Abstract
OBJECTIVE
The purpose of this study was to assess temporal trends for hysterectomy among Olmsted County, Minnesota women.
STUDY DESIGN
Using the Rochester Epidemiology Project database, we identified all county residents undergoing a hysterectomy in 1965-2002. Temporal changes in the utilization (incidence) rate, type, diagnostic indications, and age at surgery were assessed.
RESULTS
Between 1965 and 2002, 6152 women had a hysterectomy alone, whereas 3126 women had, in addition, a pelvic floor repair; the age-adjusted utilization rate for hysterectomy alone and for combined procedures declined (P < .0001) by 13% and 63%, respectively. Except for subjects aged 75-85 years, this decline affected every age group. The distribution of vaginal (56%) and abdominal (44%) procedures differed across indications. Uterine leiomyomata, precancerous conditions, and genital prolapse accounted for 28%, 23%, and 12% of all procedures, respectively.
CONCLUSION
Among community women, the utilization rate, age distribution, and indications for a hysterectomy changed substantially between 1965 and 2002.
Keywords: Epidemiology, hysterectomy, indications, population utilization rate
Hysterectomy ranks second only to cesarean section as the most common surgical procedure performed in reproductive age women in the US. In 2000, 633,000 hysterectomies were performed in this country.1 Indeed, by age of 60 years, approximately one third of women in the US have had a hysterectomy.2,3 The National Hospital Discharge Survey (NHDS)4 and the Nationwide Inpatient Sample of the Healthcare Cost and Utilization Project5,6 have tracked nationwide trends in the utilization of hysterectomy for many years. The former (ie, NHDS) is conducted in a sample of nonfederal short stay hospitals, most recently updated for 2001, while the latter is conducted in a 20% stratified sample of community hospitals. These surveys suggest that the overall rate of hysterectomy in this country declined slightly from 7.1 hysterectomies per 1000 women in 1980 to 6.6 per 1000 in 1987. Between 1988 and 1993, this rate decreased further to 5.5 per 1000 women annually2 and plateaued thereafter (ie, between 1994 and 1998).4
While useful, these figures have incompletely characterized temporal trends in the utilization, types, and indications for hysterectomy. An accurate appraisal of the current incidence of hysterectomy is important to plan future healthcare needs, particularly since several factors might be anticipated to reduce the utilization of hysterectomy. These factors include an increasing adoption of medical treatments (eg, hormonal therapy for endometriosis and other menstrual disorders) and minimally invasive procedures (eg, endoscopic and laser therapy) for management of gynecologic disorders that previously were treated with surgical intervention,7-9 the need for previous authorization for surgical procedures, peer review, and quality assurance,10-13 and a trend among women in the US to childbearing,14 with a resultant desire preserve fertility. An understanding of the temporal trends in hysterectomy is also necessary to understand temporal trends in the incidence of surgery for pelvic organ prolapse.
To explore these issues, we examined secular trends in the utilization rates, types, and indications for hysterectomy as a single procedure as well as hysterectomy combined with pelvic floor repair among Olmsted County, Minnesota, women from Jan 1, 1965 to Dec 31, 2002, taking advantage of the population-based data resources of the Rochester Epidemiology Project.
