Abstract
Met5-enkephalin (ME)-induced cardioprotection occurs via epidermal growth factor receptor (EGFR) transactivation with the subsequent activation of phosphatidylinositol 3-kinase (PI3K). In the present study, we investigated whether there is a sex difference in ME-elicited PI3K signaling. Neonatal murine cardiomyocytes were isolated by collagenase digestion and subjected to 90 min hypoxia and 180 min reoxygenation at 37°C (n = 5 to 7 replicates). PI3K/Akt signaling was interrogated using pharmacological inhibitors and small interfering RNA (siRNA). Cell death was assessed by propidium iodide. More than 300 cells were examined for each treatment. The data are presented as means ± SE. There was not a sex difference in the basal content of total Akt. ME (100 μM) elicited comparable protection in both sexes. Wortmannin and the nonselective Akt inhibitor IV completely abolished ME-induced protection in male cardiomyocytes but only attenuated protection in female cardiomyocytes. Isoform-selective knockdown of Akt in males with siRNAs against Akt1/2 completely abolished ME-induced cardioprotection, whereas the siRNAs against Akt3 only attenuated protection of ∼40%. In contrast, in females the siRNAs against Akt1/2 attenuated and against Akt3 eliminated ME-induced cardioprotection. There is not a sex difference in the degree of ME-induced protection, and there is a sex difference in the cardioprotective signaling pathways after the administration of ME; ME-induced cardioprotection in males primarily utilizes a PI3K/Akt1/2 pathway and in females primarily utilizes a PI3K/Akt3 pathway. The incomplete loss of protection in females following the blockade of PI3K suggests that additional factors may facilitate the maintenance or function of activated Akt.
Keywords: peptides, opioid, pharmacological preconditioning, intracellular signaling
There is abundant epidemiologic evidence of sex differences in coronary artery disease risk in humans, with premenopausal females enjoying protection relative to men (4). There is also laboratory evidence showing a sex difference in the tolerance to experimentally induced myocardial ischemia-reperfusion (39), as well as clinical data demonstrating sex differences in response to treatment for acute coronary syndromes. Recently, it has been reported that female sex is an independent predictor of adverse outcome after conventional coronary artery bypass grafting using cardiopulmonary bypass (1, 7, 17), and young women experiencing myocardial infarction have a higher mortality than men (42). Whether these negative outcomes are due to underlying comorbidities, the undertreatment of women, or the lack of efficacy of current treatments for women has not been fully clarified.
Opioids confer tolerance to myocardial ischemia-reperfusion (27), but it is not known whether the mechanism of opioid-induced protection is different in males and females. In male animals, the phosphatidylinositol 3-kinase (PI3K)/Akt pathway contributes to ischemic and pharmacological cardioprotection (12, 31, 32). In male rabbits, Met5-enkephalin (ME) induces the phosphorylation of Akt at serine residue 473 (Akt473), and both the phosphorylation of Akt473 and the protection of cardiomyocytes are abolished by the blockade of PI3K with LY-294002 (10). The sex differences in PI3K/Akt signaling-pathway elements have been described, with increased content of nuclear phospho-Akt473 reported in premenopausal female versus male human hearts and in female versus male murine hearts (8). In addition, nuclear phospho-Akt473 content is higher in premenopausal women compared with postmenopausal women. Based on the involvement of PI3K/Akt in opioid-induced cardioprotection in males and differences in PI3K/Akt signaling elements associated with sex and/or gonadal steroids, we hypothesized that there is a sex dependence of PI3K/Akt involvement in ME-induced cardioprotection. Studies were carried out in isolated murine cardiomyocytes subjected to simulated ischemia-reperfusion.
Materials and Methods
The animals used in these studies were allowed access to phytoestrogen-free food (No. 2014; Harlan Teklad, Madison, WI) and water ad libitum. With local Institutional Animal Care and Use Committee approval, all animals received humane treatment in compliance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Research, National Research Council, National Academy Press, 1996).
Cell isolation and culture
Ventricular myocytes from neonatal mice postnatal day (PND) 7 to 8 were prepared by the modifications of methods described previously (22). Neonatal mice were sexed by measuring the anogenital distance; pups with an intermediate anogenital distance were excluded (18). Three to four hearts from same-sex mice were pooled for cell isolation and culture. The pups were then anesthetized with an intraperitoneal injection of 50 mg/kg pentobarbital sodium, and the hearts were removed aseptically, retaining the ventricles only. The ventricles were kept in iced Hanks' balanced salt solution (HBSS) without Ca2+ and Mg2+ containing (in g/l) 0.4 KCl, 0.06 KH2PO4, 8.0 NaCl, and 0.05 Na2HPO4 (pH 7.4). The ventricles were then washed three times with HBSS and minced into small fragments. The cells were dissociated at 25°C for 15 min with 0.625% wt/vol trypsin (Cat. No. 27250-018; Invitrogen, GIBCO) in HBSS without Ca2+ and Mg2+ (pH 7.4). The cells released after the first digestion were discarded; the cells from subsequent digestions (∼7) were added to an equal volume of cold HBSS with Ca2+ and Mg2+ containing (in g/l) 0.14 CaCl2, 0.4 KCl, 0.06 KH2PO4, 0.047 MgCl2, 0.049 MgSO4, 8.0 NaCl, 0.35 NaHCO3, 0.05 Na2HPO4, and 1.0 d-glucose (pH 7.4) until all cardiac cells were isolated. The resulting mixture was centrifuged for 8 min at 200 g, and the cells were resuspended in FBS-medium 199 (M199) supplemented with estrogen-free 15% FBS (vol/vol; Colcaco), 100 U/ml penicillin, 100 μg/ml streptomycin, and 25 μM cytosine arabinoside (to inhibit fibroblast attachment and proliferation). To exclude nonmuscle cells, the isolated cells were first plated in tissue culture dishes at 37°C for 1 h under a water-saturated atmosphere of 5% CO2-95% O2. The suspended cells (cardiomyocytes) were then collected and plated at a density of 1.0 × 105 cells/cm2 and incubated under the same conditions as above for 24 h.
