Abstract
During embryonic development in chick, axons pause in a plexus region for approximately one day prior to invading the limb. We have previously shown that this “waiting period” is governed by maturational changes in the limb. Here we provide a detailed description of the spatiotemporal pattern of Raldh2 expression in lumboscaral motoneurons and in the limb, and show that retinoid signaling in the limb contributes significantly to terminating the waiting period. Raldh2, indicative of retinoid signaling, first appears in hindlimb mesenchyme near the end of the waiting period. Transcripts are more abundant in connective tissue associated with predominantly fast muscles than predominantly slow muscles, but are not expressed in muscle cells themselves. The tips of ingrowing axons are always found in association with domains of Raldh2, but development of Raldh2 expression is not regulated by the axons. Instead, retinoid signaling appears to regulate axon entry into the limb. Supplying exogenous retinoic acid to proximal limb during the waiting period caused both motor and sensory axons to invade the limb prematurely and altered the normal stereotyped pattern of axon ingrowth without obvious effects on limb morphogenesis or motoneuron specification. Conversely, locally decreasing retinoid synthesis reduced axon growth into the limb. Retinoic acid significantly enhanced motor axon growth in vitro, suggesting that retinoic acid may directly promote axon growth into the limb in vivo. In addition, retinoid signaling may indirectly affect the waiting period by regulating the maturation of other gate keeping or guidance molecules in the limb. Together these findings reveal a novel function of retinoid signaling in governing the timing and patterning of axon growth into the limb.
Introduction
During ontogeny, axons grow to their correct targets with remarkable precision. The timing and trajectory of axon growth is directed by locally distributed guidance cues in the environment, which are detected by receptors on growth cones (reviewed in Dickson, 2002). The expression of guidance cues and receptors must be coordinated so that cues are present as axons traverse a region, and axons have the requisite receptors when they encounter important cues. In some developing systems axons pause at precise points in their outgrowth, as if they are waiting for maturation of either the receptors or the environment. In the chick, for example, motor and sensory axons pause in a plexus region for nearly 24 hours before invading the limb (Hollyday, 1983; Tosney and Landmesser, 1985; Wang and Scott, 2000; but see Dieu and Newgreen, 2007), and sensory afferents in chick pause in the dorsal root entry zone for about 2 days prior to invading the gray matter of spinal cord (Davis et al., 1989; Sharma et al., 1994). Similarly, thalamic axons in mammals pause in the subplate before invading the visual cortex (Shatz et al., 1990).
The cellular and molecular bases of waiting periods are likely to be complex. We have shown that the waiting period in the hindlimb is governed by maturational changes in the limb rather than in the axons, since axons extend prematurely into transplanted older donor limbs (Wang and Scott, 2000). Some progress has been made in understanding the molecules that regulate axon entry into the limb. Perturbation of signaling through either ephrinA-EphA4 (Sahin et al., 2005) or semaphorin3A-neuropilin-1 (sema3A-Npn-1) (Huber et al., 2005) pathways allows premature entry of axons into hindlimb, indicating that inhibitory or repulsive interactions may normally contribute to the waiting period. It is also likely, however, that permissive or attractive cues appear in proximal limb near the end of the waiting period, promoting axon growth into the limb at the appropriate time. The factors that regulate expression of these “gate keeping molecules,” regardless of whether they are inhibitory or attractive, are unknown.
One mechanism that could potentially regulate gate keeping molecules is retinoid signaling. Retinoids, derivatives of vitamin A, are important signaling molecules with broadranging effects on patterning and differentiation during embryonic development (Lee et al., 2004; McCaffery et al., 2003; Ross et al., 2000). For example, early in development retinoid signaling from lateral plate mesoderm is essential to initiate limb development (Mic et al., 2004; Niederreither et al., 2002; but see Stratford et al., 1999) and pattern the anterior-posterior limb axis via establishment of the zone of polarizing activity (ZPA) and induction of Sonic hedgehog (Shh) expression (Helms et al., 1996; Lu et al., 1997; Niederreither et al., 2002; but see Mic et al., 2004). Retinaldehyde dehydrogenases (RALDHs) are major retinoic acid (RA)-synthesizing enzymes, and their expression domains are associated with regions of RA synthesis and signaling (Berggren et al., 2001; McCaffery and Drager, 1994), although an RALDH-independent pathway of RA synthesis has recently been reported (Chambers et al., 2007). In mammals, all-trans-RA mediates most retinoid signaling. In the chick, however, 3,4-didehydroretinoic acid (dd-RA) is more abundant and is likely to be more important (Thaller and Eichele, 1990). Here we refer to both dd-RA and at-RA simply as RA, since they appear to be equally effective in chick (Repa et al., 1996; Thaller and Eichele, 1990).
Interestingly, in the wing retinaldehyde dehydrogenase type 2 (RALDH2), which is present in lateral plate mesoderm prior to limb outgrowth, decreases to undetectable levels as the limb begins to grow out, but reappears in proximal limb regions around stage (St.) 23 (E3.5–4) (Berggren et al., 2001; Berggren et al., 1999), about the time that brachial axons leave the plexus region at the end of the waiting period (Hollyday, 1983). Thus, the spatiotemporal pattern of RALDH2 in the wing suggests that retinoids could play a role in governing the waiting period.
RA-signaling occurs by RA diffusing through cell membranes and binding to nuclear receptors, which function as ligand-activated transcription factors to activate or repress expression of myriad different genes (reviewed in Balmer and Blomhoff, 2002; Bastien and Rochette-Egly, 2004; Ross et al., 2000; Williams et al., 2004). Thus, RA could trigger axon growth into the limb indirectly by influencing expression of gate keeping molecules in the limb. Alternatively or additionally, RA itself might directly promote axon growth into the limb. RA has been shown to enhance or direct outgrowth of a variety of central and peripheral neurons in vitro and in vivo (reviewed in Clagett-Dame et al., 2006; Dmetrichuk et al., 2006), although nearly all these studies involved neurite regeneration rather than de novo outgrowth.
Here we have investigated the possibility that retinoid signaling plays a role in regulating axon growth during the waiting period in chick hindlimb. We show that expression of Raldh2, indicating the potential for retinoid signaling, appears in the right time and place to govern axon entry into the hindlimb. Perturbing the normal spatiotemporal pattern of retinoid signaling in the limb altered both the timing and pattern of axon ingrowth, without obvious effects on the early differentiation of motoneurons and limb morphogenesis. In vitro, RA promoted the outgrowth of motor, but not sensory axons, suggesting that retinoic acid could directly enhance axon entry into the limb in vivo. To our knowledge, this is the first direct demonstration that RA can affect motor axon growth. These findings expand the impressive list of developmental events regulated by retinoid signaling, and identify this signaling pathway as a major mechanism governing the waiting period for axon entry into the limb.
Materials and methods
General
Fertile White leghorn chick eggs from a local supplier were incubated in a humidified, force-draft incubator at 38°C. Embryos were staged according to Hamburger and Hamilton (1951) at the time of surgery and at sacrifice.
Embryo Manipulation
Bead implantation in the hindlimb
To initiate retinoid signaling in the limb precociously, we implanted beads soaked in all-trans-retinoic acid [0.05-5 mg/ml dimethysulfoxide (DMSO), Sigma] in the limb, preparing the beads (AG1X-2 beads, 75-140 µm in diameter, formate form, Bio-Rad) as previously described (Eichele et al., 1984; Shen et al., 1997). Three RA-treated beads were inserted into the anterior hindlimb bud slightly distal to the crural plexus region of St. 21–22.5 [embryonic day (E) 3.5–4] embryos (Fig. 5A).
Figure 5.
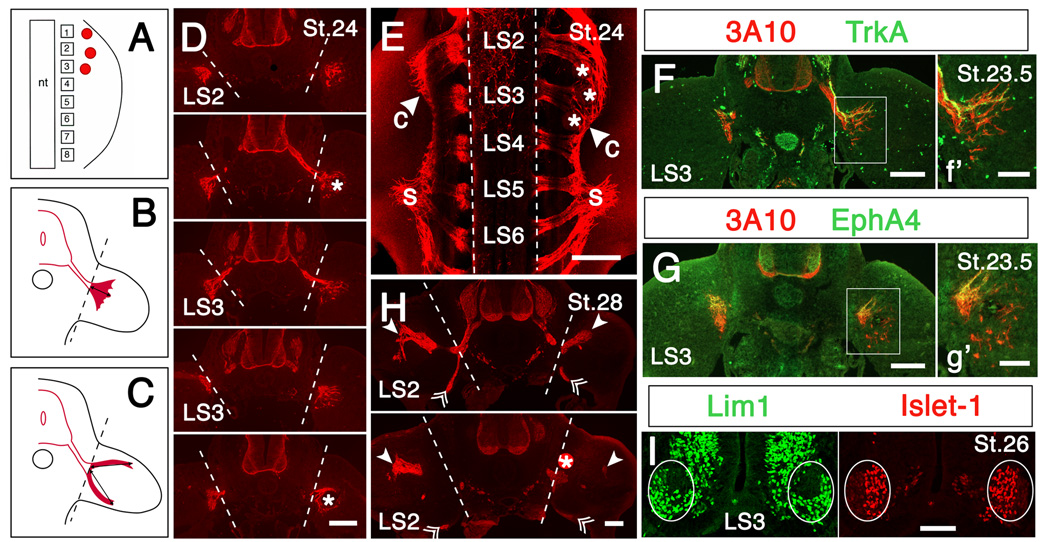
Effects of perturbing retinoid signaling on axon growth into limb. (A) Cartoon showing the placement of retinoic acid (RA)-, citral-, disulfiram- or DMSO-soaked beads (red dots) in anterior limb bud. Squares with numbers represent lumbosacral somites; nt: neural tube. Treated limbs are shown on the right in all panels. (B, C) Cartoons showing quantification of axon growth into the limb in RA-treated (B) and citral- or disulfiram-treated (C) limbs, as described in Materials and Methods. Dashed lines indicate the base of the limb; lines tipped with arrowheads indicate the length of axon incursion into the limb that was measured. (D-G) Early exposure to exogenous RA promotes premature growth of axons into the limb and broadens the crural plexus; axons in RA-treated limbs extend farther into the limb and enter the limb from more caudal segments than in the contralateral control limbs. (D) Sections through ventral spinal cord and proximal hindlimb from segment LS2 to caudal LS3 of an embryo implanted with 3 RA-beads (0.5 mg/ml) at St. 21. Note that in this embryo axons extended significantly farther into the RA-treated limb than in the contralateral control limb (treated/control = 1.58). Dashed lines indicate the base of the limb. (E) Whole-mount view of spinal cord and proximal hindlimbs of an embryo implanted with 3 RA-beads (5 mg/ml) at St. 22; c: crural plexus; s: sciatic plexus. Note that axons in the crural plexus have extended farther from the spinal cord on the RA-treated side, and enter the limb from more caudal segments than on the control side (arrowhead). Dashed lines indicate the edge of the spinal cord. (F, G) Sections through ventral spinal cord and proximal hindlimbs at segment LS3 in an embryo implanted with RA-beads (5 mg/ml) at St. 21.5. (f’, g’) Higher power views of boxed regions of F and G, respectively. (F) TrkA staining shows that both sensory axons, as well as motor axons, grow precociously into RA-treated limbs. (G) EphA4+ axons are confined to the dorsal plexus in RA-treated limbs, as in controls. (H) Two sections through segment LS2 in an embryo implanted with 2 citral-soaked beads (0.5 g/ml) at St. 22.5. Solid arrowheads and double arrowheads indicate the farthest extent of axon growth in the dorsal and ventral nerve branch, respectively. Note that ingrowth of dorsal axons is reduced in the citral-treated limb (treated/control = 0.74). Dashed lines indicate the base of the limb. All axons are labeled with antibody 3A10 (red) in panels D - H. Asterisks indicate RA- or citral-soaked beads. (I) Section through the ventral spinal cord at segment LS3 of an embryo implanted with RA-beads (5 mg/ml) at St. 21, stained with anti-Lim1 and anti-Islet-1 to identify the lateral and medial portions of the lateral motor column (enclosed in an oval), respectively. Scale bars: (D-H): 200 µm; (d’, e’, I): 100 µm.