MATERIALS AND METHODS
Medical care in Olmsted County, located in the southeastern part of Minnesota, is virtually self-contained within the community, thus allowing population-based epidemiologic research into the incidence and determinants of diverse diseases and therapeutic interventions. Women’s medical care is provided mainly by Mayo Clinic and the Olmsted Medical Group. Mayo Clinic has maintained a common medical record with its 2 large affiliated hospitals (Rochester Methodist and St Marys) for 100 years. This dossier-type record thus contains both inpatient and outpatient data, and specific records are easily retrieved for review since diagnoses and surgical procedures are indexed.15 The medical records of the other providers who serve the local population, most notably the Olmsted Medical Center (Olmsted Medical Group and its affiliated Olmsted Community Hospital), are similarly indexed by the Rochester Epidemiology Project.16 Thus, the details of almost all of the medical care provided to Olmsted County residents are available for study through this medical record linkage system. This population has been extensively studied for the purpose of reporting the epidemiology of surgical procedures.17-19
Case identification
Following approval by the Institutional Review Boards of both Mayo Clinic and Olmsted Medical Center, the Rochester Epidemiology Project database was used to identify all women who underwent hysterectomy either as a single procedure or along with pelvic floor repair procedures between Jan 1, 1965 and Dec 31, 2002, and were Olmsted County residents. A total of 615 patients from the hysterectomy file (6%) who refused to authorize use of their medical records for research were excluded from the study.20 The procedure type and indications were identified electronically using the Berkson coding system from Jan 1, 1965 to Dec 31, 1987 and the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) procedural codes from Jan 1, 1988 to Dec 31, 2002, as delineated in Table 1. Since there was no ICD-9-CM code for laparoscopic hysterectomy before Oct 1996, we used the laparoscopic code 54.21 to idenalltifyofany procedure performed in conjunction with vaginal hysterectomy, subtotal, or other hysterectomy before 1996. This methodology for identifying laparoscopic hysterectomy has been used previously.21 For the purpose of study, hysterectomies were broadly categorized as abdominal or vaginal. In 100 randomly selected women, the procedure type in the electronic record system was manually compared to the procedure type listed in the surgical note; these 2 sources agreed in 99% of cases. The procedures were then matched with their corresponding indication codes as contained in the electronic medical records of the operation, and the pathologic diagnosis. Where there were multiple diagnoses, the principal indication was assigned using the Center for Disease Control and Prevention (CDC) established hierarchic system of diagnosis.2 First, if cancer of the reproductive tract was listed as 1 of the diagnoses, it was assigned as the primary indication for hysterectomy. Second, if both hysterectomy and the debulking of cancer of the urinary or intestinal tract were listed, the debulking procedure was assigned as the primary indication. Third, if a precancerous condition (eg, endometrial hyperplasia or carcinoma in situ of the cervix) was listed in the absence of a diagnosis of cancer, then the precancerous condition was assigned as the primary diagnosis. Fourth, if cancer or a precancerous condition was not listed, the diagnoses were scanned for 1 of the 3 most common indications for hysterectomy (ie, uterine leiomyoma, endometriosis, or uterine prolapse),22 and the first of these diagnoses listed was assigned as the primary diagnosis. Fifth, the remaining records were placed in the “other” diagnostic category.
TABLE 1.
Diagnostic codes of indications for hysterectomy
| Berkson procedure codes | |
| Abdominal hysterectomy | |
| Subtotal hysterectomy | 4694; 4695* |
| Total abdominal hysterectomy | 4680; 4690* |
| Radical abdominal hysterectomy | 4720 |
| Vaginal hysterectomy | 4700 & 4710 |
| International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes | |
| Abdominal hysterectomy | |
| Subtotal or supracervical hysterectomy | 68.3 |
| Total abdominal hysterectomy-TAH | 68.4 |
| Vaginal hysterectomy | 68.5, 68.59; 69.51 |
| Laparoscopic hysterectomy | 68.51 |
| Radical hysterectomy | 68.6; 68.7 |
| Others and unspecified hysterectomy | 68.9 |
4695: with salpingo-oophorectomy (unilateral or bilateral); 4690: with salpingo-oophorectomy (unilateral or bilateral).
Statistical analysis
To be considered an incident case, the patient must have been residing in Olmsted County at the time of the surgical procedure. For estimating hysterectomy utilization rates (incidence density) the entire population of Olmsted County women was considered to be at risk for the procedure; the denominator and age-specific person-years were derived from decennial census data with linear interpolation between census years.23 The hysterectomy utilization rates were directly age-adjusted to the population structure of US white females in 2000 as the standard population since this is the population that most resembles Olmsted County (99% white in 1970, 98% in 1980, 96% in 1990, and 90% in 2000). Standard errors and 95% confidence intervals (95% CI) for the rates were estimated, assuming that the incidence cases followed a Poisson distribution.23,24 Temporal trends in the procedure type, indications, and patient’s age were analyzed using separate Poisson regression models for any hysterectomy, abdominal hysterectomy, and vaginal hysterectomy. A multiple logistic regression model evaluated whether the choice between abdominal and vaginal hysterectomy was influenced by age, the indication for the hysterectomy, or calendar period. For each indication, the odds ratios (and 95% CIs) for abdominal versus vaginal hysterectomy were estimated relative to the reference group (ie, the odds of having an abdominal [versus vaginal] hysterectomy for uterine leiomyomata between 1965 and 1974 in women ≤ 50 years of age).
