Abstract
Background
In clinical trials, peptides derived from food proteins have shown an effect on blood pressure. This biological mechanism is mainly due to inhibition of angiotensin-I-converting enzyme (ACE), thereby regulating blood pressure through the renin-angiotensin system. A meta-analysis of these trials is needed to better quantify their effect, sources of variation, and possible publication bias.
Objective
To perform a meta-analysis of placebo-controlled clinical trials on peptides derived from food proteins and their effect on blood pressure.
Design
Trials identified using a defined search strategy in PubMed were included in the meta-analysis, and their pooled effect was estimated with a random effects model.
Results
Pooled effect of peptides was −5.13 mmHg (95% CI: −7.12, −3.14) for systolic blood pressure, and −2.42 mmHg (95% CI: −3.82, −1.03) for diastolic blood pressure. There were indications of publication bias for diastolic blood pressure data.
Conclusions
Peptides derived from food proteins may lead to significantly reduced blood pressure and could therefore be a supplement or alternative to pharmaceutical treatment for mild hypertension. Their effect seems more pronounced, or at least comparable, to that of other food components studied by randomized controlled trials. A high proportion of the reported trials was carried out using the well-known ACE inhibiting tripeptides – Valine-Proline-Proline (VPP) and Isoleucine-Proline-Proline (IPP).
Keywords: angiotensin-I-converting enzyme (ACE), clinical trials, hypertension
High blood pressure, which is estimated to affect one-third of the Western population, is an important risk factor for coronary heart disease, stroke and renal disease (1). Lifestyle modifications, including weight loss, quitting smoking, reducing sodium and alcohol intake, increasing physical activity and changing diet, are recommended for both treatment and prevention (2). Besides the recommendation of general lifestyle changes, efforts have been made to produce functional foods that contain components – nutraceuticals – which have a blood pressure reducing effect, and could be a supplement or alternative to the pharmaceutical treatment of hypertension. Bioactive peptides with angiotensin-I-converting enzyme (ACE) inhibitory and antihypertensive effects have been the focus of special attention. They have been isolated from several food sources, biochemically characterized, and currently, some commercial food products with clinically proven effects are used (3). Specifically, milk and dairy products have been shown to have good effects (4). Results from extensive randomized, blinded and placebo-controlled clinical trials are needed to scientifically support claimed effects. Therefore, it is important to systematically summarize the findings from such clinical trials on antihypertensive bioactive peptides derived from foods.
Meta-analysis has been defined as ‘the statistical analysis of a large collection of analysis results from individual studies for the purpose of integrating the finding’ (5). It goes beyond an expert literature review where the results from various studies are discussed and compared, since it synthesizes the results of the individual studies into a new, pooled, result using statistical methodology (6). This enhances the precision of the estimates of treatment effects, thereby leading to improvement in clinical strategies (7). Even though the number of expert literature reviews on antihypertensive peptides from food sources is extensive, to my knowledge a meta-analysis of placebo-controlled clinical trials has not been performed. A meta-analysis would contribute to an improved understanding of the effectiveness of peptides in hypertension and whether these could be a feasible supplement to pharmaceutical treatment. The possible presence of publication bias in these trials was also investigated.
Methods
Selection of studies
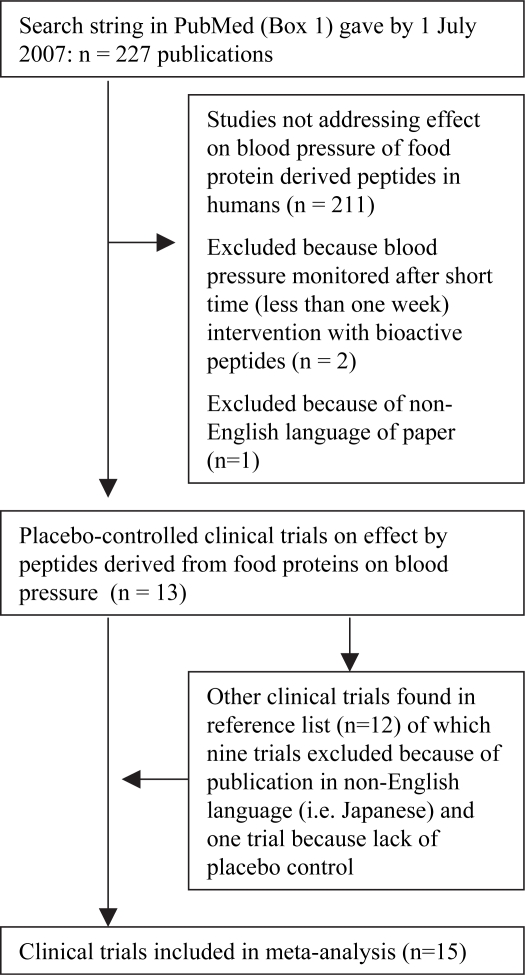
As part of finding a defined search string in PubMed for relevant clinical trails, literature reviews and selected clinical trials on bioactive peptides derived from food proteins were studied. Several searches of databases and the Internet were also carried out, providing an overview of the subject. It was also discovered that some reports have only been published in Japanese. To avoid problems and limitations with the translation of these reports, it was decided to perform this meta-analysis solely on trials published in English. The strategy was to develop a defined search string that would find all relevant clinical trials registered in PubMed. The literature reference list of the selected trials would then be carefully examined to identify any supplementary trials not registered in PubMed. The defined search string in PubMed with generated translations of expressions is shown in Box 1. By 1 July 2007 it gave 227 hits of which 13 trials were found relevant for further examination by meta-analysis. By examining the literature reference list in those trials, two additional trials were included for meta-analysis (Fig. 1). Aihara et al. (8) and Mizuno et al. (9) performed separate studies on patients with high-normal blood pressure and mild hypertension. Therefore, it was decided to extract those separate data as independent trials.
