Abstract
Several stress conditions are characterized by activation of 5′-AMP-protein kinase (AMPK) and the development of leucine resistance in skeletal muscle. The present study determined whether direct activation of the AMPK by AICAR prevents the characteristic leucine-induced increase in protein synthesis by altering mTOR (mammalian target of rapamycin) signal transduction. Rats were injected with AICAR or saline and 1 h thereafter received an oral gavage of leucine (or saline). Efficacy of AICAR was verified by increased AMPK phosphorylation. AICAR decreased basal in vivo muscle (gastrocnemius) protein synthesis and completely prevented the leucine-induced increase, independent of a change in muscle adenine nucleotide concentration. AICAR also prevented the hyperphosphorylation of eukaryotic initiation factor (eIF) 4E binding protein (4E-BP1), ribosomal protein S6 kinase (S6K1), S6, and eIF4G in response to leucine suggesting a decrease in mTOR activity. Moreover, AICAR prevented the leucine-induced redistribution of eIF4E from the inactive eIF4E·4E-BP1 to the active eIF4E·eIF4G complex. This ability of AICAR to produce muscle leucine resistance could not be attributed to a change in phosphorylation of tuberous sclerosis complex (TSC)2, the formation of a TSC1·TSC2 complex, or the binding of raptor with mTOR, or the phosphorylation of eukaryotic elongation factor-2. However, the inhibitory actions of AICAR were associated with a reduction in the phosphorylation of proline-rich Akt substrate (PRAS)-40 and increased phosphorylation of raptor, and these represent potential mechanisms by which AICAR might be expected to inhibit leucine-induced increases in mTOR activity and protein synthesis under in vivo conditions.
Keywords: leucine, protein synthesis, PRAS40, raptor, AMPK
INTRODUCTION
The rate of muscle protein synthesis is controlled by the capacity (e.g., abundance of ribosomes and mRNA) and efficiency (e.g., initiation, elongation and termination) of the synthetic pathway. Several stress conditions, exemplified by sepsis and the related condition of endotoxemia, decrease muscle protein synthesis principally by slowing the rate of peptide-chain initiation (1). This impairment is mediated by a decrease in the kinase activity of mTOR3 (mammalian target of rapamycin), as assessed by reduced phosphorylation of the down stream substrates eukaryotic initiation factor (eIF)-4E binding protein (4E-BP1) and the 70-kDa ribosomal protein S6 kinase (S6K1) (2-5). The canonical mTOR signaling pathway integrates nutrient, hormonal signals, and the intracellular energy state to control mRNA translation. Furthermore, several stresses (e.g.,sepsis, alcohol intoxication, glucocorticoid excess) not only decrease the constitutive rate of muscle protein synthesis, but also acutely limit the normal anabolic response to the branched-chain amino acid leucine, an effect partially mediated by an inability to activate mTOR (3, 6-10).
The 5′-AMP-activated protein kinase (AMPK) is an important sensor for monitoring cellular energy status (11). AMPK regulates carbohydrate, lipid and protein metabolism in muscle via its effects on multiple signaling pathways, and thereby suppresses ATP-demanding processes and activates ATP-repleting pathways. The acute metabolic consequences of AMPK activation on protein balance are mediated in part by the tuberous sclerosis complex gene products (TSC)-1 and TSC2 which are up stream of mTOR (12). Both increased TSC2 phosphorylation and enhanced formation of the TSC1·TSC2 heterodimer can negatively regulate mTOR activity (12, 13). In addition, AMPK activation regulates gene expression of metabolic pathways influencing protein balance (14, 15).
A variety of cellular stressors, including 2-deoxyglucose, nutrient deprivation and exercise, activate AMPK by raising the cellular AMP/ATP ratio. In addition, AMPK can also be activated without altering endogenous adenine nucleotide concentration by the pharmacological agent 5-aminoimidazole-4-carboxamide-1-β-D-ribonucleoside (AICAR) (11). Administration of AICAR in vivo blunts the anabolic actions of electrical stimulation in skeletal muscle (16) and the cardiac hypertrophy produced by pressure overload (17). Similarly, because AMPK is an obligate effector of many cellular responses to nutrient limitation, its activation may blunt the ability of the branched-chain amino leucine to stimulate protein synthesis. In this regard, during the preparation of this manuscript, Du et al (18) reported that incubation of C2C12 myoblasts with AICAR prevented leucine-induced phosphorylation of mTOR and S6K1.
In general, both AICAR and leucine appear to have their effect on skeletal muscle predominantly via alterations in mTOR signaling, as opposed to altering the eIF2/2B system and the formation of 43S preinitiation complex (19-21). Therefore, the current in vivo study focused on the ability of AICAR to modulate the anabolic effects of leucine by regulating steps in peptide-chain initiation involving the binding of mRNA to the 43S preinitiation complex, which is mediated by a functional eIF4F complex. Accordingly, we tested the hypothesis that direct AMPK activation by AICAR attenuates or prevents the ability of leucine to stimulate in vivo protein synthesis via inhibition of mTOR activity in skeletal muscle.
MATERIALS and METHODS
Animal preparation and experimental protocol
The animal protocol was approved by the Institutional Animal Care and Use Committee of The Pennsylvania State University College of Medicine and adheres to the National Institutes of Health guidelines for the use of experimental animals. Specific pathogen-free male Sprague-Dawley rats (175-200 g, Charles River Breeding Laboratories, Cambridge, MA) were housed in a controlled environment and provided food (Harlan Teklad #2018 rodent diet; Madison, WI) and water ad libitum for one week before the start of the study. The commercial diet consisted of approximately 18% protein, 6% fat, and 3.8% fiber.
On the morning of the study, food was removed and rats were injected subcutaneously with AICAR (1 mg/g body weight; Toronto Research Chemicals, Ontario, Canada) to stimulate AMPK. Time-matched control rats were injected with an equal volume of vehicle (sterile 0.9% saline). Rats were orally gavaged with leucine (1.35 g/kg body weight) or an equal volume of saline 60 min after AICAR. Hence, this study contained 4 experimental groups: Saline (Sal) + Sal, Sal + Leu (Leu), AICAR + Sal, and AICAR + Leu. There was no significant difference in the average body weight for rats among these groups (data not shown). The dose and timing for AICAR were selected because they have been reported to decrease basal muscle protein synthesis (20), and the leucine protocol shown to maximally stimulate muscle protein synthesis via increases in mTOR activity and cap-dependent translation (3, 8, 19, 22).
After leucine gavage, rats were anesthetized with intraperitoneal (IP) pentobarbital and a catheter inserted to the carotid artery for blood collection. Exactly 20 min after leucine gavage, protein synthesis was determined using the flooding-dose method (23). Rats were injected with L-[2,3,4,5,6-3H]phenylalanine (Phe; 150 mmol/L, 30 μCi/ml; 1ml/100 g body weight) via the jugular vein and arterial blood collected into heparinized syringes at 2, 6 and 10 min for measurement of plasma Phe-specific radioactivity. Thereafter, a portion of the representative fast-twitch gastrocnemius was freshly homogenized and the remainder freeze-clamped and stored at -70 °C. Muscle was powdered under liquid nitrogen and a portion used to estimate the rate of incorporation of [3H]Phe into protein, exactly as previously described (3, 5, 24).
Plasma hormones and tissue adenine nucleotides
Prior to the injection of [3H]Phe, arterial blood was collected and the plasma used to determine insulin (Linco Research, St. Charles, MO), total insulin-like growth factor (IGF)-I (Immunodiagnostic Systems Limited, Fountain Hills, AZ) and testosterone (DSL, Webster, TX) by enzyme-linked immunosorbent assay. Branched chain amino acids were determined using reverse-phase HPLC after precolumn derivatization of amino acids with phenylisothiocyanate. Powdered gastrocnemius was extracted in cold PCA, neutralized, and used for the determination of ATP, AMP and creatine phosphate (CP) by standard fluorometric methods (25).