RESULTS
During the 38-year study period 9893 hysterectomies (with or without pelvic floor repair procedures) were performed in this population, but 615 patients (6%) did not authorize use of their medical records for research purposes. Thus, 9278 hysterectomies were included in this analysis. Of these, 6152 (66%) hysterectomies were performed as a single procedure, while 3126 (34%) hysterectomies were combined with a pelvic floor repair procedure (PFR).
Temporal trends for hysterectomy alone versus hysterectomy combined with pelvic floor repair
The age-adjusted utilization rate for any hysterectomy (alone or with PFR) declined (P < .0001) by 37%, from 745 per 100,000 person years in 1965-1974 to 472 per 100,000 in 1995-2002 (Table 2). The age-adjusted annual utilization rate of hysterectomy alone declined steadily over time by 13%, from 395 per 100,000 women in 1965-1974 to 342 per 100,000 in 1995-2002. By contrast, the age-adjusted utilization of combined hysterectomy and PFR procedures declined by 63%, from 349 per 100,000 in 1965-1974 to 130 per 100,000 in 1995-2002. With the exception of women aged 75-85 years, the incidence of any hysterectomy (ie, with or without a PFR) declined in every age group. However, a significant interaction (P < .001) between age and calendar period was detected, signifying that the actual magnitude of temporal changes varied among age groups. Indeed, Table 2 suggests that the utilization of any hysterectomy was most pronounced (ie, by 66%) among women aged 25-34 years. In contrast, smaller reductions were observed among most older women (eg, 9% among women aged 55-64 years). However, among women aged 75-84 years, the annual incidence of any hysterectomy actually increased by 45%, from 308 per 100,000 in 1965-1974 to 446 per 100,000 in 1995-2002.
TABLE 2.
Annual age-adjusted utilization of any hysterectomy among Olmsted County, Minnesota women by age group, 1965-2002
| Age-group (y) | 1965-1974 rate* | 1975-1984 rate* | 1985-1994 rate* | 1995-2002 rate* | All years rate* |
|---|---|---|---|---|---|
| <25 | 18.9 | 7.2 | 8.5 | 10.5 | 11.3 |
| 25-34 | 690.7 | 478.3 | 362.8 | 236.0 | 429.1 |
| 35-44 | 1952.5 | 1423.0 | 1149.6 | 1017.7 | 1301.4 |
| 45-54 | 1526.6 | 1393.0 | 1129.6 | 1103.6 | 1252.5 |
| 55-64 | 585.0 | 515.7 | 503.6 | 527.2 | 530.9 |
| 65-74 | 563.2 | 543.7 | 532.4 | 494.7 | 532.4 |
| 75-84 | 307.7 | 355.9 | 342.4 | 446.1 | 368.2 |
| ≥85 | 177.8 | 106.2 | 205.9 | 164.3 | 166.8 |
| Total | 744.8 | 603.6 | 508.1 | 472.2 | 562.2 |
| (95% CI)† | (713.9, 775.7) | (578.1, 629.1) | (487.5, 528.6) | (452.4, 492.0) | (550.6, 573.8) |
The decline in utilization rates was more pronounced in younger than in older women. Among women aged 75-84 years, the utilization rate increased by 45% between 1965-1974 and 1995-2002.
Annual incidence of the procedure per 100,000 Olmsted County women.
Age-adjusted incidence per 100,000 women directly adjusted to US white women in 2000 (and 95% CI for the age-adjusted rate).
Temporal trends for abdominal versus vaginal hysterectomy
Of the 9278 hysterectomies performed during the study period, 5151 (56%) were performed vaginally, while 4127 (44%) were abdominal procedures. Subtotal (ie, supracervical, n = 58) and radical hysterectomies (n = 79) comprised a negligible proportion of abdominal hysterectomies. Fifty women had a laparoscopic-assisted vaginal hysterectomy. Between the initial and final epochs, the age-adjusted utilization rate declined (P < .0001) by 40% for abdominal hysterectomy, from 323.1 per 100,000 annually in 1965-1974 to 194.2 per 100,000 in 1995-2002 (Table 3), while the annual utilization of vaginal hysterectomy declined (P < .0001) by 34%, from 421.7 per 100,000 in 1965-1974 to 278 per 100,000 in 1995-2002 (Table 4).
