Abstract
The neural circuits that regulate sleep and arousal as well as their integration with circadian circuits remain unclear, especially in Drosophila. This issue intersects with that of photoreception, because light is both an arousal signal in diurnal animals and an entraining signal for the circadian clock. To identify neurons and circuits relevant to light-mediated arousal as well as circadian phase-shifting, we developed genetic techniques that link behavior to single cell-type resolution within the Drosophila central brain. We focused on the unknown function of the 10 PDF-containing large ventral lateral neurons (l-LNvs) of the Drosophila circadian brain network and show here that these cells function in light-dependent arousal. They also are important for phase shifting in the late-night (dawn), indicating that the circadian photoresponse is a network property and therefore non-cell-autonomous. The data further indicate that the circuits underlying light-induced arousal and circadian photoentrainment intersect at the l-LNvs and then segregate.
Keywords: circadian clock, diurnal, phase resetting, sleep/arousal, photoresponse
Light promotes arousal in diurnal species. Drosophila arousal–sleep circuits remain largely uncharacterized. Exceptions are the mushroom body with a role in sleep–arousal (1, 2) as well as neurons of the pars intercerebralis (PI) and the ellipsoid body (EB) of the central complex (CC) with roles in locomotion (3, 4). Importantly, there is no indication of how the poorly understood fly sleep–movement circuits are coordinated or communicate with the well-studied brain circadian network, or whether these ≈150 circadian neurons impact directly on sleep or arousal.
Light is also a powerful entrainment stimulus, a “zeitgeber,” that synchronizes animal circadian clocks with the 24-h rotation of the earth. In a nonparametric view of light entrainment, the discontinuities at dawn and dusk are key entrainment stimuli that synchronize the circadian clock (5). These are mimicked by short light pulses given during the night. It is generally believed that a short light pulse in the late night causes a phase advance because it mimics premature dawn, whereas a short light pulse in the early night mimics delayed dusk and therefore causes a phase delay. Genetic studies in Drosophila have identified CRYPTOCRHOME (CRY), a protein photopigment essential for circadian photoentrainment. They have also identified anatomically distinct clock neurons, evening cells (E cells) and morning cells (M cells), as the key pacemakers timing the onset of the evening locomotor activity peak and the morning activity peak, respectively (6–13). E cells include the 5th LNv, dorsal lateral neurons (LNds), and a few dorsal neurons (DN1s). E cells keep time in the daytime, when the evening activity peak begins. The small LNvs (s-LNvs) are the M cells and keep time in the dark, when the morning activity peak begins.
However, many nonpacemaker clock neurons express CRY. As a consequence, the cells that underlie light-induced phase resetting, i.e., how the internal phase of pacemaker neurons is synchronized to the external light/dark (LD) cycles, are not well understood. This also reflects the fact that there are few tools available to selectively manipulate individual types of clock cells. For example, transgenic expression of CRY in PDF-expressing LNvs rescued a cry loss-of-function genotype, suggesting that LNvs are important circadian light sensors (9). However, this result did not distinguish between the 2 types of PDF-expressing neurons, the pacemaker s-LNvs and the l-LNvs. Because the s-LNvs keep time in constant darkness, they must ultimately experience the phase shift, but it remains uncertain whether this is a cell-autonomous response to light or a network property, e.g., requiring signaling from the l-LNvs.
The 10 l-LNvs (5 on each side of the brain) are among the first circadian neurons identified in Drosophila (14), and there are some indications that they may function as coincident detectors for light (9, 12, 15–17). However, they have never been assigned a circadian or behavioral function, because there are no tools available to selectively address their function. Intrigued by the possibility that the l-LNvs might be circadian photoreceptors as well as a possible relationship between light and arousal, we set out to study the functions of l-LNvs in Drosophila circadian and diurnal behavior. We therefore developed a method to stimulate or eliminate only these cells. This intersectional mosaic technique can label, activate, or eliminate central brain neural circuits with single-cell type and even single-cell spatial resolution. Moreover, use of a temperature-sensitive dTRPA1 channel and mild temperature shifts allows the acute activation of individual neurons. Behavioral assays of these same flies indicate that the l-LNvs are indeed a source of light-mediated arousal and important for circadian phase-shifting but only at dawn.
Results
l-LNvs Are a Major Subset of Peptidergic Arousal Circuits in the Fly Brain.
To address the function of the l-LNvs, we first exploited the driver line c929Gal4. It expresses in a broad set of peptidergic neurons expressing PHM, including the l-LNvs as the only clock neurons [Fig. 1A and supporting information (SI) Fig. S1A] (18). To alter the temporal firing pattern of this entire set, we used the c929Gal4 driver to overexpress NaChBacGFP, a bacterial-derived voltage-gated sodium channel (19). The strategy was then to compare this strain (929:NaChBac) with addition of pdfGal80 transgenes, which completely suppress GAL4 transcription activity in all l-LNvs (Fig. S1B) (12).
Fig. 1.
l-LNvs are part of the peptidergic arousal pathways in Drosophila. (A) Genetic scheme for the 2 mutants is shown. (B and C) Constitutive firing of PHM+ peptidergic cells dramatically increased activity at night in LD conditions. LD activity/rest pattern was monitored from groups of flies with 3 different genotypes: control 929:dORKΔNC1 (n = 14), PHM+ 929:NaChBac (n = 20), and PHM+ PDF− 929:NaChBac;pdfGal80 (n = 29). Experiments were repeated >3 times. dORKΔNC1GFP is a nonconducting K+ channel and was used as control. UAS-NaChBacGFP flies showed no abnormal nocturnal activity or sleep like that seen in 929:NaChBacGFP flies (data not shown). (B) Double-plotted actograms are shown. Light phase is highlighted in yellow and dark phase is highlighted in gray. Red brackets indicate increase of nighttime activity in mutant flies (Left) and black arrows (Center and Right) indicate rescue of early night behavior by pdfGal80. (C) The daytime and nighttime activity of the genotypes are compared. (D) Heat-induced acute firing of peptidergic cells and l-LNvs caused a transient increase of locomotor activity and a long-term suppression of sleep homeostasis. The rest–activity behavior was monitored from groups of flies with 3 different genotypes: control (n = 23), 929:dTrpA1 (n = 20), and 929:dTrpA1;pdfGal80 (n = 19). (E) Constant light abolished the arousal-promoting effect of hyperexciting l-LNvs and other peptidergic cells. LD and LL sleep was monitored from groups of flies with 3 different genotypes: control 929:dORKΔNC1 (n = 20), PHM+ 929:NaChBac (n = 17), and PHM+ PDF− 929:NaChBac;pdfGal80 (n = 16).