To inhibit synthesis of RA, beads (SM2 beads, 200 µm in diameter, Bio-Rad or AG1X-2 beads) were soaked in either citral (3,7-dimethyl-2,6-octadienal, 0.5-0.88 g/ml DMSO, Aldrich) (Song et al., 2004) or disulfiram (tetraethylthiuram disulfide, 25 mg/ml DMSO, Sigma) (Stratford et al., 1996; Vallari and Pietruszko, 1982), and a single large or 2–3 small beads were inserted into anterior limb buds of St. 22–23.4 (E4-4.5) embryos.
Control embryos were implanted with DMSO-soaked beads. After implantation of beads, the embryos were incubated until the desired stages, when they were killed, cryosectioned and stained to visualize axons, as described below.
Motoneuron deletion
To determine whether motor axons affect Raldh2 expression in the hindlimb (cf. Berggren et al., 2001), we unilaterally removed the lumbosacral neural tube from St.16-17 (E2.5) embryos, leaving the contralateral half intact as a control.
In situ hybridization and staining
In situ hybridization for Raldh2 and Shh was carried out on cryosections, as previously described (Wang and Scott, 2004) using digoxigenin-labeled probes generated from plasmids kindly provided by Dr. Malcolm Maden (King’s College, London) and Dr. Gary Schoenwolf (University of Utah), respectively. For whole-mount in situs, embryos were treated with methanol and 6% H2O2 prior to hybridization, and the time for each step was lengthened.
To analyze axon growth and to investigate the spatiotemporal relationship of RA-signaling, axons and muscles, sections were stained with various neuron or muscle-specific antibodies, often followed by in situ hybridization for Raldh2. The following primary antibodies were used: 3A10 [1: 50, Developmental Studies Hybridoma Bank (DSHB)]; anti-axonin-1 (23.4-5, 1:10, DSHB); anti-IsIet-1 (39.4D5, 1: 10, DSHB); anti-Lim1 (1: 20000, kindly provided by Dr. T. M. Jessell, Columbia University); PAX7 (1: 10, DSHB); 13F4 (1: 10, DSHB); NA8 (1:10, DSHB); anti-EphA4 (1: 100, Invitrogen); and anti-TrkA (1: 7000; kindly provided by Dr. Frances Lefcort, Montana State University). Antibodies were visualized with species-specific Alexa-488 and Alexa-568 conjugated secondary antibodies (1: 400, Invitrogen).
To view the morphology of the pelvic girdle, embryos were stained with Alcian blue as previous described (Wang and Scott, 1999).
Co-culture of neuronal explants and RA beads
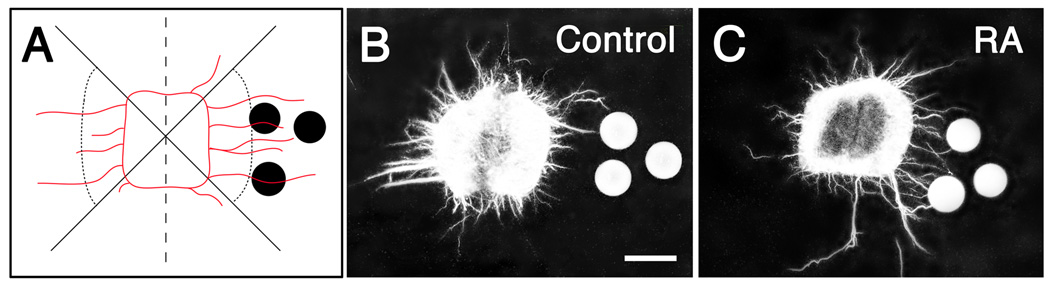
Explants of motoneurons were dissected as “open-book” preparations of ventral spinal cord from lumbosacral regions of St. 23–25 embryos, and placed in coverglass wells in 35mm Petri dishes, as described by Kuhn (2003). The explant was covered with a 60 µl drop of collagen solution [2 parts 5X DMEM (Gibco), 1 part 0.2 M Hepes (Research Organics, Inc.) and 7 parts collagen (3 mg/ml HCL, pH 3.0, Roche)], and 3 RA-treated beads or control beads were placed 150–200 µm from one lateral edge of the explant (Fig. 6). Collagen was allowed to gel for 20 min at 37°C; 500µl DMEM + N2 (Gibco) was then added to the well. Explants were cultured for 24 to 48 hr, fixed in 4% paraformaldehyde and stained with 3A10. In initial studies we verified that the majority of axons that grew out of ventral spinal cord explants were indeed motoneurons either by retrogradely labeling motoneurons with DiI prior to dissecting the explant or by labeling explants with antibody SC1, which selectively marks DRGs and motoneurons at early stages (Tanaka et al., 1991).
Figure 6.
Retinoic acid promotes growth of motor axons in vitro. (A) Cartoon showing the placement of beads and quantification of axon growth. To assess axon growth, explants were divided into quadrants (solid lines) and the numbers of axons or axon bundles that extended ≥150 µm (dotted line) from the explant edge were counted in the lateral quadrants. (B, C) Representative explants from a single experiment showing that more axons extend in quadrants with RA-beads than without beads, whereas control beads have no effect on axon growth. Dashed line: ventral midline. Scale bar: 200 µm.
Lumbosacral dorsal root ganglia (DRGs) were dissected from St. 23–25 embryos, and co-cultured with beads as described above, except that DRGs were cultured in F12 (Gibco) + N2. 10 ng/ml each of NGF (Gibco), BDNF (Regeneron) and NT3 (Regeneron) were added to DRG cultures derived from St. 25 embryos.
Quantification of axon growth and Raldh2 expression
Axon growth in treated limbs
To quantify the effects of exogenous RA on axon growth, cryosections of spinal cord and limb were stained with 3A10 to visualize axons, and the length of axon growth into the limb in the middle of the “fan” of axons was measured from captured images of every 4th or 6th section throughout the crural plexus region, as illustrated in Fig. 5B. In addition, the total area of axons in the limb in each of these sections was measured using the threshold function of ImageJ software (NIH), and the areas for all sections of individual embryos summed for control and treated limbs. The same number of sections was analyzed in the treated and contralateral control limb of individual embryos. For embryos treated with citral or disulfiram, the distance from the middle of plexus to the tip of the dorsal and ventral nerve branches was measured (Fig. 5C). Nerve lengths and arborization areas of treated and control limbs were compared for significance using a paired t-test.
Raldh2 and axon areas in denervated limbs
Similar methods were used to measure the area of axons and Raldh2 expression in denervated and contralateral control limbs of St. 25–27.5 embryos. Raldh2 expression was analyzed only in embryos in which the total axon area in the denervated limb was less than 12% that of the contralateral control limb (Table 2).
Table 2.
Relative levels of Raldh2 expression in denervated hindlimbs
| Embryo | Stage | Axons % of control | Raldh2 % of control |
|---|---|---|---|
| RA171 | 25 | 7.7 | 102.2 |
| RA136 | 26 | 7.9 | 148.9 |
| RA172 | 26 | 11.2 | 104.7 |
| RA168 | 27.5 | 2.5 | 138.0 |
| Average | 7.3 ± 3.6 | 123.4 ± 23.5 | |
The lumbosacral neural tube was removed unilaterally from St. 16–17 (E2.5) embryos. Subsequently, axons and Raldh2 were measured in images of every 4th or 6th section through denervated and control limbs, as described in Materials and Methods. Areas were summed, and values in each denervated limb were expressed as a percentage of the corresponding control values. The same number of sections was analyzed in individual embryos. Denervation did not reduce Raldh2 expression. Averages ± s.d.
Raldh2 in fast and slow muscle
To determine relative levels of expression of Raldh2 in different muscles, cross-sections of the mid-thigh of St. 30–35 embryos were stained with antibody 13F4 to label all muscle cells, followed by in situ hybridization for Raldh2. The cross-sectional areas of individual fast and slow muscles and Raldh2 expression in each muscle were measured with ImageJ software, as above. Raldh2 in individual muscles was then expressed as a percentage of the area of that muscle (Table 1). Muscles were scored as fast or slow based on earlier reports (Crow and Stockdale, 1986; Rafuse et al., 1996) and our analysis of sections stained with antibody NA8 to identify slow muscles (data not shown).
Table 1.
Relative levels of Raldh2 expression in hindlimb muscles
| Slow Muscles | Raldh2 | Fast Muscles | Raldh2 |
|---|---|---|---|
| Sartorius | 3.3 ± 2.3 | Lateral adductor | 29.8 ± 15.8 |
| Medial adductor | 4.8 ± 5.2 | Ischioflexorius | 30.0 ± 27.0 |
| Femorotibialis internus | 5.5 ± 7.7 | Caudilioflexorius | 34.7 ± 17.8 |
| Anterior iliotibialis | 9.7 ± 5.8 | Ambiens | 40.5 ± 24.7 |
| Iliofibularis | 25.9 ± 17.8 | Femorotibialis externus & medialis | 40.8 ± 22.3 |
| Posterior iliotibialis | 48.9 ± 19.5 | ||
| Average slow | 9.9 ± 12.3 | Average fast | 37.6 ± 21.5 |
The cross-sectional area and Raldh2 in each muscle was measured from a cryosection through the mid-thigh in 9 embryos (St. 30 – 35). Raldh2 was expressed as a percentage of the muscle area, as described in Material and Methods. Values shown are the mean ± s.d. Muscles were categorized as predominantly fast or slow based on published studies (Crow and Stockdale, 1986; Rafuse et al., 1996) and analysis of sections stained with NA8, to identify slow muscle fibers (data not shown). On average Raldh2 was significantly less abundant in slow muscles (p < 001, t-test).
RA effects in vitro
To assess the effects of RA on motor axon growth in vitro, cultures were stained with 3A10 to visualize axons and confocal images were collected at 10 µm intervals and saved as a single image. A line was drawn through the ventral midline of the explant, and the explant divided in quadrants, as illustrated in Fig. 6A. The numbers of axons or axon bundles that extended at least 150 µm from the explant edge were counted in the two lateral quadrants. Axon growth from DRGs was assessed in the same manner (see also Honig and Zou, 1995).
Results
Spatiotemporal pattern of Raldh2 expression in hindlimb
The spatiotemporal pattern of RALDH2 expression in the wing (Berggren et al., 2001; Berggren et al., 1999) suggested that retinoids could potentially play a role in governing the waiting period. Here we tested that possibility directly in the hindlimb, where the waiting period has been most extensively studied. Since ontogeny of retinoid signaling has not been described in detail in either the hindlimb or lumbosacral neurons in the chick, we first characterized the pattern of Raldh2 expression in the thigh and in lumbosacral (LS) motor and sensory neurons from St. 18 (E3), shortly after the first motor axons reach the plexus region (Tosney and Landmesser, 1985) (shown schematically in Fig. 5A), through St. 30 (E7), when lumbosacral motoneuron cell death (eg. Calderó et al., 1998) and muscle patterning in the thigh (Schroeter and Tosney, 1991) are nearly complete.