Immunoprecipitation and Western blot analysis
The tissue preparation was the same as described by our laboratories (1-3, 6, 8, 26). A muscle homogenate was prepared using a 1:5 ratio of ice-cold homogenization buffer consisting of (in mmol/L) 20 HEPES pH 7.4, 2 EGTA, 50 NaF, 100 KCl, 0.2 EDTA, 50 β-glycerophosphate, 1 DTT, 0.1 PMSF, 1 benzamidine, 0.5 sodium vanadate, plus a protease inhibitor cocktail table from Roche, and clarified by centrifugation. The samples were subjected to SDS-PAGE and the proteins electrophoretically transferred to PVDF membranes. The blots were incubated with either primary antibodies (unless otherwise noted from Cell Signaling, Beverly, MA) to total (C-20) and Thr1462-phosphorylated TSC2, total GβL (G protein β-subunit-like protein; mLST8), total and phosphorylated (Ser792) raptor, total S6K1 (Santa Cruz Biotechnology, Santa Cruz, CA), phospho-specific S6K1 (Thr389;), total 4E-BP1 (Bethyl Laboratories, Montgomery, TX), phospho-specific 4E-BP1 (Thr37/46), total and phosphorylated (Ser1108) eIF4G, total and phosphorylated-S6 (Ser240/Ser244), total and phosphorylated (Thr56) eukaryotic elongation factor (eEF)-2, and total and phosphorylated (Thr246) PRAS40 (Biosource, Camarillo, CA), In addition, to determine activation of AMPK, total and phosphorylated AMPKα (Thr172) and acetyl-CoA carboxylase (ACC; Ser79) were used (14). Blots were washed with TBS-T (1X TBS including 0.1% Tween-20) and incubated with secondary antibody (horseradish peroxidase conjugated goat anti-mouse or goat anti-rabbit) IgG at room temperature. The blots were developed with enhanced chemiluminescence Western blotting reagents, as per the manufacturer’s (Amersham) instructions, and exposed to X-ray film in a cassette equipped with a DuPont Lightning Plus intensifying screen. After development, the film was scanned (Microtek ScanMaker IV) and analyzed using National Institutes of Health Image 1.6 software.
The eIF4E·4EBP1and eIF4E·eIF4G complexes were quantified as described (3, 6, 8, 26). From an aliquot of supernatant, eIF4E was immunoprecipitated using an anti-eIF4E monoclonal antibody (kindly provided by Drs. Jefferson and Kimball; Hershey, PA). Antibody-antigen complexes were collected using magnetic beads, subjected to SDS-PAGE, and proteins transferred to a PVDF membrane. Blots were incubated with a mouse anti-human eIF4E antibody, rabbit anti-rat 4E-BP1 antibody, or rabbit anti-eIF4G antibody.
To maintain protein-protein interaction muscle samples were homogenized in CHAPS buffer consisting of (in mmol/L) 40 HEPES, pH 7.5, 120 NaCl, 1 EDTA, 10 pyrophosphate, 10 β-glycerol phosphate, 50 NaF, 1.5 sodium vanadate, 0.3% CHAPS, and 1 protease inhibitor cocktail tablet. The homogenate was mixed on a platform rocker and clarified by centrifugation. An aliquot of the resulting supernatant was combined with either anti-TSC2, anti-mTOR or anti-raptor antibody and immune complexes isolated with a goat anti-rabbit BioMag IgG beads (PerSeptive Diagnostics). Beads were collected, washed with CHAPS buffer, precipitated by centrifugation, and subjected to SDS-PAGE and analysis as above.
Statistical analysis
Data for each condition are summarized as means ± SE where the number of rats per treatment group is indicated in the legends to the figure or table. Unless otherwise indicated, statistical evaluation of the data was performed using 2-way ANOVA with post-hoc Student-Neuman-Keuls test when the interaction was significant. When necessary, data were logarithmically transformed to normalize the distribution of residuals and to obtain variance homogeneity. Differences were considered significant when P < 0.05.
RESULTS
Plasma amino acid and hormone concentrations
The administration of AICAR in control rats increased the plasma leucine concentration by 77%, compared to time-matched values from Sal + Sal rats (Table 1). Provision of oral leucine increased the plasma leucine concentration 3.7-fold compared to control values. The leucine concentrations achieved in the AICAR + Leucine group were not statistically different from those determined in the Sal + Leu group.
TABLE 1.
Plasma concentration of branched-chain amino acids and insulin in rats treated with AICAR, leucine, both, or neither1
| Leucine | Isoleucine | Valine | Insulin | |
|---|---|---|---|---|
| μmol/L | μmol/L | μmol/L | pmol/L | |
| Sal + Sal | 165 ± 6c | 57 ± 4c | 118 ± 5b | 547 ± 41b |
| AICAR + Sal | 293 ± 16b | 105 ± 4a | 194 ± 10a | 304 ± 44c |
| Sal + Leu | 773 ± 45a | 57 ± 6c | 80 ± 5c | 814 ± 93a |
| AICAR + Leu | 968 ± 93a | 82 ± 9b | 142 ± 13b | 160 ± 46c |
Values are means ± SEM; n = 8-10 per group. Means in a column without a common letter differ, P < 0.05.
Additionally, AICAR increased the plasma concentrations of the other two branched-chain amino acids, isoleucine and valine (84% and 65%, respectively; Table 1). In control rats, oral leucine did not alter plasma isoleucine, but decreased the valine concentration by 32%. Finally, the plasma concentrations of isoleucine and valine in AICAR + Leu group were greater than those determined in the Sal + Leu rats.
AICAR alone decreased the plasma insulin concentration by 45% compared with time-matched values from control rats (Table 1). Conversely, leucine alone increased insulin 49% compared to control values. In contrast, no hyperinsulinemia was detected in rats in the AICAR + Leu group. Finally, neither AICAR nor leucine significantly altered the plasma concentration of either IGF-I or testosterone at the time point assessed (data not shown).
Activation of AMPK, adenine nucleotide concentration, and protein synthesis in muscle
AICAR administered in vivo activated AMPK in gastrocnemius as indicated by the increased Thr172 phosphorylation (control = 1.00 ± 0.07 vs AICAR = 2.33 ± 0.36 fold of Sal control; P < 0.05). Furthermore, AICAR increased Ser79 phosphorylation of ACC, a well-established substrate of AMPK (control = 1.00 ± 0.11 vs AICAR = 1.79 ± 0.21 fold of Sal control; P < 0.05).
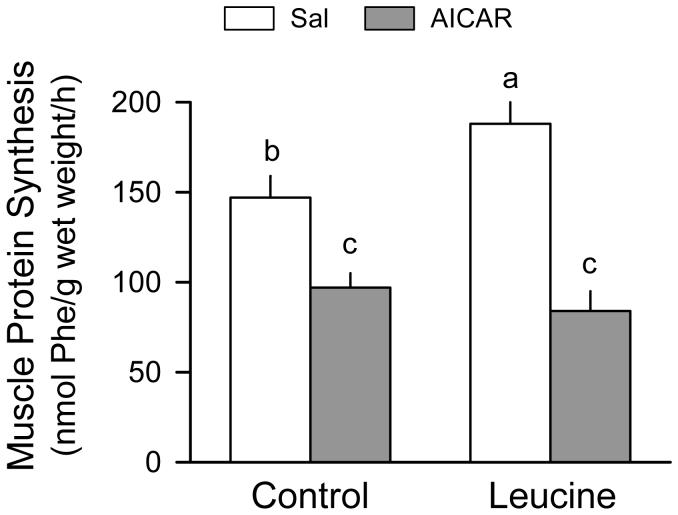
The in vivo-determined rate of muscle protein synthesis was reduced 34% by AICAR (Fig. 1). Protein synthesis was increased 28% in the Sal + Leu group compared with values from the Sal + Sal group. In contrast, no leucine-induced increase in protein synthesis was detected in gastrocnemius from AICAR-treated rats.