The 929:NaChBac strain has a dramatic increase in nighttime activity (Fig. 1B Center; note black arrow), but addition of pdfGal80 almost completely rescued the abnormal early nighttime activity (Fig. 1B Right; compare black arrow with black arrow in Center). Quantitative analysis of activity confirmed that the broad excitation by peptidergic neuron stimulation was substantially rescued by the presence of pdfGal80 transgenes (Fig. 1C). A sleep assay showed similar results, i.e., a dramatic reduction of especially nighttime sleep by PHM+ neuronal firing with substantial rescue by the suppression of l-LNv firing (Fig. 1E). Therefore, activity and/or sleep circuits lie downstream of the l-LNvs.
To avoid possible complications from persistent neuronal firing throughout development as well as in adult stages, we replaced the NaChBac channel with the recently identified Drosophila heat-activated dTRPA1 channel. It is normally expressed in only approximately a dozen central brain neurons and not in clock circuits, mushroom bodies or other brain cells known or suspected to be relevant to locomotor activity and sleep (20). Flies were entrained for 5 days at 25 °C, below the reported dTRPA1 activation temperature, and diurnal behavior was then assayed for 2 days at 30 °C, above this temperature.
Although the temperature shift rapidly induced locomotor activity in both 929:dTrpA1 and 929:dTrpA1;pdfGal80 flies, the pdfGal80 flies showed lower activity during the first 2 h immediately after heat activation (see Fig. S2, red arrows and legend). More importantly, only the 929:dTrpA1;pdfGal80 flies significantly recovered the inactivity characteristic of early night on day 2 (Fig. 1D, Fig. S2B, green arrows). We interpret this difference between strains on day 2 vs. day 1 to indicate that hyperfiring of l-LNvs is required only for suppression of the larger sleep drive that accrued by day 2 but not yet on day 1. Indeed, the sleep data indicate that the 929:dTrpA1 flies sleep considerably less than their 929:dTrpA1;pdfGal80 counterparts (36). The data further suggest that it takes 2 days of sleep in 929:dTRPA1;pdfG80 flies to achieve steady-state. Because the day 2 dTRPA1 results resemble more closely the effect of NaChBac expression shown above (Fig. 1B), they not only confirm that dTRPA1 causes persistent hyperexcitation of l-LNvs but also suggest that the hyperactive l-LNvs increase arousal by antagonizing sleep homeostasis rather than by merely stimulating the motor program.
The Arousal Function of the l-LNvs Is Light-Dependent.
Because hyperexciting l-LNv causes nighttime but not daytime hyperactivity, l-LNvs may be normally more day-active and contribute to higher activity as well as lower sleep in the day than at night. A prediction is that 929:NaChBac and 929:NaChBac;pdfGal80 flies should show similar sleep levels in constant light, which is the case (Fig. 1E).
To assay whether l-LNv firing alone is sufficient for arousal, we developed an intersectional technique. It uses 2 expression systems, Gal4-UAS/Gal80 and LexA-LexAop (21, 22), which are combined with flippase (FLP/FRT) to generate mosaic clones within the overlap of the 2 expression systems (for details, see Materials and Methods and Fig. 2) (23). Because FRT sites are expected to be lost in a subset of LexA-expressing cells, Gal4 should be expressed in only a fraction of the overlap between the 2 patterns, and the GFP tag on the NaChBac channel allows facile identification of NaChBac-expressing cells by immunostaining. To adapt the strategy to the l-LNvs, a strain was generated expressing pdfLexA, LexAop-FLP, and tub>Gal80> as well as the transgenes c929Gal4 and UAS-NaChBacGFP described above (Fig. 3A). As anticipated, the system is able to express the NaChBacGFP channel in a few l-LNvs (Fig. 3B). Because the flipping event does not occur at 100% efficiency, individual flies have different numbers of labeled constitutively excited l-LNvs (see below).
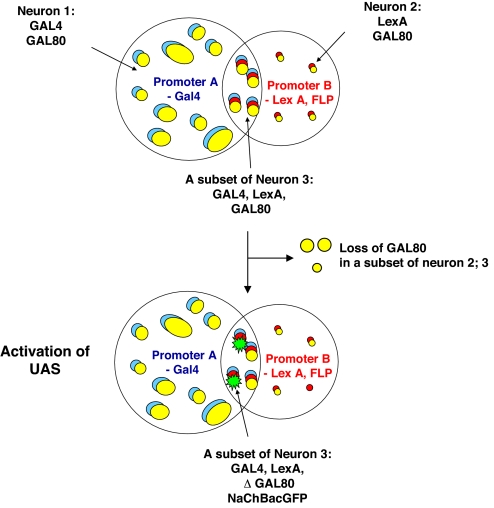
Fig. 2.
A subset of l-LNvs can be labeled by the intersectional technique. The flies carries 5 transgenes: promoter A-Gal4, UAS-NaChBacGFP, tub>GAL80>, promoter B-LexA, and LexAop-Flp. GAL80 is flanked by 2 FRT sites (>) and is ubiquitously expressed by the tubulin promoter (yellow). “Neuron 1” are groups of neurons expressing GAL4 proteins (blue). “Neuron 2” are groups of neurons expressing LexA transcription factors (red). “Neuron 1” and “Neuron 3” intersect at “Neuron 2,” which express both GAL4 and LexA proteins (blue and red). GAL80 proteins (yellow) suppress the GAL4-induced expression of genes under UAS control with no effect on LexA-induced expression. FLP causes the Gal80 coding region to be removed in a subset of neurons 2 and 3. As a result, a subset of neuron 3 will lose Gal80 coding sequence, thereby activating GAL4-driven UAS-NaChBacGFP transcription (green).