Fig 1. .
Selection of randomized placebo-controlled trials for meta-analysis.
Data abstraction
Number, age and sex-ratio of participants, duration of intervention, baseline systolic (SBP) and diastolic blood pressure (DBP), change at end of intervention and daily amount of active component or placebo product were obtained from each trail (Tables 1 [10–22] and 2). In a trial by Mizuno et al. (9), participants were given different amounts of active product. Only data from the group of participants given the highest amount were abstracted for use in the meta-analysis.
Table 1.
Baseline characteristics of clinical trials included for meta-analysis with mean and standard deviation values
| Active intervention |
Placebo control |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Source | n | SBP (mmHg) | DBP (mmHg) | Age | Male (%) | n | SBP (mmHg) | DBP (mmHg) | Age | Male (%) |
| Hata et al. (10) | 17 | 159 (46) | 89 (39) | 77 (31) | 23 | 13 | 151 (34) | 87 (33) | 73 (38) | 31 |
| Kawasaki et al. (11) | 17 | 146 (10) | 91 (7) | 46 (13) | 94 | 12 | 146 (8) | 92 (6) | 49 (10) | 92 |
| Kawase et al. (12) | 10 | 124 (10) | NA | NA | 100 | 10 | 124 (10) | NA | NA | 100 |
| Fujita et al. (13) | 30 | 150 (9) | 94 (10) | 62 (8) | 60 | 31 | 149 (7) | 93 (9) | 61 (10) | 58 |
| Seppo et al. (14) | 10 | 148 (13) | 94 (6) | 49 (6) | 30 | 7 | 148 (13) | 93 (3) | 46 (13) | 29 |
| Seppo et al. (15) | 22 | 155 (13) | 97 (6) | 51 (7) | 46 | 17 | 152 (13) | 96 (6) | 48 (7) | 53 |
| Mizushima et al. (16) | 23 | 148 (10) | 95 (10) | 44 (10) | 100 | 23 | 145 (13) | 92 (10) | 48 (9) | 100 |
| Toumilehto et al. (17) | 30 | 153 (10) | 98 (7) | 51 (10) | NA | 29 | 157 (11) | 98 (7) | 54 (9) | NA |
| Aihara et al.* (8) | 20 | 137 (6) | 85 (11) | 53 (11) | 65 | 20 | 137 (5) | 85 (5) | 50 (12) | 65 |
| Aihara et al.** (8) | 20 | 147 (9) | 92 (9) | 52 (12) | 80 | 20 | 149 (7) | 93 (12) | 52 (10) | 80 |
| Jauhiainen et al. (18) | 53 | 149 (7) | 94 (6) | 51 (12) | 66.0 | 55 | 150 (9) | 93 (6) | 55 (11) | 62 |
| Mizuno et al.* (9) | 12 | 134 (3) | 82 (5) | NA | NA | 12 | 133 (3) | 82 (2) | NA | NA |
| Mizuno et al.** (9) | 21 | 149 (5) | 88.6 (5) | NA | NA | 20 | 150 (5) | 89 (5) | NA | NA |
| Sano et al. (19) | 72 | 138 (7) | 85 (5) | 51 (10) | 40.3 | 72 | 138 (6) | 85 (5) | 50 (10) | 38.9 |
| Cadée et al. (20) | 24 | 138 (12) | 87 (10) | 56 (11) | 25 | 24 | 137 (15) | 85 (10) | 56 (12) | 16.7 |
| Lee et al. (21) | 27 | 144 (9) | 91 (6) | 55 (10) | 52 | 26 | 141 (12) | 90 (6) | 48 (12) | 62 |
| Pins et al. (22) | 15 | 137 (11) | 84 (7) | 46 (13) | 47 | 15 | 135 (9) | 82 (6) | 47 (14) | 47 |
*Data from participants with high-normal blood pressure.
**Data from participants with mild hypertension.
Table 2.