TABLE 3.
Utilization of abdominal hysterectomy among Olmsted County, Minnesota women by age group, 1965-2002
| Age group (y) | 1965-1974 rate* | 1975-1984 rate* | 1985-1994 rate* | 1995-2002 rate* | All years rate* |
|---|---|---|---|---|---|
| <25 | 14.5 | 6.3 | 5.5 | 5.3 | 8.0 |
| 25-34 | 305.1 | 220.9 | 154.4 | 102.1 | 189.7 |
| 35-44 | 791.2 | 649.1 | 500.0 | 365.9 | 539.0 |
| 45-54 | 772.5 | 675.3 | 545.4 | 498.2 | 601.1 |
| 55-64 | 227.9 | 284.9 | 242.9 | 230.2 | 246.1 |
| 65-74 | 152.1 | 262.8 | 237.3 | 202.9 | 216.4 |
| 75-84 | 136.8 | 148.7 | 177.7 | 182.1 | 1634.4 |
| ≥85 | 88.9 | 93.0 | 134.3 | 69.2 | 97.8 |
| Total | 323.1 | 288.4 | 234.3 | 194.2 | 250.6 |
| (95% CI)† | (302.7, 343.4) | (270.8, 306.1) | (220.3, 248.4) | (181.4, 206.9) | (242.9, 258.4) |
Annual incidence of the procedure per 100,000 Olmsted County women.
Age-adjusted incidence per 100,000 women directly adjusted to US white women in 2000 (and 95% CI for the age-adjusted rate).
TABLE 4.
Utilization of vaginal hysterectomy among Olmsted County, Minnesota women by age group, 1965-2002
| Age group (y) | 1965-1974 rate* | 1975-1984 rate* | 1985-1994 rate* | 1995-2002 rate* | All years rate* |
|---|---|---|---|---|---|
| <25 | 4.4 | 1.0 | 3.0 | 5.3 | 3.3 |
| 25-34 | 385.6 | 257.3 | 208.4 | 133.9 | 239.4 |
| 35-44 | 1161.3 | 773.8 | 649.6 | 651.8 | 762.4 |
| 45-54 | 754.1 | 717.7 | 585.9 | 605.4 | 651.9 |
| 55-64 | 357.2 | 230.8 | 260.8 | 297.0 | 284.8 |
| 65-74 | 411.1 | 280.8 | 295.1 | 291.6 | 316.0 |
| 75-84 | 170.9 | 207.2 | 164.7 | 264.0 | 203.9 |
| ≥85 | 88.9 | 13.3 | 71.6 | 95.1 | 69.0 |
| Age-adjusted (95% CI)† | 421.7(398.4,444.9) | 315.1 (296.7, 333.5) | 274.0 (258.9, 289.0) | 278.0 (262.8, 293.2) | 311.6 (303.0, 320.3) |
Annual incidence of the procedure per 100,000 Olmsted County women.
Age-adjusted incidence per 100,000 women directly adjusted to US white women in 2000 (and 95% CI for the age-adjusted rate).
Similar to the results for any hysterectomy, the separate models for vaginal and abdominal hysterectomy both revealed a significant interaction (P < .001) between age and calendar period, signifying that the actual magnitude of temporal changes varied among age groups. Moreover, in contrast to the overall trends, the utilization rate for abdominal hysterectomy did not change over time in women aged 55-64 years but increased by approximately 33% in women aged 65-74 years and also among women aged 75-84 years. With the exception of women aged ≥ 75 years, the incidence of vaginal hysterectomy decreased (P < .001) over time in all groups.
Temporal trends in indications for hysterectomy
All calendar periods except for the latest were 10 years in duration. Allowing for differences in duration across epochs, the total number of abdominal or vaginal procedures during a calendar period was relatively stable between 1965 and 2002 (Table 5). Uterine leiomyomata and precancerous conditions were the 2 leading indications for both abdominal and vaginal hysterectomy. Malignant diseases and uterine prolapse comprised the third leading indication for abdominal and vaginal hysterectomy, respectively. The distribution of indications for hysterectomy also changed across time (Table 5). Between 1965-1974 and 1994, an increasing proportion of vaginal and abdominal procedures were conducted for precancerous conditions. However, this trend was reversed between 1995-2002.
