Abstract
HIV infection is characterized by a brisk immune activation that plays an important role in the CD4 depletion and immune dysfunction of patients with AIDS. The mechanism underlying this activation is poorly understood. In the current study, we tested the hypothesis that this activation is the net product of two distinct pathways: the inflammatory response to HIV infection and the homeostatic response to CD4 T cell depletion. Using ex vivo BrdU incorporation of PBMCs from 284 patients with different stages of HIV infection, we found that CD4 proliferation was better predicted by the combination of CD4 depletion and HIV viral load (R2 = 0.375, P < 0.001) than by either parameter alone (CD4 T cell counts, R2 = 0.202, P < 0.001; HIV viremia, R2 = 0.302, P < 0.001). Interestingly, CD8 T cell proliferation could be predicted by HIV RNA levels alone (R2 = 0.334, P < 0.001) and this predictive value increased only slightly (R2 = 0.346, P < 0.001) when CD4 T cell depletion was taken into account. Consistent with the hypothesis that CD4 T cell proliferation is driven by IL-7 as a homeostatic response to CD4 T cell depletion, levels of phosphorylated STAT-5 were found to be elevated in naive subsets of CD4 and CD8 T cells from patients with HIV infection and in the central memory subset of CD4 T cells. Taken together these data demonstrate that at least two different pathways lead to immune activation of T cells in patients with HIV infection and these pathways differentially influence CD4 and CD8 T cell subsets.
Keywords: CD4 T cell homeostasis, IL-7, STAT-5
HIV infection is characterized by chronic immune activation and CD4 T cell depletion leading to dysfunction of the immune system. While a direct infection of CD4 T cells by HIV partially explains the CD4 T cell depletion, it is clear that the overall disruption of immune function in patients with HIV infection is the sum of multiple factors (1). Immune activation is a major contributor to the pathogenesis of HIV disease and is manifested in many ways varying from increased T cell proliferation, as reflected in an increased number of cycling cells as measured by DNA incorporation of BrdU or intranuclear expression of Ki67, to increased expression of surface activation markers such as HLA-DR and CD38 (2–4). The importance of immune activation in patients with HIV infection is reflected in the observation that increased expression of CD38 on CD4 and CD8 T cells has been found to be a better correlate of clinical disease progression than CD4 T cell counts or HIV-RNA levels (5, 6).
Despite the presence of immunodeficiency, virtually all cellular components of the immune system, B cells, NK cells, T cells and macrophages show evidence of immune activation (5, 7–9). Elevated CD4 and CD8 T cell proliferation can be observed in vitro and in vivo through examination of Ki67, measurement of DNA content, or labeling with BrdU (10–15). Increased levels of proinflammatory cytokines including forms of IFN-α, TNF-α and IL-6 are also present in patients with HIV infection (16–18). Whether this is directly or indirectly part of the host response to HIV is unclear. In addition, in HIV infection and other lymphopenic conditions, depletion of CD4 T cells triggers homeostatic responses that can result in increased circulating levels of IL-7 (19–22). IL-7 is a member of the type I cytokine family and signals through a heterodimer receptor composed of the IL-7Rα chain and the common cytokine signaling γ-chain present in receptors for IL-2, IL-4, IL-7, IL-9, IL-15 and IL-21 (23). The IL-7R complex signals through the Janus kinases, JAK1 and JAK3, leading to phosphorylation of the signal transducer and activator of transcription-5 (STAT-5). Phosphorylated dimers of STAT-5 translocate to the nucleus and induce gene transcription and cell cycle progression (24, 25).
An unresolved paradox in the immune systems of patients with HIV infection is that while both CD4 and CD8 T cells are activated, one sees depletion of the CD4 T cell pool and expansion of the CD8 T cell pool. Although the direct cytopathic effects of HIV on CD4 T cells may explain part of this difference, the low number of cells actively infected at any given point in time makes this an unsatisfactory explanation of the huge dichotomy seen between these two subsets. One alternative hypothesis is that different pathways of activation are triggered in these two subsets with different end results. In vitro and in vivo studies have shown that proliferation of CD4 and CD8 T cell subsets correlate with plasma levels of HIV RNA, however, the role of the T cell homeostatic response to CD4 T cell depletion has not been fully analyzed. We hypothesized that CD4 T cell depletion leads to a homeostatic response occurring in an inflammatory environment generated and maintained by the virus. In such a setting, CD4 T cells could be induced to proliferate by both forces with the net result being activation induced cell death. In the present study, we sought to determine the relative contributions of viral load and its associated inflammatory environment and CD4 T cell depletion and its associated homeostatic response on CD4 and CD8 T cell activation in patients with HIV infection by examining the relationships between CD4 counts, HIV RNA levels and BrdU incorporation. By virtue of being a selective marker of cells in S-phase and thus a measurement of cells actively proliferating, examination of BrdU incorporation allows a detailed analysis of that element of immune activation reflected in increased T cell proliferation.
Results
Study Cohort.
The study cohort consisted of 284 patients with HIV infection seen during the period of February 1998 to February 2007. Their CD4 T cell counts ranged from 11 to 1938 cells per microliter, and their HIV RNA levels from <50 to 860,608 copies per milliliter. At the time of enrollment, the median CD4 T cell count was 470 cells per microliter, the median CD8 T cell count was 839 cells per microliter and the median HIV RNA level was 56 copies per milliliter. Patients were monitored for a median of 7.5 visits. The median CD4 T cell and CD8 T cell counts for the cohort of normal volunteers (n = 373) were 846 cells per microliter and 392 cells per microliter respectively. The normal volunteers were monitored for a median of 2 visits [The datasets used for each analysis are depicted in supporting information (SI) Table S1].