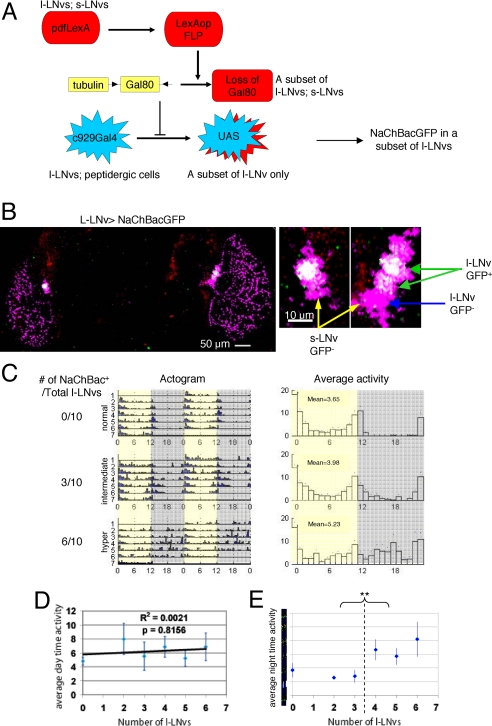
Fig. 3.
Stimulation of l-LNvs is sufficient to promote arousal at night. (A) The combination of GAL4-UAS/GAL80, LexA-LexAop expression system with the FLP/FRT system in the same fly. The l-LNv> NaChBacGFP fly carries 5 transgenes: c929Gal4, UAS-NaChBacGFP, tub>GAL80>, pdfLexA, and LexAopFlp (for details, see Materials and Methods). (B) A small subset of l-LNvs can be labeled by the intersectional technique. A brain from a fly w; c929Gal4,UAS-NaChBacGFP/tub>GAL80>; pdfLexA,LexAopFLP/+ was fixed and stained with antibody against GFP (green) and PDF (magenta). The higher magnification (on the right) shows 3 of 10 l-LNvs in this fly expressing NaChBacGFP channel (white). No GFP staining can be detected outside the l-LNvs. (C) Examples of activity/rest pattern in LD and genotype of l-LNvs from 3 individual flies. (D and E) Increasing the excitability of l-LNvs at night directly promotes activity. Twenty-eight flies expressing no GFP outside the l-LNvs were divided into subgroups based on the number of l-LNvs expressing NaChBacGFP. The mean daytime (D) or nighttime (E) activity was then calculated within each group. The correlation between the number of l-LNvs labeled and the mean activity during daytime or nighttime is plotted separately. The number of l-LNvs expressing NaChBacGFP channel is highly correlated with the nighttime activity, without affecting daytime activity. A threshold effect was observed, i.e., exciting 4 or more l-LNvs is sufficient to induce nighttime hyperactivity (E). **, P < 0.01.
To correlate behavioral phenotype with the number of firing l-LNvs, we first assayed the diurnal behavioral patterns of individual flies under standard LD conditions. Although most showed normal behavior in the day, some were hyperactive at night. Examples of 2 hyperactive flies and 1 normal fly are shown in Fig. 3C (Middle and Bottom for an intermediate and a hyperactive fly, respectively; Top shows a fly with no hyperactivity). We then determined the NaChBacGFP expression patterns, which were restricted to l-LNvs as expected. Importantly, flies with more nighttime activity had more labeled l-LNvs [Fig. 3C: 0 (Top), 3 (Middle), and 6 (Bottom)]. To address this issue quantitatively, we grouped individual flies based on the number of labeled l-LNvs and averaged the total daytime activity and total nighttime activity of each group. Although l-LNv firing did not significantly affect daytime activity (presumably because the l-LNvs already promote activity during the day), average nighttime activity was highly correlated with the number of NaChBacGFP-expressing LNvs (Fig. 3 D and E). Although there may be a threshold effect (constitutively activating 4 or more l-LNvs may be sufficient to induce a maximal level of abnormal nighttime activity; Fig. 3E), the data indicate that increasing the excitibility of a few l-LNvs promotes activity at night.
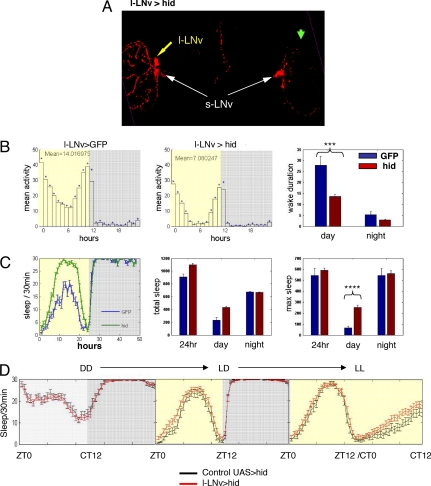
To test whether l-LNvs are necessary for normal light-mediated activity/arousal in the context of otherwise normal brain firing, we used a similar intersectional approach to effect mosaic expression of a FRT-containing UAS-hid transgene within the l-LNvs; (see Materials and Methods and Fig. 4 legend). Based on immunostaining, the cell death gene reduced the number of l-LNvs from 10 to an average of 2–4 (Fig. 4A). These flies had a consistent and significant decrease in female daytime activity (Fig. 4B) and a reciprocal increase in daytime sleep (Fig. 4C). To test whether the high sleep phenotype is light-dependent, we compared the l-LNv>hid flies with a different control strain carrying but not expressing the UAS>stop>hid transgene. Although these control flies generally sleep more than the different l-LNv>GFP control flies used in Fig. 4C, we could still observe a high sleep phenotype in l-LNv>hid flies. Importantly, this phenotype immediately disappeared after transfer from LD to constant darkness (DD) (day 1 of DD) and was even more evident in constant light (LL), indicating again that l-LNv-mediated arousal is light-dependent (Fig. 4D). Males are less responsive to a reduction of l-LNv number, perhaps because of their already high levels of daytime sleep. This may reflect sex-specific regulation of l-LNv neuronal activity.