Daily intervention of bioactive components from different food sources and duration of clinical trials included for meta-analysis
| Source | Daily intervention | Active component | Food source | Placebo | Duration (weeks) |
|---|---|---|---|---|---|
| Hata et al. (10) | 100 ml fermented milk | 1.5 mg VPP and 1.1 mg IPP peptide | Milk | 100 ml artificially acidified milk | 8 |
| Kawasaki et al. (11) | 100 ml beverage with hydrolyzed sardine muscle | 3 mg VY peptide | Fish | 100 ml beverage without hydrolyzed sardine muscle | 4 |
| Kawase et al. (12) | 400 ml fermented milk with whey protein concentrate | Whey protein concentrate | Milk | 400 ml artificially acidified milk | 8 |
| Fujita et al. (13) | 10 tablets with active Katsuobushi oligopeptide | 1.5 g Katsuobushi oligopeptide | Bonito (fish) | 10 tablets with inactive Katsuobushi powder | 5 |
| Seppo et al. (14) | 150 ml fermented milk with strain that increase production of bioactive peptides | 1.5 mg VPP and 1.1 mg IPP peptide | Milk | 150 ml ordinary fermented milk | 8 |
| Seppo et al. (15) | 150 ml fermented milk with process to increase production of bioactive peptides | 2.5 mg VPP and 2.25 mg IPP peptide | Milk | 150 ml ordinary fermented milk | 8 |
| Mizushima et al. (16) | 160 g fermented milk | 1.92 mg VPP and 1.15 mg IPP peptide | Milk | 160 g artificially acidified milk | 4 |
| Toumilehto et al. (17) | 150 ml fermented milk with Lb. helveticus | 2.5 mg VPP and 2.5 mg IPP peptide | Milk | 150 ml ordinary fermented milk | 10 |
| Aihara et al. (8) | 12 g dried product from fermented milk | 8.3 mg VPP and 4.7 mg IPP peptide | Milk | 12 g dried product made without fermentation | 4 |
| Jauhiainen et al. (18) | 300 ml milk fermented with Lb. helveticus | 30 mg VPP and 22.5 mg IPP peptide | Milk | 300 ml ordinary fermented milk | 10 |
| Mizuno et al. (9) | Two tablets of hydrolyzed casein | 3.6 mg of VPP + IPP peptide | Milk | Tablets without hydrolyzed casein | |
| Sano et al. (19) | 200 ml fruit juice with hydrolyzed casein | 1.47 mg VPP and 1.6 mg IPP peptide | Milk | 200 ml fruit juice without hydrolyzed casein | 12 |
| Cadée et al. (20) | Tablets containing C12 Peptide (DMV International, The Netherlands) | 3.8 g C12 peptide | Milk | Placebo tablets | 4 |
| Lee et al. (21) | 125 ml skimmed milk based beverage with whey powder | Whey powder | Milk | 125 ml skimmed milk based beverage | 12 |
| Pins et al. (22) | Hydrolyzed whey powder mixed with water | Hydrolyzed whey powder | Milk | Unhydrolyzed whey powder mixed with water | 6 |
*Data from participants with high-normal blood pressure.
**Data from participants with mild hypertension.
Statistical analysis
The effect of intervention or placebo was not estimated identically in the trials. This effect was estimated either with a within or between subject comparison of the participants’ blood pressure at the start and end of trial (Table 3). Statistically, let X ij be the blood pressure for the intervention group, where i=1 or 2 which is at start or end of trial, respectively, and j=1, 2, … , n is the participants with active intervention. Further, let Y ij be the blood pressure for the placebo control group, where i=1 or 2 which is at start or end of trial, respectively, and j=1, 2, … , m is the participants with placebo control.
Table 3.
Calculating the effect of trials based on within or between subject comparison of intervention and placebo control treatment
| Intervention |
Placebo control |
Effect of trial |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SBP |
DBP |
SBP |
DBP |
SBP |
DBP |
|||||||||
| Study | n | Mean | SD | Mean | SD | n | Mean | SD | Mean | SD | Effect | SE | Effect | SE |
| Within subject comparison of blood pressure | ||||||||||||||
| Hata et al. (10) | 17 | −14.1 | 12.8 | −6.9 | 10.3 | 13 | −4.4 | 13.0 | −2.2 | 6.9 | −9.7 | 4.7 | −4.7 | 3.3 |
| Seppo et al. (14) | 10 | −14.9 | 9.4 | −8.8 | 6.8 | 7 | −4.1 | 11.4 | −2.0 | 5.2 | −10.8 | 5.0 | −6.9 | 3.1 |
| Seppo et al. (15) | 19 | −15.4 | 8.3 | −9.3 | 4.8 | 17 | −9.4 | 13.2 | −5.5 | 7.4 | −6.0 | 3.6 | −3.8 | 2.1 |
| Mizushima et al. (16) | 23 | −5.2 | 11.3 | −2.0 | 8.0 | 23 | −3.7 | 10.6 | −0.3 | 8.3 | −1.5 | 3.2 | −1.7 | 2.4 |
| Toumilehto et al. (17) | 30 | −15.8 | 13.2 | −10.3 | 6.5 | 29 | −13.5 | 11.9 | −9.8 | 7.5 | −2.3 | 3.3 | −0.5 | 1.8 |
| Aihara et al.* (8) | 20 | −5.0 | 8.9 | −6.0 | 6.7 | 20 | −2.0 | 8.9 | −1.0 | 8.9 | −3.0 | 2.8 | −5.0 | 2.5 |
| Aihara et al.** (8) | 20 | −10.0 | 15.7 | −7.5 | 15.7 | 20 | 0.5 | 11.2 | −0.5 | 8.9 | −10.5 | 4.3 | −7.0 | 4.0 |
| Jauhianinen et al. (18) | 53 | −5.1 | 9.4 | −1.1 | 5.6 | 55 | −3.1 | 11.5 | −2.1 | 6.1 | −2.0 | 2.0 | 1.0 | 1.1 |
| Cadée et al. (20) | 24 | −10.7 | 7.8 | −6.9 | 5.9 | 24 | −3.6 | 11.8 | −2.7 | 7.8 | −7.1 | 2.9 | −4.2 | 2.0 |
| Between subject comparison of blood pressure | ||||||||||||||
| Kawasaki et al. (11) | 17 | 137.1 | 12.0 | 85.3 | 8.6 | 12 | 142.5 | 11.5 | 91.0 | 8.7 | −5.4 | 4.4 | −5.7 | 3.2 |
| Kawase et al. (12) | 10 | 118.0 | 9.5 | − | − | 10 | 125.0 | 14.−2 | − | − | −7.0 | 5.4 | − | − |
| Fujita et al. (13) | 30 | 137.5 | 11.5 | 86.5 | 8.2 | 31 | 148.5 | 9.9 | 92.0 | 8.4 | −11.0 | 2.7 | −5.5 | 2.1 |
| Mizuno et al.* (9) | 12 | 131.1 | 5.8 | 79.8 | 6.0 | 12 | 133.1 | 5.5 | 79.2 | 4.3 | −2.0 | 2.3 | 0.6 | 2.1 |
| Mizuno et al.** (9) | 21 | 135.0 | 10.9 | 83.9 | 8.8 | 20 | 147.2 | 10.8 | 87.3 | 7.3 | −12.2 | 3.4 | −3.4 | 2.5 |
| Sano et al. (19) | 72 | 132.3 | 7.3 | 81.2 | 4.8 | 72 | 136.4 | 8.0 | 83.7 | 5.6 | −4.1 | 1.3 | −2.5 | 0.9 |
| Lee et al. (21) | 27 | 143.7 | 13.5 | 90.4 | 6.5 | 26 | 137.0 | 14.4 | 87.7 | 6.6 | 6.7 | 3.8 | 2.7 | 1.8 |
| Pins et al. (22) | 15 | 126.0 | 6.2 | 77.0 | 5.4 | 15 | 133.0 | 7.4 | 80.0 | 5.8 | −7.0 | 2.5 | −3.0 | 2.1 |
*Data from participants with high-normal blood pressure.