CD4 Depletion Drives CD4 T Cell Proliferation in both Normal Volunteers and Patients with HIV Infection.
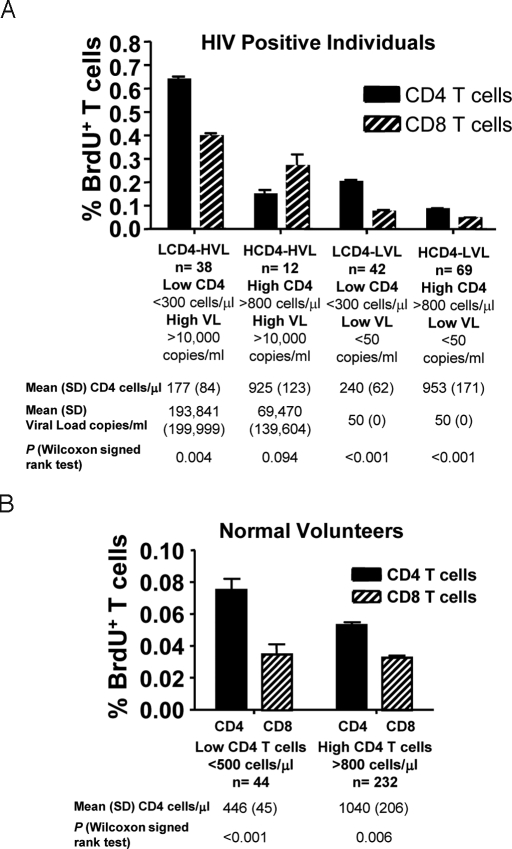
To examine the separate effects of CD4 depletion and HIV viral load on T cell proliferation, we measured spontaneous ex vivo incorporation of BrdU in the CD4 and CD8 T cells from the whole blood of patients with HIV infection; characterized as having high (>800 cells per microliter) or low (<300 cells per microliter) levels of CD4 T cells and high (>10,000 copies per milliliter) or low (<50 copies per milliliter) levels of HIV RNA (Fig. 1A). Similar determinations were performed on cells from normal volunteers with high (>800 cells per microliter) or low (<500 cells per microliter) CD4 T cell counts (Fig. 1B).
Fig. 1.
CD4 T cell proliferation is driven by homeostatic response to CD4 T cell depletion in HIV infected individuals and normal volunteers, while CD8 T cell proliferation is driven by the viral load. (A) Ex vivo BrdU labeling of PBMCs in a cohort of 284 HIV positive individuals. T cells were analyzed by flow cytometry gating in CD3+CD4+ and CD3+CD8+ T cells. The groups of patients were divided based upon low (LCD4: <300 cells per microliter) and high (HCD4: >800 cells per microliter) CD4 T cells with high (HVL: >10,000 copies per milliliter) and with low (LVL: <50 copies per milliliter) viral loads. (B) Ex vivo BrdU labeling of PBMCs from normal volunteers (n = 373) as above. The normal volunteers were divided into individuals with low (LCD4: <500 cells per microliter) and high (HCD4: >800 cells per microliter) CD4 T cell counts. Values for CD4 T cell counts and viral load are expressed as Mean (SD) across randomizations. The P values represent the differences between CD4 and CD8 T cell proliferation.
Both normal volunteers and patients with HIV infection and HIV RNA levels <50 copies per milliliter showed an increase in CD4 proliferation when those with higher CD4 counts were compared with those with lower CD4 counts [0.053 vs. 0.075% (P < 0.001)] for normal volunteers and [0.085 vs. 0.203% (P < 0.001)] for patients (Fig. 1 A and B). The absolute CD4 T cells counts had little absolute effect on CD8 proliferation of the 2 HIV groups with <50 copies per milliliter [0.075 vs. 0.045% (P < 0.001)] and in normal volunteers [0.033 vs. 0.035% (P > 0.05)].
In both HIV infected groups with HIV-RNA levels <50 copies per milliliter and normal volunteers, CD4 T cell proliferation was higher than CD8 T cell proliferation (P < 0.001 in the HIV groups and P = 0.006 and P < 0.001 in normal volunteers, respectively Fig. 1 A and B). This effect was also observed in the group of patients with low CD4 counts and high viral loads (P = 0.004). In contrast, CD8 T cell proliferation trended higher than CD4 T cell proliferation in the HIV patients with high CD4 T cell counts and high viral load.
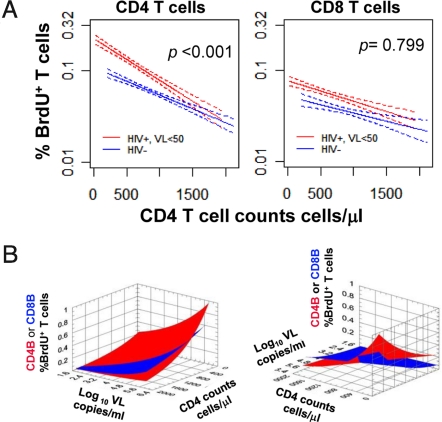
The relationship between CD4 T cell depletion and CD4 and CD8 T cell proliferation was further analyzed in those patients with viral loads <50 copies per milliliter and in normal volunteers (Fig. 2A). Lower CD4 T cell counts were associated with increased rates of CD4 T cell proliferation in both HIV infected and healthy volunteers. The slopes describing these relationships indicated higher rates of CD4 T cell proliferation for patients with HIV infection. Similar rates of CD4 T cell proliferation at very high CD4 T cell counts (1,500 cells per microliter) were observed for HIV infected patients and controls. Of note, there was no intercept for the CD8 lines. Thus, a persistent force independent of CD4 counts, probably “ongoing” viral replication leads to higher rates of CD8 proliferation in all patients with HIV infection. This includes those patients with “undetectable” HIV RNA levels.