Fig. 4.
l-LNvs are part of the light-mediated arousal pathway. (A) Ectopic expression of hid, a cell death gene, lead to loss of l-LNvs. Brains from l-LNv> hid flies were fixed and stained with PDF antibody. This fly had only 2 l-LNvs in the left hemisphere and no l-LNvs in the right hemisphere (yellow arrow). The PDF signal is largely reduced in the left optical lobe and almost completely lost in the right optical lobe (green arrow). s-LNvs appear normal (white arrows). (B) Loss of l-LNvs significantly reduced the daytime activity in female flies. The actogram, average activity, and mean duration of wakefulness for control (c929Gal4,UASGFP/+;pdfLexA,LexAopFLP/+, n = 9) and l-LNv> hid flies (n = 18) are shown. ***, P < 0.001. (C) Loss of l-LNvs significantly increases the daytime sleep in female flies. Sleep graph, total sleep, and maximum sleep for the 2 genotypes are shown. ****, P < 0.0001. In B and C, blue box is control and red box is flies with fewer l-LNvs. Female flies expressing hid (n = 32 for control and n = 52 for hid flies total) consistently showed less activity and more sleep during the day. (D) l-LNv>hid flies have no arousal/sleep phenotype in the first subjective day of DD compared with that of LD, whereas the arousal/sleep phenotype is more obvious in LL condition. Control flies (UAS>hid/+;pdfLexA,LexAopFLP/+, n = 34) and l-LNv>hid flies (n = 10) were entrained in LD for 5 days, released into DD for 2 days, reentrained in LD for 2 days, and released into LL. The sleep graph in DD, LD, and LL for both genotypes is shown.
The l-LNv-Mediated Light Arousal Circuits Are Distinct from the Circadian Pacemaker Cells.
To test whether other clock neurons can also give rise to an arousal phenotype like the l-LNvs, we stimulated M cells or E cells and assayed the resulting arousal/sleep phenotypes. We first used the R6Gal4 driver, which expresses strongly in s-LNvs but no other clock cells (24). R6Gal4:NaChBac had no arousal/sleep phenotype (Fig. 5A; because the R6Gal4 driver also labels a few noncircadian cells, the experiment was done with and without PDF-gal80 to address the specific role of the s-LNvs). Therefore, s-LNv stimulation alone appears insufficient for a sleep–arousal phenotype and is therefore different from l-LNv stimulation (see Discussion).
Fig. 5.
The l-LNv-mediated light arousal circuits are distinct from the circadian pacemaker cells. (A) Activation of s-LNvs does not dramatically change sleep/arousal. R6Gal4:NaChBac flies express NaChBac in s-LNvs and other noncircadian cells. R6Gal4:NaChBac;pdfGal80 flies express NaChBac only in the noncircadian cells. These 2 genotypes show indistinguishable arousal/sleep phenotype. (B) E cell stimulation and inhibition (cry39Gal4:NaChBac;pdfGal80 and cry39Gal4:Kir2.1;pdfGal80, respectively) show opposite (but milder) effects than l-LNvs, namely, stimulation of E cells enhances sleep, especially at night in LD, and the effect is more obvious in DD. The amount of sleep per 30 min of each genotype in LD and DD is plotted.
We then tested E cell stimulation and inhibition (cry39Gal4:NaChBac; pdfGal80 and cry39Gal4:Kir2.1; pdfGal80, respectively). These circadian cells showed mild and opposite effects from those of the l-LNvs in LD: Stimulation of E cells enhanced sleep, especially at night, whereas hyperpolarization of E cells reduced nighttime sleep (Fig. 5B). Importantly, these sleep-promoting effects were much more obvious in DD (Fig. 5B), which is yet another difference from the l-LNv manipulations (compare with Fig. 4D). Another cry E cell driver line, cry13Gal4, gave similar results (data not shown). We conclude that l-LNvs are unusual if not unique circadian cells, which generate a straightforward light-mediated arousal/sleep phenotype.
l-LNvs Are also Part of the Circadian Photoreception Circuits at Dawn.
Sleep and arousal assays do not necessarily report circadian status. Moreover, the only reagent previously used to specifically manipulate LNvs (the driver line pdf-gal4) does not differentiate between small and large LNvs. We therefore assayed additional circadian parameters in flies with few or no l-LNvs. These flies had normal rhythmic behavior with a period of ≈24 h in constant darkness (Fig. 6B; the DD actogram is not shown), supporting previous conclusions that the s-LNvs are the only key pacemakers in the dark (11–13).
Fig. 6.
l-LNvs are also the major sources of photic information for the circadian clock at dawn. (A) The projections of l-LNvs pass very close to s-LNv cell bodies and processes. A fly brain with w;c929Gal4/UAS-mCD8GFP was stained with anti-GFP (green) and anti-PDF antibody (red). The confocal image shows the GFP and PDF staining in l-LNvs (yellow arrows). The s-LNvs are labeled only with PDF antibody (white arrows). A higher-magnification image on the right shows that the processes of l-LNvs (green) and those of s-LNvs (red) are intermingled. (B) l-LNvs are essential for phase advance at ZT21. l-LNv>hid flies were exposed to a 10-min light pulse (LP) at ZT21 on the last LD cycle and then released into DD (LP group). The LP group was then divided into 2 subgroups based on the number of l-LNvs that remained in the brain. The control flies are l-LNv>hid flies that were released into DD without light exposure (Non-LP group; n = 19). The phase of these flies in DD was compared with the phase of LP l-LNv>hid flies. (Upper) Flies containing 0–2 l-LNvs completely abolished the phase advance in response to LP (n = 8). (Lower) Flies with 3–6 l-LNvs can still respond to LP (n = 6). Black arrows indicate the phase of the flies with 3–6 l-LNvs is slightly advanced compared with that of non-LP control group. (C) The 10 l-LNvs are specifically important for light-induced phase advance at late night but not phase delay at early night. Standard phase responsive assay was performed at ZT15 or ZT21 on both female control flies (UAS>hid; pdfLexA,LexAopFLP) and mutant l-LNv>hid flies (0–6 l-LNvs remaining). Mosaic flies with fewer l-LNvs show normal or even slightly greater responses to light at ZT15. On the contrary, the response at ZT21 was dramatically reduced. (D) Neurocircuits underlying Drosophila arousal and phase resetting at dawn overlap with the l-LNvs as a specific neuronal point of intersection and then segregate. At dawn, l-LNvs transmit dark–light transition to fly brains to promote arousal and reset clock to ZT0. We propose 2 separate circuits: l-LNvs communicate with ellipsoid body (EB body) in central complex or PI region through PDF signaling while they reset the clock of E cells including LNds through s-LNvs.