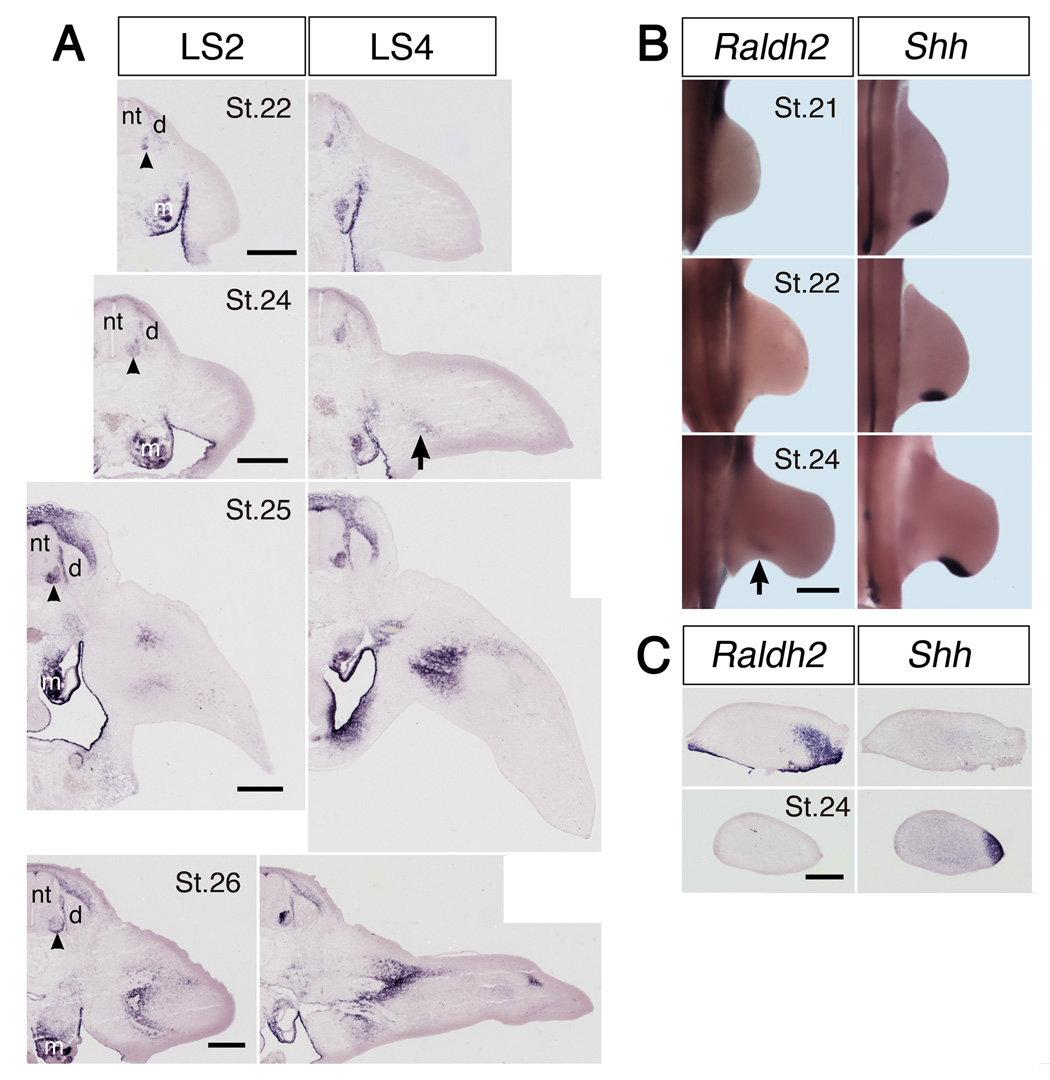
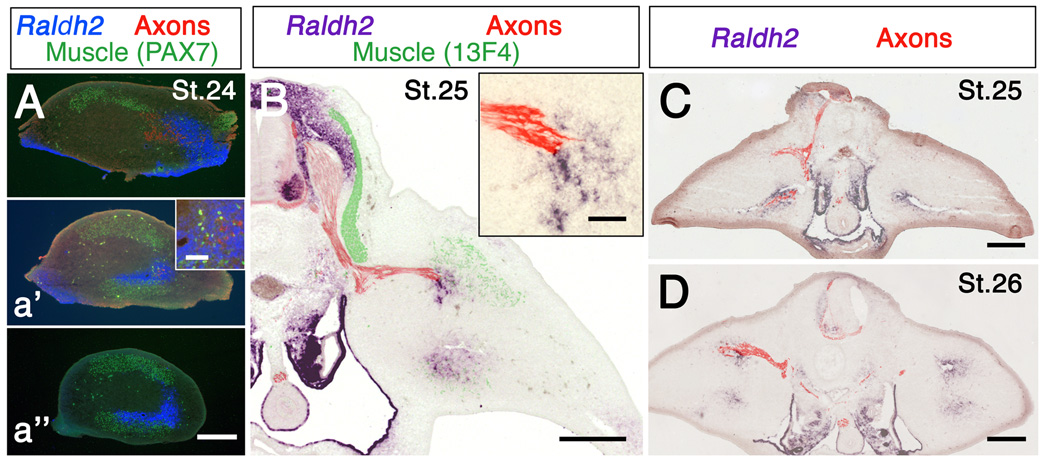
Raldh2 transcripts were absent from hindlimb buds at St.18, the earliest we examined (data not shown), but were abundant in adjacent somites. Transcripts first appeared in the limb in early St. 24 (E4), with expression initially restricted to proximal posterior thigh (Fig. 1, Fig. 2A, and Fig. 4A). Raldh2 transcripts appeared slightly later in anterior thigh, around St. 24.5–25 (E4-4.5). At later stages, expression extended distally, but remained concentrated primarily in antero-dorsal and postero-ventral domains (Fig. 1).
Figure 1.
Spatiotemporal pattern of Raldh2 and Shh expression in chick hindlimb. (A) In situ hybridization for Raldh2 in sections of hindlimb at the level of lumbosacral (LS) dorsal root ganglia LS2 and LS4 at the embryonic stages indicated. (B) Whole mount in situ hybridization for Raldh2 and Shh in hindlimbs at the embryonic stages indicated. (C) Raldh2 and Shh expression in adjacent cross-sections through proximal (top) and more distal (bottom) hindlimb of a single embryo. Anterior is to the left and dorsal to the top. Note that Raldh2 transcripts first appear in proximal hindlimb at around stage (St.) 24 (arrow), and are initially restricted to a ventral domain in posterior limb, distinct from that of Shh. Raldh2 is also expressed in motoneurons (arrowheads) and mesonephros (m), but not in dorsal root ganglia (d). nt: neural tube. Scale bars: (A) 300 µm; (B) 500 µm; (C) 400 µm.
Figure 2.
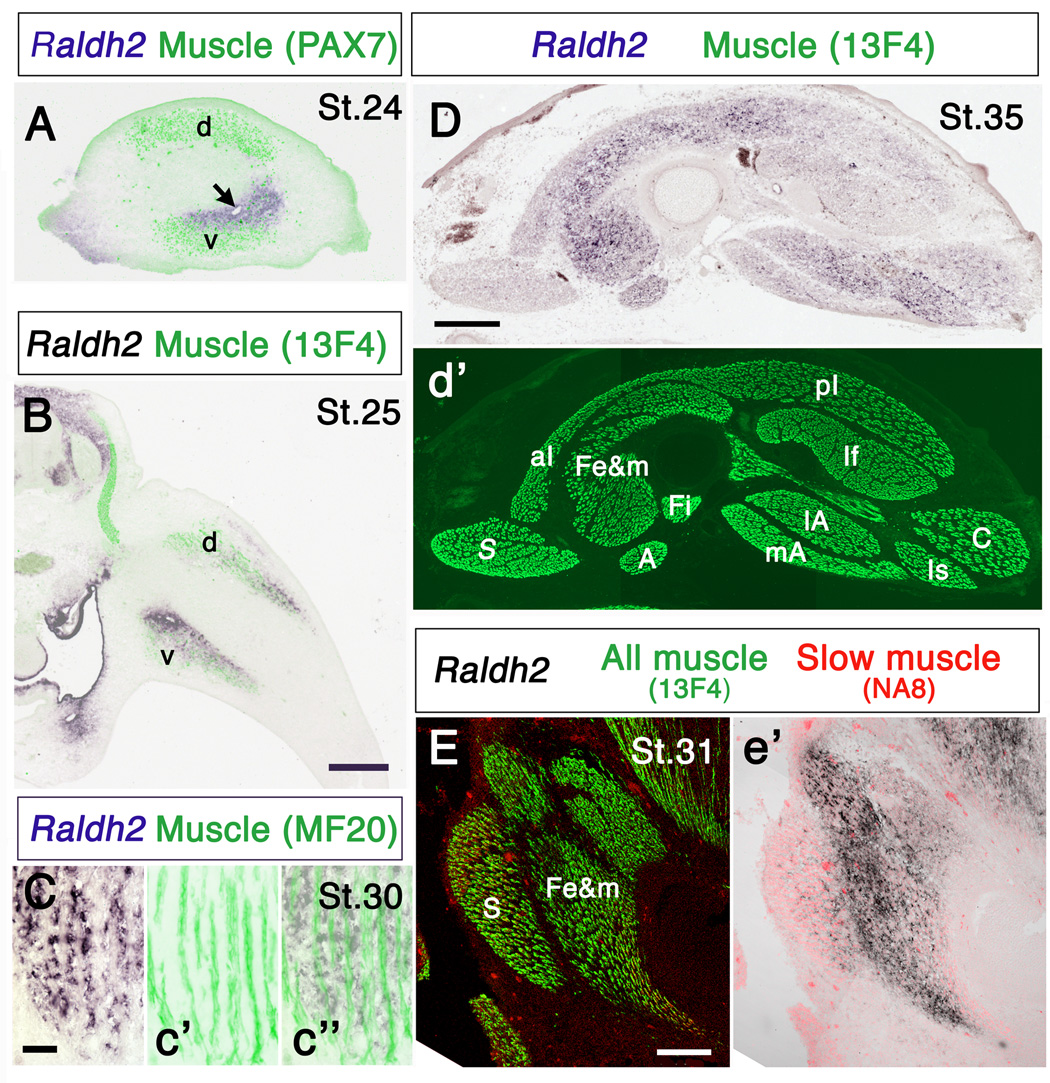
Comparison of the spatiotemporal patterns of Raldh2 expression and developing muscles in chick hindlimb. (A) Cross-section through proximal limb showing Raldh2 expression and the dorsal (d) and ventral (v) muscle masses. Note that at St. 24 Raldh2 partially overlaps the ventral muscle mass, but is not expressed dorsally. Arrow indicates a blood vessel. (B) Section through the limb at the level of dorsal root ganglion LS3, showing Raldh2 expression and the dorsal (d) and ventral (v) muscle masses. (C) Raldh2 expression in a longitudinal section of a thigh muscle of a St. 30 embryo Note that Raldh2 is restricted to connective tissue associated with muscle, but is not expressed in the muscle cells themselves. Left panel: Raldh2; c’: muscle fibers in the same section stained with antibody MF20; c”: merged images. (D) Raldh2 expression in a cross-section through the thigh of a St.35 embryo; (d’) the same section stained with antibody 13F4 to visualize all muscles. (E) Raldh2 expression and fast and slow muscles in a longitudinal section through the anterior thigh; (e’) only slow muscles and Raldh2 are shown. Note that Raldh2 is expressed predominantly in connective tissue associated with fast muscles, and is nearly absent from slow muscles. Relative levels of Raldh2 expression in fast and slow muscles are summarized in Table 1. Anterior is to the left and dorsal to the top in A, D and E. A: ambiens; aI: anterior iliotibialis; C: caudilioflexorius; Fe&m: femorotibialis externus and medialis; Fi: femorotibialis internus; If: iliofibularis; Is: ischioflexorius; lA: lateral adductor; mA: medial adductor; pI: posterior iliotibialis; S: sartorius. Scale bars: (A, B) 300 µm; (C) 50 µm; (D) 500 µm; (E) 200 µm.