Fig. 2.
Modeling of CD4 and CD8 T cell proliferation in response to viral load and CD4 T cell depletion. (A) The relationship between CD4 T cell count and CD4 (CD4 BrdU+ T cells) or CD8 (CD8 BrdU+ T cells) proliferation was studied in the subgroup of HIV infected patients with HIV RNA <50 copies per milliliter and normal volunteers. Linear regressions of T cell proliferation vs. CD4 T cell count are depicted in the solid lines with the 95% confidence interval bands indicated by the dotted lines. Data from the patients are depicted in red; data for the normal volunteers are depicted in blue. The P values indicate the differences between the slopes (ANCOVA type analysis). (B) The relationships between CD4 T cell counts (cells per microliter), HIV RNA levels (log10 viral load) and CD4 (Red curve) or CD8 (Blue curve) T cell proliferation depicted from two different views.
HIV Viral Load Drives both CD4 and CD8 Proliferation in Patients with HIV Infection.
A comparison of rates of T cell proliferation in patients with HIV infection separated into cohorts based upon viral load revealed higher rates of both CD4 and CD8 proliferation as a function of increased viral load (Fig. 1A). For CD4 proliferation, the cohort of patients with low (<300 cells per microliter) CD4 counts demonstrated approximately a 3-fold increase in rates of BrdU incorporation when patients with HIV RNA levels of <50 copies per milliliter were compared with those with HIV RNA levels >10,000 copies per milliliter (0.203 vs. 0.639%, P < 0.001). In contrast, viral load had less of an effect on CD4 proliferation in patients with high (>800 cells per microliter) CD4 T cell counts with only a 1.7-fold increase in BrdU incorporation observed in this subset of patients as a consequence of higher viral loads (0.085 vs. 0.148%, P < 0.001). For CD8 proliferation, both cohorts of patients with high (>10,000 copies per milliliter) viral loads had rates of BrdU incorporation that were ≈5- and 6-fold higher than their respective CD4 cohorts with low (<50 copies per milliliter) viral loads (0.269 vs. 0.045%, P < 0.001 and 0.396 vs. 0.075%, P < 0.001; for high CD4 and low CD4 T cell count groups respectively).
Multivariate Analysis of the Association Between CD4 T Cell Depletion, Viral Load and CD4, CD8 T Cell Proliferation.
Taken together these data indicate that CD4 proliferation is the net result of immune activation driven by viral load and homeostatic forces while CD8 proliferation is mainly driven by viral load. The critical role of CD4 T cell depletion in CD4 T cell proliferation is suggested by those patients in whom CD4 T cell counts are >800 cell/μl yet whose viral loads are >10,000 copies per milliliter. In these patients, CD8 proliferation numerically outpaced CD4 proliferation (0.269% vs. 0.148%, P = 0.094, Fig. 1A). To better define the above relationships, we performed a multivariate analysis with mixed-effects linear models, using CD4 count and viral load as covariates and CD4 and CD8 proliferation as reflected in BrdU incorporation as the readouts (Table 1 and Table S1). Together CD4 count and viral load could account for 37.5% of the variance in CD4 T cell proliferation and 34.6% of the variance in CD8 T cell proliferation. For CD4 proliferation the predictive value of viral load alone was 30.2% and increased to 37.5% with the addition of CD4 count. In contrast, for CD8 proliferation the predictive value of viral load alone was 33.4% and increased very little (to 34.6%) with the addition of CD4 count to the model. These relationships are depicted graphically in Fig. 2B in which one can observe that CD4 proliferation is generally greater than CD8 proliferation other than in the setting of high CD4 count and high viral load.
Table 1.
Mutivariate analysis of CD4 and CD8 T cell proliferation with the covariates CD4 T cell count and viral load
| Output | Analysis model | 〈R2〉 (P) | Coef of CD4(95% CI),(cell per microliter)−1 | Coeficient of VL(95% CI), (copies per milliliter)−1 | P (univariated vs. multivariate) |
|---|---|---|---|---|---|
| CD4B | Bivariate | 0.375 (p <0.001) | -0.00049 (−0.00054, −0.00043) | 0.14446 (0.13411, 0.15481) | |
| Univariate | 0.202 (p <0.001) | -0.00076 (−0.00082, −0.00070] | <0.001 | ||
| Univariate | 0.302(<0.001) | 0.17338 (0.16326,0.18350) | <0.001 | ||
| CD8B | Bivariate | 0.346 (<0.001) | −0.00026 (−0.00034, −0.00019) | 0.22752 (0.21378,0.24126) | |
| Univariate | 0.093 (<0.001) | −0.00070 (−0.00078, −0.00062) | <0.001 | ||
| Univariate | 0.334 (<0.001) | 0.24384 (0.23080,0.25688) | <0.001 |
In Vivo Contribution of Cytokines to T Cell Proliferation.