However, s-LNv cell bodies are in close proximity to l-LNv processes (Fig. 6A), which suggests that the l-LNvs may signal light information to the s-LNv pacemaker cells. Indeed, the phase advance response to light at ZT21 was almost completely abolished in flies with <2 l-LNvs, and flies with more l-LNvs showed an intermediate ZT21 phase response (Fig. 6B). On the contrary, the circadian response to a light pulse at ZT15, the time of maximal phase delay, was essentially indistinguishable between the l-LNv-deficient and wild-type control genotypes (Fig. 6C; P > 0.2). This suggests that the l-LNvs are important for the circadian photoresponse at dawn and that light information perceived by individual l-LNvs is summed to generate a complete advance phase shift.
Consistent with a role of l-LNvs in phase resetting, l-LNv>NaChBac mosaic flies show complex behavioral phenotypes in DD (Fig. S3), suggesting that stimulation of l-LNvs may cause spontaneous phase shifts without light. This also confirms that NaChBacGFP overexpression changes the temporal firing pattern of l-LNvs, i.e., l-LNvs now fire in the dark as well as in the light (35).
Discussion
Our study reveals a previously uncharacterized light–arousal pathway and focuses on the PDF+ l-LNvs. These 10 cells are identified as brain cells important for the diurnal behavior of Drosophila, i.e., they contribute to higher arousal and lower sleep in a light-dependent fashion. The experiments also suggest that l-LNvs are essential for a light-mediated circadian phase advance at dawn. The arousal as well as the circadian assays exploited an intersectional method to manipulate individual central brain neurons, and acute stimulation of l-LNvs in vivo was achieved with a temperature-activated dTRPA1 channel.
One recent study that recorded electrophysiologically from l-LNvs showed that neuronal firing is light-stimulated (17). This report also showed that light-mediated firing during the light phase was substantially (2/3) reduced in a mutant cry background, consistent with direct photoreception by l-LNvs in our behavioral studies. However, the eyes are not irrelevant for circadian resetting (15). Interestingly, the l-LNvs are the only clock neurons that show extensive connections with the optic lobes in both hemispheres of fly brains. They can also receive light information from the H-B eyelet, an extraretinal photoreceptor also important for photoentrainment. The l-LNvs are therefore ideally positioned to be coincidence detectors, i.e., to receive light signals from the eyes and/or the H-B eyelet and integrate this rhodopsin-mediated information with their own CRY-mediated photoreception.
The l-LNvs are necessary for light-induced circadian phase resetting at dawn but not for locomotor activity rhythms, either in the dark or in the light. This indicates that the l-LNvs are necessary for resetting the phase of the neighboring s-LNvs, the free-running pacemaker cells in the dark. The circadian network is therefore important for circadian photoreception, in contrast to the prevailing cell-autonomous view. In contrast, the l-LNvs appear to be the only circadian cells relevant to light-mediated arousal, suggesting that the circuits that underlie light-induced arousal and circadian photoentrainment intersect at the l-LNvs and then segregate (Fig. 6D).
The intersectional methods described here should facilitate further study of Drosophila neurons and circuits affecting vigilance, sleep, and diurnal activity as well as other fly behaviors. They also provide a substantial advance over previous approaches, because 30% to ≈80% of flies showed GFP or hid expression in a subset of l-LNvs. We suspect that the LexA system generates higher flippase levels. As an additional motivation to generate these methods, we found that heat-shocking 1st- or 2nd-instar larvae at 37 °C for 1 h (standard heat-shock flip procedure) resulted in ≈10% of adult flies with fewer s-LNvs and arrhythmic behaviors in constant darkness (data not shown). This indicates some residual damage to adult neuronal circuits and suggests that other sensitive behavioral assays might similarly benefit from the absence of larval heat shock. Moreover, the universal tub>stop>Gal80 allows use of well-characterized Gal4 and UAS lines, which differs from the Gal4 split technique (26). Last, the use of mild-temperature activation and dTRPA1 should be of further use for other behavioral and sleep studies in Drosophila (36).
Fly sleep is now defined as 5 min of nonmovement (32, 34). Although we mostly assayed locomotor activity, we always obtained parallel and opposite results measuring sleep (data not shown). Although we favor a direct stimulatory effect of the l-LNvs on arousal and locomotor activity, we cannot exclude a direct inhibitory effect on sleep neurons. Thus, additional assays are needed to rigorously distinguish between (i) a direct stimulating effect of l-LNv firing on arousal circuits and a secondary inhibitory effect on sleep and (ii) a direct inhibitory effect of l-LNv firing on sleep promoting neurons and a secondary effect on arousal and activity.
Because of this ambiguity, the arousal–sleep circuit downstream of l-LNvs is unknown. We speculate that l-LNvs promote the neural activity of CC, a higher center for locomotor behavior (4). This is because the EB of the CC probably expresses the PDF receptor (PDFR) (Fig. 6D) (B. Lear, L. Zhang, and R. Allada, unpublished work; and 36). We therefore propose that l-LNv firing regulates the activity of the EB through PDF signaling. Although we do not detect direct projections from l-LNvs to these regions (data not shown), secreted PDF may still be able to reach EB PDFR. Because l-LNvs increase firing rate in response to light (17), PDF secretion from these cells may be light-regulated. This is consistent with the fact that both PDF null and PDF-receptor hypomorphic mutants showed increased daytime sleep (36). Although the s-LNvs may participate in the communication between l-LNvs and other brain regions, the R6 driver results (Fig. 5A) make us favor a divergence between the l-LNvs and the rest of the circadian circuitry for light-mediated arousal (Fig. 6D).
Light-mediated PDF release from the LNvs may be relevant to the late-night phase shift. Indeed, pdfGal4:UAS-Cry rescue of cryb phase shifting was most effective in the late night, and CRY levels in the l-LNvs normally reach a peak in the late night (9, 25). Although shaggy (SGG) overexpression with timGal4 led to a reduced phase delay at ZT15 as well as a reduced phase advance at ZT21, addition of the pdfGal80 transgene rescued only the ZT21 phase advance (8). This is also consistent with a circadian role of l-LNvs at dawn (Fig. 6 B and C) and a broader circuit view of circadian entrainment: The l-LNvs are directly photosensitive in the late night and signal through PDF receptors. Although the precise expression pattern of PDFR in different neurons remains unknown, many clock neurons show responses to PDF (27). Therefore, the light information perceived by l-LNvs may be transmitted to the clock network by PDF and through routes that may even include some nonclock cells. Another complication is that CRY expression by tim-Gal4:UAS-cry;pdfGal80 fully rescued the phase response curve of cryb flies, which was interpreted to suggest that only E cells are directly photoresponsive (8). However, it is possible that the strong CRY expression driven by timGal4 was not completely suppressed by pdfGal80 in the LNvs, especially in the late night when CRY reaches peak levels. The neural basis of Drosophila circadian photoreception therefore remains an important topic for future investigation.