TABLE 5.
Indications for abdominal and vaginal hysterectomy among Olmsted County, Minnesota women, 1965-2002
| Indication | Abdominal (n)*(%)† | Vaginal(n)* (%)† | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1965-1974 | 1975-1984 | 1985-1994 | 1995-2002 | All years | 1965-1974 | 1975-1984 | 1985-1994 | 1995-2002 | All years | |
| Cancer of the reproductive tract |
154 (15) | 181 (17) | 181 (16) | 122 (13) | 638 (15) | 144 (11) | 61 (5) | 41 (3) | 65 (5) | 311 (6) |
| Debulking of urinary or GI cancer |
27 (3) | 12 (1) | 32 (3) | 33 (4) | 104 (2) | 8 (0.6) | 2 (0.2) | 4 (0.3) | 8 (0.6) | 22 (0.4) |
| Precancerous conditions | 201 (20) | 249 (23) | 355 (32) | 167 (18) | 972 (24) | 207 (16) | 297 (25) | 421 (32) | 279 (21) | 1204 (23) |
| Uterine leiomyomata | 313 (31) | 321 (30) | 295 (26) | 338 (37) | 1267 (31) | 315 (24) | 317 (27) | 327 (25) | 389 (29.5) | 1348 (26) |
| Endometriosis | 117 (11) | 124 (11) | 96 (9) | 100 (11) | 437 (11) | 54 (4) | 51 (4) | 58 (4) | 105 (8) | 268 (5) |
| Uterine/vaginal prolapse | 10(1) | 5 (0.5) | 18 (2) | 12 (1) | 45 (1) | 439 (33) | 243 (20.5) | 208 (16) | 207 (16) | 1097 (21) |
| Menstrual disorders | 100 (10) | 81 (7) | 77 (7) | 91 (10) | 349 (8) | 107 (8) | 187 (16) | 246 (18.5) | 233 (18) | 773 (15) |
| Menopausal disorders | 9 (1) | 11 (1) | 4 (0.4) | 18 (2) | 42 (1) | 1 (0.8) | 7 (0.6) | 10 (0.8) | 19 (1) | 37 (1) |
| Inflammatory diseases of pelvic organs |
60 (6) | 81 (7) | 46 (4) | 15 (2) | 202 (5) | 41 (13) | 14 (10) | 8 (0.6) | 5 (0.4) | 68 (1) |
| Others | 27(3) | 16 (1.5) | 13 (1) | 15 (2) | 71 (2) | 4 (0.3) | 5 (0.4) | 5 (0.4) | 9 (0.7) | 23 (0.4) |
| Total | 1018 (100) | 1081 (100) | 1117 (100) | 911 (100) | 4127 (100) | 1320 (100) | 1184 (100) | 1328 (100) | 1319 (100) | 5151 (100) |
n = number of procedures.
% = percentage of the procedure per column.
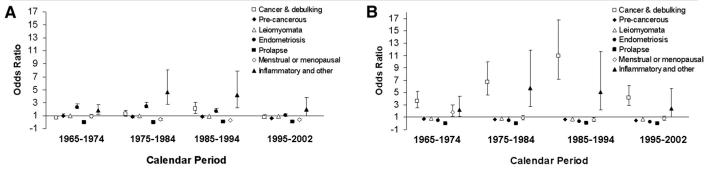
The odds for having an abdominal versus vaginal hysterectomy also changed over time. Figure 1A and B depicts the odds ratios for having an abdominal versus vaginal hysterectomy for specific indications among women aged ≤ 50 years (Figure 1A) and > 50 years (Figure 1B). In these figures, the ence group is women ≤ 50 years who had a hysterectomy for uterine leiomyomata between 1965-1974. In women aged ≤ 50 years (Figure 1A), endometriosis and inflammatory conditions were generally associated with an increased odds for abdominal hysterectomy, while uterine prolapse, menstrual, or menopausal disorders were generally associated with a lower odds for abdominal (ie, an increased odds for vaginal hysterectomy) relative to the reference group. The most striking difference between women ≤ 50 and > 50 years of age is that older women undergoing a hysterectomy for cancer or for debulking were more likely than younger women to have an abdominal hysterectomy (Figure 1B).