**Data from participants with mild hypertension.
Box 1.
Search string in PubMed with generated translations to find clinical trials on effect of peptides derived from food proteins on blood pressure
| Search string in PubMed |
|
|---|---|
| (blood pressure OR hypertension) | |
| AND | |
| (clinical trial[ptyp] OR randomized controlled trial[ptyp] OR clinical trial, phase I[ptyp] OR clinical trial, phase II[ptyp] OR clinical trial, phase III[ptyp] OR controlled clinical trial[ptyp] OR placebo) | |
| AND | |
| (beverages OR food OR milk proteins OR vegetables OR meat OR fishes) | |
| AND | |
| (peptides OR ferment* OR sour OR hydrol*) | |
| Expression | Translations in PubMed |
| Blood pressure | “blood pressure’’[MeSH Terms] OR (“blood pressure determination”[TIAB] NOT Medline[SB]) OR “blood pressure determination”[MeSH Terms] OR blood pressure[Text Word] |
| Hypertension | “hypertension”[MeSH Terms] OR hypertension[Text Word] |
| Placebo | (“placebos’’[TIAB] NOT Medline[SB]) OR “placebos”[MeSH Terms] OR placebo[Text Word] |
| Beverages | “beverages”[MeSH Terms] OR beverages[Text Word] |
| Food | “food”[MeSH Terms] OR food[Text Word] |
| Milk Proteins | “milk proteins”[MeSH Terms] OR milk proteins[Text Word] |
| Vegetables | “vegetables’’[MeSH Terms] OR Vegetables[Text Word] |
| Meat | “meat”[MeSH Terms] OR meat[Text Word] |
| Fishes | “fishes”[MeSH Terms] OR fishes[Text Word] |
| Peptides | “peptides”[MeSH Terms] OR peptides[Text Word] |
For within subject comparison, the mean effect of intervention is defined as
| 1 |
and for placebo
| 2 |
For between subject comparison, the mean effect of intervention is defined as
| 3 |
and for placebo
| 4 |
Estimates of the mean effect of intervention and placebo with standard deviations (SDI and SDP) were obtained from the studies. For meta-analysis, the trial effect for both within subject and between subject comparisons could then be estimated by
| 5 |
with standard error of trial effect given by
| 6 |
Both fixed and random effects models were examined to calculate the mean pooled effect size with confidence intervals. The homogeneity among studies was tested using Cochran's Q-test (23). In this paper, results presented are based on a random effects model. To calculate a pooled effect size, each study was assigned a weight. The random effects weighted model by DerSimonian and Laird (24) based on the inverse variance was used. Publication bias was visually examined after construction of a funnel plot, where standard error is plotted against net changes in blood pressure. In addition, Egger regression and its corresponding test for publication bias were performed (25). The effect and quality of individual trials were examined by making cumulative and exclusion sensitivity forest plots (7). Meta-regression with trial effects as dependent variable and combined amounts of tripeptides Valine-Proline-Proline (VPP) and Isoleucine-Proline-Proline (IPP) in trials as independent variable weighted by the inverse variance from the random effect model was performed.
Statistical calculations for meta-analysis and generation of plots were performed using the MIX software, version 1.54 (26), and weighted least square regression for meta-regression was preformed using SPSS 15.0.1 for Windows (Chicago, IL, USA).
Results
Study characteristics
Characteristics of the included trials for meta-analysis are given in Table 1 and 2. A total of 826 participants completed the trials. In the study by Kawase et al. (12) DBP was not measured. All trials have been published in international peer-reviewed journals. Mean duration of intervention was 7.4 weeks with a minimum intervention of 4 weeks. More than half of the trials used intervention with the bioactive peptides IPP and VPP. In most of the trials, participants were aged more than 50 years on average, with a higher proportion of males than females.