The dual functions of l-LNvs in light-arousal and phase resetting ensure that sleep–wake patterns and the circadian clock are both phase-locked to lights-on at dawn. Similar neural mechanisms may be used to synchronize behavioral phase and the internal clock to lights-off at dusk, i.e., clock cells anatomically distinct from l-LNvs may promote sleep as well as reset the internal clock of pacemaker cells to ZT12 at dusk. Despite indications that some E cells may be important for dusk photoreception (8), these cells and circuits remain unknown. A dual photoreceptor paradigm for circadian photoreception also complements the classic 2-oscillator model of Pittendrigh and Daan (1976) as previously described in Drosophila (6–8, 11, 12).
Materials and Methods
Fly Stocks.
Standard medium was used to rear flies. Twelve-hour LD cycles and 23 °C to 25 °C were used to raise flies. w;c929GAL4/cyo was used to label peptidergic neurons expressing 1 of the 2 Drosophila α-amidation enzymes PHM (18). w;UAS-NaChBacGFP, w;UAS-dTrpA1, or w;UAS-kir2.1 was used to increase or decrease the excitability of neurons (20, 28). Transgenic flies carrying w;UAS-NLSGFP or yw;UAS-dORKΔNC1GFP, a nonconducting K+ channel (29), were used as control. pdfGal80 was used to suppress the expression of Gal4-UAS system in PDF+ neurons (LNvs) (12). To specifically label l-LNvs, yw;Tub>GAL80> was used (kindly provided by Gary Struhl (Columbia University, New York, NY)). We also generated yw;PDFLexA and yw;;LexAop-FLP fly lines to induce the expression of flippase in PDF+ neurons (LNvs). To verify the expression of flippase induced by LexA-LexAop system, we assayed GFP staining in PDF+ LNvs in yw;UAS-mCD8GFP/PDFLexA; Tub>CD2,y+>GAL4/LexAop-FLP flies (30). R6Gal4 labels s-LNvs and other nonclock neurons (24). cry39Gal4 or cry13Gal4 both label l-LNvs, s-LNvs, the 5th LNvs, LNds, and some of the DN1s (12, 16). cry39Gal4 also labels some of the DN3s.
Generation of New Transgenic Fly Lines.
First, the 2.4-kb Pdf promoter (12) was cloned into pCasPeR3 between BamHI and BglII sites. A XbaI site was added to the 3′ end of LexAVP16-SV40 3′UTR by PCR from pBS-LexAVP16 plasmid (22). The PCR fragment was then subcloned into the XbaI site of the pBS vector. LexAVP16-SV40 was then released by NotI digestion and inserted into the NotI site in pCaspeR3, downstream of Pdf promoter. Second, the coding region of flippase was cloned into pLOT vector between NotI and BglII sites (pLexAop-FLP) (22). Third, to construct the UAS>CD2, y+stop>hid plasmid, the coding region of a cell death gene, hid, was PCR amplified by oligos hid5′Xba and hid3′Spe. The PCR fragment was subcloned into the XbaI site of the pUAST vector to generate pUAST-hid. The NheI fragment containing the >CD2, y+stop> cassette was released from plasmid FC15 and was inserted into the XbaI site of the pUAST-hid. The plasmids were used to generate transgenic flies (yw background) through standard embryo microinjection procedures (BestGene). Flies bearing 1 copy of each of the transgenes were generated and verified for their ability to induce the expression of flippase by assaying GFP staining in PDF+ LNvs in yw;UAS-mCD8GFP/PDFLexA;LexAop-FLP/tub>y+>GAL4 flies (data not shown).
Genetic Intersectional Method.
The combination of GAL4-UAS/GAL80, LexA-LexAop expression system with the FLP/FRT system in the same fly allows GAL4-mediated expression only in cells that constitute the restricted overlap between the Gal4 and LexA patterns (the logic “AND” gate) (31) (Fig. 2 and Fig. 3A). Specifically, Gal4-UAS expression is ubiquitously suppressed by GAL80, which is driven by a tubulin promoter. GAL80 proteins suppress the GAL4-induced expression with no effect on LexA-induced expression. The LexA-expression system produces FLP, which in turn binds to FRT sites (>) flanking the Gal80 coding region and induces loss of Gal80. After the GAL80 proteins are completely depleted, the expression of UAS driven by GAL4 will occur.
To generate flies with fewer l-LNvs as shown in Fig. 4A, we combined the c929Gal4 and pdf LexA drivers with flippase (FLP/FRT) to express the cell death gene hid in a subset of l-LNvs. Specifically, the UAS-hid transgene contains FRT sites that flank a stop codon (UAS> CD2, y+stop >hid); therefore these flies normally do not translate hid. The FLP is expressed in LNvs and causes the FRT sites to undergo recombination. This leads to a permanent loss of the CD2, y+stop region, which allows for translation of UAS-hid in some of the l-LNvs and their progeny (Fig. 4A). This should then lead to a reduction in l-LNv numbers. Indeed, ≈74% of the flies contained only 2–4 l-LNvs rather than the normal 10 based on PDF staining. These flies will be referred as l-LNv>hid.
Behavioral Analysis.
Individual flies were housed separately in 65 × 5-mm glass tubes (Trikinetics) containing 5% agarose with 2% sucrose. Flies (0- to 3-day-old) were collected and entrained under standard LD conditions, 12-h light phase followed by 12-h dark phase, for 5–8 days. The locomotor activity pattern of each fly was monitored by an automated method (DAM System; Trikinetics), and the behavioral data from day 2 were collected and analyzed by using MATLAB software. To analyze activity, data were collected with 30-min bin, and mean activity was generated by using MATLAB software. For sleep analysis, data from days 6–8 were collected by using 1-min bin. The sleep-like resting state is defined as no movement for 5 min (32–34). Total sleep measures the amount of sleep per 24 h, whereas maximum sleep measures the duration of the longest sleep episode in each genotype.