Figure 4.
Relationship between axons and Raldh2 expression in hindlimb. (A-a’’) Raldh2, muscle masses and axons in cross-sections through progressively more distal regions of hindlimb. At St. 24 axons from the sciatic plexus have begun to grow into posterior limb, but have not yet left the more anterior crural plexus. Note that axons are associated with Raldh2 in A and a’, and that Raldh2 extends distal to the axons (a’’). Inset in a’ shows axons and Raldh2 at higher magnification. (B) Raldh2, muscles and axons in a section though spinal cord and limb at the level of dorsal root gangion LS2. Inset shows Raldh2 and axons at higher magnification. (C, D) Raldh2 and axons in embryos in which the right half of the neural tube was removed at St. 17 and St. 16, respectively. Note that axons are missing from denervated limbs, but Raldh2 expression is unchanged. Scale bars: 300 µm except insets. Insets: 50 µm.
Because retinoid signaling determines the distribution of Sonic hedgehog (Shh) early in embryogenesis (Helms et al., 1996; Lu et al., 1997; Niederreither et al., 1999; Stratford et al., 1996), and Shh transcripts are first expressed in the zone of polarizing activity (ZPA) in posterior limb mesenchyme in a domain resembling that of Raldh2 (Riddle et al., 1993), we directly compared the patterns of Raldh2 and Shh expression throughout early limb development. Interestingly, expression domains of these two signaling molecules never overlapped. Shh transcripts first appeared in hindlimb bud around St. 20 (data not shown), and were consistently expressed in a more posterior and distal domain than Raldh2 (Fig.1B, C). Thus, although retinoid signaling from lateral plate mesoderm is required for Shh expression in the limb, Raldh2 in the limb bud itself has a unique spatiotemporal pattern of expression, distinct from that of Shh.
Raldh2 expression also did not uniquely coincide with other obvious tissue components of the developing limb. For example, Raldh2 was expressed near developing blood vessels (Fig. 2A, arrow) (cf. Berggren et al., 2001), but this relationship was not unique, since Raldh2 was also expressed in regions of the limb without large blood vessels. Raldh2 was absent from the cartilaginous precursors of the limb skeleton, and only partially overlapped developing muscles (Fig. 2A, B, E). Myogenic cells migrate into limb bud from the dermamyotome beginning at about St. 16 (Jacob et al., 1979), and aggregate in a dorsal and a ventral muscle mass (reviewed in Francis-West et al., 2003). The position of these two muscle masses is indirectly regulated by retinoic acid, at least in mouse forelimb (Mic and Duester, 2003). However, at early stages of muscle development in the chick the two domains of Raldh2 expression and the two muscle masses are not entirely coincident. In general, Raldh2 is expressed closer to the condensing cartilage and somewhat more distally than myogenic cells (Fig. 2A, B). At later stages, when distinct myotubes had differentiated, Raldh2 transcripts are expressed in the mesenchymal cells surrounding myotubes, but not in the myotubes themselves (Fig. 2C) (cf. Berggren et al., 2001).
Interestingly, Raldh2 initially appeared in regions of the ventral muscle mass destined to become fast muscle, but was absent from dorsal slow muscle regions [compare Fig. 4b of Crow and Stockdale (1986) with Fig. 2A and Fig. 4A]. At later stages (St. 30–35; E7-9), as muscle masses cleaved and individual thigh muscles became distinct, Raldh2 was associated predominantly with fast muscles, and was expressed at significantly lower levels (p < 0.001, t-test) within slow muscles or slow fiber regions of mixed muscles (Fig. 2D, E; Table 1). Whether retinoid signaling participates in patterning the distribution of fast and slow muscle fibers within the limb remains to be determined, but is beyond the scope of the present study.
Raldh2 and limb innervation
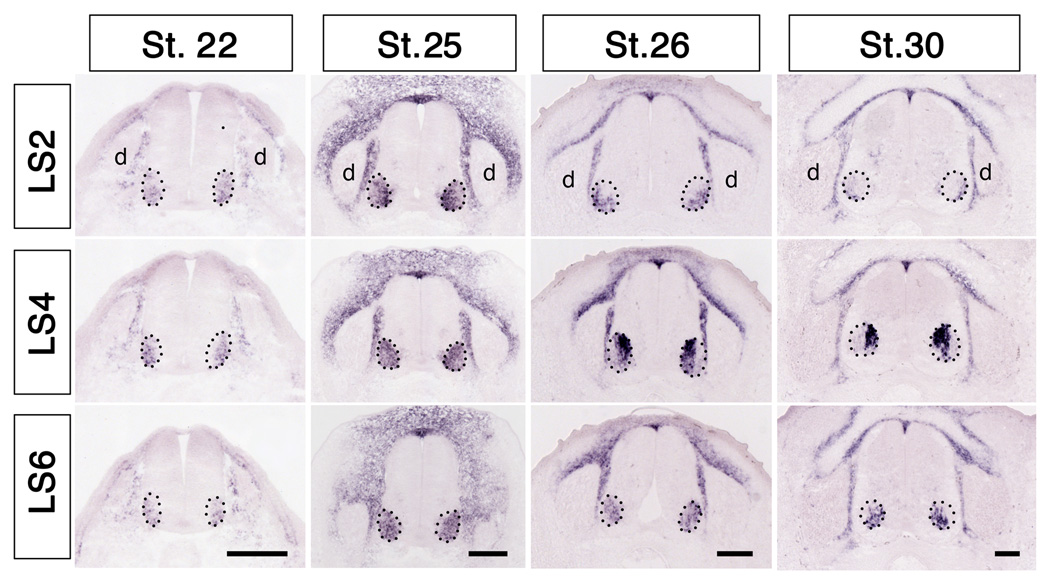
Chick hindlimb is innervated by motor and sensory neurons in lumbosacral (LS) segments LS1-8, with axons from segments LS1-3 comprising the crural plexus and axons from segments LS4-8 comprising the sciatic plexus (Fig. 5E). Raldh2 was expressed in lumbosacral (LS) motoneurons, with the distribution changing dramatically along both the rostrocaudal and mediolateral axes in concert with the segregation of the medial and lateral subdivisions of the lateral motor column (mLMC and lLMC, respectively). Transcripts first appeared in ventrolateral cord beginning around St. 20 (data not shown), and by St. 22 were distributed uniformly throughout the entire developing lateral motor column from segments LS1-LS7. Beginning around St. 24.5-25 Raldh2 expression increased markedly in mLMC in rostral segments (LS2 and LS4, Fig. 3), but remained broadly distributed in more caudal segments (LS6, Fig. 3). By St. 26, transcripts disappeared from lLMC in the most rostral segments (LS2, Fig. 3), but became restricted exclusively to mLMC at LS4, and remained broadly distributed more caudally. This pattern did not change noticeably at later stages. This spatiotemporal pattern of expression of Raldh2 matured earlier and is more complex than previously described (Berggren et al., 1999; Sockanathan and Jessell, 1998). Whereas the restriction of Raldh2 to mLMC neurons in mid-lumbar segments may arise from later-born lLMC neurons (Raldh2−) migrating through earlier-born mLMC neurons (Raldh2+) (Sockanathan and Jessell, 1998), this scenario cannot account for the distribution of Raldh2 at more rostral and caudal levels. Raldh2 was never expressed in lumbar sensory neurons, although transcripts were abundant in cells of the surrounding somite (Fig. 3), as previously reported (Berggren et al., 1999; Blentic et al., 2003).
Figure 3.
Spatiotemporal pattern of Raldh2 expression in lumbosacral (LS) spinal cord and dorsal root ganglia. Note that Raldh2 transcripts first appear in motoneurons around St. 22, where they are expressed throughout the entire lateral motor column (enclosed by dotted lines). Transcripts eventually become restricted to the medial lateral motor column in more caudal segments, and are not expressed in DRGs (d) at any stage or spinal level. Scale bars: 150 µm.
Interestingly, the initial expression of Raldh2 in anterior and posterior thigh was highly correlated with the stage and position at which axons began to enter the limb from the crural and sciatic plexus, respectively. Axons leave the sciatic plexus region and enter posterior limb at about St. 24 (Fig. 4A), slightly before axons exit the more anterior crural plexus at St. 24.5–25 (Tosney and Landmesser, 1985; Wang and Scott, 2000). In both limb regions axon growth cones were always found in domains of Raldh2 expression, with Raldh2 transcripts extending somewhat distal to the axon tips (Fig. 4A, B).
Previous studies suggested that motoneurons regulate RALDH2 expression in wing, leading to the observed association of axons with RALDH2 immunoreactivity (Berggren et al., 2001); denervation initially reduced RALDH2 immunoreactivity in the wing, which subsequently returned to normal levels within several stages (Berggren et al., 2001). In contrast, we detected no obvious change in the timing, amount, or distribution of Raldh2 transcripts in denervated hindlimbs (n = 4) (Fig. 4C, D; Table 2). Theoretically it is possible that the small residual innervation (< 12% of control) that remained in denervated limbs was sufficient to induce normal levels of Raldh2. We find this unlikely, however, since there was no correlation between the amount of axons remaining and Raldh2. Instead, it appears that the onset and patterning of Raldh2 is independent of innervation in the hindlimb. These findings are consistent with previous reports of Raldh2 mutant mice that suggest that the regulation and function of retinoid signaling may be different in fore- and hindlimbs (Mic and Duester, 2003; Niederreither et al., 2002). Thus, the close spatiotemporal expression of Raldh2 and axon growth into the hindlimb suggests another possibility, that RA promotes the entry of axons into the limb, a possibility we tested directly here.
Retinoic acid can promote axon growth into the limb
The fact that Raldh2 is expressed in limb mesenchyme, the source of guidance cues for axon growth (Lance-Jones and Dias, 1991), together with the close association of outgrowing axons and Raldh2, suggested that retinoids could be involved in governing the timing and/or distribution of axon growth into the limb. To test this possibility, we implanted beads soaked in all-trans-RA (0.05 – 5 mg/ml) into proximal limb bud opposite the crural plexus at St. 21–22.5 (Fig. 5A), and examined the resulting distribution of axons in the limb at St. 23.5–25, using the contralateral untreated limb as a control. Additional control embryos were implanted with DMSO-soaked beads.
