Abstract
Uridine adenosine tetraphosphate (Up4A) has been recently reported as an endothelium-derived vasoconstrictor and plasma levels of this dinucleotide are increased in juvenile hypertensive subjects. This study aimed to evaluate the vascular actions of Up4A, typify the putative purinergic receptors that might mediate these effects and characterize the intracellular signaling pathways that may govern Up4A responses. Up4A induced a modest endothelium-dependent relaxation of rat aortic rings contracted with phenylephrine. From baseline, Up4A induced concentration-dependent contractions that were significantly potentiated by endothelium removal or nitric oxide synthase inhibition. The contractile response induced by Up4A was not tachyphylactic and was significantly reduced in the presence of P1 or P2X receptor antagonists, L-type Ca2+ channel blocker and Rho-kinase inhibitor. Up4A-induced contraction apparently involves superoxide anion formation since it was significantly reduced by treatment with apocynin or tempol. This study presents the unique findings that the endogenous compound Up4A is able to induce relaxation in addition to contraction of rat aorta. Up4A-induced contraction is modulated by nitric oxide production, mediated by P1 and P2X receptor activation, and involves L-type Ca2+ channels, Rho-kinase pathway and superoxide formation.
Keywords: Uridine adenosine tetraphosphate, rat aorta, contraction, relaxation, purinergic receptors
1. Introduction
Extracellular nucleotides contribute to the local regulation of vascular tone (Buvinic et al., 2006; Inscho and Cook, 2002; Ralevic and Burnstock, 1991). Uridine adenosine tetraphosphate (Up4A) has been recently described as a novel non-peptidic endothelium-derived vasoconstrictive factor (Jankowski et al., 2005). This factor, isolated from human endothelial cells, increases mean arterial pressure and perfusion pressure in isolated kidneys of rats, suggesting a role for Up4A in the regulation of vascular tone.
Purinoceptors have been classified into two sub-types: P1 and P2 receptors, based on their pharmacological properties and molecular clonings (Dalziel and Westfall, 1994; Knight and Burnstock, 2001). Adenosine and its phosphates, ATP and ADP, have been identified as the endogenous ligands for P1 and P2 receptors, respectively (Fredholm et al., 1994; Malec, 1996). Four subtypes of P1 receptors, all metabotropic, have been cloned, namely A1, A2A, A2B and A3 (Ralevic and Burnstock, 1998). The P2 receptors for nucleotides exist in two major families: metabotropic (P2Y receptors) and ionotropic (P2X receptors). There are at least 8 cloned P2Y and 7 cloned P2X receptors (Boarder and Hourani, 1998; Burnstock, 2004; Communi et al., 2000; North, 2002). Most of these receptors are capable of mediating responses to several nucleotides, resulting in multiple receptors having overlapping ligand preferences (Ralevic and Burnstock, 1998).
In isolated perfused rat kidney, Up4A was found to induce vasoconstriction through P2X1, P2Y2 and P2Y4 receptor activation (Jankowski et al., 2005). The vasodilatory effect of this compound has not been tested.
Because Up4A is secreted by human endothelial cells and is present in effective concentrations in human plasma, a role for Up4A in the regulation of vascular tone and in cardiovascular disease seems to be likely. More recently it has been reported that plasma Up4A levels are increased in human juvenile hypertension and has a significant proliferative effect on human smooth muscle cells (Jankowski et al., 2007).
The present experiments were carried out in order to characterize the responses induced by Up4A in rat aorta. We hypothesized that Up4A induces contraction of rat aortic smooth muscle. In an attempt to characterize Up4A-induced contraction, P1 and P2 receptor antagonists, inhibitors of Ca2+ channels and Rho-kinase were tested. The involvement of the endothelium, nitric oxide and superoxide anion was also evaluated.
2. Material and methods
2.1. Animals
Male Sprague Dawley rats (250–300 g, Harlan Laboratories, Indianapolis, IN) were maintained on a 12h light/dark cycle with access to water and standard rat chow ad libitum. On the day of an experiment, rats were anesthetized with CO2 and the thoracic aorta was excised. All experiments were conducted in accordance with the Medical College of Georgia’s Animal Use for Research and Education Committee.
2.2. In vitro measurement of isometric force generation in rat aortic rings
After removal of fat and connective tissue, aortic rings (3 mm long) were mounted in an organ chamber (Danish MyoTechnology, Aarhus, Denmark) for isometric tension recordings (ADinstruments, Castle Hill, Australia) and bathed in physiological salt solution (PSS) of the following composition: 130.0 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 1.6 mM CaCl2, 14.9 mM NaHCO3, 0.03 mM EDTA, and 5.5 mM glucose, which was maintained at 37°C and bubbled with 95% O2 and 5% CO2. The aorta segments were stretched at a tension of 30 mN with periodic washes every 15 min. After a 1-hour equilibration period, vessels were contracted with phenylephrine (10−7 M) and subsequently challenged with acetylcholine (10−5 M) to test tissue viability and integrity of the endothelium. Aortic rings that relaxed at least 70% of contraction induced by phenylephrine were used as retaining functional endothelium [E(+)]. Endothelium denudation [E(−)] was mechanically induced by gently rubbing the intimal surface of the aortic rings with a stainless steel wire. The absence of functional endothelium was confirmed by the complete lack of relaxation to acetylcholine.
2.3. Experimental protocols
To investigate the ability of Up4A to induce contraction of rat aortic rings, cumulative concentration-effect curves to Up4A (10−9 to 3×10−5M) were constructed in endothelium intact [E(+)] and endothelium-denuded [E(−)] aortic rings.
To test whether Up4A was able to induce relaxation, E(+) and E(−) aortic rings were stimulated with phenylephrine (10−7M) until the plateau of the contraction was obtained followed by a cumulative concentration-effect curve to Up4A (10−9 to 10−5M).
To evaluate the contribution of nitric oxide to Up4A-induced contraction, concentration-effect curves to Up4A were constructed in E(+) and E(−) aortic rings in the presence and absence of the nitric oxide synthase inhibitor L-NNA (10−4 M).
To assess whether Up4A-induced contraction is reversible and induces desensitization or tachyphylaxis, two consecutive cumulative concentration-effect curves to Up4A (10−9 to 3×10−5M) were constructed in aortic rings E(−) and E(+) in the presence of L-NNA (10−4 M). For that, after the maximum response for the last concentration was achieved in the first curve, the rings were washed several times with PSS or PSS + L-NNA for approximately 60 min until the tension returned to baseline. Subsequently, the second curve to Up4A was then constructed.
To determine which receptors mediate the contraction induced by Up4A, concentration-effect curves to Up4A were constructed in the presence of the P1 purinoceptor antagonist 8-PST (10−4 M); and the P2X1/P2X3 receptor antagonist NF279 (10−4M).
