and stimulates mucosal immunity as well. Glutamine has been studied in this regard with the finding that a 2% glutamine added to standard amino acids increases the number of lymphocytes within Peyer’s Patches and lamina propria, improves - but does not completely normalize - the Th-2 cytokines, and significantly improves respiratory and intestinal IgA but not to chow levels. 50,51,52 Glutamine improves anti-viral and antibacterial defenses but not to chow levels. 50, 53 It completely returns survival of mice after gut ischemia/reperfusion to normal however. 54 We have not investigated its effect upon pIgR yet. Surprisingly, it fails to affect MAdCAM-1 expression. 55 These finds portend a promising area of research for investigators.
Others surrogates for enteral stimulation are the neuropeptides. 56 The body has three nervous systems: the central nervous system and spinal cord, the autonomic nervous system and the enteric nervous system. The enteric nervous system is huge. There is approximately 3 meters of nerve for every cubic millimeter of tissue in the gastrointestinal tract with the highest concentration of these nerve fibers next to the mucosa around and within the GALT. These nerve fibers release neuropeptides such as cholecystokinin, neurotensin, and, in humans, gastrin-releasing peptide (GRP). GRP is a peptide released early in the cycle of feeding which stimulates the release of other neuropeptides downstream including gastrin. Experimentally, bombesin is used to study GRP effects since they share the same terminal 7 amino acid sequences that provide the functional effects. The addition of bombesin to TPN returns the lymphocyte numbers within the Peyer’s Patches and lamina propria to normal, increases levels of the Th-2 cytokines toward normal, stimulates normal levels of intestinal and respiratory IgA and maintains both antiviral and anti-bacterial defenses. 57, 58, 59, 60 Just like glutamine, it does not function through MAdCAM-1 and probably functions via direct cell effects. 55 It is not protective after ischemia/reperfusion injury. The neuropeptides show promise as another field of future research which may prove to be an important pharmacologic tool.
But all of this is interesting but less relevant if it does not occur in humans. Our ability to test some of these principles is limited since can’t randomize volunteers to enteral or parenteral feeding and electively remove intestine except under circumstances such as Dr. Fukatsu’s work with bowel resection. 39 But there are other avenues of access to mucosal immunity available to clinicians. For example, we can obtain lung lavage specimens to measure airway IgA within the epithelial lining fluid (ELF) which lines the airway surfaces. A bronchoalveolar lavage specimen allows us to quantify the volume of epithelial lining fluid returned, the concentration of IgA in that lining fluid and the total amount of IgA in the specimen. We obtained specimens from patients undergoing elective operations such as an inguinal hernia repair or cholecystectomy and determined the baseline levels of ELF and IgA in otherwise normal individuals. 61 We also obtained sequential specimens from trauma patients requiring prolonged intubation and documented dramatic increases in the volume of epithelial lining fluid, the concentration of IgA in the ELF, and total IgA recovered after injury (Figure 20). This surprised us (serendipity!) and we examined whether this occurred in the mouse model.61 An abbreviated, simple injury stress of neck and abdominal incisions stimulates a significant increase in airway IgA at 8 hours in mice which returns to normal at 24 hours (Figure 21). The mechanism for this acute IgA increase appears related to TNFα since prior blockade of TNFα eliminates the response. 62 What effect does IV-TPN have on this injury response? It completely stops it! 63
Figure 20.