FIGURE. Odds ratio (95% CIs) for abdominal versus vaginal hysterectomy among Olmsted County, MN.
Women A, aged ≤ 50 years B, and > 50 years. The reference group is women ≤ 50 years who had a hysterectomy for uterine leiomyoma between 1965-1974. In women aged ≤ 50 years, endometriosis and inflammatory conditions were associated with increased odds, while uterine prolapse or menstrual or menopausal disorders were associated with lower odds for abdominal hysterectomy relative to the reference group. The most striking age-related difference was that older women undergoing a hysterectomy for cancer or for debulking were more likely than younger women to have an abdominal hysterectomy. Between 1965-2002, the utilization rate for hysterectomy in the population declined by 37%; the age distribution and indications for this procedure also changed over time.
COMMENT
Though the NHDS survey data have suggested a downward trend in hysterectomy utilization rates since the mid-1980s,2 it is unclear if those trends affected by differences in survey technique, including sampling methods, over time. Systematic searches of Medline (1996-2006) and PubMed databases using the keywords “hysterectomy” AND “population” and “hysterectomy” AND “United States” suggest that this is the first strictly population-based study in the US where detailed data on hysterectomy patterns were available over a were long period of time. Our findings reveal that the utilization rate, age distribution, and indications for hysterectomy, either alone or combined with pelvic floor repair procedures, changed between 1965 and 2002. The overall hysterectomy rate declined significantly (ie, by 36%), but this temporal trend was not uniform across age groups, being most pronounced among women aged 25-34 years. In contrast, among women aged 75-84 years, the utilization rate increased by 45% between the initial (ie, 1965-1974) and final (ie, 1995-2002) epochs. Consequently, the age distribution of women undergoing hysterectomy gradually shifted to the right over time.
Approximately 60% of the hysterectomies in Olmsted County were performed by the vaginal route, which is similar to the reported rate of 40-50% in France and Australia,25, 26 but higher than 25% rate reported elsewhere in this country.2 The 3 leading indications abdominal hysterectomy (ie, leiomyomata, precancerous conditions, and cancer of the reproductive tract) and for vaginal hysterectomy (ie, leiomyomata, precancerous conditions, and prolapse) were similar to previous national surveys.2,4,5 Our data (Figure 1A) confirm that a vaginal approach was preferred not only in younger women who had uterine or vaginal prolapse but also in women with endometriosis. Moreover, surgeons were equally likely to do a vaginal or an abdominal procedure even among younger women with leiomyomata or menstrual disorders. We suspect that physician preferences, partly attributable to a long tradition of expertise in vaginal surgery, may explain why a greater proportion of procedures in Olmsted County were performed via the vaginal route. Similarly, we suspect that the proportion of laparoscopic hysterectomies (ie, < 1% of all hysterectomies) is lower than the nationwide estimate (ie, 10%) because surgeons were able to remove the uterus vaginally without laparoscopic assistance. It is unlikely that differences in the proportion of LAVH between Olmsted County and the nationwide estimate vitiate the findings of our study.5 Indeed, there is no advantage to doing a laparoscopic-assisted hysterectomy, 27,28 particularly in centers with expertise in vaginal surgery (eg, in Olmsted County).
The most striking trend in the distribution of indications for hysterectomy between 1995 and 2002 was the substantial decline in the number of procedures for precancerous conditions. This decline may be explained by the efficacy of screening programs for identifying pre-cancerous lesions at an earlier stage when they are more amenable to newer therapeutic approaches. Though less invasive therapeutic options for uterine leiomyomata are now available,25,29 the number of hysterectomies for this indication increased in the most recent epoch (ie, 1995-2002). Though we did not specifically assess for a temporal trend among women undergoing a vaginal hysterectomy for leiomyomata, the magnitude of these changes is small.