To investigate the involvement of L-type Ca2+ channels in the Up4A-induced contraction of rat aortic rings, concentration-effect curves to Up4A in E(−) aortic rings were evaluated in the presence of the L-type Ca2+ channel blocker nifedipine (10−5M). To examine the contribution of RhoA/Rho-kinase pathway to Up4A-induced contractions, concentration-effect curves to Up4A in E(−) aortic rings were evaluated in the presence of the Rho-kinase inhibitor Y-27632 (10−7M) .
To study the role of superoxide anion in the Up4A-induced contraction, E(−) aortic rings were contracted with Up4A (10−6M) and a concentration-effect curve (3–12mM) to the superoxide anion scavenger (tempol) was performed. To confirm the involvement of superoxide anion in the Up4A-induced contraction of rat aortic ring, a concentration-effect curve to Up4A was performed in the presence and in the absence of the NADPH oxidase inhibitor apocynin (10−4M).
All inhibitors were added to the organ chambers 30 min before the start of the concentration-effect curves to Up4A, and were maintained in the organ chamber until the end of the experiment.
2.4. Drugs
Phenylephine hydrochloride [(R)-(−)-1-(3-Hydroxyphenyl)-2-methylaminoethanol hydrochloride], acetylcholine chloride, 8-(p-sulphophenyl)-theophylline (8-PST), 4’- Hidoxy-3’-methoxyacetophenone (apocynin), 4-hydroxy-2,2,6,6-tetramethylpiperidinyloxy (tempol), 1,4-Dihydro-2,6-dimethyl-4-(2-nitrophenyl)-3,5-pyridinedicarboxylic acid dimethyl ester (nifedipine) were purchased from Sigma-Aldrich, St Louis-MO, USA). The compounds [(R)-(+)-trans-N-(4-pyridyl)-4-(1-aminoethyl)-cyclohexanecarboxamide.2HCl] (Y-27632), NG-nitro-L-arginine (L-NNA), [8,8′- (carbonylbis(imino-4, 1-phenylenecarbonylimino-4,1-phenylenecarbonylimino)) bis(1,3,5-naphthalenetrisul fonic acid)] (NF279) were acquired from Calbiochem (San Diego, California, USA). Uridine adenosine tetraphosphate (Up4A) was purchased from Biolog - Life Science Institute (Bremen, Germany) and dimethyl sulfoxide (DMSO) from Fisher Scientific (Fair Lawn, NJ, USA). Stock solutions were prepared using sterile HPLC grade water with the exception of apocynin and nifedipine that were prepared using DMSO.
2.5. Data Analysis
Statistical analysis was performed using one-way ANOVA followed by the Student-Newman-Keuls post hoc test for multiple comparisons. A value of P < 0.05 was considered statistically significant. The points represent the mean ± standard error of the mean (SEM) of the contraction in mN or as % of relaxation of agonist-induced force generation.
3. Results
3.1. Up4A-induced contraction of rat aortic rings is modulated by the endothelium
The ability of Up4A (10−9 – 3×10−5M) to induce contraction was tested in E(+) and E(−) rat aortic rings. Up4A caused contraction of rat aortic rings in a concentration-dependent manner, and the contraction was significantly potentiated by endothelium removal (Fig. 1A). The maximum contraction observed to the highest concentration of Up4A (3×10−5M) corresponded to approximately 30% and 80% of the contractile response induced by phenylephrine (10−6M) in rat aortic rings with and without endothelium, respectively (data not shown).
Fig. 1.
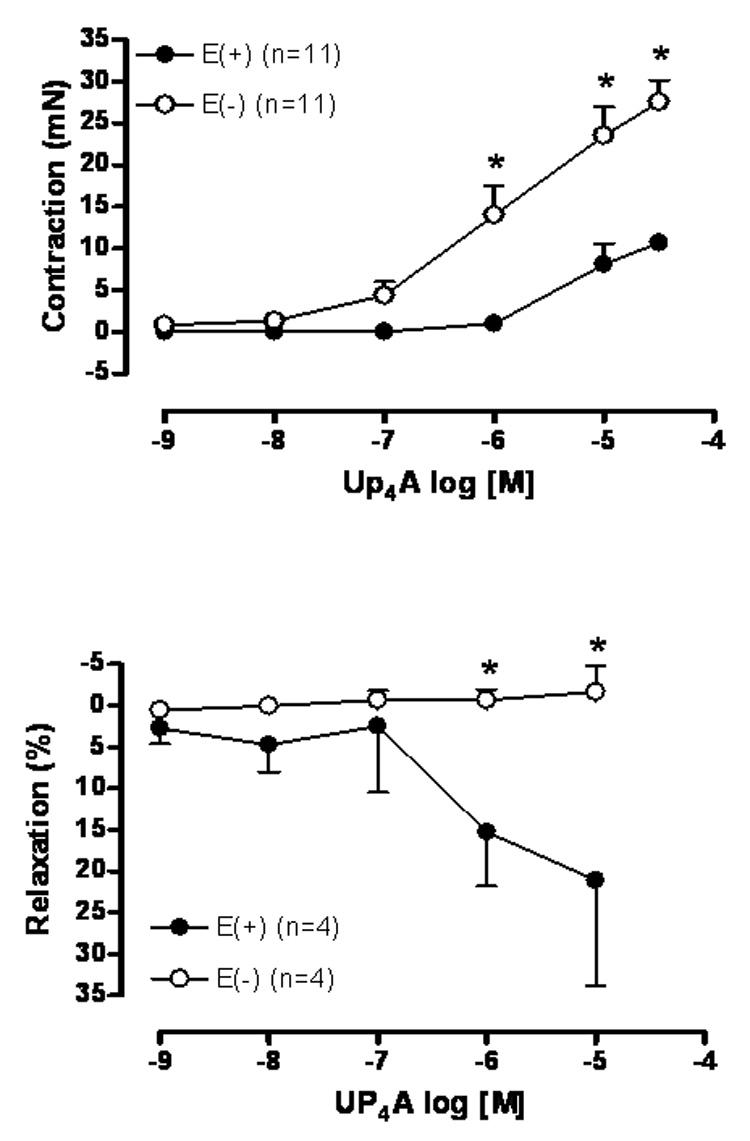
Up4A induces contraction and relaxation in rat aorta. Concentration-effect curves to Up4A (10−9 to 3×10−5M) were performed in endothelium-intact [E(+)] and endothelium-denuded [E(−)] rat aortic rings. In (A) the rings were treated from the baseline and in (B) the rings were contracted in response to phenylephrine 10−7 M. The points represent the mean ± S.E.M. of the force displacement in mN (for contraction) or as % of relaxation of the contraction induced by phenylephrine (10−7M). “n” represents the number of animals. * represents P value <0.05 compared to endothelium-denuded rat aortic rings.