To test the effect of heat-induced firing by dTRPA1 channels, we entrained flies in standard LD conditions at 25 °C for 5 days, and we raised the temperature of the incubator to 30 °C at ZT12. We then monitored animal behavior for 2 days at 30 °C. This allowed us to test the acute effect of induced firing of peptidergic neurons and l-LNvs on the locomotor activity as well as the long-term effect on sleep homeostasis. After 2 days heat treatment, we lowered the temperature to 25 °C at ZT12 to test whether the effect was reversible.
The standard phase-shifting analysis was performed (8). Flies from ZT15 or ZT21 were exposed to a 10-min light pulse on the last LD cycle and then released into DD for 5 days. The average phase difference between non-light-pulsed flies and light-pulsed flies with the same genotype from day 2 to day 5 in DD was calculated. The results from at least 2 experiments were then averaged.
Immunocytochemistry.
Fly heads were fixed in 4% paraformaldehyde in 0.008% PBS-Triton X-100 for 1 h at 4 °C. Paraformaldehyde-fixed samples were washed for 1 h in 0.1% PBS- Triton X-100 at room temperature (RT) and then dissected in PBS. Fixed brains were washed twice for 10 min each in 0.5% PBS-Triton X-100 at RT and then blocked in 10% goat serum with 0.5% PBS-Triton X-100 for 1 h at RT. Brains were incubated with primary antibodies at 4 °C overnight, and then they were washed 3 times and incubated in secondary antibodies: Alexa Fluor-633, Alexa Fluor-546, and Alexa Fluor-488 conjugates (Molecular Probes) at 1:500 dilution for 1 h at RT. Brains were washed 3 times and resuspended in mounting solution (Vectashield; Vector Laboratories). Brain samples were visualized by using a Leica TCS SP2 confocal microscope.
To correlate the mosaic expression of NaChBacGFP in l-LNvs induced by pdfLexA-LexAopFLP system with the single fly behavioral data as shown in Fig. 3, locomotor activity pattern of individual flies in LD was monitored for 8 days, and individual fly brain was fixed separately and stained with goat anti-GFP antibody and mouse anti-PDF antibody. The number of GFP-positive l-LNvs in each brain was counted. In some flies, tub>Gal80> failed to completely suppress the expression of NaChBacGFP driven by c929Gal4 in 4 large peptidergic cells on the surface, thus in Fig. 3, only flies with no GFP staining outside l-LNvs were analyzed.
Supplementary Material
Acknowledgments.
We thank Drs. Paul Taghert (Washington University, St. Louis, MO) and Paul Garrity (Brandeis University) for providing the c929Gal4, R6Gal4, and UAS-dTrpA1 flies; Dr. Gary Struhl for sharing the unpublished tub>Gal80> flies and plasmids containing >CD2,y+> cassette; Dr. Rudolf Bohm (Brandeis University) for flippase cDNA and transgenic flies yw;UAS-mCD8GFP/cyo; Tub>CD2,y+>GAL4/TM6b; Dr. Tzumin Lee (University of Massachusetts, Amherst, MA) for providing pBS-LexAVP16 and pLOT plasmids; and Dr. Hermann Steller (Rockefeller University, New York, NY) for hid cDNA. yw;UAS-NaChBacGFP/cyo and w;UAS-Kir2.1GFP flies were obtained from the Bloomington Stock Center. We thank Jose Agosto and Dr. Katherine Parisky for aid with the sleep analysis; Drs. Ravi Allada and Paul Garrity and M.R. laboratory members, especially Drs. Katharine Abruzzi, Sadanand Vodala, Emi Nagoshi, Jerome Menet, and Sebastian Kadener for discussion and critical comments on an earlier version of this manuscript; E. Dougherty for assistance in confocal microscopy; and H. Felton, Marlene Bender, and Kristyna Palm for administrative assistance. This work was supported in part by National Institutes of Health Grant P01 NS044232-06 (to M.R.)
Note.
While we were preparing our manuscript, we learned that an independent study by Todd Holmes and colleagues reached a similar conclusion on the function of l-LNvs in light-mediated arousal (35).
Footnotes
This Feature Article is part of a series identified by the Editorial Board as reporting findings of exceptional significance.
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 19567.
This article contains supporting information online at www.pnas.org/cgi/content/full/0809577105/DCSupplemental.