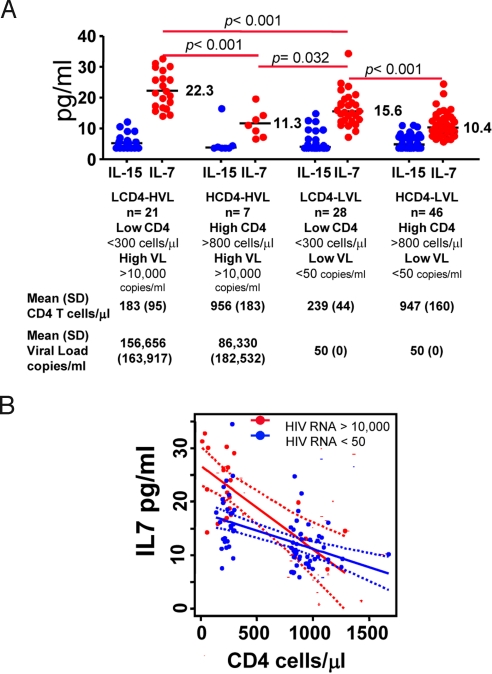
Having established the fact that CD4 proliferation is driven by both CD4 depletion (homeostasis) and viral load while CD8 proliferation is mainly driven by viral load we sought to determine the role, if any, played by cytokines known to be associated with T cell homeostasis. Levels of the γ-common cytokines IL-2, IL-7 and IL-15 were determined for a computer generated set of 102 randomly selected samples (one per patient as described in Table S1) to ensure a even split among the 4 groups of patients. As demonstrated in Fig. 3A, whereas levels of IL-7 were highest in the two groups of patient with the lowest CD4 counts, levels of IL-15 were similar among all 4 groups. For IL-2, median serum levels were similar between the groups (HCD4-HVL: 39.1 pg/ml; LCD4-LVL: 38.4 pg/ml; LCD4-HVL: 38.4 pg/ml; HCD4-LVL: 38.4 pg/ml). Using an univariate linear regression analysis, the relationships between IL-7 levels and CD4 or CD8 T cell counts, showed a strong inverse correlation between CD4 counts and serum IL-7 levels (R2 = 0.36; P < 0.001); and no correlation between CD8 counts and IL-7 levels (R2 = 0.02; P = 0.14). Since viral load has been associated with higher serum levels of IL-7 in some studies (19), we further analyzed only those patients with HIV RNA levels >50 copies per milliliter (Table S2). In this subset, the multivariate analysis indicated that CD4 T cell counts (R2 = 0.42, P < 0.001) were a better predictor of IL-7 serum levels than the viral loads (R2 = 0.22, P = 0.012). To further examine the relationship between viral loads, CD4 counts and serum IL-7 levels, an ANCOVA analysis was carried out using the 102 samples selected for cytokine measurements (Table S1). In this analysis, viral load was treated as a binary variable (>10,000 or <50 copies per milliliter) while adjusting for the CD4 counts. This analysis, depicted in Fig. 3B, indicated that at lower CD4 counts (<300 cells per microliter), patients with high viral loads tended to have higher serum levels of IL7 than patients with low viral loads (P < 0.05). No such association was seen for patients with higher CD4 counts (>800 cells per microliter).
Fig. 3.
Effect of CD4 T cell counts and viral load on IL-7 serum levels. (A) One hundred two serum samples from the 4 groups of HIV positive patients were tested by ELISA for IL-7 (red symbols) and IL-15 (blue symbols). Values for CD4 T cell counts and viral load are expressed as means (SD). Median values for the IL-7 levels are noted inside the plot. (B) Linear regression of IL-7 vs. CD4 T cell counts in patients with viral loads >10,000 (solid red line) or <50 (solid blue line) copies per milliliter. Dotted lines represent the 95% confidence interval bands.
The relative contributions of the covariates CD4 count, IL-7 and HIV-RNA copy number to CD4 and CD8 T cell proliferation, were analyzed in a subgroup of 28 patients with HIV RNA, >50 copies per milliliter (Table S3). In this cohort, CD4 T cell proliferation was most strongly correlated to CD4 counts (R2 = 0.327), compared with viral load and IL-7, suggesting that factors in addition to IL-7 may be driving homeostasis. As was expected from the previous results, CD8 T cell proliferation correlated most strongly with the HIV-RNA levels (R2 = 0.269).
To determine whether or not the proinflammatory cytokines that have been associated with ongoing HIV replication are contributing to CD8 proliferation, analyses for IFN-γ, TNF-α, IL-1β, IL-2, IL-4, IL-5, IL-8, IL-10, IL-12p70, and IL-13 were performed on the same 102 samples selected above (Table S1). Although correlations were noted between IFN-γ levels and viral load in the 28 patients with HIV-RNA levels >50 copies per milliliter (R2 = 0.22; P = 0.012), IFN-γ did not add predictive value to CD8 T cell proliferation beyond that seen for viral load alone (data not shown), suggesting that factors other than those studied here may be leading to CD8 proliferation in the setting of HIV infection (Table S2).
Increased STAT-5 Phosphorylation in T Cells of HIV Infected Individuals.