3.2. Up4A-induced relaxation of rat aortic rings is endothelium-dependent
Endothelium-intact and denuded rat aortic rings were contracted with phenylephrine (10−7M) followed by a cumulative concentration-effect curve induced by Up4A (10−9 –10−5M). Up4A induced a modest relaxation (~25%) of the contraction to phenylephrine in endothelium-intact rat aortic rings. The absence of endothelium prevented Up4A-induced relaxation (Fig. 1B).
3.3. Up4A-induced contraction of rat aortic rings is modulated by NO pathway
The vasoconstriction induced by Up4A (10−9 – 3×10−5M) was tested in endothelium-intact (Fig. 2A) and endothelium-denuded (Fig. 2B) rat aortic rings in the absence and in the presence of the non-selective NO synthase inhibitor L-NNA (10−4M) for 30 min. Incubation with L-NNA did not affect the Up4A-induced contraction in E(−) rings (Fig. 2B), but significantly increased the contraction observed in E(+) rings to a similar extent observed by endothelium removal (Fig. 2A compared to Fig. 1A).
Fig. 2.
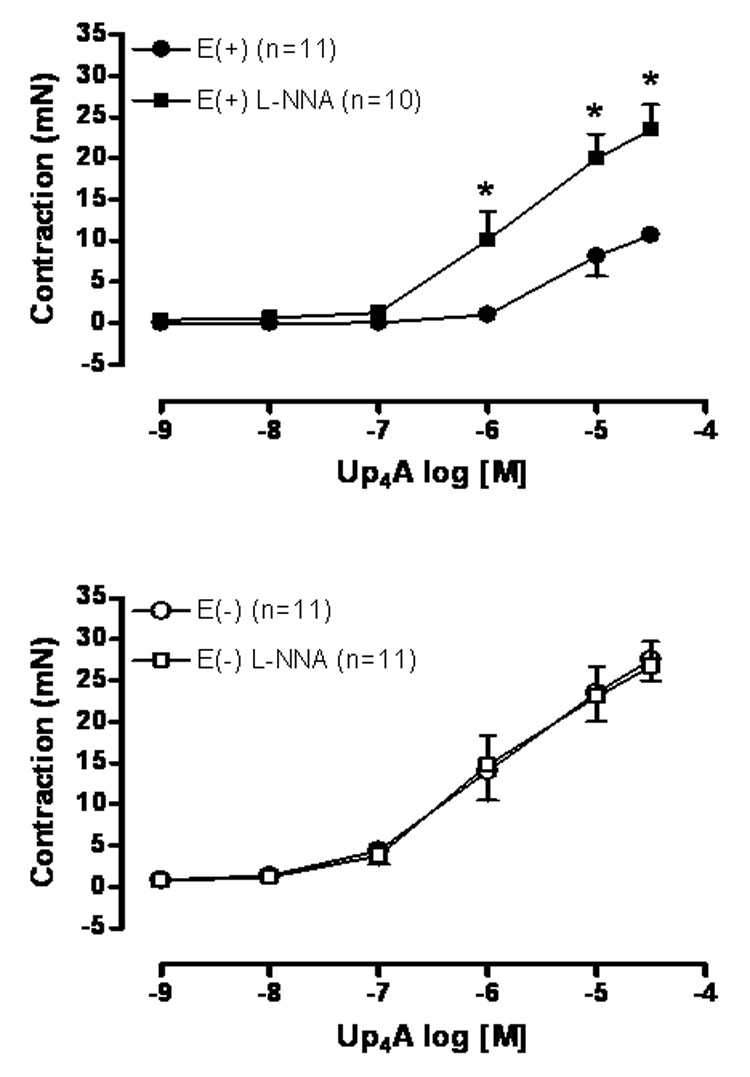
Up4A-induced contraction is significantly potentiated by endothelium removal and NO synthase inhibition. Concentration-effect curves to Up4A (10−9 to 3×10−5M) were performed in (A) endothelium-intact [E(+)] and (B) endothelium-denuded [E(−)] rat aortic rings in the absence and in the presence of L-NNA (10−4 M ) for 30 min. The points represent the mean ± S.E.M. of the force displacement in mN, “n” represents the number of animals and * represents P value <0.05 compared to the response obtained in the absence of L-NNA.
3.4. Up4A-induced contraction is reversible and does not induce tachyphylaxis in rat aortic rings
Two consecutive curves to Up4A were tested in aortic rings E(−) and E(+) treated with L-NNA. After the maximum response was obtained for the highest concentration of Up4A on the first curve, the aortic rings were freed from the agonist through several wash out events. After a period of 60 min the rings returned to the baseline tension (approximately 30 mN). The second curve to Up4A obtained after this re-stabilization period was similar to the first one obtained in both E(−) and E(+)/L-NNA (data not shown).
3.5. P1 and P2X receptors play an important role on Up4A-induced contraction in endothelium denuded rat aortic rings
To investigate the involvement of the P1 receptors in Up4A-induced contraction, we tested the ability of the P1 receptor antagonist 8-PST with known affinity for A1 and A2 receptors (Bruns, 1981; Bruns et al., 1986; Petrack et al., 1981) to inhibit endothelium denuded aortic ring Up4A-induced contraction. Fig. 3A demonstrates that the P1 receptor antagonist 8-PST (10−4M) significantly shifted Up4A -induced contraction in a parallel and competitive fashion suggesting the involvement of P1 receptors.
Fig. 3.