Figure 1.
Skeletal muscle (gastrocnemius) protein synthesis in rats treated with AICAR, leucine, both, or neither. Values in bar graph are means ± SEM; n = 8-10 rats per group. Means without a common letter differ, P < 0.05. There was no difference in the dry-to-wet weight ratios of muscle from control and AICAR-treated rats (data not shown).
There was no significant difference between the AMP, ATP or CP concentration of gastrocnemius 1 h after the injection of AICAR, compared to time-matched control values (Table 2). Likewise, the adenine nucleotide concentration of muscle was not altered by the oral administration of leucine in either control or AICAR-treated rats. As a consequence, the AMP-to-ATP ratio in skeletal muscle, which is a principal mediator of AMPK activation, was not different between the experimental groups (data not shown).
TABLE 2.
Adenine nucleotide concentration in gastrocnemius from rats treated with AICAR, leucine, both, or neither1
| AMP | ATP | CP | |
|---|---|---|---|
| μmol/g muscle | |||
| Sal + Sal | 0.115 ± 0.007 | 7.51 ± 0.22 | 22.4 ± 1.1 |
| AICAR + Sal | 0.108 ± 0.005 | 7.72 ± 0.19 | 21.9 ± 1.2 |
| Sal + Leu | 0.112 ± 0.006 | 7.69 ± 0.24 | 23.7 ± 0.9 |
| AICAR + Leu | 0.111 ± 0.007 | 7.73 ± 0.18 | 23.5 ± 1.6 |
Values are means ± SEM; n = 8-10 per group. There was no statistical difference among any of the four groups, as analyzed by ANOVA, for AMP, ATP or CP.
Alterations in mTOR signaling
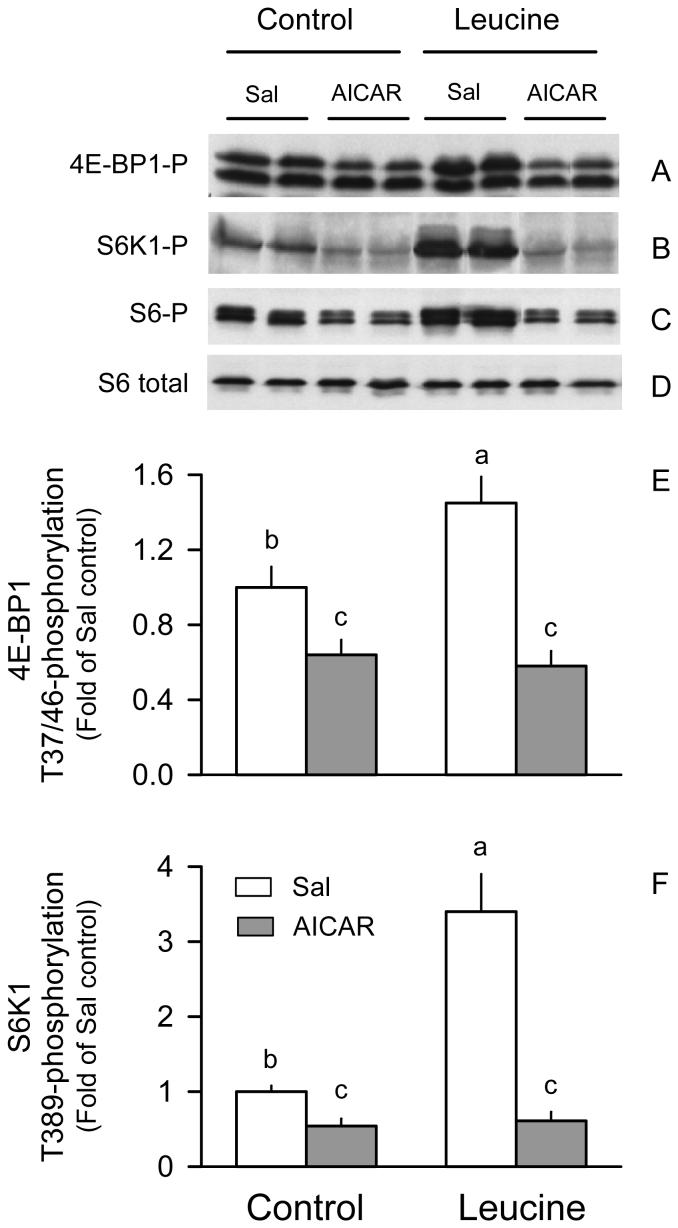
The kinase activity of mTOR was not directly examined but a functional readout of its activity was determined by quantifying phosphorylation of 4E-BP1 and S6K1 at sites (Thr37/46 and Thr389, respectively) known to be phosphorylated by mTOR (27, 28). AICAR decreased Thr37/46 phosphorylation of 4E-BP1 by 40%, compared to values from the Sal + Sal group (Fig. 2A and 2E). Conversely, oral leucine increased 4E-BP1 phosphorylation by 45% in control rats. In contrast, no such hyperphosphorylation of 4E-BP1 was detected in gastrocnemius of rats in the AICAR + Leu group.
Figure 2.
Phosphorylation of 4E-BP1 and S6K1 in gastrocnemius of rats treated with AICAR, leucine, both, or neither. Representative immunoblots of Thr37/46-phosphorylated (P) 4E-BP1, Thr389-phosphorylated S6K1, and Ser240/Ser244-phosphorylated ribosomal protein S6 in gastrocnemius (A-C, respectively). For Thr37/46 phosphorylated 4E-BP1, the β- (bottom band) and γ- (top band) isoforms are identified. The total amount of S6 protein was unchanged by the various treatments and indicates equal protein loading per lane (D). No significant differences in total 4E-BP1 and S6K1 were detected between groups (data not shown). Bar graphs quantitating the densitometric analysis of all immunoblots for phosphorylated 4E-BP1 (E) and S6K1 (F). The value from the Sal + Sal group was set at 1.0. Values in bar graph are means ± SEM; n = 8-10 rats per group. Means without a common letter differ, P < 0.05.
AICAR decreased both basal S6K1 phosphorylation and completely prevented the 3-fold leucine-induced increase (Fig. 2B and 2F). Comparable AICAR- and leucine-induced changes in the phosphorylation of other sites on S6K1 were observed with a phosphospecific antibody for Thr421/Ser424 (data not shown). AICAR- and leucine-induced changes in the Ser240/Ser244-phosphorylation of the ribosomal protein (rp) S6 were comparable to the above mentioned changes in S6K1 (Figure 2C).