References
- 1.Joiner WJ, Crocker A, White BH, Sehgal A. Sleep in Drosophila is regulated by adult mushroom bodies. Nature. 2006;441:757–760. doi: 10.1038/nature04811. [DOI] [PubMed] [Google Scholar]
- 2.Pitman JL, McGill JJ, Keegan KP, Allada RA. Dynamic role for the mushroom bodies in promoting sleep in Drosophila. Nature. 2006;441:753–756. doi: 10.1038/nature04739. [DOI] [PubMed] [Google Scholar]
- 3.Foltenyi K, Greenspan RJ, Newport JW. Activation of EGFR and ERK by rhomboid signaling regulates the consolidation and maintenance of sleep in Drosophila. Nat Neurosci. 2007;10:1160–1167. doi: 10.1038/nn1957. [DOI] [PubMed] [Google Scholar]
- 4.Strauss R, Heisenberg MA. Higher control center of locomotor behavior in the Drosophila brain. J Neurosci. 1993;13:1852–1861. doi: 10.1523/JNEUROSCI.13-05-01852.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pittendrigh CS. Circadian rhythms and the circadian organization of living systems. Cold Spring Harb Symp Quant Biol. 1960;25:159–184. doi: 10.1101/sqb.1960.025.01.015. [DOI] [PubMed] [Google Scholar]
- 6.Murad A, Emery-Le M, Emery P. A subset of dorsal neurons modulates circadian behavior and light responses in Drosophila. Neuron. 2007;53:689–701. doi: 10.1016/j.neuron.2007.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Picot M, Cusumano P, Klarsfeld A, Ueda R, Rouyer F. Light activates output from evening neurons and inhibits output from morning neurons in the Drosophila circadian clock. PLoS Biol. 2007;5:e315. doi: 10.1371/journal.pbio.0050315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stoleru D, et al. The Drosophila circadian network is a seasonal timer. Cell. 2007;129:207–219. doi: 10.1016/j.cell.2007.02.038. [DOI] [PubMed] [Google Scholar]
- 9.Emery P, et al. Drosophila CRY is a deep brain circadian photoreceptor. Neuron. 2000;26:493–504. doi: 10.1016/s0896-6273(00)81181-2. [DOI] [PubMed] [Google Scholar]
- 10.Stanewsky R, et al. The cryb mutation identifies cryptochrome as a circadian photoreceptor in Drosophila. Cell. 95:681–692. doi: 10.1016/s0092-8674(00)81638-4. [DOI] [PubMed] [Google Scholar]
- 11.Grima B, Chelot E, Xia R, Rouyer F. Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature. 2004;431:869–873. doi: 10.1038/nature02935. [DOI] [PubMed] [Google Scholar]
- 12.Stoleru D, Peng Y, Agosto J, Rosbash M. Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature. 2004;431:862–868. doi: 10.1038/nature02926. [DOI] [PubMed] [Google Scholar]
- 13.Helfrich-Forster C. Robust circadian rhythmicity of Drosophila melanogaster requires the presence of lateral neurons: A brain-behavioral study of disconnected mutants. J Comp Physiol A. 1998;182:435–453. doi: 10.1007/s003590050192. [DOI] [PubMed] [Google Scholar]
- 14.Helfrich-Forster C, Homberg U. Pigment-dispersing hormone-immunoreactive neurons in the nervous system of wild-type Drosophila melanogaster and of several mutants with altered circadian rhythmicity. J Comp Neurol. 1993;337:177–190. doi: 10.1002/cne.903370202. [DOI] [PubMed] [Google Scholar]
- 15.Helfrich-Forster C. The circadian system of Drosophila melanogaster and its light input pathways. Zoology (Jena) 2002;105:297–312. doi: 10.1078/0944-2006-00074. [DOI] [PubMed] [Google Scholar]
- 16.Klarsfeld A, et al. Novel features of cryptochrome-mediated photoreception in the brain circadian clock of Drosophila. J Neurosci. 2004;24:1468–1477. doi: 10.1523/JNEUROSCI.3661-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheeba V, Gu H, Sharma VK, O'Dowd DK, Holmes TC. Circadian- and light-dependent regulation of resting membrane potential and spontaneous action potential firing of Drosophila circadian pacemaker neurons. J Neurophysiol. 2008;99:976–988. doi: 10.1152/jn.00930.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taghert PH, et al. Multiple amidated neuropeptides are required for normal circadian locomotor rhythms in Drosophila. J Neurosci. 2001;21:6673–6686. doi: 10.1523/JNEUROSCI.21-17-06673.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nitabach MN, Sheeba V, Vera DA, Blau J, Holmes TC. Membrane electrical excitability is necessary for the free-running larval Drosophila circadian clock. J Neurobiol. 2005;62:1–13. doi: 10.1002/neu.20053. [DOI] [PubMed] [Google Scholar]
- 20.Hamada FN, et al. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 22.Lai SL, Lee T. Genetic mosaic with dual binary transcriptional systems in Drosophila. Nat Neurosci. 2006;9:703–709. doi: 10.1038/nn1681. [DOI] [PubMed] [Google Scholar]
- 23.Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- 24.Helfrich-Forster C, et al. Development and morphology of the clock-gene-expressing lateral neurons of Drosophila melanogaster. J Comp Neurol. 2007;500:47–70. doi: 10.1002/cne.21146. [DOI] [PubMed] [Google Scholar]
- 25.Yoshii T, Todo T, Wulbeck C, Stanewsky R, Helfrich-Forster C. Cryptochrome is present in the compound eyes and a subset of Drosophila's clock neurons. J Comp Neurol. 2008;508:952–966. doi: 10.1002/cne.21702. [DOI] [PubMed] [Google Scholar]
- 26.Luan H, Peabody NC, Vinson CR, White BH. Refined spatial manipulation of neuronal function by combinatorial restriction of transgene expression. Neuron. 2006;52:425–436. doi: 10.1016/j.neuron.2006.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shafer OT, et al. Widespread receptivity to neuropeptide PDF throughout the neuronal circadian clock network of Drosophila revealed by real-time cyclic AMP imaging. Neuron. 2008;58:223–237. doi: 10.1016/j.neuron.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nitabach MN, et al. Electrical hyperexcitation of lateral ventral pacemaker neurons desynchronizes downstream circadian oscillators in the fly circadian circuit and induces multiple behavioral periods. J Neurosci. 2006;26:479–489. doi: 10.1523/JNEUROSCI.3915-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nitabach MN, Blau J, Holmes TC. Electrical silencing of Drosophila pacemaker neurons stops the free-running circadian clock. Cell. 2002;109:485–495. doi: 10.1016/s0092-8674(02)00737-7. [DOI] [PubMed] [Google Scholar]
- 30.Struhl G, Greenwald I. Presenilin-mediated transmembrane cleavage is required for Notch signal transduction in Drosophila. Proc Natl Acad Sci USA. 2001;98:229–234. doi: 10.1073/pnas.011530298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo L, Callaway EM, Svoboda K. Genetic dissection of neural circuits. Neuron. 2008;57:634–660. doi: 10.1016/j.neuron.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287:1834–1837. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- 33.Agosto J, et al. Modulation of GABAA receptor desensitization uncouples sleep onset and maintenance in Drosophila. Nat Neurosci. 2008;11:354–359. doi: 10.1038/nn2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hendricks JC, et al. Rest in Drosophila is a sleep-like state. Neuron. 2000;25:129–138. doi: 10.1016/s0896-6273(00)80877-6. [DOI] [PubMed] [Google Scholar]
- 35.Sheeba V, et al. Large ventral lateral neurons modulate arousal and sleep in Drosophila. Curr Biol. 2008;18:1537–1545. doi: 10.1016/j.cub.2008.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parisky M, et al. PDF cells are a GABA-responsive wake-promoting component of the Drosophila sleep circuit. Neuron. 2008 doi: 10.1016/j.neuron.2008.10.042. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.