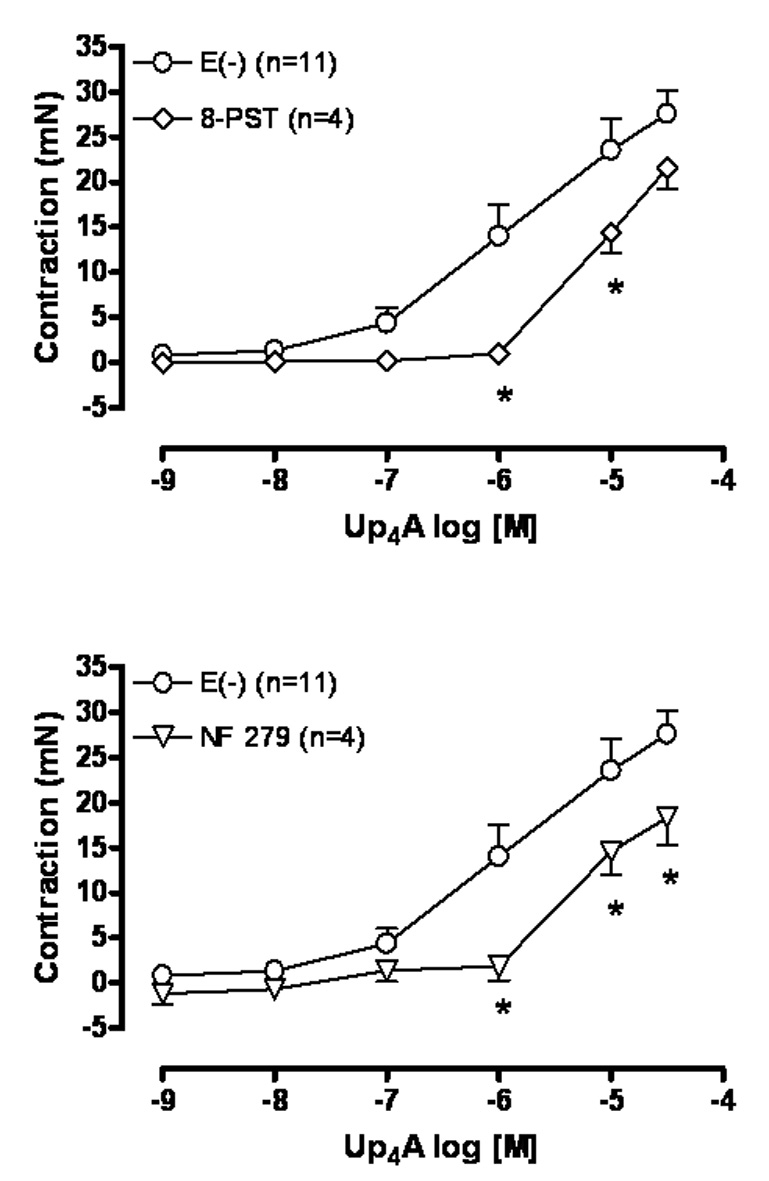
Up4A-induced contraction involves activation of P1 and P2X1 receptors. Concentration-effect curves to Up4A (10−9 to 3×10−5M) were performed in endothelium denuded [E(−)] rat aortic rings in the absence and in the presence of (A) the non-selective antagonist of adenosine A1 and A2 receptors 8-PST (10−4M), and (B) the non-selective P2X1 receptor antagonist NF279 (10−4M). The points represent the mean ± S.E.M. of the force displacement in mN, “n” represents the number of animals and * represents P value <0.05 compared to the response obtained in the absence of the antagonist.
To study the contribution of the P2X receptors in Up4A-induced contraction, we tested the ability of the non-selective P2X receptor antagonist NF279 with known affinity for P2X1 and P2X3 receptors (Rettinger et al., 2000) to inhibit Up4A-induced contraction of endothelium-denuded aortic rings. NF279 (10−4M) significantly rightward shifted the Up4A-induced contraction confirming the involvement of P2X receptors (Fig. 3B).
3.6. Up4A-induced contraction in endothelium denuded rat aortic rings involves L-type Ca2+ channels and Rho-kinase pathway
The vasoconstriction induced by Up4A (10−9 – 3×10−5M) was tested in endothelium-denuded rat aortic rings in the absence and in the presence of the selective L-type Ca2+ channel blocker nifedipine or the Rho-kinase inhibitor Y-27632. After 30 min incubation with nifedipine (10−5M) Up4A-induced contraction was blunted (Fig. 4A), whereas incubation with Y-27632 (10−7M) significantly reduced the contraction induced by Up4A in endothelium denuded aortic rings (Fig. 4B).
Fig. 4.
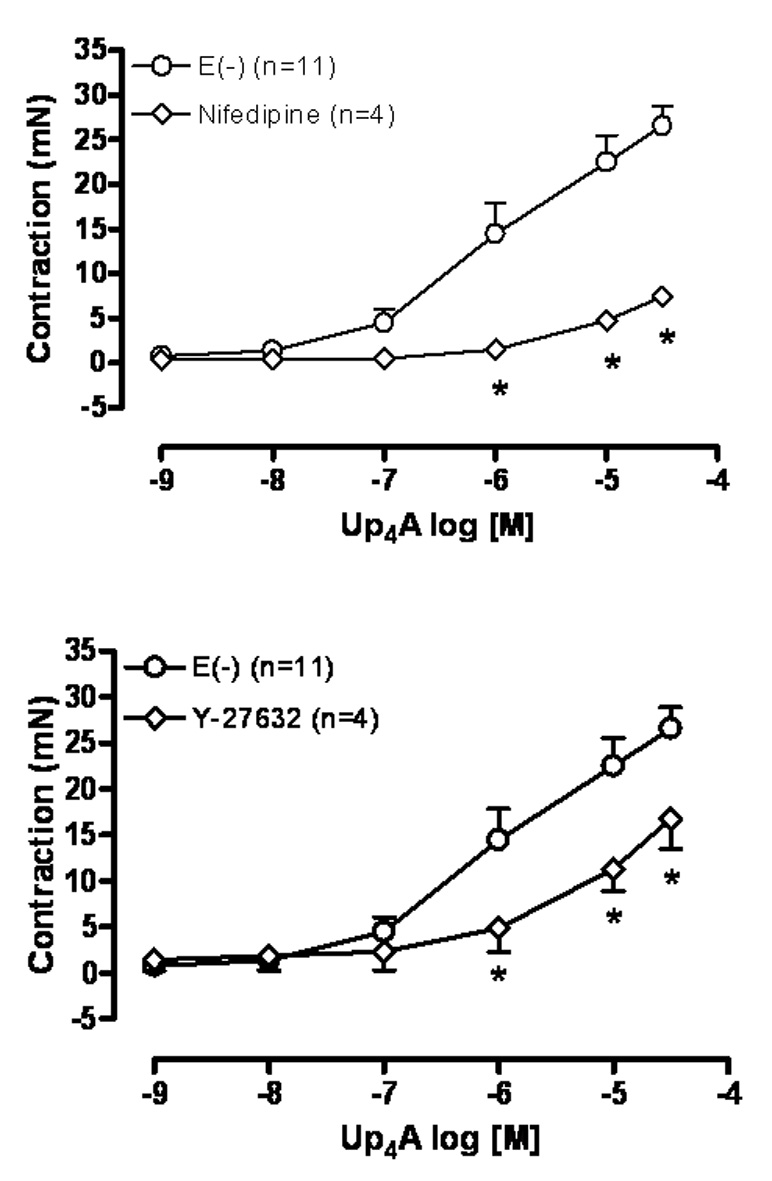
Up4A-induced contraction involves activation of L-type Ca2+ channels and the Rho-kinase pathway. CCEC(s) to Up4A (10−9 to 3×10−5M) were performed in endothelium denuded [E(−)] rat aortic ring in the absence and in the presence of (A) the selective L-type Ca2+ channel blocker nifedipine (10−5M) or (B) the selective Rho-kinase inhibitor Y-27632 (10−7M). The points represent the mean ± S.E.M. of the force displacement in mN, “n” represents the number of animals and * represents P value <0.05 compared to the response obtained in the absence of the inhibitor.