For 1995-2002, the age-adjusted annual utilization rate for hysterectomy in Olmsted County (ie, 4.7 per 1000) was lower than the corresponding national figure of 5.6 per 1000 in 1997.5 The loss of potential study subjects due to restricted access to their medical record data20 did not appear to substantially affect the observed temporal decline in utilization of hysterectomy. Thus, within the age-grouped (but deidentified) list of all hysterectomy cases, the overall age-adjusted rate in 1988-2002 was 5.11 per 1000, which is approximately 22% less than the rate (ie, 6.52 per 1000) for the previous time period (ie, 1965-1987). Moreover, the estimated utilization rate for 1998-2002 in Olmsted County, including the deidentified cases, was also comparable (ie, only 9% lower) to the national figure of 5.6 per 1000 in 1997. In contrast to these findings from the predominantly white population in Olmsted County, the NHDS data showed that hysterectomy rates between 1994-1999 were highest for black women (6.2 per 1000 women), intermediate for other races (5.9 per 1000), and lowest for white women (5.3 per 1000).4 In contrast, National Health Interview Survey suggested that Hispanic women undergo fewer hysterectomies than non-Hispanic women.30
The trends reported here have several implications from a public health perspective. Though overall hysterectomy rates have declined, the increasing utilization among older women deserves further scrutiny, particularly as the baby boomers age. It is conceivable that hysterectomy utilization rates will continue to decline as screening programs for cervical cancer are optimized and as less-invasive therapeutic options (eg, endometrial ablation, uterine artery embolization) are compared to hysterectomy in rigorous trials, and are adopted more widely in medical practice. Our data also demonstrate that there is considerable scope for increasing the proportion of procedures done by the vaginal route, with its advantages, nationwide.
Acknowledgments
This study was supported in part by research grants HD 41129, AR 30582, and AG 04875 from the National Institutes of Health, US Public Health Service.
REFERENCES
- 1.Hall MJ. 2000 national hospital discharge survey. Adv Data. 2002:119. [PubMed] [Google Scholar]
- 2.Lepine LA, Hillis SD, Marchbanks PA, et al. Hysterectomy surveillance—United States, 1980-1993. MMWR CDC Surveill Summ. 1997;46:1–15. [PubMed] [Google Scholar]
- 3.Wilcox LS, Koonin LM, Pokras R, Strauss LT, Xia Z, Peterson HB. Hysterectomy in the United States, 1988-1990. Obstet Gynecol. 1994;83:549–55. doi: 10.1097/00006250-199404000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Keshavarz H. Hysterectomy surveillance—United States, 1994-1999. MMWR Surveill Summ. 2002;51:1–8. [Google Scholar]
- 5.Farquhar CM, Steiner CA. Hysterectomy rates in the United States 1990-1997. Obstet Gynecol. 2002;99:229–34. doi: 10.1016/s0029-7844(01)01723-9. [DOI] [PubMed] [Google Scholar]
- 6.Sills ES, Saini J, Steiner CA, McGee M, 3rd, Gretz HF., 3rd Abdominal hysterectomy practice patterns in the United States. Int J Gynaecol Obstet. 1998;63:277–83. doi: 10.1016/s0020-7292(98)00144-1. [DOI] [PubMed] [Google Scholar]
- 7.Derman SG, Rehnstrom J, Neuwirth RS. The long-term effectiveness of hysteroscopic treatment of menorrhagia and leiomyomas. Obstet Gynecol. 1991;77:591–4. [PubMed] [Google Scholar]
- 8.Goldrath MH. Hysteroscopic endometrial ablation. Obstet Gynecol Clin NA. 1995;22:559–72. [PubMed] [Google Scholar]
- 9.Verkauf BS. Changing trends in treatment of leiomyomata uteri. Curr Opin Obstet Gynecol. 1993;5:301–10. [PubMed] [Google Scholar]
- 10.Gambone JC, Reiter RC, Lench JB, Moore JG. The impact of a quality assurance process on the frequency and confirmation rate of hysterectomy. Am J Obstet Gynecol. 1990;163:545–50. doi: 10.1016/0002-9378(90)91195-i. [DOI] [PubMed] [Google Scholar]