RA treatment did not grossly affect the overall development of the limb, presumably because proximal limb regions where beads were implanted had already been specified by St. 21 (Saunders, 1948), and perturbing RA signaling at this stage has little effect on limb patterning (Helms et al., 1996; Summerbell, 1983). Axons left the crural plexus and entered the limb bud prematurely in embryos treated with RA, growing beyond the plexus region as early as St. 23.5 (E4), when axons in the contralateral limb were still arrested in the crural plexus (Fig. 5D-G). Staining sections of RA-treated embryos with anti-axonin-1 (Halfter et al., 1994) (n = 4) or anti-trkA (Rifkin et al., 2000) (n = 2), which selectively label sensory axons, together with 3A10, which labels all axons, showed that both sensory and motor axons entered the limb precociously in response to exogenous RA (Fig. 5F).
In addition to accelerating the timing of axon growth into the limb, early exposure to RA altered the initial pattern of axon ingrowth. Normally axons project into the limb from each plexus region in 2 distinct nerve trunks, a ventral trunk derived from motoneurons in the mLMC and a dorsal branch dervived from the lLMC (reviewed in Landmesser, 2001), shown schematically in Fig. 5C. A similar pattern was observed when axons grow into the limb prematurely following transplantation of older donor limbs (Wang and Scott, 2000). In contrast, axons invaded the limb in a broad fan following early exposure to RA (Fig. 5D, F-G), similar to the distribution of axons that results from interfering with sema3A-Npn-1 (Huber et al., 2005) or ephrinA-EphA4 signaling (Sahin et al., 2005). Interestingly, the crural plexus was wider in RA-treated limbs than in controls, extending nearly one segment more caudally than normal, with the posteriorly directed ramus between the crural and sciatic plexii being nearly eliminated (Fig. 5E). None of these changes was observed in limbs implanted with control beads (data not shown).
To quantify the effects of early exposure to exogenous RA on axon growth, we measured the length that axons extended into the limb and the overall arborization of axons from the crural plexus in RA-treated and control limbs, as described in Materials and Methods and illustrated schematically in Fig. 5B. Because neither the concentration of RA and nor the age of the embryo appeared to affect the extent of precocious ingrowth, data from all treated embryos were pooled. Axons extended significantly farther in RA-treated limbs than in contralateral control limbs (n = 19; p < 0.001, paired t-test). The total area of arborization of axons from the crural plexus was also significantly greater in RA-treated limbs (p < 0.001, paired t-test). In contrast, there were no differences in any of these measures in limbs implanted with control beads (n = 6) (Table 3).
Table 3.
Early exogenous RA promotes axon growth into the hindlimb
| DMSO/Control | RA/Control | |
|---|---|---|
| (n = 6) | (n = 19) | |
| Length of “fan” | 1.1 ± 03# | 1.4 ± 0.3** |
| Area of “fan” | 1.0 ± 0.1# | 1.2 ± 0.2** |
The longest incursion of axons (length of “fan”) and the total arborization of axons in the crural plexus (area of “fan”) were measured in limbs in St. 23.5–25 embryos implanted with RA-soaked or control (DMSO-soaked) beads at St. 19–22.5, as described in Materials and Methods and Fig. 5B. The same number of sections was analyzed in treated and control limbs of individual embryos; values in each embryo were compared for significance with a paired t-test. Early exposure to exogenous RA significantly promoted axon growth into treated limbs
p > 0.05
p < 0.001.
Thus, early exposure of axons to RA altered both the timing and pattern of axon ingrowth into the limb. The failure of crural axons to form distinct dorsal and ventral nerve trunks when they first enter RA-treated limbs, as well as the broadening of the crural plexus region, suggests that axons did not receive their usual peripheral guidance cues, as if either the peripheral signals or the neurons themselves were altered by early exposure to RA. For example, perhaps the normal differentiation of motoneurons was perturbed, such that motoneurons did not acquire their full identity as mLMC or lLMC neurons and/or became mispositioned in the two subdivisions of the LMC. Alternatively or additionally, interfering with normal RA signaling could also alter the peripheral cues that determine axon trajectories in the limb. To determine the relative contribution of these mechanisms to the altered axon projection patterns in RA-treated embryos, we first examined whether precociously increasing RA in the limb affected the early differentiation of motoneurons. There was no obvious change in the establishment of the mLMC and lLMC, as judged by Islet-1 and Lim1 staining (Tsuchida et al., 1994) (Fig. 5I). Similarly, the pattern of Raldh2 in motoneurons developed normally in RA-treated embryos (data not shown). Importantly, EphA4+ axons, which normally arise from neurons in the lLMC (Eberhart et al., 2000), were observed only in the dorsal half of the axonal “fan” in RA-treated limbs (Fig. 5G), indicating that, unlike embryos in which Sema3A-Npn-1 was perturbed (Huber et al., 2005), the dorsal and ventral axons sorted more or less normally and were not intermixed as they left the crural plexus. It is not surprising that motoneurons appeared to differentiate normally in embryos with RA-treated limbs, since we perturbed RA levels long after RA in the periphery is required for correct patterning of the spinal cord and LMC (Maden, 2006; Novitch et al., 2003). The development of altered axon projection patterns by seemingly normal motoneurons suggests that perturbing the spatiotemporal pattern of RA signaling in the limb either disturbed or overwhelmed normal guidance cues for dorsal and ventral axons, and/or enticed axons to enter the limb before the usual complement of guidance cues was established.
Thus, early exposure to exogenous RA in the limb showed that retinoid signaling can influence the time that axons wait in and exit from the plexus region. To determine whether endogenous RA is normally required for axons to leave the plexus region on schedule, we reduced RA signaling by inhibiting the synthesis of RA in the limb with citral or disulfiram. Implanting citral-soaked beads in anterior limb often caused hemorrhaging and a large blood clot, especially with concentrations of citral higher than 0.7 g/ml. Such embryos were excluded from further study, but indicated that RA may play an essential role in the development of vasculature in the limb (cf. Berggren et al, 2001). We also excluded embryos in which the treated limb was obviously smaller than the contralateral control (cf. Tanaka et al., 1996), using as a criterion that we only analyzed embryos in which the length of the ventral nerve branch in the limb was at least 90 percent that of the contralateral control ventral branch. In embryos that met these criteria, we found that inhibiting the synthesis of RA in the dorsal limb with citral (n = 6) or disulfiram (n = 4) significantly reduced the growth of axons from the dorsal branch of the crural plexus (p < 0.002, paired t-test), without affecting axons in the ventral branch, which was farther from the implanted beads (Fig. 5H; Table 4). These results cannot be attributed to a reduction in the number of lLMC neurons (which normally project their axons into the dorsal branch), as has been observed following a perturbation of RA signaling in paraxial mesoderm (Ji et al., 2006) or spinal cord (Sockanathan and Jessell, 1998; Sockanathan et al., 2003; Vermot et al., 2005), since the mLMC and lLMC neurons appeared normal in citral-treated embryos (data not shown). There were no differences in the length of either dorsal or ventral axons in embryos implanted with control beads (n = 7) (Table 4). Thus, locally reducing endogenous RA in the limb delays or inhibits the entry of axons into the limb, providing strong evidence that retinoid signaling has an essential function in governing the timing of axon entry into the limb, i.e., in regulating the waiting period.
Table 4.
Inhibiting retinoid signaling impairs axon growth in the limb
| DMSO/Control | Inhibitor/Control | |
|---|---|---|
| (n = 7) | (n = 10) | |
| Dorsal branch (average) | 1.0 ± 0.1 | 0.8 ± 0.1** |
| Dorsal branch (longest) | 0.9 ± 0.1 | 0.8 ± 0.2* |
| Ventral branch (average) | 1.1 ± 0.2 | 1.0 ± 0.1 |
| Ventral branch (longest) | 1.0 ± 0.1 | 1.0 ± 0.1 |
The distance from the plexus region to the tip of the dorsal and ventral nerve branch was measured in St. 25–28 embryos implanted with beads soaked in an inhibitor of retinoic acid synthesis [citral (n = 6) or disulfiram (n = 4)] or with control beads (DMSO) at St. 22–23.5, as described in Materials and Methods and Fig. 5C. Embryos were excluded if the treated limb was obviously smaller than the control or the ventral branch was less than 90% that in the control limb. The same number of sections was analyzed in treated and control limbs of individual embryos, and values for each embryo were compared for significance with a paired t-test. Inhibiting retinoid synthesis significantly impaired axon growth near the beads
p < 0.01
p < 0.001.
RA is a transcriptional regulator that influences expression of a plethora of other molecules, some of which could promote or guide axon growth in the limb. In addition, RA itself can affect neurite growth (reviewed in Clagett-Dame et al., 2006). To determine whether RA could directly promote growth of young embryonic motor and sensory axons, we co-cultured explants of ventral spinal cord or dorsal root ganglia (DRGs) from St. 23–25 embryos with RA-soaked beads in collagen gels, and measured the number of axons that extended at least 150 µm toward or away from the beads, as described in Material and Methods (Fig. 6A). On average, nearly five times as many motor axons extended from the side of the explant adjacent to the RA-beads than from the side away from the beads (bead/control = 4.65 ± 2.99, n = 13 explants from 4 experiments) (Fig. 6C), whereas there was no difference in outgrowth toward or away from control beads (bead/control = 1.15 ± 0.46, n = 14 explants from 4 experiments) (Fig. 6B). Axons did not appear to grow directly toward RA-treated beads in vitro, and in many cases extended beyond the beads, suggesting that RA promotes, but does not direct, outgrowth of motor axons. In contrast, although sensory axons in vivo grew into the limb prematurely in response to early, exogenous RA, in vitro RA had no consistent effect on sensory neurons (data not shown).
Effects of RA on limb environment
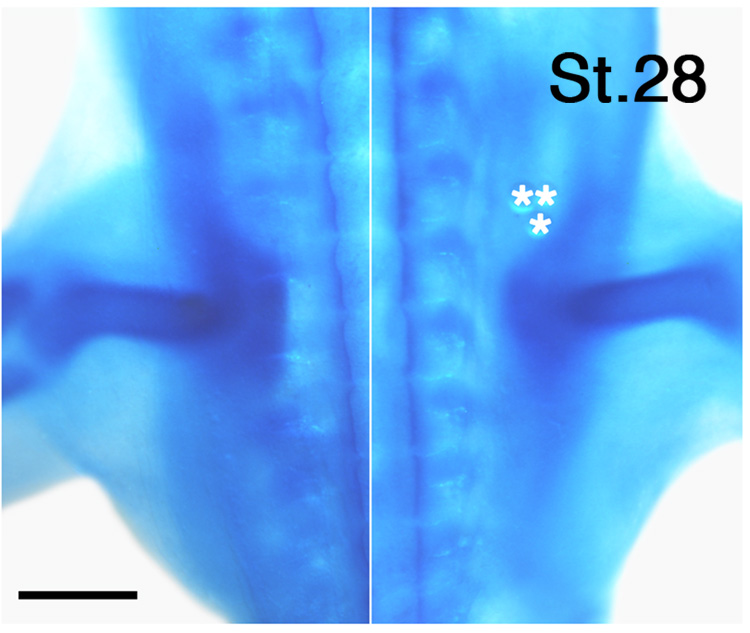
Whereas RA can directly promote the growth of motor axons, RA can also affect the limb environment. For example, RA influences genes that pattern the limb (reviewed in Lee et al., 2004; Stratford et al., 1999), and could potentially regulate expression of molecules in the limb that pattern axon growth. Interestingly, as shown in Figure 5, axons extended from crural plexus up to one segment more caudally than normal in RA-treated limbs, suggesting that exogenous RA may have reduced the normal barrier provided by the pelvic girdle precursor in this limb region (Tosney and Landmesser, 1984). Indeed, the cartilaginous pelvic girdle was somewhat reduced in RA-treated limbs (n = 8) (Fig. 7), which could permit axons to invade a broader expanse of limb. Thus, one function of retinoid signaling that occurs in advance of axon ingrowth may be sculpt the pelvic girdle precursor, thereby influencing the sites where axons enter the limb. These results are consistent with earlier observations that increasing retinoid signaling inhibits or interferes with chondrogenesis both in vitro and in vivo (Cash et al., 1997; reviewed in Lee et al., 2004).