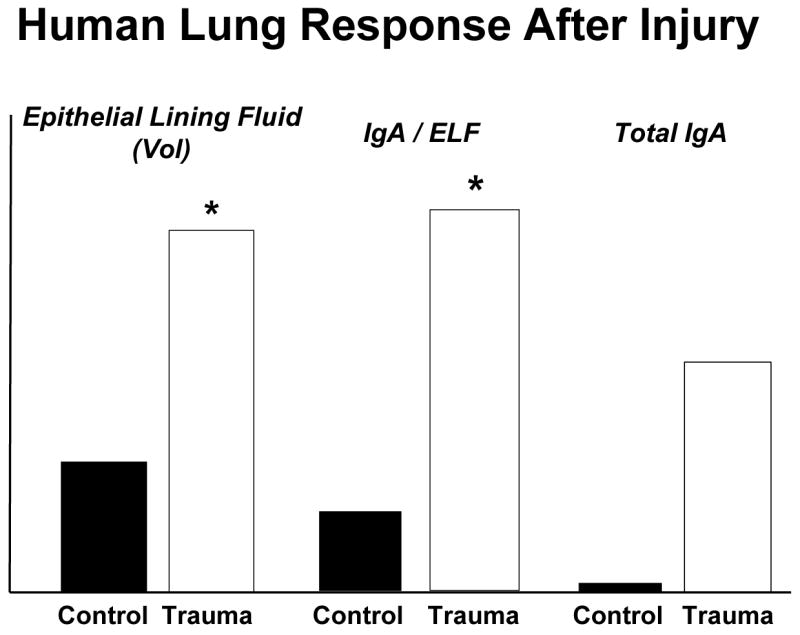
Compared to normal patients undergoing elective surgery, severely injured trauma patients significantly increase the volume of epithelial lining fluid (ELF) recovered after bronchoalveolar lavage and increase the concentration of immunoglobulin-A in the ELF. Overall, there is an increase in total IgA recovered. (adapted with permission of Kudsk et al J Trauma 2008;64(2)316–325)
Figure 21.
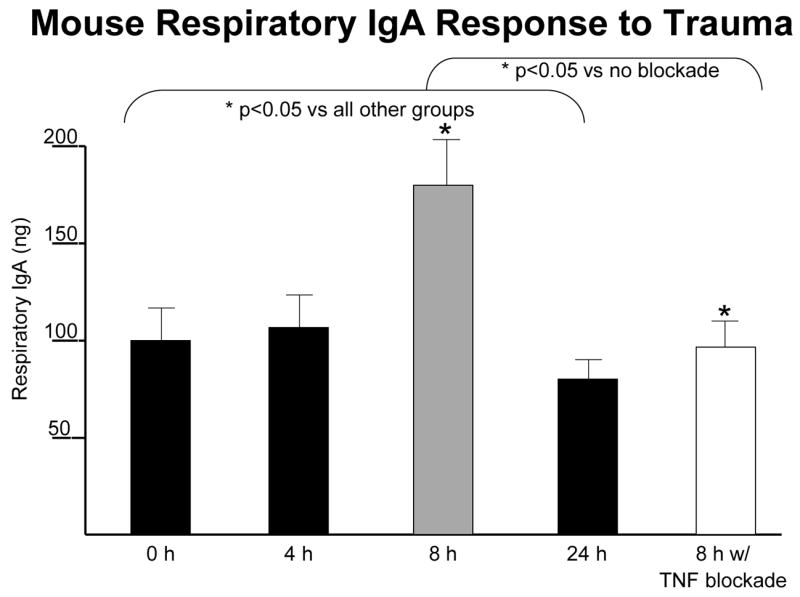
After the minor stress of neck and abdominal incisions in mice, respiratory IgA levels significantly increase eight hours after injury compared to control. This response appears to be driven by tumor necrosis factor (TNF) since TNF blockade eliminates the response. (adapted with permission of Kudsk et al J Trauma 2008;64(2)316–325).
Over the years I remember many successful “Eureka” experiments and many disappointments. I’m sure I’ve missed many pearls as well. But I am convinced that mucosal immunity is important to our field, that it explains how enteral nutritional support protects respiratory host defenses, and that it provides insights into potential new therapeutic options for us to use in our patients. However, this field requires new inquisitive minds to keep the investigations going. Please do not misinterpret this work. Parenteral nutrition is not bad, as some would have us believe. It does what is was designed to do, that is, provide adequate macro- and micro-nutrients to patients who would otherwise starve. It has saved thousands and thousands of lives of people who would have died without it. However, I believe we can do better and improve outcome by discovering, defining and understanding mechanisms in the laboratory, confirming them in humans where possible, manipulating these mechanisms in the laboratory, and applying promising results in our patients.