Simulated ischemia-reperfusion
For simulated ischemia, glucose-free medium containing (in mM) 137 NaCl, 15.8 KCl, 0.49 MgCl2, 4.0 HEPES, 20 2-deoxy-d-glucose, 20 sodium lactate, and 1 sodium dithionate (pH 6.5) was preequilibrated in 100% N2 at 37°C for 2 h. Oxygenated, glucose-replete medium was removed from the cardio-myocyte cultures, and preequilibrated glucose-free medium was immediately added. The cultures were promptly placed in a custom-made plexiglass hypoxia chamber and exposed to 100% N2 for 1.5 h at 37°C. For reperfusion, the glucose-free medium was replaced by M199, and the cells were reoxygenated in room air for 3 h.
Drugs
The agents used in this study were Akt inhibitor IV (10 μM), LY-294002 (20 μM), ME (100 μM), and wortmannin (100 nM). Akt inhibitor IV and LY-294002 were obtained from Calbiochem (San Diego, CA). Wortmannin was purchased from Sigma (St. Louis, MI), and ME was obtained from Sigma and American Peptide. ME is an endogenously produced pentapeptide that is selective for cloned δ-opioid receptors but appears to also activate κ-opioid receptors in vivo (9). LY-294002 and wortmannin block the activity of PI3K; LY-294002 competitively inhibits the ATP-binding site, whereas wortmannin covalently modifies PI3K. Akt inhibitor IV is a nonselective ATP-competitive inhibitor. All drugs except ME were dissolved in DMSO, aliquoted, and frozen until use. ME was dissolved in distilled water, aliquoted, and frozen until use. The dosages were based on previous studies, literature reports, and vendor-reported IC50 values (10, 13). On the day of the experiment, stock solutions were diluted directly into cells. ME was added to the cell cultures 15 min before the 90 min ischemia; the antagonists were administered to the cells 15 min before ME treatment.
RNA interference
The transfections of Akt1/2 (Cell Signaling) and Akt3 (Dharmacon RNA Technologies) small interfering RNAs (siRNAs) were conducted with Lipofectamine 2000 (Invitrogen) in 24-well plates according to the instructions of the manufacturer. Briefly, the cultured cardiomyocytes with 50% confluence were transfected with 100 nM Akt1/2 or Akt3 siRNA in 0.5 ml/well of transfection medium (Cell Signaling). The cells were incubated for 72 h without the replacement of the medium. Western blot analysis showed a significant reduction in Akt expression by siRNA after 48 h incubation and the maximum reduction at 72 h after transfection. After 72 h of transfection, cells were used for cell viability assays. A green fluorescent protein (GFP) siRNA (Superarray) was used as a control.
Determination of cell viability
Cell death was quantified using a Zeiss Axiovert 200 fluorescent microscope and MetaMorph imaging system (Universal Imaging). Propidium iodide (5 μM) was used to identify dead cells. Two digital images were taken of each sample field at each time point. One digital image was taken under fluorescent light (dead cells fluorescence red), and one image was taken with a bright field-transmitted light (to ascertain the total number of cells). The percentage of cell death was expressed as red fluorescent cells relative to the total cells in the same field. The serial measurements of cell viability were performed at least every 60 min. More than 300 cells were examined in each sample. All viability experiments were accompanied by an untreated oxygenated time-control group.
Western blot analysis
The myocyte proteins in the SDS sample buffer, containing 2% SDS, 10% glycerol, 80 mM Tris (pH 6.8), 0.15 M β-mercaptoethanol, and 0.02% bromphenol blue, were separated on 4–20% linear gradient SDS-polyacrylamide gels (Bio-Rad) in a minigel apparatus (Mini-Protean 3; Bio-Rad) and transferred to polyvinylidene difluoride membranes. The membranes were blocked with 5% nonfat dry milk in Tris-buffered saline Tween 20 [containing 10 mM Tris (pH 7.5), 150 mM NaCl, and 0.05% Tween 20] for 60 min at room temperature and incubated overnight at 4°C with primary antibodies (Cell Signaling) in the following concentrations: Akt, 1:4,000; phosphorylated (p)Akt (Ser473), 1:1,000; and Akt3, 1:2,000. The antigens were detected by the luminescence method (ECL-plus Western blotting detection kit; Amersham) with peroxidase-linked anti-rabbit IgG (1:4,000 in 5% dry milk). After immunoblotting was completed, immunoblot band intensity was assessed with a Kodak Image Analysis System. Data are expressed as a percent change of a single protein or phosphorylated/total protein from the control values.