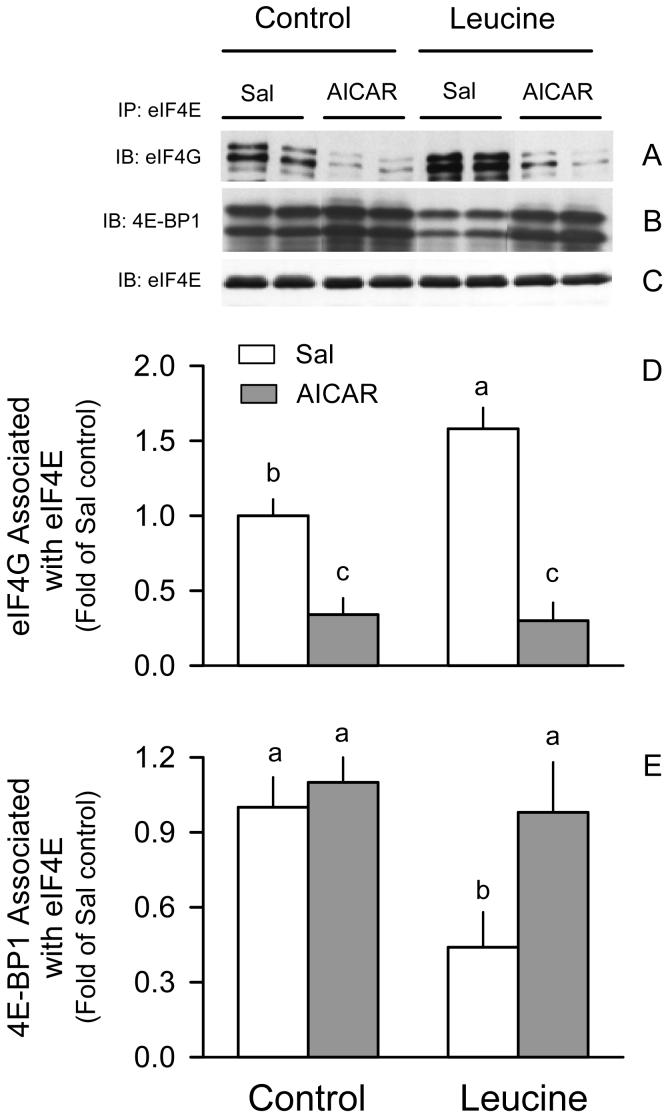
AICAR alone decreased the active eIF4E·eIF4G complex by 65% in muscle from rats in the Sal + Sal group (Fig. 3A and 3D). Contrary to expectations, we did not detect a significant increase in the inactive eIF4E·4EBP1 complex (Fig. 3B and 3E). While leucine increased eIF4E·eIF4G and produced a reciprocal decrease in the formation of the eIF4E·4EBP1 complex in control rats, AICAR completely prevented the leucine-induced redistribution of eIF4E between the inactive eIF4e·4EBP1 and active eIF4E·eIF4G complex.
Figure 3.
Distribution of eIF4E in gastrocnemus of rats treated with AICAR, leucine, both, or neither. Representative immunoblots (IB) for eIF4G (A), 4E-BP1 (B), and eIF4E (C) of the eIF4E immunoprecipitate (IP). The β- and α-isoforms for 4E-BP1 are so indicated (top and bottom bands, respectively). Bar graphs quantitate the densitometric analysis of all immunoblots for eIF4G bound to eIF4E (D) and 4E-BP1 bound to eIF4E (E). The value from the Sal + Sal group was set at 1.0. Values in bar graph are means ± SEM; n = 8-10 rats per group. Means without a common letter differ, P < 0.05.
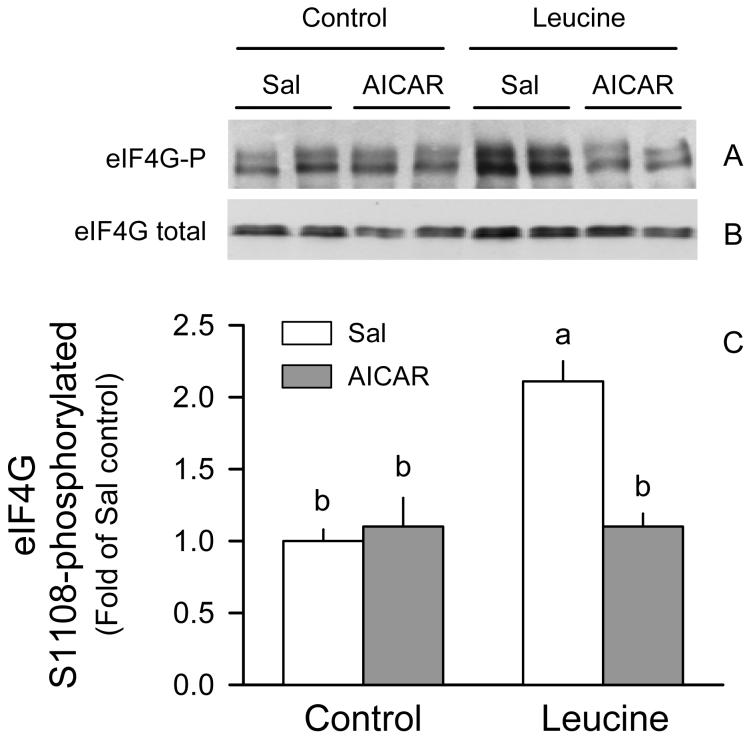
The basal level of eIF4G phosphorylation was not different between rats in the AICAR + Sal and Sal + Sal groups (Fig. 4A and 4C). However, AICAR essentially completely prevented the leucine-induced increase in Ser1108 phosphorylation of eIF4G. All of these changes were independent of a change in total eIF4G in muscle (Fig. 4B).
Figure 4.
Phosphorylation of eIF4G in gastrocnemius of rats treated with AICAR, leucine, both, or neither. Representative immunoblot of Ser1108-phosphorylated (A) and total eIF4G (B), and densitometric analysis of all immunoblots for phosphorylated eIF4G normalized for total eIF4G (C). The value from the Sal + Sal group was set at 1.0. Values in bar graph are means ± SEM; n = 8-10 rats per group. Means without a common letter differ, P < 0.05.
Potential regulators of mTOR activity
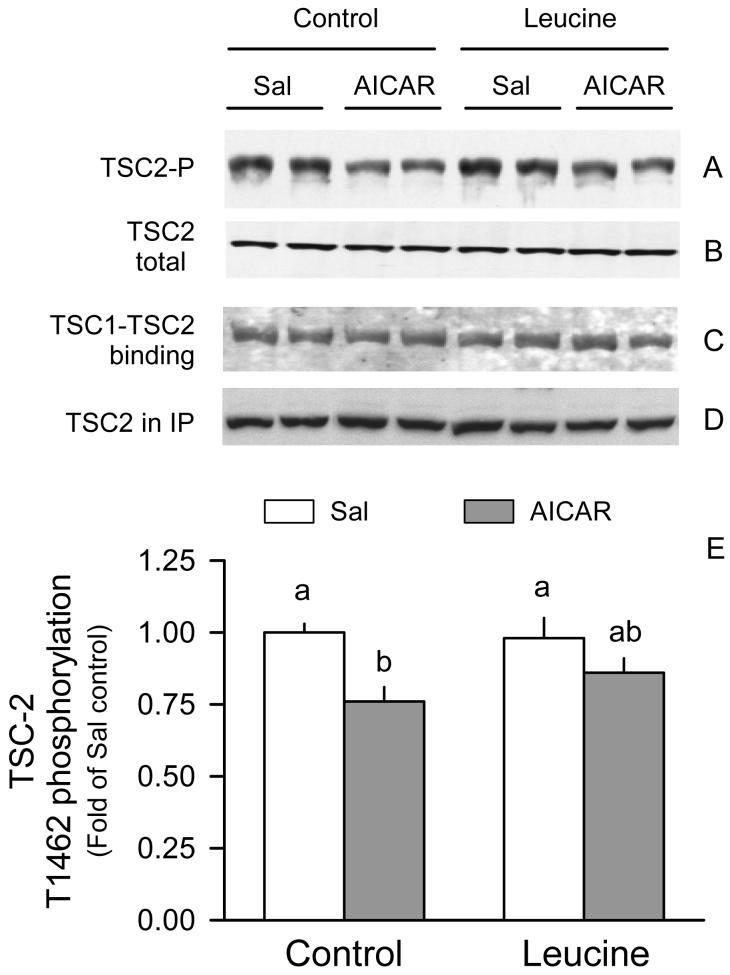
AICAR decreased TSC2 phosphorylation by 25% in gastrocnemius in control rats (Fig. 5A and 5E). However, this decreased TSC2 phosphorylation was not associated with a concomitant change in the amount of TSC1·TSC2 complex in response to AICAR (Fig. 5C). Additionally, leucine did not significantly alter TSC2 phosphorylation or TSC1·TSC2 binding in either the basal state or in rats treated with AICAR.