3.7. Up4A-induced contraction in endothelium denuded rat aortic rings involves superoxide anion formation
To investigate the involvement of superoxide anion to Up4A-induced contraction, we tested the ability of the superoxide dismutase mimetic/superoxide anion scavenger tempol, or the NADPH oxidase inhibitor apocynin to inhibit Up4A-induced contraction of endothelium denuded aortic rings. Fig. 5A demonstrates that contraction induced by Up4A is inhibited (~ 35%) by tempol (12mM). The concentration-effect curve induced by Up4A is significantly shifted to the right in the presence of apocynin (10−4M) (Fig. 5B).
Fig. 5.
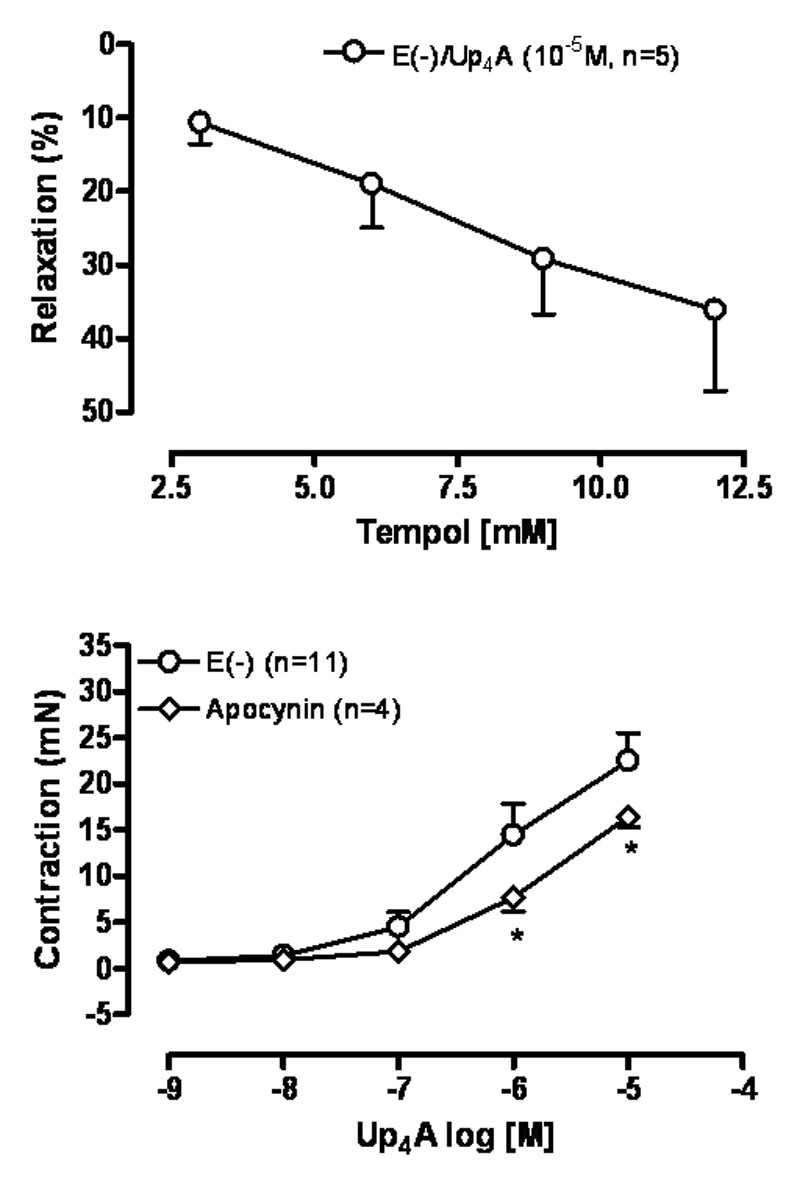
Up4A-induced contraction involves superoxide anion formation. (A) Concentration-effect curve to the superoxide anion scavenger tempol (3–12mM) was performed in endothelium-denuded [E(−)] rat aortic ring contracted with Up4A (10−5M). (B) Concentration-effect curves to Up4A (10−9 to 3×10−5M) were constructed in endothelium-denuded (E(−)) rat aortic rings in the absence and in the presence of the selective NADPH oxidase inhibitor apocynin. The points represent the mean ± S.E.M. of the force displacement in mN (for contraction) or as % of relaxation of the contraction induced by Up4A (10−5M). “n” represents the number of animals. * represents P value <0.05 compared to the response obtained in the absence of apocynin.
4. Discussion
We present here the unique findings that the novel endothelium-derived factor Up4A, originally described as a vasoconstrictor (Jankowski et al., 2005), also causes endothelium dependent relaxation of rat aortic rings.
Adenine and uracil nucleotides contribute to the local regulation of vascular tone (Olsson and Pearson, 1990; Ralevic and Burnstock, 1991). Dinucleotides have also been shown to control vascular tone (Jankowski et al., 2003; Jankowski et al., 2005; Tolle et al., 2006). However, dinucleotides containing two purines as moieties are the most common naturally-occurring signaling molecules. Up4A is the first dinucleotide containing one purine and one pyrimidine moiety found in living organisms (Jankowski et al., 2005). The potency and type of response of the dinucleotides are largely determined by the number of phosphates in the polyphosphate chain (Lewis et al., 1994; Lewis et al., 2000; Ralevic et al., 1995; Ralevic et al., 2001). In general, dinucleotides containing a phosphate chain of 2–3 compounds elicit vasorelaxation, whereas those containing a longer chain of 4 to 6 elicit vasoconstriction followed by vasodilation. Despite the characterization of Up4A as a potent vasoconstrictor of isolated perfused rat kidney, little is known about its properties in other vascular tissues. Furthermore, the ability of Up4A to induce relaxations has not been previously reported.
Two major families of purinoceptors, named P1 and P2 have been described. P1 and P2 receptor activation can cause both vasoconstriction and vasodilatation in the cardiovascular system. The responses induced by purines and pyrimidines varies extensively according to the tissue and specie studied. All P1 receptors are metabotropic (G-coupled protein) and specific agonists and antagonists are available for each different subtype (Burnstock, 2004). On the other hand, the lack of selective agonists and antagonists for P2 receptors have impeded a more detailed pharmacological characterization of the P2 receptors involved in the control of vascular tone.
We observed that Up4A induced a modest endothelium-dependent relaxation of rat aortic rings contracted with phenylephrine. The current literature would support the view that endothelium-dependent relaxation of rat aorta mediated by P1 receptors may involve the A2A (Lewis et al., 1994) and A2B (Prentice and Hourani, 1996). P2-mediated endothelium-dependent rat aorta relaxation involves P2Y1- and P2Y2-like receptors (Hansmann et al., 1997). Rat vascular endothelial cells express P2Y receptors that mediate relaxation via the nitric oxide pathway. In murine aorta, in addition to P2Y1 and P2Y2, P2Y6 is also involved in the relaxing responses (Guns et al., 2005). Further studies are necessary to characterize the receptor subtype in endothelium-dependent relaxation induced by Up4A in rat aorta.