Figure 7.
Exogenous retinoic acid alters pelvic girdle development. Whole mount view of the proximal portion of left and right limbs of an embryo implanted with RA-soaked beads (5 mg/ml; asterisks) at St. 22. Note that the anterior portion of the pelvic girdle is somewhat reduced on the RA-treated side. Scale bar: 500 µm.
Together our findings identify retinoid signaling as a major mechanism governing both the waiting period and pattern of axon entry into the limb, a mechanism that most likely involves the direct promotion of motor axon growth, as well as indirect effects on axon growth via changes in the limb environment.
Discussion
Vitamin A derivatives, known collectively as retinoids, are essential for normal patterning of diverse structures and organs during embryonic development and continue to play important roles in the adult (reviewed in Mey and McCaffery, 2004; Ross et al., 2000). Here we describe a novel function for retinoid signaling during embryonic development by showing that retinoid signaling plays an important role in governing the timing and pattern of axon entry into the limb.
The spatiotemporal pattern of Raldh2 expression in hindlimb is unique
Retinoid signaling in the chick hindlimb, deduced from the pattern of Raldh2 expression, begins shortly before axons exit the plexus region where they normally arrest growth for nearly 24 hours before invading the limb (Hollyday, 1983; Tosney and Landmesser, 1985; Wang and Scott, 2000; but see Dieu and Newgreen, 2007). Although expression patterns of several other transcriptional regulators share features in common with Raldh2, the Raldh2 pattern in the hindlimb is unique.
Raldh2 expression is initially restricted to a posterior domain, similar but not identical to Shh (see also Riddle et al., 1993). Although retinoid signaling is required to induce Shh and establish the ZPA (Helms et al., 1996; Lu et al., 1997; Mic et al., 2004; Niederreither et al., 2002), several lines of evidence suggest that the retinoid signaling that we describe here must play a different role. For example, we were unable to detect Raldh2 transcripts in posterior hindlimb prior to the stage at which expression of Shh first appears. When Raldh2 could be detected in hindlimb, transcripts were always expressed in a more proximal domain than Shh. Further, retinoid signaling is required at much earlier stages, prior to overt limb outgrowth, to induce the ZPA and Shh (Helms et al., 1996; Lu et al., 1997). Finally, blocking RA signaling at the stages we examined has no effect on patterning polarizing activity in the limb (Helms et al., 1996). Thus, at later stages after the ZPA is established, retinoid signaling in the hindlimb must have a different function.
In the hindlimb, as in the wing (Berggren et al., 2001), Raldh2 is expressed in mesenchymal cells, which provide important guidance cues for axon growth (Lance-Jones and Dias, 1991), rather than in the myotubes themselves. Similarly, Tcf4, a transcription factor that is essential for normal muscle patterning in the hindlimb, is expressed in mesodermal cells independently of the muscle cells. The two expression patterns are not identical however, as Tcf4 appears earlier than Raldh2 and has a broader, nearly uniform distribution within the limb (Kardon et al., 2003). It is possible that retinoid signaling, like Tcf4, is involved in regulating muscle patterning, but more specifically patterns subsets of muscles (e.g., fast vs. slow).
The close association of outgrowing axons and Raldh2 message suggests that axon growth and Raldh2 expression in hindlimb are related. The onset, distribution and abundance of Raldh2 transcripts were normal in denervated hindlimbs. Thus, motor axons are not required to induce or promote Raldh2 expression in hindlimb, in contrast to their role in the wing (Berggren et al., 2001). Instead, we found that perturbing retinoid signaling alters the timing and patterning of axon growth into the hindlimb. It will be of interest to determine whether retinoid signaling also regulates the waiting period in the wing, since in mouse retinoid signaling appears to have different functions in fore- and hindlimbs (Mic and Duester, 2003; Niederreither et al., 2002).
Because retinoic acid levels in vivo are normally quite tightly regulated by feedback mechanisms, such that exogenous RA alters both production and degradation of endogenous RA (Dobbs-McAuliffe et al., 2004; Martinez-Ceballos and Burdsal, 2001; Reijntjes et al., 2003; Sen et al., 2005), we are unable to assess the actual magnitude of the changes in retinoid signaling that were effected by our treatments. Nevertheless, we clearly were able to locally altered RA levels in the limb, since exogenous RA promoted precocious axon growth in the limb and altered the pattern of ingrowth, whereas RA synthesis inhibitors reduced axon growth in the limb. Together these findings provide strong support that retinoid signaling plays an important role in governing axon growth into the hindlimb.
Differential effects of RA on motor and sensory axon growth
Both motor and sensory axons leave the plexus region and enter the limb precociously in the presence of early exogenous RA in the limb. In contrast, RA enhanced neurite outgrowth only from motor neurons in vitro, with no obvious effects on sensory neurons. These observations suggest that in vivo only the motor growth cones responded directly to the exogenous RA, with sensory axons entering the limb precociously simply because their growth cones follow the outgrowing motor axons, as they do during normal development and following experimental manipulations (Landmesser and Honig, 1986; Scott, 1988; Wang and Scott, 1997).
The failure to observe a direct effect of RA on sensory neurite growth in vitro contrasts with findings in several other studies. This discrepancy most likely results from differences in the age of sensory neurons examined. Here we analyzed RA effects on neurons from St. 23.5-25 (E4.5–5) embryos, stages when DRG neurons are still being generated (Carr and Simpson, 1978) and few sensory axons have left the plexus region (Wang and Scott, 2000). In contrast, studies showing that RA enhances sensory neurite outgrowth involved DRGs from older embryos (Corcoran et al., 2000; Haskell et al., 1987) or adults (So et al., 2006). It is possible that RA increases sensory neurite outgrowth only at later stages in embryonic development, much like the age-related effects of nerve growth factor on trigeminal ganglion neuron arborization (Scott and Davies, 1993). Interestingly, RA-responsive DRG neurons selectively up-regulate expression of RARβ2 transcripts upon exposure to RA in vitro (Corcoran et al., 2000; see also Lu et al., 1997). RARβ2 appears to play a major role in the induction of neurite outgrowth in a variety of neuron types (Corcoran et al., 2000; Corcoran et al., 2002; Dmetrichuk et al., 2005; So et al., 2006). Perhaps at the early embryonic stages we studied sensory neurons were not yet capable of up-regulating RARβ2, a property they acquire only after initial exposure to RA once they enter the limb (cf. Vogel and Davies, 1991).
To our knowledge the present study is the first demonstrate directly that RA can promote growth of motor axons, either in vivo or in vitro. Motor axons fail to grow toward the periphery in vitamin A deficient (VAD) quail embryos (Maden et al., 1998a; Wilson et al., 2003), a finding that suggests that retinoids promote motor axon growth during embryonic development. However, due to the myriad effects of RA on CNS patterning (Maden, 2006; Wilson et al., 2004; Wilson et al., 2003; see also Sen et al., 2005), both the spinal cord (Wilson et al., 2004) and motoneuron development (Wilson et al., 2003) are severely compromised in VAD embryos, confounding interpretation of these observations.
Several other studies have shown that RA not only promotes (Corcoran et al., 2002; Dmetrichuk et al., 2005; Maden et al., 1998b), but also attracts (Maden et al., 1998b; see also Dmetrichuk et al., 2006) neurite outgrowth from spinal cord neurons in vitro, but the identity of the neurons was not determined. In one case the ventral cord, which would include the motoneurons, was specifically excluded from the cultures (Maden et al., 1998b). In initial studies we verified that the majority of axons that grew out of ventral spinal cord explants were indeed motoneurons either by retrogradely labeling motoneurons with DiI prior to dissecting the explant or by labeling explants with antibody SC1, which selectively marks DRGs and motoneurons at early stages (Tanaka et al., 1991) (data not shown). Thus, we are confident that the RA-enhanced neurite outgrowth from ventral cord was indeed from motoneurons. The failure to observe chemoattraction of motor axons to RA-treated beads either in vivo or in vitro could represent a true difference among neuronal populations or a technical difference in experimental design.
Our in vitro studies show that RA itself can directly promote motor axon growth, presumably by diffusing through the growth cone membrane, binding to nuclear RARs to regulate transcription of genes, which subsequently enhance axon growth (reviewed in Clagett-Dame et al., 2006). In vivo, however, it is likely that RA in the limb also triggers a cascade of signaling events in adjacent mesenchymal tissue that alter the limb environment. For example, perturbing RA levels in the limb alters expression of a number of genes involved in patterning the limb along both the anteroposterior and dorsoventral axes (reviewed in Lee et al., 2004; Stratford et al., 1999). The formation of a seemingly normal dorsal and ventral muscle masses, and the proper dorsal segregation of EphA+ axons in the limb suggest that limb patterning was not grossly perturbed in our studies. Nevertheless, it is possible that exogenous RA diminished expression of molecules reported to inhibit axon egress from the plexus region, such as Eph4 (Sahin et al., 2005) or sema3A (Huber et al., 2005), allowing axons to enter the limb precociously. Alternatively, increasing RA signaling in the limb may have triggered an increase in growth promoting substrata or chemoattractants in the limb.
Thus, it is likely that during normal development retinoid signaling, which is initiated near the end of the waiting period, regulates the timing and pattern of axon ingrowth by multiple mechanisms that involve the direct promotion of axon growth, resphaping cartilaginous barriers, and other as yet undetermined alterations of the limb environment. We interpret our findings to indicate that early exposure of the limb to retinoid signaling accelerates the appearance of these important mechanisms. An elucidation of the relative importance of these mechanisms, as well as the genes that are involved, await further study.