Figure 5.
TSC2 phosphorylation and binding to TSC1 in gastrocnemius of rats treated with AICAR, leucine, both, or neither. Total TSC2 (A) and Thr1462-phosphorylated TSC2 (B) was determined in whole muscle homogenates from the four groups of rats. TSC2 was immunoprecipitated (IP) under conditions to maintain protein-protein interactions and the amount bound to TSC1 was assessed by immunoblotting (IB) (C). TSC2 in the IP was also determined (D). Bar graph represents quantitation of the densitometric analysis of all immunoblots for TSC2 phosphorylation (E). The value from the Sal + Sal group was set at 1.0. Values in bar graph are means ± SEM; n = 8-10 rats per group. Means without a common letter differ, P < 0.05.
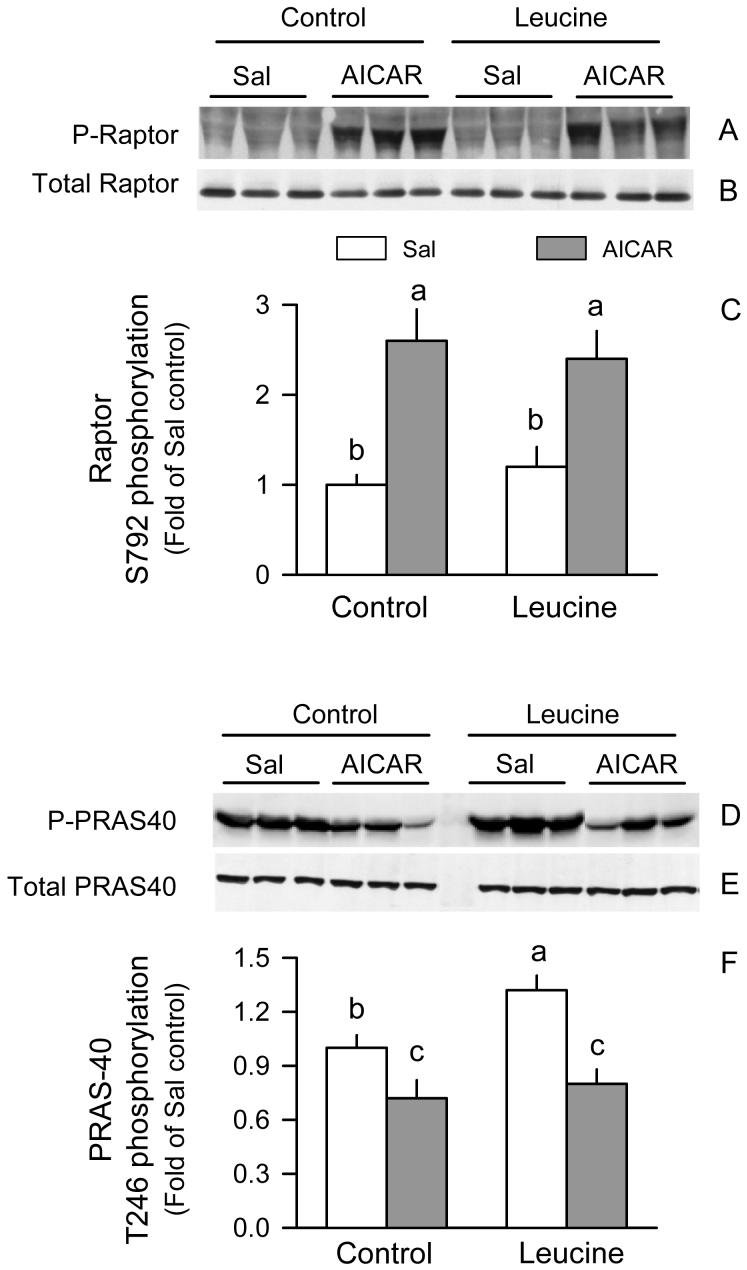
The mTOR complex 1 (mTORC1), which is composed of at least four proteins mTOR, raptor and GβL, and PRAS40 was next determined because of its predominant role in nutrient regulation of protein synthesis (29). Western blot analysis of mTOR, raptor, GβL or PRAS40 in whole muscle homogenates did not demonstrate a significance change for any of these three proteins in response to AICAR and/or leucine (data not shown). However, the extent of Ser792 phosphorylated raptor in muscle from rats in the AICAR + Sal group was 2-fold of values in the Sal + Sal group; a similar increment was seen in the AICAR + Leu group (Fig. 6A and 6C). Leucine alone did not significantly alter raptor phosphorylation. As described above, this AICAR-induced increase in raptor phosphorylation was independent of a change in total raptor in tissue homogenates (Fig.6B). Conversely, AICAR decreased Thr246 phosphorylation of PRAS40 in muscle homogenates from control rats (Fig. 6D and 6F). PRAS40 phosphorylation was increased in the Sal + Leu group but not in those rats treated with AICAR, and these changes were independent of a AICAR- or leucine-induced change in total PRAS40 in muscle (Fig. 6E).
Figure 6.
Phosphorylation of raptor and PRAS40 in gastrocnemius of rats treated with AICAR, leucine, both, or neither. Representative Western blots of total and phosphorylation raptor (Ser792) in whole muscle homogenates (A and B, respectively), and quantitation of densitometric analysis of all immunoblots for phosphorylated raptor (C). Representative Westerns blot of total and phosphorylated (Thr246) PRAS40 in whole muscle homogenates (D and E, respectively), and quantitation of densitometric analysis of all immunoblots for phosphorylated raptor (F). For bar graphs, the value from the Sal + Sal group was set at 1.0. Values in bar graph are means ± SEM; n = 8-10 rats per group. Means without a common letter differ, P < 0.05.
We did not detect any difference among the four groups in the amount of mTOR, GβL or PRAS40 bound to raptor, using homogenization conditions which maintain protein-protein interactions (data not shown). The association of raptor to mTOR was also not changed when mTOR was immunoprecipitated and raptor immunoblotted (data not shown). As a “positive control” we immunoprecipitated mTOR from muscle of rats treated with rapamycin and were able to demonstrate a >85% reduction in the interaction of raptor with mTOR (data not shown).
eEF2 phosphorylation
Select catabolic agents may also impair protein synthesis via enhanced phosphorylation (Thr56) of eEF and inhibition of translation elongation (30). However, at the time point assessed, the phosphorylation of eEF2 in muscle was not significantly altered by AICAR (control = 1.00 ± 0.11 AU vs AICAR = 0.94 ± 0.08 AU). The amount of total eEF2 was also not altered by either AICAR and/or leucine (data not shown).