Up4A-induced contraction of rat aorta was significantly increased by endothelium removal or by nitric oxide synthase inhibition. Jankowski et al. (2005) also observed that the vasoconstrictor effect of Up4A in perfused isolated kidney is increased after blockade of nitric oxide synthase (Jankowski et al., 2005). The contraction induced by certain guanine and by adenine nucleotides are modulated by the endothelium in rat aorta (Bultmann et al., 1997; Shen et al., 2000). In chorionic vessels, adenosine-induced contraction is also modulated by the endothelium (Donoso et al., 2005). Endothelium modulation of contractile responses elicited by agonists is an extensively reported phenomenon and, therefore, is not surprising to occur for Up4A.
In addition to characterizing the contractile and relaxing actions of Up4A in rat aorta, we tentatively identified the receptor subtypes and their intracellular signaling mechanisms. The contraction induced by Up4A in endothelium-denuded rat aortic rings was significantly inhibited by the non-selective P2X1/P2X3 receptor antagonist NF 279. Vascular smooth muscle cells express multiple P2X and P2Yreceptors that cause contraction. P2X1 is the primary subtype expressed on vascular smooth muscle involved in vasoconstriction. In rat aortic smooth muscle cells, the presence of P2X1, P2X2, P2X4, P2Y2 and P2Y6 have been shown [for review: (Kunapuli and Daniel, 1998)]. In addition, a study reported that purine dinucleotides containing 4 to 6 phosphates in the polyphosphate chain cause contraction mediated by P2X1 receptor present on the smooth muscle (Lewis et al., 2000). Based on these data, our results indicate that dinucleotides containing both purine and pyrimidine moieties and a long polyphosphate chain induce contraction by partially activating P2X1 receptors on the smooth muscle.
Interestingly, the non-selective P1 receptor antagonist 8-PST also inhibited Up4A-induced contraction. Adenosine is the prototype agonist for P1 receptors. P1 receptor activation is generally associated with vasodilation (Liu et al., 2002). For many years it was accepted that adenosine evoked dilatation by stimulation of A2 receptors (particularly A2A subtype) on the vascular smooth muscle via an increase in cAMP (Olsson and Pearson, 1990). In the kidney, adenosine produces vasoconstriction and vasodilatation through activation of A1 and A2, respectively (Inscho, 2003). Exceptionally, in aorta from hypertensive rats, P1 receptor activation has been shown to mediate endothelium-dependent contraction (Fahim and Mustafa, 2001; Tawfik et al., 2005). In addition, in human chorionic vessels, A2B adenosine receptor mediates contraction (Donoso et al., 2005). There have been some reports that diadenosine tetraphosphate (Ap4A) contractile response in rat urinary bladder is mediated by P1-purinoceptor whereas ATP responses were not affected by the P1 receptor antagonist theophyline (Khattab et al., 2002; Khattab and Al-Hrasen, 2006). To our knowledge, this is the first report showing the involvement of P1 receptor in vascular smooth muscle mediating contraction in aorta from normotensive rats.
The calcium channel blocker nifedipine inhibited the contraction induced by Up4A in endothelium-denuded rat aortic rings. In rat aorta, nifedipine has been reported ineffective to inhibit the contraction or increase in intracellular calcium concentration induced by P2 receptors activation (Kalthof et al., 1993; Shen et al., 2000). On the other hand, nifedipine inhibits the contraction induced by adenosine in afferent arterioles and by Ap4A and ATP in rat urinary bladder (Hansen et al., 2007; Khattab et al., 2002). These data support the presence of a P1 receptor in the smooth muscle of rat aorta mediating contraction, dependent on extracellular calcium influx.
The Rho-kinase inhibitor Y27632 (Uehata et al., 1997) inhibited Up4A-induced contraction of endothelium-denuded rat aortic rings. In rat aorta, nucleotides can induce contraction mediated by P2X, P2Y2 and P2Y6 receptor activation (Bultmann et al., 1997). Furthermore, P2Y1, P2Y2, P2Y4 and P2Y6 receptors have been shown to be coupled to Rho-kinase activation in rat aortic smooth muscle cells (Sauzeau et al., 2000). Based on this information, our results suggest the possible involvement of P2Y receptors in the contraction induced by Up4A in rat aorta.
Our data demonstrate that the membrane permeable reactive oxygen species scavenger tempol, and the NADPH oxidase inhibitor apocynin significantly reduced Up4A-induced contraction in rat aorta suggesting an important role for superoxide anion formation. To our knowledge, there have been no reports on superoxide formation after purinergic receptor activation in the vasculature. However, it has been reported that Ap4A as well as ATP-induced contractile responses in rat urinary bladder involve superoxide formation (Khattab et al., 2002; Khattab and Al-Hrasen, 2006). Furthermore, activation of ATP receptors causes increase in Ca+2 transients and production of superoxide anion in human eosinophil granulocytes, human prostate tumor spheroids and human tumor thyroid cell line (Dichmann et al., 2000; Pines et al., 2005; Sauer et al., 2001).
Tachyphylaxis, characterized by a rapidly decreasing response to a pharmacologically active agent after its initial administration, is a phenomenon that has been observed for many vasoconstrictor agents such as angiotensin II (Holloway et al., 2002; Linder et al., 2007; Thomas, 1999), endothelin 1 (Linder and Bendhack, 2002), serotonin (De Mey and Vanhoutte, 1981) and ATP (Shen et al., 2000). It has also been reported that ATP and diadenosine tetraphosphate (Ap4A) exhibit significant tachyphylaxis in isolated rat urinary bladder (Khattab et al., 2002; Shen et al., 2000). The fact that Up4A exhibits no tachyphylactic pattern indicates that the Up4A-induced constriction of rat aortic rings can not be explained by the presence of one of its degradation products. These data confirm the findings that ATP or UTP do not contribute to the UP4A-induced contraction observed previously in isolated perfused rat kidney (Jankowski et al., 2005).
A number of cardiovascular diseases involve alterations in the synthesis of vasoactive compounds. Hypertension is associated with increased vasoconstriction and decreased relaxation usually accompanied by endothelium dysfunction. It has been recently reported that the plasma of juvenile hypertensive subjects presents increased levels of Up4A (Jankowski et al., 2007). The results of the present study indicate that the endogenous compound Up4A may be involved in the control of vascular tone and, therefore, may have a strong impact in cardiovascular diseases. Understanding the pharmacological and physiological function and regulation of vascular tone by Up4A may be of extreme value to understanding the pathophysiology and treatment of these diseases.