I thank you again for the opportunity to present the 2008 Jonathon Rhoads Lecture. I consider this one of the greatest honors of my career.
Acknowledgments
Supported by: NIH R01 GM53439
References
- 1.Rombeau JL, Muldoon D, Jonathan E, Rhoads MD. Quaker sense and sensibility in the world of surgery. Philadelphia: Hanley & Belfus; 1997. [Google Scholar]
- 2.Kudsk KA. Dear Miss Milk Toast (1998 Presidential Address - ASPEN) J Parenter Enteral Nutr. 1998;22(4):191–198. doi: 10.1177/0148607198022004191. [DOI] [PubMed] [Google Scholar]
- 3.Peterson SR, Kudsk KA, Carpenter G, Sheldon GF. Malnutrition and Immunocompetence: Increased mortality following an infectious challenge during hyperalimentation. J Trauma. 1981;21:528–533. [PubMed] [Google Scholar]
- 4.Kudsk KA, Carpenter BS, Peterson S, Sheldon GF. Effect of Enteral and Parenteral Feeding in Malnourished Rats with E. coli-Hemoglobin Adjuvant Peritonitis. J Surg Res. 1981;31:105–110. doi: 10.1016/0022-4804(81)90037-8. [DOI] [PubMed] [Google Scholar]
- 5.Kudsk KA, Stone JM, Carpenter BA, Sheldon GF. Enteral and parenteral feeding influences mortality after hemoglobin-E. coli peritonitis in normal rats. J Trauma. 1983;23:605–609. doi: 10.1097/00005373-198307000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Kudsk KA, Croce MA, Fabian TC, Minard G, Tolley EA, Poret HA, Kuhl MR, Brown RO. Enteral vs. parenteral feeding: Effects on septic morbidity following blunt and penetrating abdominal trauma. Ann Surg. 1992;215(5):503–513. doi: 10.1097/00000658-199205000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore FA, Moore EE, Jones T, McCroskey BL, Peterson VM. TEN versus TPN following major abdominal trauma--reduced septic morbidity. J Trauma. 1989;29(7):916–22. doi: 10.1097/00005373-198907000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Poret HA, Kudsk KA, Croce MA, Fabian TC, Minard G, Collier PR, Brown RO, Cicala RS. The effect of enteral feeding on catecholamine response following trauma. Surg Forum. 1991;62:11–12. [Google Scholar]
- 9.Behrman SW, Kudsk KA, Brown RO, Vehe KL, Wojtysiak SL. The effect of growth hormone on nutritional markers in enterally fed immobilized trauma patents. J Parenter Enteral Nutr. 1995;19(1):41–46. doi: 10.1177/014860719501900141. [DOI] [PubMed] [Google Scholar]
- 10.Kudsk KA, Mowatt-Larssen C, Bukar J, Fabian T, Oellerich S, Dent DL, Brown R. Effect of rhIGF-I and early TPN on immune depression following severe head injury. Arch Surg. 1994;129:66–71. doi: 10.1001/archsurg.1994.01420250078010. [DOI] [PubMed] [Google Scholar]
- 11.Kudsk KA, Minard G, Croce MA, Brown RO, Lowrey TS, Pritchard E, Dickerson RN, Fabian TC. A randomized trial of isonitrogenous enteral diets following severe trauma: An immune-enhancing diet (IED) reduces septic complications. Ann Surg. 1996;224(4):531–543. doi: 10.1097/00000658-199610000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore FA, Moore EE, Kudsk KA, Brown RO, Bower RH, Koruda MJ, Baker CC, Barbul A. Clinical benefits of an immune-enhancing diet for early postinjury enteral feeding. J Trauma. 1994;37(4):607–615. doi: 10.1097/00005373-199410000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Kudsk KA, Laulederkind A, Hanna MK. Most infectious complications in parenterally fed trauma patients are not due to elevated blood glucose levels. J Parenter Enteral Nutr. 2001;25(4):174–179. doi: 10.1177/0148607101025004174. [DOI] [PubMed] [Google Scholar]