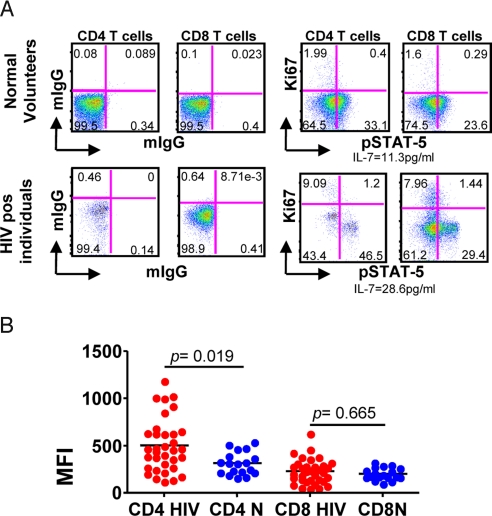
The above data suggest that IL-7, associated with low CD4 T cell counts, may be playing a role in the homeostatic induction of CD4 proliferation and may play some slight role in CD8 proliferation. To further investigate this relationship we examined peripheral blood T cells for evidence of recent IL-7 signaling. Early events after engagement of the IL-7 receptor by IL-7 include activation of Janus family kinases JAK1 and JAK3 and phosphorylation of cytoplasmic STAT-5 to generate pSTAT-5 (26–28). Thus, if IL-7 signaling of T cells is taking place in vivo one would expect freshly isolated T cells to express increased levels of pSTAT5 before cell cycle entry (27, 28). Accordingly, peripheral blood mononuclear cells from 34 HIV infected individuals and 18 normal volunteers were examined for the presence or absence of intracellular pSTAT5 by flow cytometry (Fig. 4). Although no differences in the median fluorescence intensity for pSTAT5 staining were noted between CD8 T cells from HIV infected individuals and healthy controls; increased levels of pSTAT5 were noted in the CD4 cells from HIV infected individuals when compared with controls (P = 0.019). The presence of pSTAT-5 was noted to be restricted to the Ki67 negative population of cells. This lack of pSTAT-5 in the Ki67+ cell population reflects the early, transient nature of pSTAT-5 expression. These data support the hypothesis that IL-7 is involved in the induction of CD4 proliferation in patients with HIV infection and suggest that common gamma chain using cytokines are not a major factor in the overall proliferation of CD8 T cells.
Fig. 4.
Phosphorylated STAT-5 expression in HIV positive individuals and normal volunteers. (A)Whole blood was stained by flow cytometry for pSTAT-5 in normal volunteers and HIV positive individuals. (B) Results from HIV positive (red) and normal volunteers (blue) are shown. Each dot represents an individual subject (34 HIV positive and 18 normal individuals). The mean (SD) CD4 counts for the patients and controls were 415 (264) and 704 (323); the mean (SD) CD8 counts for the patients and controls were 870 (518) and 376 (237); and the mean(SD) HIV RNA level for the patients was 35,861 (90, 228).
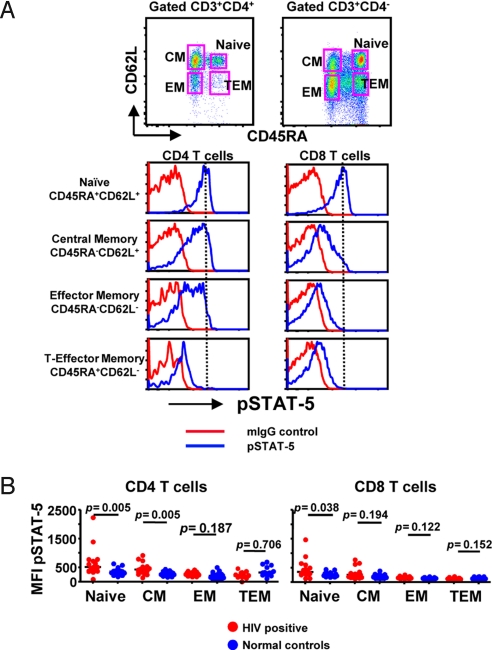
Of note, phosphorylated levels of STAT-5 were higher in CD4 T cells than CD8 T cells (P < 0.001) in both HIV positive and healthy volunteers. We did not find any correlation between the MFI of pSTAT-5 staining and serum IL-7 levels. This lack of correlation may be due to the small sample size studied or the presence of other factors. Thus, the precise relationship between these 2 measurements is difficult to ascertain. To determine whether the increased pSTAT-5 phosphorylation observed in CD4 T cells was driven by a particular subset of CD4 T cells we measured the MFI of pSTAT-5 in naive (N), central memory (CM), effector memory (EM), and terminal effector memory T cells (TEM) based on the expression of CD45RA and CD62L. Naive and central memory CD4 T cells and naive CD8 T cells, showed significantly increased levels of pSTAT-5, compared with normal volunteers (Fig. 5).
Fig. 5.
Phosphorylated STAT-5 expression in CD4 and CD8 T cell subsets from HIV positive individuals and normal volunteers. (A) Whole blood was stained by flow cytometry for pSTAT-5 in normal volunteers and HIV positive individuals, using CD45RA and CD62L as markers of naive, central memory, effector memory, and terminal effector memory CD4 or CD8 T cells. (B) Results from HIV positive (red) and normal volunteers (blue) are shown. Each dot represents an individual subject (16 HIV positive and 16 normal individuals).