Acknowledgements
This work was supported by National Institute of Health (HL74167). Dr. AE Linder is a recipient of an American Heart Association Postdoctoral Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Boarder MR, Hourani SM. The regulation of vascular function by P2 receptors: multiple sites and multiple receptors. Trends Pharmacol Sci. 1998;19:99. doi: 10.1016/s0165-6147(98)01170-5. [DOI] [PubMed] [Google Scholar]
- Bruns RF. Adenosine antagonism by purines, pteridines and benzopteridines in human fibroblasts. Biochem Pharmacol. 1981;30:325. doi: 10.1016/0006-2952(81)90062-9. [DOI] [PubMed] [Google Scholar]
- Bruns RF, Lu GH, Pugsley TA. Characterization of the A2 adenosine receptor labeled by [3H]NECA in rat striatal membranes. Mol Pharmacol. 1986;29:331. [PubMed] [Google Scholar]
- Bultmann R, Hansmann G, Tuluc F, Starke K. Vasoconstrictor and vasodilator effects of guanine nucleotides in the rat aorta. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:653. doi: 10.1007/pl00005102. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Introduction: P2 receptors. Curr Top Med Chem. 2004;4:793. doi: 10.2174/1568026043451014. [DOI] [PubMed] [Google Scholar]
- Buvinic S, Poblete MI, Donoso MV, Delpiano AM, Briones R, Miranda R, Huidobro-Toro JP. P2Y1 and P2Y2 receptor distribution varies along the human placental vascular tree: role of nucleotides in vascular tone regulation. J Physiol. 2006;573:427. doi: 10.1113/jphysiol.2006.105882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Communi D, Janssens R, Suarez-Huerta N, Robaye B, Boeynaems JM. Advances in signalling by extracellular nucleotides. the role and transduction mechanisms of P2Y receptors. Cell Signal. 2000;12:351. doi: 10.1016/s0898-6568(00)00083-8. [DOI] [PubMed] [Google Scholar]
- Dalziel HH, Westfall DP. Receptors for adenine nucleotides and nucleosides: subclassification, distribution, and molecular characterization. Pharmacol Rev. 1994;46:449. [PubMed] [Google Scholar]
- De Mey C, Vanhoutte PM. Effect of age and spontaneous hypertension on the tachyphylaxis to 5-hydroxytryptamine and angiotensin II in the isolated rat kidney. Hypertension. 1981;3:718. doi: 10.1161/01.hyp.3.6.718. [DOI] [PubMed] [Google Scholar]
- Dichmann S, Idzko M, Zimpfer U, Hofmann C, Ferrari D, Luttmann W, Virchow C, Jr, Di Virgilio F, Norgauer J. Adenosine triphosphate-induced oxygen radical production and CD11b up-regulation: Ca(++) mobilization and actin reorganization in human eosinophils. Blood. 2000;95:973. [PubMed] [Google Scholar]
- Donoso MV, Lopez R, Miranda R, Briones R, Huidobro-Toro JP. A2B adenosine receptor mediates human chorionic vasoconstriction and signals through arachidonic acid cascade. Am J Physiol Heart Circ Physiol. 2005;288:H2439. doi: 10.1152/ajpheart.00548.2004. [DOI] [PubMed] [Google Scholar]
- Fahim M, Mustafa SJ. Evidence for the presence of A(1) adenosine receptors in the aorta of spontaneously hypertensive rats. Br J Pharmacol. 2001;134:1760. doi: 10.1038/sj.bjp.0704433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, Abbracchio MP, Burnstock G, Daly JW, Harden TK, Jacobson KA, Leff P, Williams M. Nomenclature and classification of purinoceptors. Pharmacol Rev. 1994;46:143. [PMC free article] [PubMed] [Google Scholar]
- Guns PJ, Korda A, Crauwels HM, Van Assche T, Robaye B, Boeynaems JM, Bult H. Pharmacological characterization of nucleotide P2Y receptors on endothelial cells of the mouse aorta. Br J Pharmacol. 2005;146:288. doi: 10.1038/sj.bjp.0706326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen PB, Friis UG, Uhrenholt TR, Briggs J, Schnermann J. Intracellular signalling pathways in the vasoconstrictor response of mouse afferent arterioles to adenosine. Acta Physiol (Oxf) 2007;191:89. doi: 10.1111/j.1748-1716.2007.01724.x. [DOI] [PubMed] [Google Scholar]
- Hansmann G, Bultmann R, Tuluc F, Starke K. Characterization by antagonists of P2-receptors mediating endothelium-dependent relaxation in the rat aorta. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:641. doi: 10.1007/pl00005101. [DOI] [PubMed] [Google Scholar]
- Holloway AC, Qian H, Pipolo L, Ziogas J, Miura S, Karnik S, Southwell BR, Lew MJ, Thomas WG. Side-chain substitutions within angiotensin II reveal different requirements for signaling, internalization, and phosphorylation of type 1A angiotensin receptors. Mol Pharmacol. 2002;61:768. doi: 10.1124/mol.61.4.768. [DOI] [PubMed] [Google Scholar]
- Inscho EW. Modulation of renal microvascular function by adenosine. Am J Physiol Regul Integr Comp Physiol. 2003;285:R23. doi: 10.1152/ajpregu.00181.2003. [DOI] [PubMed] [Google Scholar]
- Inscho EW, Cook AK. P2 receptor-mediated afferent arteriolar vasoconstriction during calcium blockade. Am J Physiol Renal Physiol. 2002;282:F245. doi: 10.1152/ajprenal.0038.2001. [DOI] [PubMed] [Google Scholar]
- Jankowski J, Jankowski V, Seibt B, Henning L, Zidek W, Schluter H. Identification of dinucleoside polyphosphates in adrenal glands. Biochem Biophys Res Commun. 2003;304:365. doi: 10.1016/s0006-291x(03)00596-5. [DOI] [PubMed] [Google Scholar]
- Jankowski V, Meyer AA, Schlattmann P, Gui Y, Zheng XL, Stamcou I, Radtke K, Tran TN, van der Giet M, Tolle M, Zidek W, Jankowski J. Increased uridine adenosine tetraphosphate concentrations in plasma of juvenile hypertensives. Arterioscler Thromb Vasc Biol. 2007;27:1776. doi: 10.1161/ATVBAHA.107.143958. [DOI] [PubMed] [Google Scholar]
- Jankowski V, Tolle M, Vanholder R, Schonfelder G, van der Giet M, Henning L, Schluter H, Paul M, Zidek W, Jankowski J. Uridine adenosine tetraphosphate: a novel endothelium- derived vasoconstrictive factor. Nat Med. 2005;11:223. doi: 10.1038/nm1188. [DOI] [PubMed] [Google Scholar]
- Kalthof B, Bechem M, Flocke K, Pott L, Schramm M. Kinetics of ATP-induced Ca2+ transients in cultured pig aortic smooth muscle cells depend on ATP concentration and stored Ca2+ J Physiol. 1993;466:245. [PMC free article] [PubMed] [Google Scholar]