- 14.Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359–67. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 15.Krinsley JS. Effect of an intensive glucose management protocol on the mortality of critically ill adult patients. Mayo Clin Proc. 2004;79(8):992–1000. doi: 10.4065/79.8.992. [DOI] [PubMed] [Google Scholar]
- 16.Speery JL, Frankel HL, Vanek SL, et al. Early hyperglycemia predicts multiple organ failure and mortality but not infection. J Trauma. 2007;63:487–94. doi: 10.1097/TA.0b013e31812e51fc. [DOI] [PubMed] [Google Scholar]
- 17.Langkamp-Henken B, Donovan TB, Pate LM, Maull CD, Fabian TC, Kudsk KA. Increased intestinal permeability following blunt and penetrating trauma. Crit Care Med. 1995;23(4):660–664. doi: 10.1097/00003246-199504000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Ziegler TR, Smith RJ, O’Dwyer ST, Demling RH, Wilmore DW. Increased intestinal permeability associated with infection in burn patients. Arch Surg. 1988;123(11):1313–9. doi: 10.1001/archsurg.1988.01400350027003. [DOI] [PubMed] [Google Scholar]
- 19.Deitch EA. Intestinal permeability is increased in burn patients shortly after injury. Surgery. 1990;107(4):411–6. [PubMed] [Google Scholar]
- 20.Janu P, Li J, Minard G, Kudsk KA. Systemic interleukin-6 (IL-6) correlates with intestinal permeability. Surg Forum. 1996;47:7–9. [Google Scholar]
- 21.Langkamp-Henken B, Kudsk KA, Proctor KG. Fasting-induced reduction of intestinal reperfusion injury. J Parenter Enteral Nutr. 1995;19:127–132. doi: 10.1177/0148607195019002127. [DOI] [PubMed] [Google Scholar]
- 22.McGhee JR, Mestecky J, Dertzbaugh MT, Eldridge JH, Hirasawa M, Kiyono H. The mucosal immune system: from fundamental concepts to vaccine development. Vaccine. 1992;10:75–88. doi: 10.1016/0264-410x(92)90021-b. [DOI] [PubMed] [Google Scholar]
- 23.Reese SR, Genton L, Ikeda S, Le TC, Kudsk KA. L-selectin and A4B7 Integrin but not ICAM-1 Regulate Lymphocyte Distribution in Gut-Associated Lymphoid Tissue of Mice. Surgery. 2005;137(2):209–215. doi: 10.1016/j.surg.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Fukatsu K, Zarzaur B, Johnson C, Wu Y, Wilcox H, Kudsk KA. Decreased MAdCAM-1 expression in Peyer’s patches: A mechanism for impaired mucosal immunity during lack of enteral nutrition. Surg Forum. 2000;LI:211–214. [Google Scholar]
- 25.Czerkinsky C, Prince SJ, Michalek SM, et al. IgA antibody-producing cells in peripheral blood after antigen ingestion: evidence for a common mucosal immune system in humans. Proc Natl Acad Sci U S A. 1987;84:2449–2453. doi: 10.1073/pnas.84.8.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sano Y, Gomez FE, Kang W, Lan J, Maeshima YL, Hermsen JL, Ueno C, Kudsk KA. Intestinal Polymeric Immunoglobulin Receptor is Affected by Type and Route of Nutrition. JPEN. 2007;31(5):351–357. doi: 10.1177/0148607107031005351. [DOI] [PubMed] [Google Scholar]