Experimental protocols
Each experimental series consisted of n = 5 to 7 replicates (i.e., 5 to 7 separate cell dissociations/culture). To test whether there is a sex difference in ME-induced cardioprotection following the blockade of PI3K, posthypoxic cellular viability was assessed in the presence and absence of the dissimilar PI3K inhibitors LY-294002 and wortmannin. For each inhibitor, four groups were studied: control, ME, LY-294002, and LY-294002 + ME and control, ME, wortmannin, and wortmannin + ME, respectively.
Since PI3K inhibition indiscriminately affects all three Akt isozymes as well as other pleckstrin-homology domain-containing signaling molecules that are dependent on phosphatidylinositol 3,4,5-trisphosphate, several other strategies were used to assess the sex-specific involvement of Akt in ME-induced cardioprotection. First, the presence of a between-sex difference in total Akt1/2 or Akt3, or in the ratio of phosphorylated to total pan-Akt following ME stimulation, was tested. Second, posthypoxic cellular viability in the presence and absence of the nonselective and selective Akt inhibitors was assessed. The groups were the control, ME, Akt inhibitor IV (nonselective), and Akt inhibitor IV + ME.
Because commercially available pharmacological inhibitors of Akt3 were not available at the time of this study, RNA interference was used to assess sex differences in both Akt1/2- and Akt3-mediated posthypoxic survival. The groups were the control siRNA, ME, Akt1/2 siRNA, and Akt1/2 siRNA + ME and the control siRNA, ME, Akt3 siRNA, and Akt3 siRNA + ME.
Data analysis
Data analysis was performed with a personal computer-based statistical software package (Prism 4.0; GraphPad Software, San Diego, CA). The primary measured end point for cell viability experiments was cell death, defined as the uptake of propidium iodide. For each group, the percentage of dead cells was plotted versus the duration of reoxygenation. The area underneath these injury curves was calculated for each individual experiment. For all the data, the differences between groups were assessed by one-way ANOVA with repeated measures with a Student-Newman-Keuls post hoc test. Statistical significance was assumed for P values ≤ 0.05. The results are expressed as means ± SE.
Results
The potential for a sex difference in PI3K signaling in ME-induced cardioprotection was assessed first. As shown in Figs. 1 and 2, both LY-294002 and wortmannin completely abolished ME-induced protection in male cardiomyocytes but only modestly attenuated protection in female cardiomyocytes. Wortmannin is an irreversible inhibitor of PI3K that covalently modifies the ATP-binding site of the enzyme, whereas LY-294002 is a competitive inhibitor of ATP. The observation that both of these dissimilar PI3K inhibitors yielded similar data with respect to ME treatment and posthypoxic cardiomyocyte viability suggests that there is indeed a sex difference in the utilization of the PI3K signaling pathway following opioid conditioning. However, both agents have been suggested to inhibit other kinases as well (41); therefore, the response to Akt inhibitor IV, which is a benzimidazole compound that nonselectively inhibits Akt phosphorylation/activation without affecting PI3K, was also examined.
Fig. 1.

Competitive inhibition of phosphatidylinositol 3-kinase (PI3K) with LY-294002 (LY) abolishes Met5-enkephalin (ME)-induced cardioprotection in males and attenuates protection in females. AUC, area under the curve; NS, not significant. Data are expressed as means ± SE.
Fig. 2.

Irreversible inhibition of PI3K with wortmannin (Wort) also abolishes ME-induced cardioprotection in males and attenuates protection in females. Data are expressed as means ± SE.
The administration of the Akt inhibitor IV also revealed that inhibition of PI3K/Akt signaling fully abolished ME-induced cellular protection in male cardiomyocytes but only modestly attenuated it in female cardiomyocytes. These data are shown in Fig. 3. Thus the data obtained from treatment with wortmannin, LY-294002, and the Akt inhibitor IV are all internally consistent.
Fig. 3.

Pharmacological inhibition of pan-Akt eliminates ME-induced protection in males and attenuates cardioprotection in females. Data are expressed as means ± SE.
Interestingly, the administration of ME resulted in a ∼20% increase in phosphorylated to total Akt in male cardiomyocytes, whereas ME elicited a ∼200% increase in the phosphorylation of Akt in female cardiomyocytes. There was no difference in the basal (unstimulated) content of total Akt between males and females (Fig. 4). These data, in combination with the PI3K and nonselective Akt inhibition data, suggest that there are Akt isoform-specific sex differences in ME-induced cardioprotection.
Fig. 4.