DISCUSSION
The in vivo administration of AICAR and activation of AMPK decreases basal protein synthesis and largely prevents the increment expected in response to leucine in fast-twitch skeletal muscle. These responses are comparable to those seen in other stress conditions such as sepsis and endotoxemia (3, 6, 7, 15). In vivo-administered AICAR decreased 4E-BP1 phosphorylation in muscle under basal conditions. Although hypophosphorylated 4E-BP1 interacts more avidly with eIF4E than hyperphosphorylated forms of the protein (31), we did not observe a concomitant increase in the inactive eIF4E·4EBP1 complex as anticipated. However, AICAR did markedly decrease the binding of eIF4E with eIF4G. While we cannot explain this dichotomy, a change in eIF4E·eIF4G without a reciprocal change in eIF4E·4EBP1 has been reported previously (24, 32). As most mammalian mRNAs are translated in a cap-dependent fashion involving eIF4E, in conjunction with a host of other proteins (31), the AICAR-induced dephosphorylation of 4E-BP1 and the reduction in the active eIF4E·eIF4G complex is consistent with the reduction in basal muscle protein synthesis. AICAR also decreased the phosphorylation of S6K1, another down stream target of mTOR, and this change is consistent with the reduction in protein synthesis observed under a wide variety of conditions (33) and with the smaller phenotype of knockout mice lacking S6K1 (34). However, AICAR did not decrease basal Ser1108-phosphorylation of eIF4G. As eIF4G is also down stream of mTOR, this finding was not expected under conditions where both 4E-BP1 and S6K1 phosphorylation was reduced. Although the reason for this apparent discrepancy is currently unclear, such a differential response has been reported at early time points in myocytes treated with AICAR (13).
A possible mechanism by which AMPK might cause mTOR dysregulation involves its inhibition of AKT-dependent phosphorylation of TSC2 and/or the binding of TSC2 with its homolog TSC1 which increases the binding of GDP to Rheb (Ras homolog enriched in brain) and thereby inhibits mTOR activity. Previous studies using cultured myocytes have demonstrated such changes in TSC2 (13). However, under in vivo conditions, AMPK activation in muscle resulted in a relatively small decrease in TSC2 phosphorylation under basal conditions, and this change was not associated with a change in either TSC1·TSC2 binding or the total amount of TSC1 or TSC2 protein.
Alternatively, mTOR activity can be regulated by its binding to various proteins, including raptor, which is sensitive to nutrient and hormonal regulation (29). Incubation of cultured myocytes with AICAR increases the binding of raptor to mTOR (13), which may imply AICAR promotes a “closed conformation” in the mTOR·raptor complex rendering it less active. However, in other cells AICAR does not alter mTOR·raptor complex function (35) and whether raptor enhances or represses mTOR activity remains controversial (36, 37). Regardless, our present data fail to confirm any change in mTOR·raptor binding in skeletal muscle in response to AICAR. Furthermore, there was no AICAR-induced change in either the total amount of GβL, a positive regulator of mTOR activity (38), or its binding to mTORC1.
The mTORC1 complex also binds PRAS40 via its interaction with raptor (39, 40). Over expression of PRAS40 inhibits mTOR autophosphorylation and kinase activity. However, the ability of in vivo administered AICAR to inhibit basal muscle protein synthesis was not associated with a change in either the total amount of PRAS40 or its binding with raptor in the mTORC1. PRAS40 is also a phospho-protein and the extent of its phosphorylation is inversely proportional to the concentration of PRAS40 (39, 40), suggesting PRAS40 is also a down stream substrate for mTOR. Our data, showing that AICAR decreases basal PRAS40 phosphorylation, are consistent with this view.
Recently, AICAR-induced activation of AMPK in myocytes and other cells has been reported to increase the phosphorylation of raptor, via a TSC2 independent mechanism, and thereby impair mTORC1 kinase activity (35). In the current study the leucine-induced stimulation of muscle protein synthesis was no associated with a change in raptor phosphorylation. However, ACIAR markedly increased Ser792-phosphorylation of raptor in muscle of control rats. Because raptor phosphorylation was similarly increased in both control + AICAR and leucine + AICAR rats, the enhanced phosphorylation of this mTORC1 complex protein remains a possible mechanism by which AICAR produces leucine resistance in skeletal muscle.
In addition to its role as a precursor molecule, leucine also regulates protein synthesis by activating intracellular signaling pathways stimulating translation initiation (40). Our current data which show increased phosphorylation of 4E-BP1, S6K1, S6 and eIF4G as well as increased eIF4E·eIF4G complex after leucine are consistent with reports indicating stimulation in cap-dependent translation and mTOR activity (21). However, increased mTOR activity could not be ascribed to a change in mTOR·raptor binding, phosphorylation of raptor, or the association of PRAS40 with raptor. The inability of amino acids to alter PRAS40·raptor binding in cultured cells has been reported and differs from the marked decrease in PRAS40·raptor seen in insulin-treated cells (39). Furthermore, oral administration of leucine to rats also failed to alter TSC2 phosphorylation or its binding with TSC1, results consistent with studies showing that nutrients can activate mTOR by a TSC-independent pathway (41).
Several catabolic conditions, including sepsis, alcohol abuse, and glucocorticoid excess in aged rats, produce a leucine resistance in striated muscle (3, 6-10). AICAR also prevents mTOR activation and hypertrophy in cardiac muscle in response to pressure overload (17) and in skeletal muscle following electrical stimulation (16). Our results indicate that AICAR-induced activation of AMPK also antagonizes the normal protein anabolic response to leucine. The ability of AICAR to produce leucine resistance is associated with inhibition of mTOR activity, as evidenced by the failure of leucine to enhance 4E-BP1 and S6K1 phosphorylation. Further, this AICAR-induced defect in 4E-BP1 phosphorylation seems the likely cause for the inability of leucine to alter the distribution of eIF4E between the active and inactive complexes. Moreover, AICAR essentially prevented the ability of leucine to increase eIF4G phosphorylation. A similar reduction in leucine-stimulated eIF4G phosphorylation has been reported in muscle of rats given the mTOR inhibitor rapamycin (42). Conversely, the AICAR-induced decrement in mTOR activity could not be attributed to a change in TSC2 phosphorylation, the binding of TSC2 with TSC1, or to the amount of mTOR·raptor or PRAS40·raptor complex. It remains possible that AICAR caused leucine resistance by impairing the subsequent movement of the mTOR·raptor complex to the eIF3-preinitiation complex (48) or via activation of human vacuolar protein sorting (hVps)-34 which is a class III phosphatidylinositol 3-kinase (43), however these possibilities were not pursed in the current investigation.
The ability of AICAR to inhibit protein synthesis appears to be mediated primarily by inhibition of translation initiation because in the current study AICAR did not alter the expression or phosphorylation of eEF2. Hyperphosphorylation of eEF2 renders this protein inactive and thereby inhibits protein synthesis (30). However, our data are in apparent contrast to those reported in rat muscle 40 min after AICAR administration (16). The difference between our study and this earlier report maybe related to the relatively transient effect of AICAR on this particular parameter. This is exemplified by in vitro studies showing AICAR increases eEF2 phosphorylation time-dependently, with eEF2 phosphorylation being increased at 15-30 min but comparable to basal levels at 60 min (13).
The AICAR-induced decrease in muscle protein synthesis also could not be attributed to a difference in the circulating concentrations of the branched-chain amino acids. Leucine, isoleucine and valine concentrations were as high in rats administered AICAR + leucine as in animals from the Sal + leucine group. However, AICAR attenuated the leucine-induced hyperinsulinemia. Therefore, the lack of a permissive effect of insulin with leucine in the AICAR + Leucine treated rats might partially explain the inability of muscle protein synthesis to increase after AICAR. The role of the transient leucine-induced hyperinsulinemia in mediating the anabolic effect of leucine on muscle protein synthesis is currently unclear (see discussion in reference #44), and the relative importance of this diminished insulin response in AICAR treated rats versus a direct effect on muscle per se will need to be defined in future studies. However, it is noteworthy that AICAR has been shown to inhibit both basal and leucine-stimulated mTOR activity in cultured myocytes (18), suggesting that at least a portion of the AICAR effect is mediated directly within muscle.