- Khattab M, O AL-S, H EL-K. Comparative study of the contractile activity evoked by ATP and diadenosine tetraphosphate in isolated rat urinary bladder. Pharmacol Res. 2002;45:93. doi: 10.1006/phrs.2001.0916. [DOI] [PubMed] [Google Scholar]
- Khattab MM, Al-Hrasen MN. Contractile activity of ATP and diadenosine tetraphosphate on urinary bladder in the rats: role of superoxide anion and urothelium. Auton Autacoid Pharmacol. 2006;26:149. doi: 10.1111/j.1474-8673.2006.00357.x. [DOI] [PubMed] [Google Scholar]
- Knight GE, Burnstock G. Identification of P1 and P2 purinoceptors in the aorta of the lizard (Agama sp.) Comp Biochem Physiol C Toxicol Pharmacol. 2001;128:413. doi: 10.1016/s1532-0456(00)00214-3. [DOI] [PubMed] [Google Scholar]
- Kunapuli SP, Daniel JL. P2 receptor subtypes in the cardiovascular system. Biochem J. 1998;336(Pt 3):513. doi: 10.1042/bj3360513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CD, Hourani SM, Long CJ, Collis MG. Characterization of adenosine receptors in the rat isolated aorta. Gen Pharmacol. 1994;25:1381. doi: 10.1016/0306-3623(94)90162-7. [DOI] [PubMed] [Google Scholar]
- Lewis CJ, Gitterman DP, Schluter H, Evans RJ. Effects of diadenosine polyphosphates (Ap(n)As) and adenosine polyphospho guanosines (Ap(n)Gs) on rat mesenteric artery P2X receptor ion channels. Br J Pharmacol. 2000;129:124. doi: 10.1038/sj.bjp.0702993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder AE, Bendhack LM. Endothelin-1-induced contraction is impaired in the tail artery of renal hypertensive rats. Vascul Pharmacol. 2002;39:77. doi: 10.1016/s1537-1891(02)00282-3. [DOI] [PubMed] [Google Scholar]
- Linder AE, Thakali KM, Thompson JM, Watts SW, Webb RC, Leite R. Methyl-beta-cyclodextrin prevents angiotensin II-induced tachyphylactic contractile responses in rat aorta. J Pharmacol Exp Ther. 2007;323:78. doi: 10.1124/jpet.107.123463. [DOI] [PubMed] [Google Scholar]
- Malec D. Purinergic receptors. Pol J Pharmacol. 1996;48:457. [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Olsson RA, Pearson JD. Cardiovascular purinoceptors. Physiol Rev. 1990;70:761. doi: 10.1152/physrev.1990.70.3.761. [DOI] [PubMed] [Google Scholar]
- Petrack B, Czernik AJ, Ansell J, Cassidy J. Potentiation of arginine-induced glucagon secretion by adenosine. Life Sci. 1981;28:2611. doi: 10.1016/0024-3205(81)90718-9. [DOI] [PubMed] [Google Scholar]
- Pines A, Perrone L, Bivi N, Romanello M, Damante G, Gulisano M, Kelley MR, Quadrifoglio F, Tell G. Activation of APE1/Ref-1 is dependent on reactive oxygen species generated after purinergic receptor stimulation by ATP. Nucleic Acids Res. 2005;33:4379. doi: 10.1093/nar/gki751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice DJ, Hourani SM. Activation of multiple sites by adenosine analogues in the rat isolated aorta. Br J Pharmacol. 1996;118:1509. doi: 10.1111/j.1476-5381.1996.tb15567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Roles of P2-purinoceptors in the cardiovascular system. Circulation. 1991;84:1. doi: 10.1161/01.cir.84.1.1. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413. [PubMed] [Google Scholar]
- Ralevic V, Hoyle CH, Burnstock G. Pivotal role of phosphate chain length in vasoconstrictor versus vasodilator actions of adenine dinucleotides in rat mesenteric arteries. J Physiol. 1995;483(Pt 3):703. doi: 10.1113/jphysiol.1995.sp020615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V, Jankowski J, Schluter H. Structure-activity relationships of diadenosine polyphosphates (Ap(n)As), adenosine polyphospho guanosines (Ap(n)Gs) and guanosine polyphospho guanosines (Gp(n)Gs) at P2 receptors in the rat mesenteric arterial bed. Br J Pharmacol. 2001;134:1073. doi: 10.1038/sj.bjp.0704341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettinger J, Schmalzing G, Damer S, Muller G, Nickel P, Lambrecht G. The suramin analogue NF279 is a novel and potent antagonist selective for the P2X(1) receptor. Neuropharmacology. 2000;39:2044. doi: 10.1016/s0028-3908(00)00022-8. [DOI] [PubMed] [Google Scholar]
- Sauer H, Klimm B, Hescheler J, Wartenberg M. Activation of p90RSK and growth stimulation of multicellular tumor spheroids are dependent on reactive oxygen species generated after purinergic receptor stimulation by ATP. Faseb J. 2001;15:2539. doi: 10.1096/fj.01-0360fje. [DOI] [PubMed] [Google Scholar]
- Sauzeau V, Le Jeune H, Cario-Toumaniantz C, Vaillant N, Gadeau AP, Desgranges C, Scalbert E, Chardin P, Pacaud P, Loirand G. P2Y(1), P2Y(2), P2Y(4), and P2Y(6) receptors are coupled to Rho and Rho kinase activation in vascular myocytes. Am J Physiol Heart Circ Physiol. 2000;278:H1751. doi: 10.1152/ajpheart.2000.278.6.H1751. [DOI] [PubMed] [Google Scholar]
- Shen JZ, Zheng XF, Kwan CY. Evidence for P(2)-purinoceptors contribution in H(2)O(2)-induced contraction of rat aorta in the absence of endothelium. Cardiovasc Res. 2000;47:574. doi: 10.1016/s0008-6363(00)00123-1. [DOI] [PubMed] [Google Scholar]
- Tawfik HE, Schnermann J, Oldenburg PJ, Mustafa SJ. Role of A1 adenosine receptors in regulation of vascular tone. Am J Physiol Heart Circ Physiol. 2005;288:H1411. doi: 10.1152/ajpheart.00684.2004. [DOI] [PubMed] [Google Scholar]
- Thomas WG. Regulation of angiotensin II type 1 (AT1) receptor function. Regul Pept. 1999;79:9. doi: 10.1016/s0167-0115(98)00140-2. [DOI] [PubMed] [Google Scholar]
- Tolle M, Giebing G, Tietge UJ, Jankowski J, Jankowski V, Henning L, Horl MP, Weiss W, Zidek W, van der Giet M. Diguanosine pentaphosphate: an endogenous activator of Rho-kinase possibly involved in blood pressure regulation. J Hypertens. 2006;24:1991. doi: 10.1097/01.hjh.0000244948.87911.05. [DOI] [PubMed] [Google Scholar]
- Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]