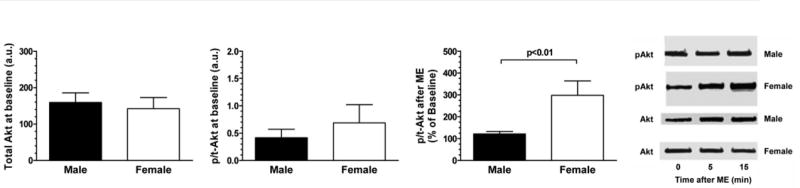
Basal and stimulated Akt content. There was no difference in basal (unstimulated) content of total Akt between males and females. However, administration of ME resulted in a maximal ∼20% increase in phosphorylated to total Akt in male cardiomyocytes at 15 min, whereas ME elicited ∼200% increase in phosphorylation of Akt in female cardiomyocytes at 15 min. Data are expressed as means ± SE. p/t, Phosphorylated/total; au, arbitrary units.
A full spectrum of isoform-selective pharmacological inhibitors of Akt was not available, and therefore RNA interference was used to assess the respective contribution(s) of Akt isoforms to opioid-induced cardioprotection in males and females (Fig. 5). After Akt1/2 siRNA transfection, ME-induced protection against hypoxia-reoxygenation cell death in males was completely eliminated, but it was only partially attenuated in females. Conversely, knockdown of Akt3 was ineffective in abolishing ME-induced protection in males but eliminated it in females (Fig. 6).
Fig. 5.

Akt knockdown by small interfering RNA (siRNA). Assessment of knockdown with sexes pooled revealed ∼50% knockdown of Akt1/2 and ∼70% knockdown of Akt3 (top, left). Analyses by sex showed that siRNA directed against Akt1/2 knocked down Akt1/2 expression to an equivalent extent in both males and females (46 ± 3% vs. 43 ± 3% of control siRNA, respectively) without affecting expression of Akt3 (bottom, left). Similarly, expression of Akt3 was reduced to a comparable degree in both sexes by siRNA directed against Akt3 (50 ± 1 males vs. 53 ± 2 females, %control siRNA) with no change in expression of Akt1/2 (bottom, right). Immunoblot for Akt by sex is shown (top, right).
Fig. 6.

Cardiomyocyte viability following Akt siRNA transfection. Knockdown of Akt1/2 eliminated ME-induced protection against hypoxia-reoxygenation cell death in males but only partially attenuated protection in females. Conversely, knockdown of Akt3 was ineffective in eliminating ME-induced protection in males but abrogated it in females. Control siRNA = green fluorescent protein (GFP). Shaded area indicates hypoxic period (0–90 min). Data are expressed as means ± SE.
The isoform-specific Western blot analysis of pan-Akt following siRNA transfection, expressed as phosphorylated/total (p/t), showed a mirror image of the following viability data: robust phosphorylation in the presence of control siRNA (GFP sequence) in both males and females, only modest phosphorylation of Akt in males in the presence of Akt3 siRNA, and robust phosphorylation of Akt in females only in the presence of Akt1/2 siRNA (Fig. 7). Thus these data show that in males ME elicits p/t conversion mostly in Akt1/2, whereas in females ME elicits p/t conversion mostly in Akt3.
Fig. 7.

Akt Western blot analysis following Akt siRNA transfection. Isoform-specific Western blot analysis of p/t pan-Akt following stimulation with ME mirrors the viability data shown in Fig. 5. The data demonstrate robust phosphorylation in the presence of control siRNA (GFP sequence) in both males and females, only modest phosphorylation of Akt in males in the presence of Akt3 siRNA, and robust phosphorylation of Akt in females only in the presence of Akt1/2 siRNA. The data suggest that ME elicits p/t conversion mostly in Akt1/2 in males vs. mostly in Akt3 in females. Control siRNA = GFP. Data are expressed as means ± SE.
Discussion
The principal finding of the current study is that in isolated PND 7 to 8 murine cardiomyocytes, ME-induced protection against simulated ischemia is abrogated by the blockade of the PI3K/Akt signaling pathway in males but not in females. Furthermore, ME-induced cardioprotection in males primarily utilizes a PI3K-Akt1/2 dominant pathway and in females primarily utilizes a PI3K-Akt3 dominant pathway.
ME elicits cytoprotection in male cardiomyocytes via Src-dependent EGFR transactivation, with consequent activation of the PI3K/Akt and MAPK/extracellular signal-regulated kinase (ERK) (MEK)/ERK1/2 pathways and the involvement of protein kinase C (PKC) and ATP-sensitive potassium channels (10). This signaling pathway has been termed the reperfusion injury salvage kinase pathway and is thought to confer protection largely via the inhibition of the mitochondrial permeability-transition pore opening (20, 21, 47). The current study extends earlier findings to now demonstrate sex-dependent differences in opioid-induced cytoprotective signaling. Sex-specific differences in the mechanisms of ischemic injury and tolerance have also been reported for the brain (16, 26, 29) and the kidney (23).
Shinmura et al. (38) reported that sex difference does not affect the magnitude of opioid-induced late preconditioning in isolated rat hearts, but this study did not address sex-dependent differences in the mechanism of protection. The current data also suggest that the magnitude of opioid-induced cytoprotection is similar in males and females; however, male and female cells appear to utilize different signaling pathways to achieve ME-induced ischemic tolerance. Many previous studies examining the mechanisms of opioid-induced preconditioning have implicated Akt in the protective signaling cascade, but all of these studies were performed in male animals, and the dissection of the roles of individual Akt isoforms was not addressed (10, 19, 35, 45).