Quantitative data synthesis
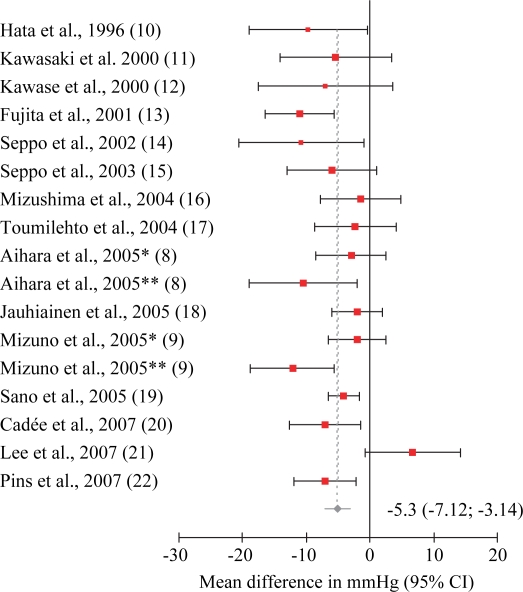
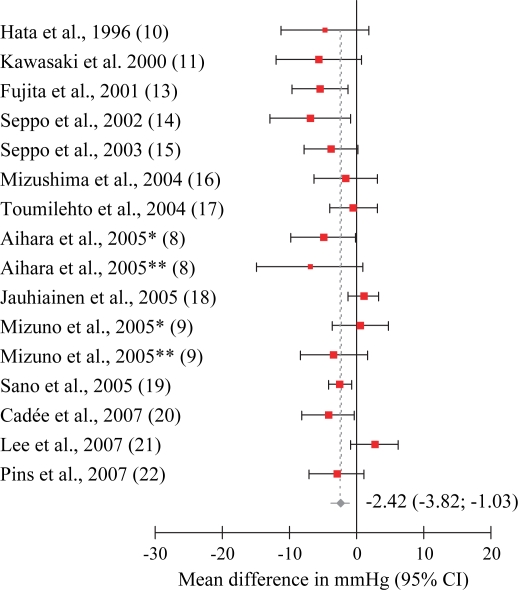
Cochran's Q-test for homogeneity among studies gave Q=30.52 for SBP data with a p-value of 0.02, and Q=29.10 for DBP data with a p-value of 0.08. This indicated heterogenicity among the studies, and a random effects model was used for meta-analysis. Exclusion sensitivity plots showed that excluding single studies did not affect pooled results from meta-analysis by more than approximately 0.5 mmHg (results not shown). The weighted pooled effect size for trials with bioactive peptides derived from food proteins was −5.13 mmHg (95% CI: −7.12, −3.14) for SBP (Fig. 2), and −2.42 mmHg (95% CI: −3.82, −1.03) for DBP (Fig. 3). Meta-regression relating combined amounts per day of IPP and VPP (mg) with effect on blood pressure was performed, but no statistically significant effect at the 5% level was found. Difference between studies with Western or Asian population was also examined. Asian studies had a somewhat larger reduction in blood pressure, but there was no statistically significant difference at the 5% level. No statistically significant effect was found for the proportion of males in the studies.
Fig 2. .
Standard forest plot from meta-analysis on systolic blood pressure. Cochran's Q-test for homogeneity was Q=30.52, p-value = 0.02.
Fig 3. .
Standard forest plot from meta-analysis on diastolic blood pressure. Cochran's Q-test for homogeneity was Q=29.10, p-value = 0.08.
Publication bias
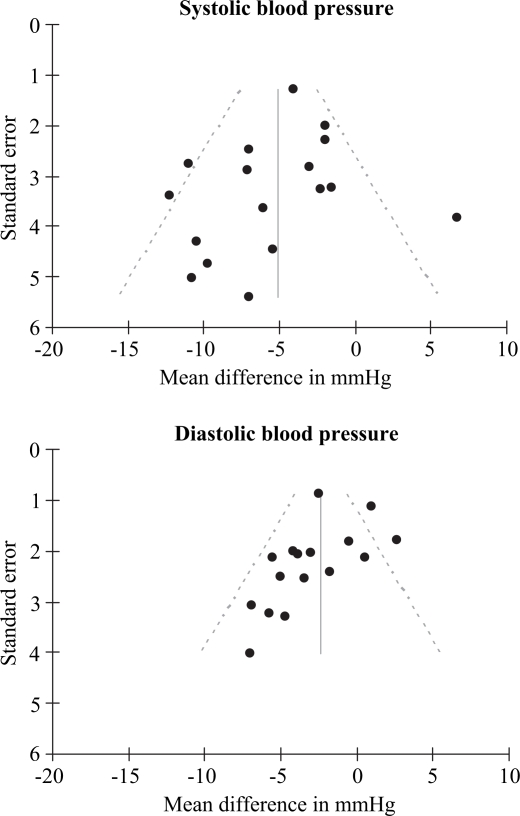
Funnel plots of SBP and DBP (Fig. 4) indicated signs of publication bias for DBP. This was partly confirmed by the Egger regression test for publication bias, which gave p-values of 0.31 and 0.08 for SBP and DBP results, respectively. There was no indication of repeated publications of the same trials.
Fig 4. .
Funnel plots of mean effects on systolic and diastolic blood pressure to detect signs of publication bias.
Discussion
Results from the meta-analysis of the clinical trails showed that peptides derived from food proteins can significantly reduce SBP and DBP, with pooled mean effects of −5.13 mmHg (95% CI: −7.12, −3.14) and −2.42 mmHg (95% CI: −3.82, −1.03), respectively. There were some signs of heterogeneity and publication bias among the trials, but exclusion sensitivity analysis did not indicate that one specific trail had a major impact on the pooled effect estimate. A high proportion of the reported trials were carried out using the ACE-inhibiting tripeptides VPP and IPP.
On studying the research literature on bioactive peptides derived from food proteins, it is striking that the number of research papers and literature reviews is extensive compared to the number of controlled clinical trials. Nutraceuticals or functional foods are not subject to the same strict legislation and documentation requirement using randomized controlled clinical trials, as is the case for pharmaceuticals. Much work is based on in vitro assays or to a certain extent animal (rat) experiments. Extensive searches in other databases, such as the Cochrane Library or the Internet, did not yield additional English-language placebo-controlled clinical trials published in peer-reviewed journals. However, there are some other trials written in Japanese. Based on official English translations of abstracts, many of the trials used IPP and VPP peptides as active components and their effect on blood pressure was comparable to those reported in this meta-analysis.