- 27.Koretz R. Does nutritional intervention in protein-energy malnutrition improve morbidity or mortality? J Ren Nutr. 1999 Jul;9(3):119–21. doi: 10.1016/s1051-2276(99)90047-x. [DOI] [PubMed] [Google Scholar]
- 28.Li J, Kudsk KA, Gocinski B, Dent D, Glezer J, Langkamp-Henken B. Effects of parenteral and enteral nutrition on gut-associated lymphoid tissue. J Trauma. 1995;39(1):44–52. doi: 10.1097/00005373-199507000-00006. [DOI] [PubMed] [Google Scholar]
- 29.King BK, Li J, Kudsk KA. A temporal study of TPN-induced changes in gut-associated lymphoid tissue and mucosal immunity. Arch Surg. 1997;132:1303–1309. doi: 10.1001/archsurg.1997.01430360049009. [DOI] [PubMed] [Google Scholar]
- 30.Johnson CD, Kudsk KA, Fukatsu K, Renegar KB, Zarzaur BL. Route of nutrition influences generation of antibody-forming cells (AFCs) and initial defense to an active viral infection in the upper respiratory tract. Ann Surg. 2003;(4):565–573. doi: 10.1097/01.SLA.0000059991.89316.B8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kudsk KA, Li J, Renegar KB. Loss of upper respiratory tract immunity with parenteral feeding. Ann Surg. 1996;223(6):629–638. doi: 10.1097/00000658-199606000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janu P, Li J, Renegar KB, Kudsk KA. Recovery of gut-associated lymphoid tissue (GALT) and upper respiratory tract (URT) immunity following parenteral nutrition (TPN) Ann Surg. 1997;225(6):707–717. doi: 10.1097/00000658-199706000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abraham E. Intranasal immunization with bacterial polysaccharide containing liposomes enhances antigen-specific pulmonary secretory antibody response. Vaccine. 1992;10:461–68. doi: 10.1016/0264-410x(92)90395-z. [DOI] [PubMed] [Google Scholar]
- 34.King BK, Kudsk KA, Li J, Wu Y, Renegar KB. Route and type of nutrition influence mucosal immunity to bacterial pneumonia. Ann Surg. 1999;229(2):272–278. doi: 10.1097/00000658-199902000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kudsk KA. Current aspects of mucosal immunology and its influence by nutrition. Am J Surg. 2002;183(4):390–398. doi: 10.1016/s0002-9610(02)00821-8. [DOI] [PubMed] [Google Scholar]
- 36.Ikeda S, Kudsk KA, Fukatsu K, Johnson C, Le T, Reese S, Zarzaur B. Enteral Feeding Preserves Mucosal Immunity Despite in vivo MAdCAM-1 Blockage of Lymphocyte Homing. Ann of Surg. 2003;237(5):677–685. doi: 10.1097/01.SLA.0000064364.40406.EA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fukatsu K, Zarzaur B, Johnson C, Wu Y, Wilcox H, Kudsk KA. Decreased MAdCAM-1 expression in Peyer’s patches: A mechanism for impaired mucosal immunity during lack of enteral nutrition. Surg Forum. 2000;LI:211–214. [Google Scholar]
- 38.Zarzaur BL, Fukatsu K, Johnson CJ, Eng E, Kudsk KA. A temporal study of diet-induced changes in peyer patch MAdCAM-1 expression. Surgical Forum. 2001;52:194–196. [Google Scholar]
- 39.Okamato K, Fukatsu K, Ueno C, Shinto E, et al. T lymphocyte numbers in human gut associated lymphoid tissue are reduced without enteral nutrition. JPEN J Parenter Enteral Nutr. 2005;29(1):56–8. doi: 10.1177/014860710502900156. [DOI] [PubMed] [Google Scholar]