Although Przyklenk et al. (36) reported that sex difference did not affect myocardial infarct size in intact dogs; others have reported an innate tolerance to myocardial ischemia in female mice, rats, and rabbits (3, 39, 43, 44). Sex differences in salvage kinases and associated signaling elements have been reported to exist in cardiac tissue. For example, Bae and Zhang (3) reported that ischemia resulted in enhanced activation of cardiac Akt and PKC-∊ in females versus males even though the baseline content of the phosphorylated kinase was similar. Camper-Kirby et al. (8) found an increased content of nuclear phospho-Akt473 in the premenopausal female versus the postmenopausal female and male human hearts and in the female versus male murine hearts. Similarly, in the current data, there was no difference in the baseline (unfractionated) content of Akt between males and females, and the p/t-Akt ratio was augmented in females following the administration of ME compared with males. Sex differences in Akt activation following the onset of hypoxic incubation/substrate deprivation were not assessed in the current study, nor were the subcellular content and/or translocation of Akt. However, an incomplete loss of opioid-induced protection in females following the blockade of PI3K was observed, which suggests that additional factors may facilitate the maintenance or function of activated Akt.
The favorable effect of sex difference on myocardial ischemic tolerance is often considered to be due to the actions of the gonadal steroid estrogen on survival kinases. Indeed, estrogen or selective estrogen-receptor modulators have been reported to be cardioprotective, both in terms of reducing the risk of acute myocardial infarction as well as the tolerance to induced ischemia (5, 30, 43). Furthermore, estrogen is reported to activate PI3K/Akt signaling (33, 37), and both the estrogen salutary effect and PI3K/Akt activation are abrogated by ovariectomy or estrogen receptor blockade (24, 25, 34). However, the current study was performed in vitro using estrogen-free media. Thus the current results are indicative of a sex difference that is not the result of recent estrogen exposure.
Because dietary phytoestrogens have been reported to elicit tolerance to cerebral and myocardial ischemia (28, 48), the cardiomyocytes from animals whose dams were given a phytoestrogen-free diet were used in the current study. Although estrogen-free conditions were used in the cell culture and phytoestrogen-free rodent chow was provided, the developing fetus is exposed to gonadal steroids. Maternal estrogens are largely bound to α-fetoprotein. Fetal ovaries are not a source of estradiol (46), although estradiol is made in the fetal testes through the aromatization from testosterone (6). The largest source of estrogen in both sexes is the placenta, although the murine placenta does not participate in gonadal steroid production during the second half of pregnancy (2, 14). The adrenal gland does contain aromatase, and it is possible that estrogen could be produced by the fetal adrenals, although this has not been described. In contrast, the murine fetal testis does produce testosterone with testosterone production surging at approximately murine fetal day 16 and at parturition (11, 15). Thus PND 7 to 8 cardiomyocytes of both sexes will have been exposed to varying levels of estradiol during fetal life, with neonatal male cardiomyocytes exposed to testosterone surges not experienced by neonatal female cardiomyocytes. Whether this prenatal-gonadal steroid exposure affects postnatal-cardiomyocyte hypoxic tolerance was not addressed in the current study and requires future examination.
Because neonatal cardiomyocytes were used in the current study rather than adult cardiomyocytes, the possibility that adult tissue would behave differently from what was observed in the current study cannot be excluded. However, murine cardiomyocyte hyperplasia is thought to be largely complete by PND 7 to 8 (the age of myocytes used in the current study). Soonpaa et al. (40) reported that cardiac DNA labeling with tritiated thymidine was <10% at PND 7 and 0% at PND 10, and growth arrest homeobox gene expression was absent in embryonic cardiac tissue but expressed at adult levels by PND 7. Thus our PND 7 to 8 cardiomyocytes were likely comprised of almost exclusively binucleated and terminally differentiated cells.
Finally, because the Akt knockdown was incomplete, it is possible that the degree of inhibition was sufficient to abrogate signaling in one sex but not enough for the other, i.e., there is a dose response or sensitivity threshold such that, for example, female cardiomyocytes are more susceptible than male cardiomyocytes to reductions in Akt3.
In conclusion, the current results demonstrate that in the absence of concurrent hormonal stimulation (i.e., estrogen-free medium), there is not a sex difference in the degree of ME-induced protection against hypoxia-reoxygenation; there is a sex difference in the utilization of specific cardioprotective signaling pathways after the administration of ME, with ME-induced cardioprotection in males predominantly utilizing a PI3K-Akt1/2 pathway and in females predominantly utilizing a PI3K-Akt3 pathway; and incomplete loss of protection in females following the blockade of PI3K suggests that additional factors may facilitate the maintenance or function of activated Akt.
Acknowledgments
Grants: This work was supported by a Veteran Affairs Merit Review Grant (Medical Research Service, Department of Veterans Affairs; to D. M. Van Winkle).