Cochrane's Q-test indicated heterogenicity among the studies. The trials were performed by different groups with different active components. The number of trials was also too limited to perform meta-regression to test for many sources of heterogenicity, and the effect of daily intake of VPP and IPP was not significant. Meta-regression can be carried out to relate the results of trials to the average participant's characteristics, such as blood pressure or age, but an interpretation of such results is difficult and should be carried out with care. This is because the relationship with participant averages across trials may not be the same as the relationship for participants within trials (27). Therefore, it was not performed in this meta-regression. Egger regression, funnel plots (Fig. 4) and cumulative Forrest plots (results not shown) did indicate signs of publication bias, especially for DBP results. One might speculate that some studies with low accuracy, e.g. with few included participants and with little or negative effects, have not been published in international peer-reviewed journals. Typically, this could have been minor research or student projects. Therefore, the random effects model was used because of the possible presence of heterogenicity and its appropriateness to test whether a treatment will have an effect ‘on average’ (28).
Results from this meta-analysis gave a significant reduction on both SDP and DBP at p<0.01. However, their blood pressure reducing effect seems limited in the range of 2 − 7 mmHg.
Many of the clinical trials were also carried out using the well-known ACE inhibiting tripeptides, IPP and VPP. Additional clinical trials on non-specific food protein hydrolysates could provide a better understanding if the effect on blood pressure is limited to some specific peptide structures or is a general property of many peptides derived from food proteins. Lee et al. (21) was the only study in which active treatment did not, on average, reduce blood pressure. They used a hydrolysate, and the content of specific bioavailable ACE-inhibiting peptides, such as Valine-Tyrosine (VY), IPP or VPP, was not identified. That could indicate that the presence of some specific bioactive peptides is important for clinical effect, but further investigations are needed to draw such conclusions. Stratified meta-analysis of trials with or without tripeptides IPP and VPP gave −4.59 mmHg (95% CI: −6.61, −2.57) or −5.42 mmHg (95% CI: −10.10, −0.76) for SBP and −2.20 mmHg (95% CI: −3.73, −0.67) or −2.87 mmHg (95% CI: −6.17, 0.44) for DBP, respectively.
Another interesting result from this stratified meta-analysis was that the Cochrane Q-test was significant for trials without VPP and IPP (p=0.02 for both SBP and DBP), but not for trials with IPP and VPP (p=0.16 and 0.08 for SBP and DBP, respectively). Bioinformatic modelling has, for instance, indicated that the pre-hydrolysis of proteins in foods may influence bioavailability and the ACE-inhibitory effect after digestion (29).
The effect of peptides may also be related to the association found between protein intake and reduced blood pressure from meta-analysis of epidemiological data on dietary protein intake (30). Research on bioactive peptides is extensive within the dairy field, and most of the clinical trials used milk-based products (Table 2). However, lack of controlled clinical trials examining the effect of different proteins makes it difficult to say if the original protein source for bioactive peptides is important for their effect on blood pressure. This area needs further studies.
Antihypertensive peptides have limitations as pharmaceuticals since they are susceptible to degradation by proteolytic enzymes in the stomach and intestines during digestion and because of their limited bioavailability (31). However, compared to other blood pressure reducing components derived from food sources, bioactive peptides seem to be competitive. For instance, meta-analysis of fibre supplementation gave a non-significant reduction of SBP by 1.2 mmHg and a significant 1.5 mmHg reduction of DBP (32, 33). Recent meta-analyses of potassium and magnesium supplementation found no significant effect on blood pressure (34, 35), but is somewhat contradicted by other previous meta-analyses (36)–(38). Calcium supplementation has shown to significantly effect SBP by −1.86 mmHg (95% CI: −2.91, −0.81) and DBP by −0.99 mmHg (95% CI: −1.61, −0.37) in a meta-analysis by van Mierlo et al. (39), but this significant effect has been contradicted by Dickinson et al. (40), who claimed it was due to poor quality, heterogeneity and biases among trials. For individuals with elevated blood pressure, a Cochrane review on salt reduction found a significant reduction in SBP of −5.06 mmHg (95% CI −5.81, −4.31) and DBP of −2.70 mmHg (95% CI −3.16, −2.24). Blood pressure reduction in individuals with normal blood pressure was somewhat less (41). In meta-analysis, fish oil supplementation showed an effect on SBP of −2.1 mmHg (95% CI: −3.2, −1.0) and DBP of −1.6 mmHg (95% CI: −2.2, −1.0) (42). Meta-analysis of clinical trials with garlic preparations showed a significant reduction in blood pressure, but the investigators concluded that there was insufficient evidence to recommend it as a routine clinical therapy for the treatment of hypertensive subjects (43).
Even though this meta-analysis only showed a limited effect of peptides derived from food proteins on blood pressure, it could be an alternative or supplement to pharmacological treatment in combination with other lifestyle changes. In more severe hypertension, when a reduction of >10 mmHg is needed (44), the limited effect of these bioactive peptides might not provide adequate treatment. Clinical trials comparing bioactive peptides with pharmaceuticals are needed to gain better insight into how effective bioactive peptides could be as an alternative to medical treatment. The role of food-derived peptides in diminishing the risk of hypertension remains to be established.
Conflict of interest and funding
The author has not received any funding or benefits from industry to conduct this study.