Finally, previous studies in myoblasts suggested the ability of leucine to stimulate protein synthesis is mediated by decreased AMPK activity resulting from a concomitant elevation in the ATP concentration (18). However, such an effect of leucine on AMPK activity and ATP concentration in skeletal muscle under in vivo conditions was not observed in the current study. Therefore, while the anabolic effect of leucine on mTOR-stimulated protein synthesis appears mediated via a suppression of AMPK activity in myoblasts incubated under conditions of low serum and energy substrates, such a mechanism is clearly not operational in skeletal muscle under in vivo conditions.
In conclusion, we describe herein that stimulation of AMPK activity by the chemical activator AICAR produces a muscle leucine resistance under in vivo conditions. The diminution of the anabolic response to leucine by AICAR is associated with a reduction in mTOR activity (evidenced by the decreased phosphorylation of 4E-BP1, S6K1, eIF4G, and PRAS40), maintained enhanced raptor phosphorylation, and a reduction in the active eIF4E·eIF4G complex. Moreover, these changes were independent of changes in TSC2 phosphorylation, TSC1·TSC2 heterodimer formation, as well as the binding of either mTOR or PRAS40 with raptor. Finally, our data suggest that some mechanisms regulating mTOR activity in cell culture may not necessarily be operational under in vivo conditions.
Footnotes
This work was supported by NIH grants GM48032, AA 11290 (C.H.L.), and GM39277 (T.C.V).
- ACC
- acetyl-CoA carboxylase
- AICAR
- 5-aminoimidazole-4-carboxamide-1-β-D-ribonucleoside
- AMPK
- 5′-AMP-activated protein kinase
- ANOVA
- analysis of variance
- AU
- arbitrary units
- CP
- creatine phosphate
- DTT
- dithiotreitol
- EDTA
- ethylenediaminetetraacetic acid
- EGTA
- ethylene glycol tetraacetic acid
- eEF
- eukaryotic elongation factor
- eIF
- eukaryotic initiation factor
- 4E-BP1
- eukaryotic initiation factor 4E binding protein-1
- GβL
- G protein β-subunit-like protein
- HEPES
- 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- IGF
- insulin-like growth factor
- IP
- intraperitoneal
- Leu
- leucine
- mTOR
- mammalian target of rapamycin
- PAGE
- polyacrylamide gel electrophoresis
- Phe
- phenylalanine
- PRAS40
- proline-rich Akt substrate 40
- Sal
- saline
- SDS
- sodium dodecyl sulfate
- Ser
- serine
- S6K1
- 70-kDa ribosomal protein S6 kinase
- Thr
- theronine
- TSC
- tuberous sclerosis complex
Literature Cited
- 1.Lang CH, Frost RA, Vary TC. Regulation of muscle protein synthesis during sepsis and inflammation. Am J Physiol Endocrinol Metab. 2007;293:E453–E459. doi: 10.1152/ajpendo.00204.2007. [DOI] [PubMed] [Google Scholar]
- 2.Lang CH, Frost RA, Jefferson LS, Kimball SR, Vary TC. Endotoxin-induced decrease in muscle protein synthesis is associated with changes in eIF2B, eIF4E, and IGF-I. Am J Physiol Endocrinol Metab. 2000;278:E1133–E1143. doi: 10.1152/ajpendo.2000.278.6.E1133. [DOI] [PubMed] [Google Scholar]
- 3.Lang CH, Frost RA. Endotoxin disrupts the leucine-signaling pathway involving phosphorylation of mTOR, 4E-BP1, and S6K1 in skeletal muscle. J Cell Physiol. 2005;203:144–55. doi: 10.1002/jcp.20207. [DOI] [PubMed] [Google Scholar]
- 4.Orellana RA, Jeyapalan A, Escobar J, Frank JW, Nguyen HV, Suryawan A, Davis TA. Amino acids augment muscle protein synthesis in neonatal pigs during acute endotoxemia by stimulating mTOR-dependent translation initiation. Am J Physiol Endocrinol Metab. 2007;293:E1416–E1425. doi: 10.1152/ajpendo.00146.2007. [DOI] [PubMed] [Google Scholar]
- 5.Svanberg E, Frost RA, Lang CH, Isgaard J, Jefferson LS, Kimball SR, Vary TC. IGF-I/IGFBP-3 binary complex modulates sepsis-induced inhibition of protein synthesis in skeletal muscle. Am J Physiol Endocrinol Metab. 2000;279:E1145–E1158. doi: 10.1152/ajpendo.2000.279.5.E1145. [DOI] [PubMed] [Google Scholar]
- 6.Lang CH, Frost RA. Differential effect of sepsis on ability of leucine and IGF-I to stimulate muscle translation initiation. Am J Physiol Endocrinol Metab. 2004;287:E721–E730. doi: 10.1152/ajpendo.00132.2004. [DOI] [PubMed] [Google Scholar]
- 7.Lang CH, Pruznak AM, Frost RA. TNFalpha mediates sepsis-induced impairment of basal and leucine-stimulated signaling via S6K1 and eIF4E in cardiac muscle. J Cell Biochem. 2005;94:419–31. doi: 10.1002/jcb.20311. [DOI] [PubMed] [Google Scholar]
- 8.Lang CH, Frost RA. Glucocorticoids and TNFalpha interact cooperatively to mediate sepsis-induced leucine resistance in skeletal muscle. Mol Med. 2006;12:291–9. doi: 10.2119/2006-00071.Lang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Connor PM, Kimball SR, Suryawan A, Bush JA, Nguyen HV, Jefferson LS, Davis TA. Regulation of translation initiation by insulin and amino acids in skeletal muscle of neonatal pigs. Am J Physiol Endocrinol Metab. 2003;285:E40–E53. doi: 10.1152/ajpendo.00563.2002. [DOI] [PubMed] [Google Scholar]
- 10.Rieu I, Sornet C, Grizard J, Dardevet D. Glucocorticoid excess induces a prolonged leucine resistance on muscle protein synthesis in old rats. Exp Gerontol. 2004;39:1315–21. doi: 10.1016/j.exger.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Hardie DG, Sakamoto K. AMPK: a key sensor of fuel and energy status in skeletal muscle. Physiology. 2006;21:48–60. doi: 10.1152/physiol.00044.2005. [DOI] [PubMed] [Google Scholar]
- 12.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–90. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 13.Williamson DL, Bolster DR, Kimball SR, Jefferson LS. Time course changes in signaling pathways and protein synthesis in C2C12 myotubes following AMPK activation by AICAR. Am J Physiol Endocrinol Metab. 2006;291:E80–E89. doi: 10.1152/ajpendo.00566.2005. [DOI] [PubMed] [Google Scholar]
- 14.Krawiec BJ, Nystrom GJ, Frost RA, Jefferson LS, Lang CH. AMP-activated protein kinase agonists increase mRNA content of the muscle-specific ubiquitin ligases MAFbx and MuRF1 in C2C12 cells. Am J Physiol Endocrinol Metab. 2007;292:E1555–E1567. doi: 10.1152/ajpendo.00622.2006. [DOI] [PubMed] [Google Scholar]
- 15.Nystrom GJ, Lang CH. Sepsis and AMPK activation by AICAR differentially regulate FoxO-1, -3, and -4 mRNA in striated muscle. Int J Clin Exp Med. 2007;1:1–14. [PMC free article] [PubMed] [Google Scholar]
- 16.Thomson DM, Fick CA, Gordon SE. AMPK Activation Attenuates S6K1, 4E-BP1, and eEF2 Signaling Responses to High-frequency Electrically Stimulated Skeletal Muscle Contractions. J Appl Physiol. 2008;164:625–32. doi: 10.1152/japplphysiol.00915.2007. [DOI] [PubMed] [Google Scholar]