References
- 1.Abramov D, Tamariz MG, Sever JY, Christakis GT, Bhatnagar G, Heenan AL, Goldman BS, Fremes SE. The influence of gender on the outcome of coronary artery bypass surgery. Ann Thorac Surg. 2000;70:800–805. doi: 10.1016/s0003-4975(00)01563-0. [DOI] [PubMed] [Google Scholar]
- 2.Arensburg J, Payne AH, Orly J. Expression of steroidogenic genes in maternal and extraembryonic cells during early pregnancy in mice. Endocrinology. 1999;140:5220–5232. doi: 10.1210/endo.140.11.7144. [DOI] [PubMed] [Google Scholar]
- 3.Bae S, Zhang L. Gender differences in cardioprotection against ischemia/reperfusion injury in adult rat hearts: focus on Akt and protein kinase C signaling. J Pharmacol Exp Ther. 2005;315:1125–1135. doi: 10.1124/jpet.105.090803. [DOI] [PubMed] [Google Scholar]
- 4.Barrett-Conner E. Sex differences in coronary heart disease. Why are women so superior? The 1995 Ancel Keys lecture. Circulation. 1997;95:252–264. doi: 10.1161/01.cir.95.1.252. [DOI] [PubMed] [Google Scholar]
- 5.Booth EA, Marchesi M, Kilbourne EJ, Lucchesi BR. 17beta-Estradiol as a receptor-mediated cardioprotective agent. J Pharmacol Exp Ther. 2003;307:395–401. doi: 10.1124/jpet.103.054205. [DOI] [PubMed] [Google Scholar]
- 6.Boukari K, Ciampi ML, Guiochon-Mantel A, Young J, Lombes M, Meduri G. Human fetal testis: source of estrogen and target of estrogen action. Hum Reprod. 2007;22:1885–1892. doi: 10.1093/humrep/dem091. [DOI] [PubMed] [Google Scholar]
- 7.Brandrup-Wognsen G, Berggren H, Hartford M, Hjalmarson A, Karlsson T, Herlitz J. Female sex is associated with increased mortality and morbidity early, but not late, after coronary artery bypass grafting. Eur Heart J. 1996;17:1426–1431. doi: 10.1093/oxfordjournals.eurheartj.a015078. [DOI] [PubMed] [Google Scholar]
- 8.Camper-Kirby D, Welch S, Walker A, Shiraishi I, Setchell KD, Schaefer E, Kajstura J, Anversa P, Sussman MA. Myocardial Akt activation and gender: increased nuclear activity in females vs. males. Circ Res. 2001;88:1020–1027. doi: 10.1161/hh1001.090858. [DOI] [PubMed] [Google Scholar]
- 9.Cao Z, Liu L, Van Winkle DM. Activation of δ- and κ-opioid receptors by opioid peptides protects cardiomyocytes via KATP channels. Am J Physiol Heart Circ Physiol. 2003;285:H1032–H1039. doi: 10.1152/ajpheart.01004.2002. [DOI] [PubMed] [Google Scholar]
- 10.Cao Z, Liu L, Van Winkle DM. Met5-enkephalin-induced cardioprotection occurs via transactivation of EGFR and activation of PI3K. Am J Physiol Heart Circ Physiol. 2005;288:H1955–H1964. doi: 10.1152/ajpheart.00256.2004. [DOI] [PubMed] [Google Scholar]
- 11.Dalterio S, Bartke A. Fetal testosterone in mice: effect of gestational age and cannabinoid exposure. J Endocrinol. 1981;91:509–514. doi: 10.1677/joe.0.0910509. [DOI] [PubMed] [Google Scholar]
- 12.Dawn B, Bolli R. Role of nitric oxide in myocardial preconditioning. Ann NY Acad Sci. 2002;962:18–41. doi: 10.1111/j.1749-6632.2002.tb04053.x. [DOI] [PubMed] [Google Scholar]
- 13.Defeo-Jones D, Barnett SF, Fu S, Hancock PJ, Haskell KM, Leander KR, McAvoy E, Robinson RG, Duggan ME, Lindsley CW, Zhao Z, Huber HE, Jones RE. Tumor cell sensitization to apoptotic stimuli by selective inhibition of specific Akt/PKB family members. Mol Cancer Ther. 2005;4:271–279. [PubMed] [Google Scholar]
- 14.Delbes G, Levacher C, Duquenne C, Racine C, Pakarinen P, Habert R. Endogenous estrogens inhibit mouse fetal leydig cell development via estrogen receptor-α. Endocrinology. 2005;146:2454–2461. doi: 10.1210/en.2004-1540. [DOI] [PubMed] [Google Scholar]
- 15.Delbes G, Levacher C, Habert R. Estrogen effects on fetal and neonatal testicular development. Reproduction. 2006;132:527–538. doi: 10.1530/rep.1.01231. [DOI] [PubMed] [Google Scholar]
- 16.Du L, Bayir H, Lai Y, Zhang X, Kochanek PM, Watkins SC, Graham SH, Clark RSB. Innate gender-based proclivity in response to cytotoxicity and programmed cell death pathway. J Biol Chem. 2004;279:38563–38570. doi: 10.1074/jbc.M405461200. [DOI] [PubMed] [Google Scholar]
- 17.Edwards FH, Carey JS, Grover FL, Bero JW, Hartz RS. Impact of gender on coronary bypass operative mortality. Ann Thorac Surg. 1998;66:125–131. doi: 10.1016/s0003-4975(98)00358-0. [DOI] [PubMed] [Google Scholar]