Discussion
Human HIV infection disrupts the immune system through generalized immune activation and CD4 T cell depletion. In the present study, we demonstrate that immune activation as reflected in T cell proliferation is driven by both homeostatic and viral factors that differentially affect the CD4 and CD8 T cell pools. CD4 T cell proliferation is driven by the homeostatic response to CD4 T cell depletion and by viremia, whereas CD8 T cell proliferation is mainly a result of the levels of HIV viremia. We have been able to demonstrate for the CD4 T cell pool that the homeostatic proliferation induced by CD4 T cell depletion is accelerated by the inflammatory environment generated by the virus.
T cell homeostasis is an important mechanism to assure survival and maintenance of the T cell repertoire through life. Key regulators of this process are the gamma-common cytokines IL-2, IL-7 and IL-15 (21, 22). Increased serum levels of IL-7 have been described in patients with HIV infection and in other lymphopenic conditions such as postbone marrow transplant and idiopathic CD4 lymphopenia (19, 20, 29). In this study, the strong correlations observed between CD4 depletion, increased T cell proliferation and IL-7 levels (levels of IL-2 and IL-15 were not significantly different between the groups) suggest that homeostatic forces represent an important factor in the immune activation seen in patients with HIV infection. This statement is supported by the increased levels of pSTAT-5 seen in naive and central memory CD4 T cells and in naive CD8 T cells. The elevated levels of pSTAT-5 observed in the naive CD4 and CD8 T cell compartments compared with the levels in cells from controls could represent in vivo signals triggered by the increased levels of IL-7 observed in HIV infection (ref. 19 and Fig. 3B). The lack of STAT-5 phosphorylation in the other T cell subsets is consistent with the hypothesis that their proliferation is primarily driven by different forces. These forces are likely directly induced by the virus and may include things such as TcR specific expansions and/or bystander inflammatory cytokines. Given that the differentiation pathway to effector T cells leads to a loss of CD127 and a refractoriness of CD8 effector T cells to IL-7 signaling (30), these results are not surprising. pSTAT-5 was observed predominantly in Ki67-negaive cells, which supports the notion that phosphorylation of STAT-5 is an early and transient marker of cell-cycle progression.
HIV viremia has been associated with a clear effect on the activation and proliferation of both CD4 and CD8 T cells (14). In the present study, viral load alone predicted 30.2% and 33.4% of CD4 and CD8 T cell proliferation respectively. These are substantially higher predictive values than the 10% that has been associated with the ability of viral load to predict CD4 decline (31). The downstream pathways leading to this proliferation remain unclear but are likely a combination of both specific TcR engagement and non specific bystander activation via inflammatory cytokines such as plasmacytoid DC secreted type I IFN, IFN-γ and TNF-α, and/or microbial translocation of LPS (7, 17, 32). HIV viremia perpetuates an inflammatory milieu (33), likely causing a bystander effect mediated by cytokines, as has been described in vitro with human CD4 T cells (33, 34). The effect of inflammation on the proliferation of CD4 and CD8 T cells is quickly reduced when viremia is suppressed by HAART. Unfortunately, none of the cytokines tested in this study showed a relationship to CD8 T cell proliferation, suggesting that other cytokines or mechanisms are contributing to this aspect of immune activation.
These results suggesting two forces driving CD4 proliferation are consistent with in vivo labeling experiments of HIV infected individuals with BrdU, which showed a biphasic kinetics of CD4 and CD8 T cells, including “rapidly” and “slowly” proliferating populations (13, 35). In this situation the CD4 T cells driven by homeostatic forces into the slowly proliferating pool may be more easily recruited into the “rapidly” proliferating pool where they undergo additional proliferation by bystander mechanisms and ultimately die. After initiation of HAART, the “rapidly” proliferating (and dying) CD4 T cell population, is selectively and immediately reduced while there is little effect on the homeostatic forces driving the “slowly” proliferating T cells. This could help to explain why one sees a rapid increased followed by a slow, steady increase in the size of the CD4 T cell pool after the initiation of HAART.
The results from the normal volunteer cohort, revealed a clear inverse correlation between CD4 T cell proliferation and CD4 T cell counts with less of an effect of CD4 T cell counts on the proliferation of the CD8 T cells (Figs. 1B and 2A). These data illustrate the increased importance of homeostasis in maintenance of the CD4 T cell pool and are consistent with studies in SIV infected and uninfected sooty mangabeys (36). Levels of CD4 proliferation in HIV positive patients approached that of HIV negative controls as the CD4 T cell counts increased; confirming the more potent effects of homeostatic than virologic forces on the CD4 T cell pool. In contrast, CD8 T cell proliferation was elevated in all patients with HIV infection, demonstrating a strong correlation with viremia even in HIV infected patients with HIV RNA levels <50 copies per milliliter. The precise pathways leading to CD8 proliferation remain unclear. CD8 T cells with non-HIV specificities have also been shown to express an activated phenotype in HIV infected individuals (37, 38), suggesting bystander activation in the setting of HIV infection.
These data contribute to our understanding of the effects that HIV infection has on the CD4 and CD8 T cell pools. In the case of CD4 T cells one has virus-specific immune responses and inflammatory forces in the presence of homeostatic forces with the net result being activation and slow CD4 T cell depletion. In the case of CD8 T cells, one has virus-specific immune responses and inflammatory forces with the net result being CD8 T cell expansion. Further study of the precise mechanisms involved may help to identify new targets for therapeutic intervention.
Methods
Patient Selection.