- 17.Li HL, Yin R, Chen D, Liu D, Wang D, Yang Q, Dong YG. Long-term activation of adenosine monophosphate-activated protein kinase attenuates pressure-overload-induced cardiac hypertrophy. J Cell Biochem. 2007;100:1086–99. doi: 10.1002/jcb.21197. [DOI] [PubMed] [Google Scholar]
- 18.Du M, Shen QW, Zhu MJ, Ford SP. Leucine stimulates mammalian target of rapamycin signaling in C2C12 myoblasts in part through inhibition of adenosine monophosphate-activated protein kinase. J Anim Sci. 2007;85:919–27. doi: 10.2527/jas.2006-342. [DOI] [PubMed] [Google Scholar]
- 19.Anthony JC, Anthony TG, Kimball SR, Vary TC, Jefferson LS. Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation. J Nutr. 2000;130:139–45. doi: 10.1093/jn/130.2.139. [DOI] [PubMed] [Google Scholar]
- 20.Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem. 2002;277:23977–80. doi: 10.1074/jbc.C200171200. [DOI] [PubMed] [Google Scholar]
- 21.Kimball SR, Jefferson LS. Amino acids as regulators of gene expression. Nutr Metab. (Lond) 2004:1–3. doi: 10.1186/1743-7075-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crozier SJ, Kimball SR, Emmert SW, Anthony JC, Jefferson LS. Oral leucine administration stimulates protein synthesis in rat skeletal muscle. J Nutr. 2005;135:376–82. doi: 10.1093/jn/135.3.376. [DOI] [PubMed] [Google Scholar]
- 23.Garlick PJ, McNurlan MA, Preedy VR. A rapid and convenient technique for measuring the rate of protein synthesis in tissues by injection of [3H]phenylalanine. Biochem J. 1980;192:719–23. doi: 10.1042/bj1920719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vary TC, Jefferson LS, Kimball SR. Amino acid-induced stimulation of translation initiation in rat skeletal muscle. Am J Physiol. 1999;277:E1077–E1086. doi: 10.1152/ajpendo.1999.277.6.E1077. [DOI] [PubMed] [Google Scholar]
- 25.Lowry OH, Passonneau JV. A flexible system of enzymatic analysis. Academic Press; New York: 1972. [Google Scholar]
- 26.Lang CH, Frost RA. Sepsis-induced suppression of skeletal muscle translation initiation mediated by tumor necrosis factor alpha. Metabolism. 2007;56:49–57. doi: 10.1016/j.metabol.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 27.Gingras AC, Raught B, Gygi SP, Niedzwiecka A, Miron M, Burley SK, Polakiewicz RD, Wyslouch-Cieszynska A, Aebersold R, Sonenberg N. Hierarchical phosphorylation of the translation inhibitor 4E-BP1. Genes Dev. 2001;15:2852–64. doi: 10.1101/gad.912401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah OJ, Anthony JC, Kimball SR, Jefferson LS. 4E-BP1 and S6K1: translational integration sites for nutritional and hormonal information in muscle. Am J Physiol Endocrinol Metab. 2000;279:E715–E729. doi: 10.1152/ajpendo.2000.279.4.E715. [DOI] [PubMed] [Google Scholar]
- 29.Corradetti MN, Guan KL. Upstream of the mammalian target of rapamycin: do all roads pass through mTOR? Oncogene. 2006;25:6347–60. doi: 10.1038/sj.onc.1209885. [DOI] [PubMed] [Google Scholar]
- 30.Browne GJ, Finn SG, Proud CG. Stimulation of the AMP-activated protein kinase leads to activation of eukaryotic elongation factor 2 kinase and to its phosphorylation at a novel site, serine 398. J Biol Chem. 2004;279:12220–31. doi: 10.1074/jbc.M309773200. [DOI] [PubMed] [Google Scholar]
- 31.Gingras AC, Gygi SP, Raught B, Polakiewicz RD, Abraham RT, Hoekstra MF, Aebersold R, Sonenberg N. Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism. Genes Dev. 1999;13:1422–37. doi: 10.1101/gad.13.11.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr. 2000;130:2413–9. doi: 10.1093/jn/130.10.2413. [DOI] [PubMed] [Google Scholar]
- 33.Jastrzebski K, Hannan KM, Tchoubrieva EB, Hannan RD, Pearson RB. Coordinate regulation of ribosome biogenesis and function by the ribosomal protein S6 kinase, a key mediator of mTOR function. Growth Factors. 2007;25:209–26. doi: 10.1080/08977190701779101. [DOI] [PubMed] [Google Scholar]
- 34.Pende M, Um SH, Mieulet V, Sticker M, Goss VL, Mestan J, Mueller M, Fumagalli S, Kozma SC, Thomas G. S6K1(-/-)/S6K2(-/-) mice exhibit perinatal lethality and rapamycin-sensitive 5′-terminal oligopyrimidine mRNA translation and reveal a mitogen-activated protein kinase-dependent S6 kinase pathway. Mol Cell Biol. 2004;24:3112–24. doi: 10.1128/MCB.24.8.3112-3124.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–26. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S, Tokunaga C, Avruch J, Yonezawa K. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110:177–89. doi: 10.1016/s0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- 37.Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–75. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 38.Kim DH, Sarbassov DD, Ali SM, Latek RR, Guntur KV, Erdjument-Bromage H, Tempst P, Sabatini DM. GbetaL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol Cell. 2003;11:895–904. doi: 10.1016/s1097-2765(03)00114-x. [DOI] [PubMed] [Google Scholar]
- 39.Fonseca BD, Smith EM, Lee VH, MacKintosh C, Proud CG. PRAS40 is a target for mammalian target of rapamycin complex 1 and is required for signaling downstream of this complex. J Biol Chem. 2007;282:24514–24. doi: 10.1074/jbc.M704406200. [DOI] [PubMed] [Google Scholar]
- 40.Thedieck K, Polak P, Kim ML, Molle KD, Cohen A, Jeno P, Arrieumerlou C, Hall MN. PRAS40 and PRR5-like protein are new mTOR interactors that regulate apoptosis. PLoS ONE. 2007;2:e1217. doi: 10.1371/journal.pone.0001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith EM, Finn SG, Tee AR, Browne GJ, Proud CG. The tuberous sclerosis protein TSC2 is not required for the regulation of the mammalian target of rapamycin by amino acids and certain cellular stresses. J Biol Chem. 2005;280:18717–27. doi: 10.1074/jbc.M414499200. [DOI] [PubMed] [Google Scholar]
- 42.Vary TC, Anthony JC, Jefferson LS, Kimball SR, Lynch CJ. Rapamycin blunts nutrient stimulation of eIF4G, but not PKCepsilon phosphorylation, in skeletal muscle. Am J Physiol Endocrinol Metab. 2007;293:E188–E196. doi: 10.1152/ajpendo.00037.2007. [DOI] [PubMed] [Google Scholar]
- 43.Gulati P, Gaspers LD, Dann SG, Joaquin M, Nobukuni T, Natt F, Kozma SC, Thomas AP, Thomas G. Amino acids activate mTOR complex 1 via Ca2+/CaM signaling to hVps34. Cell Metab. 2008;7:456–65. doi: 10.1016/j.cmet.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anthony JC, Lang CH, Crozier SJ, Anthony TG, MacLean DA, Kimball SR, Jefferson LS. Contribution of insulin to the translational control of protein synthesis in skeletal muscle by leucine. Am J Physiol Endocrinol Metab. 2002;282:E1092–E1101. doi: 10.1152/ajpendo.00208.2001. [DOI] [PubMed] [Google Scholar]