References
- 1.Kearney P, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–23. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 2.Krousel-Wood MA, Munther P, He J, Whelton PK. Primary prevention of essential hypertension. Med Clin North Am. 2004;88:223–38. doi: 10.1016/s0025-7125(03)00126-3. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto N, Ejiri M, Mizuno S. Biogenic peptides and their potential use. Curr Pharm Des. 2003;9:1345–55. doi: 10.2174/1381612033454801. [DOI] [PubMed] [Google Scholar]
- 4.Lopez-Fandiño R, Otte J, van Camp J. Physiological, chemical and technological aspects of milk-protein-derived peptides with antihypertensive and ACE-inhibitory activity. Int Dairy J. 2006;16:1277–93. [Google Scholar]
- 5.Glass GV. Primary, secondary and meta-analysis of research. Educ Res. 1976;5(10):3–8. [Google Scholar]
- 6.Berman NG, Parker RA. Meta-analysis: Neither quick nor easy. BMC Med Res Methodol. 2002;2:10. doi: 10.1186/1471-2288-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F. Methods for meta-analysis in medical research. Chichester, UK: John Wiley & Sons; 2000. [Google Scholar]
- 8.Aihara K, Kajimoto O, Hirata H, Takahashi R, Nakamura Y. Effect of powdered fermented milk with Lactobacillus helveticus on subjects with high-normal blood pressure or mild hypertension. J Am Coll Nutr. 2005;24:257–65. doi: 10.1080/07315724.2005.10719473. [DOI] [PubMed] [Google Scholar]
- 9.Mizuno S, Matsuura K, Gotou T, Nishimura S, Kajimoto O, Yabune M, et al. Antihypertensive effect of casein hydrolysate in a placebo-controlled study in subjects with high-normal blood pressure and mild hypertension. Br J Nutr. 2005;94:84–91. doi: 10.1079/bjn20051422. [DOI] [PubMed] [Google Scholar]
- 10.Hata Y, Yamamoto M, Ohni M, Nakajima K, Nakamura Y, Takano T. A placebo-controlled study of the effect of sour milk on blood pressure in hypertensive subjects. Am J Clin Nutr. 1996;64:767–71. doi: 10.1093/ajcn/64.5.767. [DOI] [PubMed] [Google Scholar]
- 11.Kawasaki T, Seki E, Osajima K, Yoshida M, Asada K, Matsui T, et al. Antihypertensive effect of Valyl-Tyrosine, a short chain peptide derived from sardine muscle hydrolyzate, on mild hypertensive subjects. J Hum Hypertens. 2000;14:519–23. doi: 10.1038/sj.jhh.1001065. [DOI] [PubMed] [Google Scholar]
- 12.Kawase M, Hashimoto H, Hosoda M, Morita H, Hosono A. Effect of administration of fermented milk containing whey protein concentrate to rats and healthy men on serum lipids and blood pressure. J Dairy Sci. 2000;83:255–63. doi: 10.3168/jds.S0022-0302(00)74872-7. [DOI] [PubMed] [Google Scholar]
- 13.Fujita H, Yamagami T, Ohshima K. Effect of an ace-inhibitory agent, Katsuobushi oligopeptide, in the spontaneously hypertensive rat and in borderline and mildly hypertensive subjects. Nutr Res. 2001;21:1149–58. [Google Scholar]
- 14.Seppo L, Kerojoki O, Suomalainen T, Korpela R. The effect of a Lactobacillus helveticus LBK-16 H fermented milk on hypertension − a pilot study on humans. Milchwissenschaft. 2002;57:124–7. [Google Scholar]
- 15.Seppo L, Jauhiainen T, Poussa T, Korpela R. A fermented milk high in bioactive peptides has a blood pressure-lowering effect in hypertensive subjects. Am J Clin Nutr. 2003;77:326–30. doi: 10.1093/ajcn/77.2.326. [DOI] [PubMed] [Google Scholar]
- 16.Mizushima S, Ohshige K, Watanabe J, Kimura M, Kadowaki T, Nakamura Y, et al. Randomized controlled trial of sour milk on blood pressure in borderline hypertensive men. Am J Hypertens. 2004;17:701–6. doi: 10.1016/j.amjhyper.2004.03.674. [DOI] [PubMed] [Google Scholar]
- 17.Tuomilehto J, Lindström J, Hyyrynen J, Korpela R, Karhunen M-L, Mikkola L, et al. Effect of ingesting sour milk fermented using Lactobacillus helveticus bacteria producing tripeptides on blood pressure in subjects with milk hypertension. J Hum Hypertens. 2004;18:795–802. doi: 10.1038/sj.jhh.1001745. [DOI] [PubMed] [Google Scholar]
- 18.Jauhiainen T, Vapaatalo H, Poussa T, Kyrönpalo S, Rasmussen M, Korpela R. Lactobacillus helveticus fermented milk lowers blood pressure in hypertensive subjects in 24-h ambulatory blood pressure measurement. Am J Hypertens. 2005;18:1600–5. doi: 10.1016/j.amjhyper.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Sano J, Ohki K, Higuchi T, Aihara K, Mizuno S, Kajimoto O, et al. Effect of casein hydrolysate, prepared with protease derived from Aspergillus oryzae, on subjects with high-normal blood pressure or mild hypertension. J Med Food. 2005;8:423–30. doi: 10.1089/jmf.2005.8.423. [DOI] [PubMed] [Google Scholar]
- 20.Cadée JA, Chang C-Y, Chen C-W, Huang C-N, Chen S-L, Wang C-K. Bovine casein hydrolysate (C12 peptide) reduces blood pressure in prehypertensive subjects. Am J Hypertens. 2007;20:1–5. doi: 10.1016/j.amjhyper.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Lee Y-M, Skurk T, Hennig M, Hauner H. Effect of a milk drink supplemented with whey peptides on blood pressure in patients with mild hypertension. Eur J Nutr. 2007;46:21–7. doi: 10.1007/s00394-006-0625-8. [DOI] [PubMed] [Google Scholar]