Acknowledgements
We thank Ms. Tasia Robertson for technical assistance, and Drs. Thomas M. Beres, Kathryn B. Moore, and Tatajana Piotrowski for helpful comments on the manuscript. We also thank Drs. T.M. Jessell, F. Lefcort for antibodies, and Drs. M. Maden and G. Schoenwolf for plasmids. Antibodies 3A10, 23.4-5, and 39.4D5 developed by Drs. T.M. Jessell and J. Dodd, and PAX7, NA8 and 13F4, developed by Drs. A. Kawakami, E. Bandman and N. Le Douarin, respectively, were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA 52242. This work was supported by NIH NS16067 to S.A.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balmer JE, Blomhoff R. Gene expression regulation by retinoic acid. J. Lipid Res. 2002;43:1773–1808. doi: 10.1194/jlr.r100015-jlr200. [DOI] [PubMed] [Google Scholar]
- Bastien J, Rochette-Egly C. Nuclear retinoid receptors and the transcription of retinoid-target genes. Gene. 2004;328:1–16. doi: 10.1016/j.gene.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Berggren K, Ezerman EB, McCaffery P, Forehand CJ. Expression and regulation of the retinoic acid synthetic enzyme RALDH-2 in the embryonic chicken wing. Dev. Dyn. 2001;222:1–16. doi: 10.1002/dvdy.1166. [DOI] [PubMed] [Google Scholar]
- Berggren K, McCaffery P, Drager U, Forehand CJ. Differential distribution of retinoic acid synthesis in the chicken embryo as determined by immunolocalization of the retinoic acid synthetic enzyme, RALDH-2. Dev. Biol. 1999;210:288–304. doi: 10.1006/dbio.1999.9286. [DOI] [PubMed] [Google Scholar]
- Blentic A, Gale E, Maden M. Retinoic acid signalling centres in the avian embryo identified by sites of expression of synthesising and catabolising enzymes. Dev. Dyn. 2003;227:114–127. doi: 10.1002/dvdy.10292. [DOI] [PubMed] [Google Scholar]
- Calderó J, Prevette D, Mei X, Oakley RA, Li L, Milligan C, Houenou L, Burek M, Oppenheim RW. Peripheral target regulation of the development and survival of spinal sensory and motor neurons in the chick embryo. J. Neurosci. 1998;18:356–370. doi: 10.1523/JNEUROSCI.18-01-00356.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr VM, Simpson SBJ. Proliferative and degenerative events in the early development of chick dorsal root ganglia. I. Normal development. J. Comp. Neurol. 1978;182:727–740. doi: 10.1002/cne.901820410. [DOI] [PubMed] [Google Scholar]
- Cash DE, Bock CB, Schughart K, Linney E, Underhill TM. Retinoic acid receptor alpha function in vertebrate limb skeletogenesis: a modulator of chondrogenesis. J. Cell Biol. 1997;136:445–457. doi: 10.1083/jcb.136.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers D, Wilson L, Maden M, Lumsden A. RALDH-independent generation of retinoic acid during vertebrate embryogenesis by CYP1B1. Development. 2007;134:1369–1383. doi: 10.1242/dev.02815. [DOI] [PubMed] [Google Scholar]
- Clagett-Dame M, McNeill EM, Muley PD. Role of all-trans retinoic acid in neurite outgrowth and axonal elongation. J. Neurobiol. 2006;66:739–756. doi: 10.1002/neu.20241. [DOI] [PubMed] [Google Scholar]
- Corcoran J, Shroot B, Pizzey J, Maden M. The role of retinoic acid receptors in neurite outgrowth from different populations of embryonic mouse dorsal root ganglia. J. Cell Sci. 2000;113:2567–2574. doi: 10.1242/jcs.113.14.2567. [DOI] [PubMed] [Google Scholar]
- Corcoran J, So PL, Barber RD, Vincent KJ, Mazarakis ND, Mitrophanous KA, Kingsman SM, Maden M. Retinoic acid receptor beta2 and neurite outgrowth in the adult mouse spinal cord in vitro. J. Cell Sci. 2002;115:3779–3786. doi: 10.1242/jcs.00046. [DOI] [PubMed] [Google Scholar]
- Crow MT, Stockdale FE. Myosin expression and specialization among the earliest muscle fibers of the developing avian limb. Dev. Biol. 1986;113:238–254. doi: 10.1016/0012-1606(86)90126-0. [DOI] [PubMed] [Google Scholar]
- Davis BM, Frank E, Johnson FA, Scott SA. Development of central projections of lumbosacral sensory neurons in the chick. J. Comp. Neurol. 1989;279:556–566. doi: 10.1002/cne.902790405. [DOI] [PubMed] [Google Scholar]
- Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002;298:1959–1964. doi: 10.1126/science.1072165. [DOI] [PubMed] [Google Scholar]
- Dieu T, Newgreen D. Chicken wings and the brachial plexus. Neuro. Res. 2007;29:225–230. doi: 10.1179/174313206X153905. [DOI] [PubMed] [Google Scholar]
- Dmetrichuk JM, Carlone RL, Spencer GE. Retinoic acid induces neurite outgrowth and growth cone turning in invertebrate neurons. Dev. Biol. 2006;294:39–49. doi: 10.1016/j.ydbio.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Dmetrichuk JM, Spencer GE, Carlone RL. Retinoic acid-dependent attraction of adult spinal cord axons towards regenerating newt limb blastemas in vitro. Dev. Biol. 2005;281:112–120. doi: 10.1016/j.ydbio.2005.02.019. [DOI] [PubMed] [Google Scholar]
- Dobbs-McAuliffe B, Zhao Q, Linney E. Feedback mechanisms regulate retinoic acid production and degradation in the zebrafish embryo. Mech. Develop. 2004;121:339–350. doi: 10.1016/j.mod.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Eberhart J, Swartz M, Koblar SA, Pasquale EB, Tanaka H, Krull CE. Expression of EphA4, ephrin-A2 and ephrin-A5 during axon outgrowth to the hindlimb indicates potential roles in pathfinding. Dev. Neurosci. 2000;22:2237–2250. doi: 10.1159/000017446. [DOI] [PubMed] [Google Scholar]
- Eichele G, Tickle C, Alberts BM. Microcontrolled release of biologically active compounds in chick embryos: beads of 200-microns diameter for the local release of retinoids. Anal. Biochem. 1984;142:542–555. doi: 10.1016/0003-2697(84)90504-9. [DOI] [PubMed] [Google Scholar]
- Francis-West PH, Antoni L, Anakwe K. Regulation of myogenic differentiation in the developing limb bud. J. Anat. 2003;202:69–81. doi: 10.1046/j.1469-7580.2003.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfter W, Yip YPL, Yip JW. Axonin 1 is expressed primarily in subclasses of avian sensory neurons during outgrowth. Dev. Brain Res. 1994;78:87–101. doi: 10.1016/0165-3806(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J. Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Haskell BE, Stach RW, Werrbach-Perez K, Perez-Polo JR. Effect of retinoic acid on nerve growth factor receptors. Cell Tissue Res. 1987;247:67–73. doi: 10.1007/BF00216548. [DOI] [PubMed] [Google Scholar]
- Helms JA, Kim CH, Eichele G, Thaller C. Retinoic acid signaling is required during early chick limb development. Development. 1996;122:1385–1394. doi: 10.1242/dev.122.5.1385. [DOI] [PubMed] [Google Scholar]
- Hollyday M. Development of motor innervation of chick limb. In: Fallon J, Caplan A, editors. Limb Development and Regeneration. New York: Alan R. Liss, Inc.; 1983. pp. 183–193. [Google Scholar]
- Honig MG, Zou J-Y. The effects of taret tissues on the outgrowth of chick cutaneous and muscle sensory neurons. Dev. Biol. 1995;167:1–14. doi: 10.1006/dbio.1995.1048. [DOI] [PubMed] [Google Scholar]
- Huber AB, Kania A, Tran TS, Gu C, De Marco Garcia N, Lieberam I, Johnson D, Jessell TM, Ginty DD, Kolodkin AL. Distinct roles for secreted semaphorin signaling in spinal motor axon guidance. Neuron. 2005;48:949–964. doi: 10.1016/j.neuron.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Jacob M, Christ B, Jacob HJ. The migration of myogenic cells from the somites into the leg region of avian embryos. An ultrastructural study. Anat. Embryol. (Berl.) 1979;157:291–309. doi: 10.1007/BF00304995. [DOI] [PubMed] [Google Scholar]
- Ji SJ, Zhuang B, Falco C, Schneider A, Schuster-Gossler K, Gossler A, Sockanathan S. Mesodermal and neuronal retinoids regulate the induction and maintenance of limb innervating spinal motor neurons. Dev. Biol. 2006;297:249–261. doi: 10.1016/j.ydbio.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Kardon G, Harfe BD, Tabin CJ. A Tcf4-positive mesodermal population provides a prepattern for vertebrate limb muscle patterning. Dev. Cell. 2003;5:937–944. doi: 10.1016/s1534-5807(03)00360-5. [DOI] [PubMed] [Google Scholar]
- Kuhn TB. Methods in Cell Biology. Vol. 71. Elsevier Science; 2003. Growing and working with spinal motor neurons; pp. 67–87. [DOI] [PubMed] [Google Scholar]
- Lance-Jones C, Dias M. The influence of presumptive limb connective tissue on motoneuron axon guidance. Dev. Biol. 1991;143:93–110. doi: 10.1016/0012-1606(91)90057-a. [DOI] [PubMed] [Google Scholar]
- Landmesser L, Honig MG. Altered sensory projections in the chick hind limb following the early removal of motoneurons. Dev. Biol. 1986;118:511–531. doi: 10.1016/0012-1606(86)90023-0. [DOI] [PubMed] [Google Scholar]
- Landmesser LT. The acquisition of motoneuron subtype identity and motor circuit formation. Int. J. Dev. Neurosci. 2001;19:175–182. doi: 10.1016/s0736-5748(00)00090-3. [DOI] [PubMed] [Google Scholar]
- Lee GS, Kochhar DM, Collins MD. Retinoid-induced limb malformations. Curr. Pharm. Des. 2004;10:2657–2699. doi: 10.2174/1381612043383728. [DOI] [PubMed] [Google Scholar]
- Lu HC, Revelli JP, Goering L, Thaller C, Eichele G. Retinoid signaling is required for the establishment of a ZPA and for the expression of Hoxb-8, a mediator of ZPA formation. Development. 1997;124:1643–1651. doi: 10.1242/dev.124.9.1643. [DOI] [PubMed] [Google Scholar]
- Maden M. Retinoids and spinal cord development. J. Neurobiol. 2006;66:726–738. doi: 10.1002/neu.20248. [DOI] [PubMed] [Google Scholar]
- Maden M, Gale E, Zile M. The role of vitamin A in the development of the central nervous system. J. Nutr. 1998a;128:471S–475S. doi: 10.1093/jn/128.2.471S. [DOI] [PubMed] [Google Scholar]
- Maden M, Keen G, Jones GE. Retinoic acid as a chemotactic molecule in neuronal development. Int. J. Dev. Neurosci. 1998b;16:317–322. doi: 10.1016/s0736-5748(98)00046-x. [DOI] [PubMed] [Google Scholar]
- Martinez-Ceballos E, Burdsal CA. Differential expression of chicken CYP26 in anterior versus posterior limb bud in response to retinoic acid. J. Exp. Zool. 2001;290:136–147. doi: 10.1002/jez.1043. [DOI] [PubMed] [Google Scholar]
- McCaffery P, Drager UC. Hot spots of retinoic acid synthesis in the developing spinal cord. Proc. Natl. Acad. Sci., USA. 1994;91:7194–7197. doi: 10.1073/pnas.91.15.7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffery PJ, Adams J, Maden M, Rosa-Molinar E. Too much of a good thing: retinoic acid as an endogenous regulator of neural differentiation and exogenous teratogen. Eur. J. Neurosci. 2003;18:457–472. doi: 10.1046/j.1460-9568.2003.02765.x. [DOI] [PubMed] [Google Scholar]