From February 1998 to February 2007, patients and controls studied in National Institute of Allergy and Infectious Diseases/Critical Care Medicine Department intramural IRB approved HIV clinical research studies had routine measurement of T cell turnover/immune activation including cell surface staining for HLA-DR, CD38, CD25, nuclear antigen Ki67 and spontaneous incorporation of BrdU. During this time, a total of 341 patients and 373 controls underwent a total of 5,240 and 1,453 evaluations, respectively (Table S1 summarizes the datasets for the analysis and the description of the analytical methods). The majority of the patients studied had chronic HIV infection (<5% with acute infection) and ≈75% received a variety of standard of care antiretroviral regimens over the 10-year period of the study. By pooling together a set of treated and untreated patients and thus creating a heterogeneous group for study we were able to highlight the independence of the two covariates of interest (viral load and CD4 count). An additional 52 samples were obtained from 34 patients with HIV infection and 18 controls for analysis of STAT-5 phosphorylation.
Ex Vivo BrdU Labeling.
Whole blood was labeled as described in ref. 14.
Cytokine Measurements.
Serum samples from the HIV positive groups were tested by ELISA for IL-2 (Pierce, Rockford, IL), IL-7 and IL-15 (R&D Systems) and IFN-alpha (Amersham Biosciences). A 10 multiplex kit (Meso Scale Discovery) was used for detection of IFN-γ, IL-1β, IL-2, IL-4, IL-5, IL-8, IL-10, IL-12p70, IL-13, and TNF-α.
Flow Cytometry.
Detection of phosphorylated STAT-5 (pSTAT-5) was performed by flow cytometry as described in ref. 39. The following mAbs were used anti-CD3, CD4, Ki67, CD45RA, CD62L and pSTAT-5 (BD Biosciences). The cells were then acquired with a FACS-ARIA (BD Biosciences) and analyzed with FlowJo software (Ashland).
Supporting Information.
The datasets used for analysis and statistical methods are described in SI Text.
Supplementary Material
Acknowledgments.
We thank the patients of the National Institute of Allergy and Infectious Diseases HIV-Clinic for their participation in this study, the normal volunteers of the National Institutes of Health Blood Bank, and Dr. Anthony Fauci for his guidance and support. This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health; National Cancer Institute, National Institutes of Health Contract N01-CO-12400 (to Science Applications International).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810032105/DCSupplemental.
References
- 1.Hazenberg MD, Hamann D, Schuitemaker H, Miedema F. T cell depletion in HIV-1 infection: How CD4+ T cells go out of stock. Nat Immunol. 2000;1:285–289. doi: 10.1038/79724. [DOI] [PubMed] [Google Scholar]
- 2.Ho HN, et al. Circulating HIV-specific CD8+ cytotoxic T cells express CD38 and HLA-DR antigens. J Immunol. 1993;150:3070–3079. [PubMed] [Google Scholar]
- 3.Kestens LG, et al. Selective increase of activation antigens HLA-DR and CD38 on CD4+ CD45RO+ T lymphocytes during HIV-1 infection. Clin Exp Immunol. 1994;95:436–441. doi: 10.1111/j.1365-2249.1994.tb07015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahalingam M, et al. T cell activation and disease severity in HIV infection. Clin Exp Immunol. 1993;93:337–343. doi: 10.1111/j.1365-2249.1993.tb08182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giorgi JV, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179:859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 6.Hazenberg MD, et al. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS. 2003;17:1881–1888. doi: 10.1097/00002030-200309050-00006. [DOI] [PubMed] [Google Scholar]
- 7.Brenchley JM, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 8.Lane HC, et al. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1983;309:453–458. doi: 10.1056/NEJM198308253090803. [DOI] [PubMed] [Google Scholar]
- 9.Fauci AS, Mavilio D, Kottilil S. NK cells in HIV infection: Paradigm for protection or targets for ambush. Nat Rev Immunol. 2005;5:835–843. doi: 10.1038/nri1711. [DOI] [PubMed] [Google Scholar]
- 10.Sachsenberg N, et al. Turnover of CD4+ and CD8+ T lymphocytes in HIV-1 infection as measured by Ki-67 antigen. J Exp Med. 1998;187:1295–1303. doi: 10.1084/jem.187.8.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang ZQ, et al. Kinetics of CD4+ T cell repopulation of lymphoid tissues after treatment of HIV-1 infection. Proc Natl Acad Sci USA. 1998;95:1154–1159. doi: 10.1073/pnas.95.3.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho DD, et al. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 13.Kovacs JA, et al. Identification of dynamically distinct subpopulations of T lymphocytes that are differentially affected by HIV. J Exp Med. 2001;194:1731–1741. doi: 10.1084/jem.194.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lempicki RA, et al. Impact of HIV-1 infection and highly active antiretroviral therapy on the kinetics of CD4+ and CD8+ T cell turnover in HIV-infected patients. Proc Natl Acad Sci USA. 2000;97:13778–13783. doi: 10.1073/pnas.250472097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sieg SF, et al. Peripheral S-phase T cells in HIV disease have a central memory phenotype and rarely have evidence of recent T cell receptor engagement. J Infect Dis. 2005;192:62–70. doi: 10.1086/430620. [DOI] [PubMed] [Google Scholar]