- 18.Greenham LW, Greenham V. Sexing mouse pups. Lab Anim. 1977;11:181–184. doi: 10.1258/002367777780936620. [DOI] [PubMed] [Google Scholar]
- 19.Gross ER, Hsu AK, Gross GJ. The JAK/STAT pathway is essential for opioid-induced cardioprotection: JAK2 as a mediator of STAT3, Akt, and GSK-3β. Am J Physiol Heart Circ Physiol. 2006;291:H827–H834. doi: 10.1152/ajpheart.00003.2006. [DOI] [PubMed] [Google Scholar]
- 20.Hausenloy DJ, Tsang A, Yellon DM. The reperfusion injury salvage kinase pathway: a common target for both ischemic preconditioning and postconditioning. Trends Cardiovasc Med. 2005;15:69–75. doi: 10.1016/j.tcm.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischaemia-reperfusion injury: targeting the reperfusion injury salvage kinase (RISK)-pathway. Cardiovasc Res. 2004;61:448–460. doi: 10.1016/j.cardiores.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 22.Karliner JS, Honbo N, Epstein CJ, Xian M, Lau YFC, Gray MO. Neonatal mouse cardiac myocytes exhibit cardioprotection induced by hypoxic and pharmacologic preconditioning and by transgenic overexpression of human Cu/Zn superoxide dismutase. J Mol Cell Cardiol. 2000;32:1779–1786. doi: 10.1006/jmcc.2000.1212. [DOI] [PubMed] [Google Scholar]
- 23.Kher A, Meldrum KK, Wang M, Tsai BM, Pitcher JM, Meldrum DR. Cellular and molecular mechanisms of sex differences in renal ischemia-reperfusion injury. Cardiovasc Res. 2005;67:594–603. doi: 10.1016/j.cardiores.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Kim JK, Levin ER. Estrogen signaling in the cardiovascular system. Nucl Recept Signal. 2006;4:e013. doi: 10.1621/nrs.04013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim JK, Pedram A, Razandi M, Levin ER. Estrogen prevents cardiomyocyte apoptosis through inhibition of reactive oxygen species and differential regulation of p38 kinase isoforms. J Biol Chem. 2006;281:6760–6767. doi: 10.1074/jbc.M511024200. [DOI] [PubMed] [Google Scholar]
- 26.Kitano H, Young JM, Cheng J, Wang L, Hurn PD, Murphy SJ. Gender-specific response to isoflurane preconditioning in focal cerebral ischemia. J Cereb Blood Flow Metab. 2007;27:1377–1386. doi: 10.1038/sj.jcbfm.9600444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuzume K, Wolff RA, Amakawa K, Kuzume K, Van Winkle DM. Sustained exogenous administration of Met5-enkephalin protects against infarction in vivo. Am J Physiol Heart Circ Physiol. 2003;285:H2463–H2470. doi: 10.1152/ajpheart.00341.2003. [DOI] [PubMed] [Google Scholar]
- 28.Lu KT, Chiou RYY, Chen LG, Chen MH, Tseng WT, Hsieh HT, Yang YL. Neuroprotective effects of resveratrol on cerebral ischemia-induced neuron loss mediated by free radical scavenging and cerebral blood flow elevation. J Agric Food Chem. 2006;54:3126–3131. doi: 10.1021/jf053011q. [DOI] [PubMed] [Google Scholar]
- 29.McCullough LD, Zeng Z, Blizzard KK, Debchoudhury I, Hurn PD. Ischemic nitric oxide and poly (ADP-ribose) polymerase-1 in cerebral ischemia: male toxicity, female protection. J Cereb Blood Flow Metab. 2005;25:502–512. doi: 10.1038/sj.jcbfm.9600059. [DOI] [PubMed] [Google Scholar]
- 30.Ogita H, Node K, Asanuma H, Sanada S, Liao Y, Takashima S, Asakura M, Mori H, Shinozaki Y, Hori M, Kitakaze M. Amelioration of ischemia- and reperfusion-induced myocardial injury by the selective estrogen receptor modulator, raloxifene, in the canine heart. J Am Coll Cardiol. 2002;40:998–1005. doi: 10.1016/s0735-1097(02)02056-9. [DOI] [PubMed] [Google Scholar]
- 31.Oldenburg O, Cohen MV, Downey JM. Mitochondrial KATP channels in preconditioning. J Mol Cell Cardiol. 2003;35:569–575. doi: 10.1016/s0022-2828(03)00115-9. [DOI] [PubMed] [Google Scholar]
- 32.Oldenburg O, Qin Q, Sharma AR, Cohen MV, Downey JM, Benoit JN. Acetylcholine leads to free radical production dependent on KATP channels, Gi proteins, phosphatidylinositol 3-kinase and tyrosine kinase. Cardiovasc Res. 2002;55:544–552. doi: 10.1016/s0008-6363(02)00332-2. [DOI] [PubMed] [Google Scholar]