- 40.Kang W, Gomez EF, Lan J, Sano Y, Ueno C, Kudsk KA. Parenteral Nutrition (PN) Impairs Gut-associated Lymphoid Tissue (GALT) and Mucosal Immunity by Reducing Lymphotoxin β Receptor (LTβR) Expression. Annals of Surgery. 2006;244(3):392–399. doi: 10.1097/01.sla.0000234797.42935.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang W, Kudsk KA, Sano Y, Lan J, Yang-Xin F, Gomez FE, Maeshima Y. Effects of Lymphotoxin β Receptor Blockade on Intestinal Mucosal Immunity. JPEN. 2007;31(5):358–365. doi: 10.1177/0148607107031005358. [DOI] [PubMed] [Google Scholar]
- 42.Wu Y, Kudsk KA, DeWitt RC, Tolley EA, Li J. Route and type of nutrition influence IgA-mediated intestinal cytokines. Ann Surg. 1999;229(5):662–668. doi: 10.1097/00000658-199905000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fukatsu K, Kudsk KA, Wu Y, Zarzaur BL, Hanna MK, DeWitt RC. TPN decreases IL-4 and IL-10 mRNA expression in lamina propria cells but glutamine supplementation preserves the expression. Shock. 2001;15(4):318–322. doi: 10.1097/00024382-200115040-00012. [DOI] [PubMed] [Google Scholar]
- 44.Sano Y, Gomez FE, Kang W, Lan J, Maeshima YL, Hermsen JL, Ueno C, Kudsk KA. Intestinal Polymeric Immunoglobulin Receptor is Affected by Type and Route of Nutrition. JPEN. 2007;31(5):351–357. doi: 10.1177/0148607107031005351. [DOI] [PubMed] [Google Scholar]
- 45.Fukatsu K, Lundberg AH, Hanna MK, Wu Y, Wilcox HG, Granger DN, Gaber AO, Kudsk KA. Route of nutrition influences intercellular adhesion molecule-1 expression and neutrophil accumulation in intestine. Arch Surg. 1999;134:1055–1060. doi: 10.1001/archsurg.134.10.1055. [DOI] [PubMed] [Google Scholar]
- 46.Kudsk KA. Effect of route and type of nutrition on intestine-derived inflammatory responses. Am J Surgery. 2003;185:16–21. doi: 10.1016/s0002-9610(02)01146-7. [DOI] [PubMed] [Google Scholar]
- 47.Moore EE, Moore FA, Franciose RJ, Kim FJ, Biffl WL, Banerjee A. The postischemic gut serves as a priming bed for circulating neutrophils that provoke multiple organ failure. J Trauma. 1994;37(6):881–7. doi: 10.1097/00005373-199412000-00002. [DOI] [PubMed] [Google Scholar]
- 48.Fukatsu K, Kudsk KA, Zarzaur BL, Sabek O, Wilcox HG, Johnson CD. Increased ICAM-1 and β2 integrin expression in parenterally fed mice after a gut ischemic insult. Shock. 2002;18(2):119–24. doi: 10.1097/00024382-200208000-00005. [DOI] [PubMed] [Google Scholar]
- 49.Fukatsu K, Zarzaur BL, Johnson CD, Lundberg AH, Wilcox HG, Kudsk KA. Enteral nutrition prevents remote organ injury and mortality following a gut ischemic insult. Ann Surg. 2001;233(5):660–668. doi: 10.1097/00000658-200105000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li J, Kudsk KA, Janu P, Renegar KB. Effect of glutamine-enriched TPN on small intestine gut-associated lymphoid tissue (GALT) and upper respiratory tract immunity. Surgery. 1997;121(5):542–549. doi: 10.1016/s0039-6060(97)90109-4. [DOI] [PubMed] [Google Scholar]
- 51.Kudsk KA, Wu Y, Fukatsu K, Zarzaur BL, Johnson CD, Wang R, Hanna MK. Glutamine-enriched total parenteral nutrition maintains intestinal interleukin-4 and mucosal immunoglobulin A levels. J Parenter Enteral Nutr. 2000;24(5):270–275. doi: 10.1177/0148607100024005270. [DOI] [PubMed] [Google Scholar]