- 16.Smed-Sorensen A, et al. Differential susceptibility to human immunodeficiency virus type 1 infection of myeloid and plasmacytoid dendritic cells. J Virol. 2005;79:8861–8869. doi: 10.1128/JVI.79.14.8861-8869.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herbeuval JP, et al. Differential expression of IFN-alpha and TRAIL/DR5 in lymphoid tissue of progressor versus nonprogressor HIV-1-infected patients. Proc Natl Acad Sci USA. 2006;103:7000–7005. doi: 10.1073/pnas.0600363103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tilton JC, et al. Diminished production of monocyte proinflammatory cytokines during human immunodeficiency virus viremia is mediated by type I interferons. J Virol. 2006;80:11486–11497. doi: 10.1128/JVI.00324-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Napolitano LA, et al. Increased production of IL-7 accompanies HIV-1-mediated T-cell depletion: Implications for T-cell homeostasis. Nature medicine. 2001;7:73–79. doi: 10.1038/83381. [DOI] [PubMed] [Google Scholar]
- 20.Fry TJ, et al. A potential role for interleukin-7 in T-cell homeostasis. Blood. 2001;97:2983–2990. doi: 10.1182/blood.v97.10.2983. [DOI] [PubMed] [Google Scholar]
- 21.Fry TJ, Mackall CL. The many faces of IL-7: From lymphopoiesis to peripheral T cell maintenance. J Immunol. 2005;174:6571–6576. doi: 10.4049/jimmunol.174.11.6571. [DOI] [PubMed] [Google Scholar]
- 22.Mackall CL, Hakim FT, Gress RE. Restoration of T-cell homeostasis after T-cell depletion. Semin Immunol. 1997;9:339–346. doi: 10.1006/smim.1997.0091. [DOI] [PubMed] [Google Scholar]
- 23.Puel A, Ziegler SF, Buckley RH, Leonard WJ. Defective IL7R expression in T(−)B(+)NK(+) severe combined immunodeficiency. Nat Genet. 1998;20:394–397. doi: 10.1038/3877. [DOI] [PubMed] [Google Scholar]
- 24.Mazzucchelli R, Durum SK. Interleukin-7 receptor expression: Intelligent design. Nat Rev Immunol. 2007;7:144–154. doi: 10.1038/nri2023. [DOI] [PubMed] [Google Scholar]
- 25.Gadina M, et al. Signaling by type I and II cytokine receptors: Ten years after. Current opinion in immunology. 2001;13:363–373. doi: 10.1016/s0952-7915(00)00228-4. [DOI] [PubMed] [Google Scholar]
- 26.O'Shea JJ, Gadina M, Schreiber RD. Cytokine signaling in 2002: New surprises in the Jak/Stat pathway. Cell. 2002;109:S121–S131. doi: 10.1016/s0092-8674(02)00701-8. [DOI] [PubMed] [Google Scholar]
- 27.Yu CL, Jin YJ, Burakoff SJ. Cytosolic tyrosine dephosphorylation of STAT5. Potential role of SHP-2 in STAT5 regulation. J Biol Chem. 2000;275:599–604. doi: 10.1074/jbc.275.1.599. [DOI] [PubMed] [Google Scholar]
- 28.Seki Y, et al. IL-7/STAT5 cytokine signaling pathway is essential but insufficient for maintenance of naive CD4 T cell survival in peripheral lymphoid organs. J Immunol. 2007;178:262–270. doi: 10.4049/jimmunol.178.1.262. [DOI] [PubMed] [Google Scholar]
- 29.Malaspina A, et al. Idiopathic CD4+ T lymphocytopenia is associated with increases in immature/transitional B cells and serum levels of IL-7. Blood. 2007;109:2086–2088. doi: 10.1182/blood-2006-06-031385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boyman O, Purton JF, Surh CD, Sprent J. Cytokines and T-cell homeostasis. Curr Opin Immunol. 2007;19:320–326. doi: 10.1016/j.coi.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez B, et al. Predictive value of plasma HIV RNA level on rate of CD4 T-cell decline in untreated HIV infection. JAMA. 2006;296:1498–1506. doi: 10.1001/jama.296.12.1498. [DOI] [PubMed] [Google Scholar]
- 32.Fan J, Bass HZ, Fahey JL. Elevated IFN-gamma and decreased IL-2 gene expression are associated with HIV infection. J Immunol. 1993;151:5031–5040. [PubMed] [Google Scholar]
- 33.Boasso A, Shearer GM. Chronic innate immune activation as a cause of HIV-1 immunopathogenesis. Clin Immunol. 2008;126:235–242. doi: 10.1016/j.clim.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geginat J, Sallusto F, Lanzavecchia A. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4(+) T cells. J Exp Med. 2001;194:1711–1719. doi: 10.1084/jem.194.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hellerstein, et al. Subpopulations of long-lived and short-lived T cells in advanced HIV-1 infection. J Clin Invest. 2003;112:956–966. doi: 10.1172/JCI17533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaur A, et al. Dynamics of T- and B-lymphocyte turnover in a natural host of simian immunodeficiency virus. J Virol. 2008;82:1084–1093. doi: 10.1128/JVI.02197-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doisne JM, et al. CD8+ T cells specific for EBV, cytomegalovirus, and influenza virus are activated during primary HIV infection. J Immunol. 2004;173:2410–2418. doi: 10.4049/jimmunol.173.4.2410. [DOI] [PubMed] [Google Scholar]
- 38.Tough DF, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science. 1996;272:1947–1950. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- 39.Perez OD, Nolan GP. Simultaneous measurement of multiple active kinase states using polychromatic flow cytometry. Nat Biotechnol. 2002;20:155–162. doi: 10.1038/nbt0202-155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.