- 33.Patten RD, Karas RH. Estrogen replacement and cardiomyocyte protection. Trends Cardiovasc Med. 2006;16:69–75. doi: 10.1016/j.tcm.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Patten RD, Pourati I, Aronovitz MJ, Baur J, Celestin F, Chen X, Michael A, Haq S, Nuedling S, Grohe C, Force T, Mendelsohn ME, Karas RH. 17β-Estradiol reduces cardiomyocyte apoptosis in vivo and in vitro via activation of phospho-inositide-3 kinase/Akt signaling. Circ Res. 2004;95:692–699. doi: 10.1161/01.RES.0000144126.57786.89. [DOI] [PubMed] [Google Scholar]
- 35.Philipp S, Critz SD, Cui L, Solodushko V, Cohen MV, Downey JM. Localizing extracellular signal-regulated kinase (ERK) in pharmacological preconditioning's trigger pathway. Basic Res Cardiol. 2007;101:159–167. doi: 10.1007/s00395-005-0566-z. [DOI] [PubMed] [Google Scholar]
- 36.Przyklenk K, Ovize M, Bauer B, Kloner RA. Gender does not influence acute myocardial infarction in adult dogs. Am Heart J. 1995;129:1108–1113. doi: 10.1016/0002-8703(95)90390-9. [DOI] [PubMed] [Google Scholar]
- 37.Ren J, Hintz KK, Roughead ZKF, Duan J, Colligan PB, Ren BH, Lee KJ, Zeng H. Impact of estrogen replacement on ventricular myocyte contractile function and protein kinase B/Akt activation. Am J Physiol Heart Circ Physiol. 2003;284:H1800–H1807. doi: 10.1152/ajpheart.00866.2002. [DOI] [PubMed] [Google Scholar]
- 38.Shinmura K, Nagai M, Tamaki K, Bolli R. Gender and aging do not impair opioid-induced late preconditioning in rats. Basic Res Cardiol. 2004;99:46–55. doi: 10.1007/s00395-003-0436-5. [DOI] [PubMed] [Google Scholar]
- 39.Song X, Li G, Vaage J, Valen G. Effects of sex, gonadectomy, and oestrogen substitution on ischaemic preconditioning and ischaemia-reperfusion injury in mice. Acta Physiol Scand. 2003;177:459–466. doi: 10.1046/j.1365-201X.2003.01068.x. [DOI] [PubMed] [Google Scholar]
- 40.Soonpaa MH, Kim KK, Pajak L, Franklin M, Field LJ. Cardiomyocyte DNA synthesis and binucleation during murine development. Am J Physiol Heart Circ Physiol. 1996;271:H2183–H2189. doi: 10.1152/ajpheart.1996.271.5.H2183. [DOI] [PubMed] [Google Scholar]
- 41.Stein RC. Prospects for phosphoinositide 3-kinase inhibition as a cancer treatment. Endocr Relat Cancer. 2001;8:237–248. doi: 10.1677/erc.0.0080237. [DOI] [PubMed] [Google Scholar]
- 42.Vaccarino V, Parsons L, Every NR, Barron HV, Krumholz HM. Sex-based differences in early mortality after myocardial infarction. National registry of myocardial infarction 2 participants. N Engl J Med. 1999;341:217–225. doi: 10.1056/NEJM199907223410401. [DOI] [PubMed] [Google Scholar]
- 43.Van Eickels M, Patten RD, Aronovitz MJ, Alsheikh-Ali A, Gostyla K, Celestin F, Grohe C, Mendelsohn ME, Karas RH. 17-Beta-estradiol increases cardiac remodeling and mortality in mice with myocardial infarction. J Am Coll Cardiol. 2003;41:2084–2092. doi: 10.1016/s0735-1097(03)00423-6. [DOI] [PubMed] [Google Scholar]
- 44.Wang C, Chiari PC, Weihrauch D, Krolikowski JG, Warltier DC, Kersten JR, Pratt PF, Jr, Pagel PS. Gender-specificity of delayed preconditioning by isoflurane in rabbits: potential role of endothelial nitric oxide synthase. Anesth Analg. 2006;103:274–280. doi: 10.1213/01.ANE.0000230389.76351.0C. [DOI] [PubMed] [Google Scholar]
- 45.Weihrauch D, Krolikowski JG, Bienengraeber M, Kersten JR, Warltier DC, Pagel PS. Morphine enhances isoflurane-induced postconditioning against myocardial infarction: the role of phosphatidylinositol-3-kinase and opioid receptors in rabbits. Anesth Analg. 2005;101:942–949. doi: 10.1213/01.ane.0000171931.08371.a2. [DOI] [PubMed] [Google Scholar]
- 46.Weniger JP, Zeis A, Chouraqui J. Estrogen production by fetal and infantile rat ovaries. Reprod Nutr Dev. 1993;33:129–136. doi: 10.1051/rnd:19930205. [DOI] [PubMed] [Google Scholar]
- 47.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 48.Zhai P, Eurell TE, Cotthaus RP, Jeffery EH, Bahr JM, Gross DR. Effects of dietary phytoestrogen on global myocardial ischemia-reperfusion injury in isolated female rat hearts. Am J Physiol Heart Circ Physiol. 2001;281:H1223–H1232. doi: 10.1152/ajpheart.2001.281.3.H1223. [DOI] [PubMed] [Google Scholar]