- 22.Pins JJ, Keenan JM. Effects of whey peptides on cardiovascular disease risk factors. J Clin Hypertens. 2006;8:775–82. [PubMed] [Google Scholar]
- 23.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–29. [Google Scholar]
- 24.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 25.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bax L, Yu LM, Ikeda N, Tsuruta N, Moons KGM. MIX: comprehensive free software for meta-analysis of causal research data – Version 1.54; 2006. www.mix-for-meta-analysis.info. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson SG, Higgens JPT. How should meta-regression analysis be undertaken and interpreted? Statist Med. 2002;21:1559–73. doi: 10.1002/sim.1187. [DOI] [PubMed] [Google Scholar]
- 28.Petitti DB. New York, NY: Oxford University Press; 2000. Meta-analysis, decision analysis, and cost-effectiveness analysis. [Google Scholar]
- 29.Pripp AH. Initial proteolysis of milk proteins and its effect on formation of ACE-inhibitory peptides during gastrointestinal proteolysis: a bioinformatic, in silico, approach. Eur Food Res Technol. 2005;221:712–6. [Google Scholar]
- 30.Liu L, Ikeda K, Sullivan DH, Ling W, Yamori Y. Epidemiological evidence of the association between dietary protein intake and blood pressure: a meta-analysis of published data. Hypertens Res. 2002;25:689–95. doi: 10.1291/hypres.25.689. [DOI] [PubMed] [Google Scholar]
- 31.Vermeirssen V, van Camp J, Verstraete W. Bioavailability of angiotensin I converting enzyme inhibitory peptides. Br J Nutr. 2004;92:357–66. doi: 10.1079/bjn20041189. [DOI] [PubMed] [Google Scholar]
- 32.Strepple MT, Arends LR, van't Veer P, Grobbee DE, Geleijnse JM. Dietary fiber and blood pressure: a meta-analysis of randomized placebo-controlled trials. Arch Intern Med. 2005;165:150–6. doi: 10.1001/archinte.165.2.150. [DOI] [PubMed] [Google Scholar]
- 33.Whelton SP, Hyre AD, Pedersen B, Yi Y, Whelton PK, He J. Effect of dietary fiber intake on blood pressure: a meta-analysis of randomized, controlled clinical trials. J Hypertens. 2005;23:475–81. doi: 10.1097/01.hjh.0000160199.51158.cf. [DOI] [PubMed] [Google Scholar]
- 34.Dickinson HO, Nicolson DJ, Campbell F, Beyer FR, Mason J. Potassium supplementation for the management of primary hypertension in adults. Cochrane Database Syst Rev. 2006:19. doi: 10.1002/14651858.CD004641.pub2. CD004641. [DOI] [PubMed] [Google Scholar]
- 35.Dickinson HO, Nicolson DJ, Campbell F, Cook JV, Beyer FR, Ford GA, et al. Magnesium supplementation for the management of essential hypertension in adults. Cochrane Database Syst Rev. 2006:19. doi: 10.1002/14651858.CD004640.pub2. CD004640. [DOI] [PubMed] [Google Scholar]
- 36.Whelton PK, He J, Cutler JA, Brancati FL, Appel LJ, Follmann D, et al. Effects of oral potassium on blood pressure. Meta-analysis of randomized controlled clinical trials. JAMA. 1997;277:1624–32. doi: 10.1001/jama.1997.03540440058033. [DOI] [PubMed] [Google Scholar]
- 37.Whelton PK, He J. Potassium in preventing and treating high blood pressure. Semin Nephrol. 1999;19:494–9. [PubMed] [Google Scholar]
- 38.Mizushima S, Cappuccio FP, Nichols R, Elliott P. Dietary magnesium intake and blood pressure: a qualitative overview of the observational studies. J Hum Hypertens. 1998;12:447–53. doi: 10.1038/sj.jhh.1000641. [DOI] [PubMed] [Google Scholar]
- 39.van Mierlo LA, Arends LR, Streppel MT, Zeegers MP, Kok FJ, Grobbee DE, et al. Blood pressure response to calcium supplementation: a meta-analysis of randomized controlled trials. J Hum Hypertens. 2006;20:571–80. doi: 10.1038/sj.jhh.1002038. [DOI] [PubMed] [Google Scholar]
- 40.Dickinson HO, Nicolson DJ, Cook JV, Campbell F, Beyer FR, Ford GA, et al. Calcium supplementation for the management of primary hypertension in adults. Cochrane Database Syst Rev. 2006:19. doi: 10.1002/14651858.CD004639.pub2. CD004639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He FJ, MacGregor GA. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev. 2004 doi: 10.1002/14651858.CD004937. CD004937. [DOI] [PubMed] [Google Scholar]
- 42.Geleijnse JM, Giltay EJ, Grobbee DE, Donders AR, Kok FJ. Blood pressure response to fish oil supplementation: metaregression analysis of randomized trials. J Hypertens. 2002;20:1493–9. doi: 10.1097/00004872-200208000-00010. [DOI] [PubMed] [Google Scholar]
- 43.Silagy CA, Neil HA. A meta-analysis of the effect of garlic on blood pressure. J Hypertens. 1994;12:463–8. [PubMed] [Google Scholar]
- 44.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, et al. Seventh report of the joint national committee on prevention, detection, evaluation and treatment of high blood pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]