- 11.McCarthy EG, Finkel ML. Second consultant opinion for elective gynecologic surgery. Obstet Gynecol. 1980;56:403–10. [PubMed] [Google Scholar]
- 12.Finkel ML, Finkel DJ.The effect of a second opinion program on hysterectomy performance [see comment]. Med Care 199028776–83. [DOI] [PubMed] [Google Scholar]
- 13.Dyck FJ, Murphy FA, Murphy JK, et al. Effect of surveillance on the number of hysterectomies in the province of Saskatchewan. N Engl J Med. 1977;296:1326–8. doi: 10.1056/NEJM197706092962306. [DOI] [PubMed] [Google Scholar]
- 14.Mosher WD, Pratt WF.Fecundity and infertility in the United States: incidence and trends [comment]. Fertil Steril 199156192–3. [PubMed] [Google Scholar]
- 15.Kurland LT, Molgaard CA. The patient record in epidemiology. Sci Am. 1981;245:54–63. doi: 10.1038/scientificamerican1081-54. [DOI] [PubMed] [Google Scholar]
- 16.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 17.Gabriel SE, O’Fallon WM, Beard CM, Kurland LT, Woods JE, Melton LJ., 3rd Trends in the utilization of silicone breast implants, 1964-1991, and methodology for a population-based study of outcomes. J Clin Epidemiol. 1995;48:527–37. doi: 10.1016/0895-4356(94)00209-9. [DOI] [PubMed] [Google Scholar]
- 18.Melton LJ, 3rd, Alothman KI, Achenbach SJ, O’Fallon WM, Zincke H. Decline in bilateral orchiectomy for prostate cancer in Olmsted county, Minnesota, 1956-2000. Mayo Clin Proc. 2001;76:1199–203. doi: 10.4065/76.12.1199. [DOI] [PubMed] [Google Scholar]
- 19.Ghosh K, Melton LJ, 3rd, Suman VJ, Grant CS, Sterioff S, Brandt KR, et al. Breast biopsy utilization: a population-based study. Arch Intern Med. 2005;165:1593–8. doi: 10.1001/archinte.165.14.1593. [DOI] [PubMed] [Google Scholar]
- 20.Melton LJ., 3rdThe threat to medical-records research [see comment]. N Engl J Med 19973371466–70. [DOI] [PubMed] [Google Scholar]
- 21.Myers ER, Steege JF. Risk adjustment for complications of hysterectomy: limitations of routinely collected administrative data. Am J Obstet Gynecol. 1999;181:567–75. doi: 10.1016/s0002-9378(99)70494-1. [DOI] [PubMed] [Google Scholar]
- 22.Pokras R, Hufnagel VG. Hysterectomy in the United States, 1965-84. Am J Public Health. 1988;78:852–3. doi: 10.2105/ajph.78.7.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bergstralh EJ, Offord K, Chu CP, Beard CM, O’Fallon WM, Melton LJ., 3rd . Calculating incidence: prevalence and mortality for Olmsted County, Minnesota: an update. Mayo Clinic; Rochester (MN): 1992. [Google Scholar]
- 24.Beyer WH. CRC handbook of tables for probability and statistics. Chemical Rubber Company; Cleveland (OH): 1966. [Google Scholar]
- 25.Chapron C, Laforest L, Ansquer Y, et al. Hysterectomy techniques used for benign pathologies: results of a French multicentre study. Hum Reprod. 1999;14:2464–70. doi: 10.1093/humrep/14.10.2464. [DOI] [PubMed] [Google Scholar]
- 26.Yusuf F, Siedlecky S. Hysterectomy and endometrial ablation in New South Wales, 1981 to 1994-1995. Aust N Z J Obstet Gynaecol. 1997;37:210–6. doi: 10.1111/j.1479-828x.1997.tb02256.x. [DOI] [PubMed] [Google Scholar]
- 27.Meikle SF, Nugent EW, Orleans M.Complications and recovery from laparoscopy-assisted vaginal hysterectomy compared with abdominal and vaginal hysterectomy [see comment]. Obstet Gynecol 199789304–11. [DOI] [PubMed] [Google Scholar]
- 28.Kovac SR. Hysterectomy outcomes in patients with similar indications. Obstet Gynecol. 2000;95:787–93. doi: 10.1016/s0029-7844(99)00641-9. [DOI] [PubMed] [Google Scholar]
- 29.Wood C, Maher P, Hill D, Selwood T. Hysterectomy: a time of change. Med J Aust. 1992;157:651–3. doi: 10.5694/j.1326-5377.1992.tb137426.x. [DOI] [PubMed] [Google Scholar]
- 30.Brett KM, Higgins JA. Hysterectomy prevalence by Hispanic ethnicity: evidence from a national survey. Am J Public Health. 2003;93:307–12. doi: 10.2105/ajph.93.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]