- Mey J, McCaffery P. Retinoic acid signaling in the nervous system of adult vertebrates. Neuroscientist. 2004;10:409–421. doi: 10.1177/1073858404263520. [DOI] [PubMed] [Google Scholar]
- Mic FA, Duester G. Patterning of forelimb bud myogenic precursor cells requires retinoic acid signaling initiated by Raldh2. Dev. Biol. 2003;264:191–201. doi: 10.1016/s0012-1606(03)00403-2. [DOI] [PubMed] [Google Scholar]
- Mic FA, Sirbu IO, Duester G. Retinoic acid synthesis controlled by Raldh2 is required early for limb bud initiation and then later as a proximodistal signal during apical ectodermal ridge formation. J. Biol. Chem. 2004;279:26698–26706. doi: 10.1074/jbc.M401920200. [DOI] [PubMed] [Google Scholar]
- Niederreither K, Subbarayan V, Dolle P, Chambon P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat. Genet. 1999;21:444–448. doi: 10.1038/7788. [DOI] [PubMed] [Google Scholar]
- Niederreither K, Vermot J, Schuhbaur B, Chambon P, Dolle P. Embryonic retinoic acid synthesis is required for forelimb growth and anteroposterior patterning in the mouse. Development. 2002;129:3563–3574. doi: 10.1242/dev.129.15.3563. [DOI] [PubMed] [Google Scholar]
- Novitch BG, Wichterle H, Jessell TM, Sockanathan S. A requirement for retinoic acid-mediated transcriptional activation in ventral neural patterning and motor neuron specification. Neuron. 2003;40:81–95. doi: 10.1016/j.neuron.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Rafuse VF, Milner LD, Landmesser LT. Selective innervation of fast and slow muscle regions during early chick neuromuscular development. J. Neurosci. 1996;16:6864–6877. doi: 10.1523/JNEUROSCI.16-21-06864.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijntjes S, Gale E, Maden M. Expression of the retinoic acid catabolising enzyme CYP26B1 in the chick embryo and its regulation by retinoic acid. Gene Expr. Patterns. 2003;3:621–627. doi: 10.1016/s1567-133x(03)00112-1. [DOI] [PubMed] [Google Scholar]
- Repa JJ, Plum LA, Tadikonda PK, Clagett-Dame M. All-trans 3,4-didehydroretinoic acid equals all-trans retinoic acid in support of chick neuronal development. FASEB J. 1996;10:1078–1084. doi: 10.1096/fasebj.10.9.8801170. [DOI] [PubMed] [Google Scholar]
- Riddle RD, Johnson RL, Laufer E, Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993;75:1401–1416. doi: 10.1016/0092-8674(93)90626-2. [DOI] [PubMed] [Google Scholar]
- Rifkin JT, Todd VJ, Anderson LW, Lefcort F. Dynamic expression of neurotrophin receptors during sensory neuron genesis and differentiation. Dev. Biol. 2000;227:465–480. doi: 10.1006/dbio.2000.9841. [DOI] [PubMed] [Google Scholar]
- Ross SA, McCaffery PJ, Drager UC, De Luca LM. Retinoids in embryonal development. Physiol. Rev. 2000;80:1021–1054. doi: 10.1152/physrev.2000.80.3.1021. [DOI] [PubMed] [Google Scholar]
- Sahin M, Greer PL, Lin MZ, Poucher H, Eberhart J, Schmidt S, Wright TM, Shamah SM, O'Connell S, Cowan CW, Hu L, Goldberg JL, Debant A, Corfas G, Krull CE, Greenberg ME. Eph-dependent tyrosine phosphorylation of ephexin1 modulates growth cone collapse. Neuron. 2005;46:191–204. doi: 10.1016/j.neuron.2005.01.030. [DOI] [PubMed] [Google Scholar]
- Saunders JW., Jr The proximo-distal sequence of origin of the parts of the chick wing and the role of the ectoderm. J. Exp. Zool. 1948;108:363–403. doi: 10.1002/jez.1401080304. [DOI] [PubMed] [Google Scholar]
- Schroeter S, Tosney KW. Spatial and temporal patterns of muscle cleavage in the chick thigh and their value as criteria for homology. Am. J. Anat. 1991;191:325–350. doi: 10.1002/aja.1001910402. [DOI] [PubMed] [Google Scholar]
- Scott SA. Skin sensory innervation patterns in embryonic chick hindlimbs deprived of motoneurons. Dev. Biol. 1988;126:362–374. doi: 10.1016/0012-1606(88)90146-7. [DOI] [PubMed] [Google Scholar]
- Scott SA, Davies AM. Age-related effects of nerve growth factor on the morphology of embryonic sensory neurons in vitro. J. Comp. Neurol. 1993;337:277–285. doi: 10.1002/cne.903370208. [DOI] [PubMed] [Google Scholar]
- Sen J, Harpavat S, Peters MA, Cepko CL. Retinoic acid regulates the expression of dorsoventral topographic guidance molecules in the chick retina. Development. 2005;132:5147–5159. doi: 10.1242/dev.02100. [DOI] [PubMed] [Google Scholar]
- Sharma K, Korade Z, Frank E. Development of specific muscle and cutaneous sensory projections in cultured segments of spinal cord. Development. 1994;120:1315–1323. doi: 10.1242/dev.120.5.1315. [DOI] [PubMed] [Google Scholar]
- Shatz CJ, Ghosh A, McConnell SK, Allendoerfer KL, Friauf E, Antonini A. Pioneer neurons and target selection in cerebral cortical development. Cold Spring Harb. Symp. Quant. Biol. 1990;55:469–480. doi: 10.1101/sqb.1990.055.01.046. [DOI] [PubMed] [Google Scholar]
- Shen H, Wilke T, Ashique AM, Narvey M, Zerucha T, Savino E, Williams T, Richman JM. Chicken transcription factor AP-2: cloning, expression and its role in outgrowth of facial prominences and limb buds. Dev. Biol. 1997;188:248–266. doi: 10.1006/dbio.1997.8617. [DOI] [PubMed] [Google Scholar]
- So PL, Yip PK, Bunting S, Wong LF, Mazarakis ND, Hall S, McMahon S, Maden M, Corcoran JP. Interactions between retinoic acid, nerve growth factor and sonic hedgehog signalling pathways in neurite outgrowth. Dev. Biol. 2006;298:167–175. doi: 10.1016/j.ydbio.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Sockanathan S, Jessell TM. Motor neuron-derived retinoid signaling specifies the subtype identity of spinal motor neurons. Cell. 1998;94:503–514. doi: 10.1016/s0092-8674(00)81591-3. [DOI] [PubMed] [Google Scholar]
- Sockanathan S, Perlmann T, Jessell TM. Retinoid receptor signaling in postmitotic motor neurons regulates rostrocaudal positional identity and axonal projection pattern. Neuron. 2003;40:97–111. doi: 10.1016/s0896-6273(03)00532-4. [DOI] [PubMed] [Google Scholar]
- Song Y, Hui JN, Fu KK, Richman JM. Control of retinoic acid synthesis and FGF expression in the nasal pit is required to pattern the craniofacial skeleton. Dev. Biol. 2004;276:313–329. doi: 10.1016/j.ydbio.2004.08.035. [DOI] [PubMed] [Google Scholar]
- Stratford T, Horton C, Maden M. Retinoic acid is required for the initiation of outgrowth in the chick limb bud. Curr. Biol. 1996;6:1124–1133. doi: 10.1016/s0960-9822(02)70679-9. [DOI] [PubMed] [Google Scholar]
- Stratford T, Logan C, Zile M, Maden M. Abnormal anteroposterior and dorsoventral patterning of the limb bud in the absence of retinoids. Mech. Develop. 1999;81:115–125. doi: 10.1016/s0925-4773(98)00231-7. [DOI] [PubMed] [Google Scholar]
- Summerbell D. The effect of local application of retinoic acid to the anterior margin of the developing chick limb. J. Embryol. exp. Morph. 1983;78:269–289. [PubMed] [Google Scholar]
- Tanaka H, Matsui T, Agata A, Tomura M, Kubota I, McFarland KC, Kohr B, Lee A, Phillips HS, Shelton DL. Molecular cloning and expression of a novel adhesion molecule, SC1. Neuron. 1991;7:535–545. doi: 10.1016/0896-6273(91)90366-8. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Tamura K, Ide H. Citral, an inhibitor of retinoic acid synthesis, modifies chick limb development. Dev. Biol. 1996;175:239–247. doi: 10.1006/dbio.1996.0111. [DOI] [PubMed] [Google Scholar]
- Thaller C, Eichele G. Isolation of 3,4-didehydroretinoic acid, a novel morphogenetic signal in the chick wing bud. Nature. 1990;345:815–819. doi: 10.1038/345815a0. [DOI] [PubMed] [Google Scholar]
- Tosney KW, Landmesser LT. Pattern and specificity of axonal outgrowth following varying degrees of chick limb bud ablation. J. Neurosci. 1984;4:2518–2527. doi: 10.1523/JNEUROSCI.04-10-02518.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosney KW, Landmesser LT. Development of the major pathways for neurite outgrowth in the chick hindlimb. Dev. Biol. 1985;109:193–214. doi: 10.1016/0012-1606(85)90360-4. [DOI] [PubMed] [Google Scholar]
- Tsuchida T, Ensini M, Morton SB, Baldassare M, Edlund T, Jessell TM, Pfaff SL. Topographic organization of embryonic motor neurons defined by expression of LIM homeobox genes. Cell. 1994;79:957–970. doi: 10.1016/0092-8674(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Vallari RC, Pietruszko R. Human aldehyde dehydrogenase: mechanism of inhibition of disulfiram. Science. 1982;216:637–639. doi: 10.1126/science.7071604. [DOI] [PubMed] [Google Scholar]
- Vermot J, Schuhbaur B, Mouellic HL, McCaffery P, Garnier JM, Hentsch D, Brulet P, Niederreither K, Chambon P, Dolle P, Roux IL. Retinaldehyde dehydrogenase 2 and Hoxc8 are required in the murine brachial spinal cord for the specification of Lim1+ motoneurons and the correct distribution of Islet1+ motoneurons. Development. 2005;132:1611–1621. doi: 10.1242/dev.01718. [DOI] [PubMed] [Google Scholar]
- Vogel KS, Davies AM. The duration of neurotrophic factor independence in early sensory neurons is matched to the time course of target field innervation. Neuron. 1991;7:819–830. doi: 10.1016/0896-6273(91)90284-7. [DOI] [PubMed] [Google Scholar]
- Wang G, Scott SA. Muscle sensory innervation patterns in embryonic chick hindlimbs following dorsal root ganglion reversal. Dev. Biol. 1997;186:27–35. doi: 10.1006/dbio.1997.8583. [DOI] [PubMed] [Google Scholar]
- Wang G, Scott SA. Independent development of sensory and motor innervation patterns in embryonic chick hindlimbs. Dev. Biol. 1999;208:324–336. doi: 10.1006/dbio.1999.9212. [DOI] [PubMed] [Google Scholar]
- Wang G, Scott SA. The "waiting period" of sensory and motor axons in early chick hindlimb: Its role in axon pathfinding and neuronal maturation. J. Neurosci. 2000;20:5358–5366. doi: 10.1523/JNEUROSCI.20-14-05358.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Scott SA. An early broad competence of motoneurons to express ER81 is later sculpted by the periphery. J. Neurosci. 2004;24:9789–9798. doi: 10.1523/JNEUROSCI.3409-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SS, Mear JP, Liang HC, Potter SS, Aronow BJ, Colbert MC. Large-scale reprogramming of cranial neural crest gene expression by retinoic acid exposure. Physiol. Genomics. 2004;19:184–197. doi: 10.1152/physiolgenomics.00136.2004. [DOI] [PubMed] [Google Scholar]
- Wilson L, Gale E, Chambers D, Maden M. Retinoic acid and the control of dorsoventral patterning in the avian spinal cord. Dev. Biol. 2004;269:433–446. doi: 10.1016/j.ydbio.2004.01.034. [DOI] [PubMed] [Google Scholar]
- Wilson L, Gale E, Maden M. The role of retinoic acid in the morphogenesis of the neural tube. J. Anat. 2003;203:357–368. doi: 10.1046/j.1469-7580.2003.00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]