- 52.Fukatsu K, Kudsk KA, Wu Y, Zarzaur BL, Hanna MK, DeWitt RC. TPN decreases IL-4 and IL-10 mRNA expression in lamina propria cells but glutamine supplementation preserves the expression. Shock. 2001;15(4):318–322. doi: 10.1097/00024382-200115040-00012. [DOI] [PubMed] [Google Scholar]
- 53.DeWitt RC, Wu Y, Renegar KB, Kudsk KA. Glutamine-enriched TPN preserves respiratory immunity and improves survival to a Pseudomonas pneumonia. J Surg Res. 1999;84:13–18. doi: 10.1006/jsre.1999.5592. [DOI] [PubMed] [Google Scholar]
- 54.Ikeda S, Zarzaur BL, Johnson CD, Fukatsu K, Kudsk KA. Total parenteral nutrition supplementation with glutamine improves survival after gut ischemia/reperfusion. J Parenter Enteral Nutr. 2002;26(3):169–173. doi: 10.1177/0148607102026003169. [DOI] [PubMed] [Google Scholar]
- 55.Zarzaur BL, Ikeda S, Johnson CD, Le T, Sacks G, Kudsk KA. Mucosal immunity preservation with bombesin or glutamine is not dependent on Muscosal Addressin Cell Adhesion Molecule-1 (MAdCAM-1) expression. J Parenter Enteral Nutr. 2002;26(5):265–270. doi: 10.1177/0148607102026005265. [DOI] [PubMed] [Google Scholar]
- 56.Genton L, Kudsk KA. Interactions between the enteric nervous system and mucosal immunity: role of neuropeptides and nutrition. Amer J Surg. 2003;186(3):253–258. doi: 10.1016/s0002-9610(03)00210-1. [DOI] [PubMed] [Google Scholar]
- 57.Li J, Kudsk KA, Hamidian M, Gocinski BL. Bombesin affects mucosal immunity and gut-associated lymphoid tissue in IV-fed mice. Arch Surg. 1995;130:1164–1170. doi: 10.1001/archsurg.1995.01430110022005. [DOI] [PubMed] [Google Scholar]
- 58.Janu P, Kudsk KA, Li J, Renegar KB. Effect of bombesin on impairment of upper respiratory tract immunity induced by total parenteral nutrition. Arch Surg. 1997;132:89–93. doi: 10.1001/archsurg.1997.01430250091019. [DOI] [PubMed] [Google Scholar]
- 59.DeWitt RC, Wu Y, Renegar KB, King BK, Li J, Kudsk KA. Bombesin recovers gut-associated lymphoid tissue (GALT) and preserves immunity to bacterial pneumonia in TPN-fed mice. Ann Surg. 2000;231(1):1–8. doi: 10.1097/00000658-200001000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zarzaur BL, Wu Y, Fukatsu K, Johnson CD, Kudsk KA. The neuropeptide bombesin improves IgA-mediated mucosal immunity with preservation of gut interleukin-4 in total parenteral nutrition-fed mice. Surgery. 2002;131(1):59–65. doi: 10.1067/msy.2002.118319. [DOI] [PubMed] [Google Scholar]
- 61.Kudsk KA, Hermsen JL, Genton L, Faucher L, Gomez E. Injury stimulates an innate respiratory IgA immune response in humans. J Trauma. doi: 10.1097/TA.0b013e3181627586. in press. [DOI] [PubMed] [Google Scholar]
- 62.Hermsen JL, Sano Y, Gomez FE, Maeshima Y, Kang K, Kudsk KA. Parenteral Nutrition Inhibits a Tumor Necrosis Factor-a Mediated IgA Response to Injury. Surgical Infections. doi: 10.1089/sur.2007.029. in press. [DOI] [PubMed] [Google Scholar]
- 63.Sano Y, Hermsen JL, Kang K, Gomez FE, Lan J, Maeshima Y, Kudsk KA. Parenteral Nutrition-induced Impairment of Airway Immunoglobulin A Response after Injury. American Society for Parenteral and Enteral Clinical Nutrition Week; Chicago, IL. February 10–13,2008. [Google Scholar]